Abstract
A 2-month-old female with worsening cough, respiratory distress and an abnormal chest X-ray was referred to our institution for further evaluation of suspected scimitar syndrome. She was found to have normal pulmonary venous drainage with a large patent ductus arteriosus and severe pulmonary arterial hypertension. Chest CT was suggestive of interstitial lung disease. Wedge lung biopsy revealed alveolar simplification and patchy pulmonary interstitial glycogenosis. A definitive diagnosis of Filamin A deficiency was made with genetic studies. The patient is currently showing clinical improvement on systemic glucocorticoid therapy.
Keywords: Filamin A, Interstitial lung disease, Congenital heart defect, Pulmonary interstitial glycogenosis, Pulmonary arterial hypertension, Patent ductus arteriosus
1. Introduction
The etiology of early onset respiratory distress in term infants includes both congenital and hereditary diseases. The diagnosis of respiratory distress in infants can be challenging given the complex relationship between the respiratory and cardiovascular systems with pathologies that frequently coexist. Pulmonary complications and symptoms of congenital heart defects (CHD) occur via structural impact on the airways, pathophysiological mechanisms leading to increased lung water, and/or significant pulmonary disease [1]. Congenital heart defects are common, affecting approximately 7 infants per 1000 live births [1]. When evaluating full term infants with respiratory distress, congenital heart defects are frequently considered as the initial etiology in the differential diagnosis. Childhood interstitial lung disease (ChILD) is a heterogeneous group of respiratory disorders and is a relatively rare cause of neonatal respiratory distress. A definitive diagnosis of these disorders can be challenging, even with tissue pathology.
2. Case presentation
This patient is a female infant born at 39 3/7 weeks gestation after an uneventful pregnancy via spontaneous vaginal delivery as a first child. Birth weight was 2466 gm with Apgar scores 8 and 9 at 1 and 5 minutes, respectively. She was discharged home on day 3 of life with a documented respiratory rate of 36 and a normal lung exam. Parents reported she had an abnormal breathing pattern noted very early in life with mild intermittent labored respirations and retractions. She developed a chronic, wet cough at 1 month of age and failure to thrive (FTT). A transthoracic echocardiogram at the referring hospital revealed a large patent ductus arteriosus with intermittent bidirectional shunting, a secundum atrial septal defect with left to right shunting, associated left atrial, right atrial and right ventricular enlargement, and mild to moderate pulmonary insufficiency with right ventricular hypertrophy. A chest x-ray (Fig. 1a) showed findings suggestive of scimitar syndrome with a semi-circular density at the medial right lung base and normal right lung size. The infant was transferred to our institution at 2 months of age because of worsening respiratory distress with a supplemental oxygen requirement of 2 liter per minute (lpm) via nasal cannula. A cardiac catheterization was performed, which revealed normal pulmonary venous return to the left atrium, (ruling out scimitar syndrome), and moderate to severe pulmonary arterial hypertension (PAH) with near-systemic pulmonary artery pressures secondary to a large patent ductus arteriosus (PDA). She had a moderate elevation in pulmonary vascular resistance with mild pulmonary vasoreactivity to 100% oxygen and inhaled nitric oxide. Given these findings, she was placed on hemodynamic support with milrinone, and treated with sildenafil. A thorough infectious disease evaluation was negative for bacterial or viral infections. Head ultrasound (Fig. 1b) revealed squared frontal horns of the lateral ventricles concerning for heterotopic gray matter. She underwent a flexible bronchoscopy which revealed mild left lower lobe bronchomalacia. High resolution chest computed tomography (HRCT) (Fig. 1c) showed diffuse pulmonary hyperinflation, pruning of the peripheral pulmonary vasculature, and patchy areas of atelectasis. This pattern of findings is seen in interstitial lung disease and is most consistent with Filamin A deficiency.
Fig. 1.
Imaging studies (a) Chest radiograph with a curvilinear density in the medial right lower lung (arrow) suggestive of scimitar syndrome. (b) Coronal head ultrasound through the lateral ventricles shows squaring of the frontal horns of the lateral ventricles (arrows) consistent with nodular heterotopia. (c) Coronal reconstruction HRCT with diffuse hyperinflation and pruning of the peripheral vasculature (black arrows) as well as patchy atelectasis (white arrows). (d) Axial T2 TSE brain MRI image through the lateral ventricles. This image shows multiple foci of subependymal gray matter heterotopia (arrows).
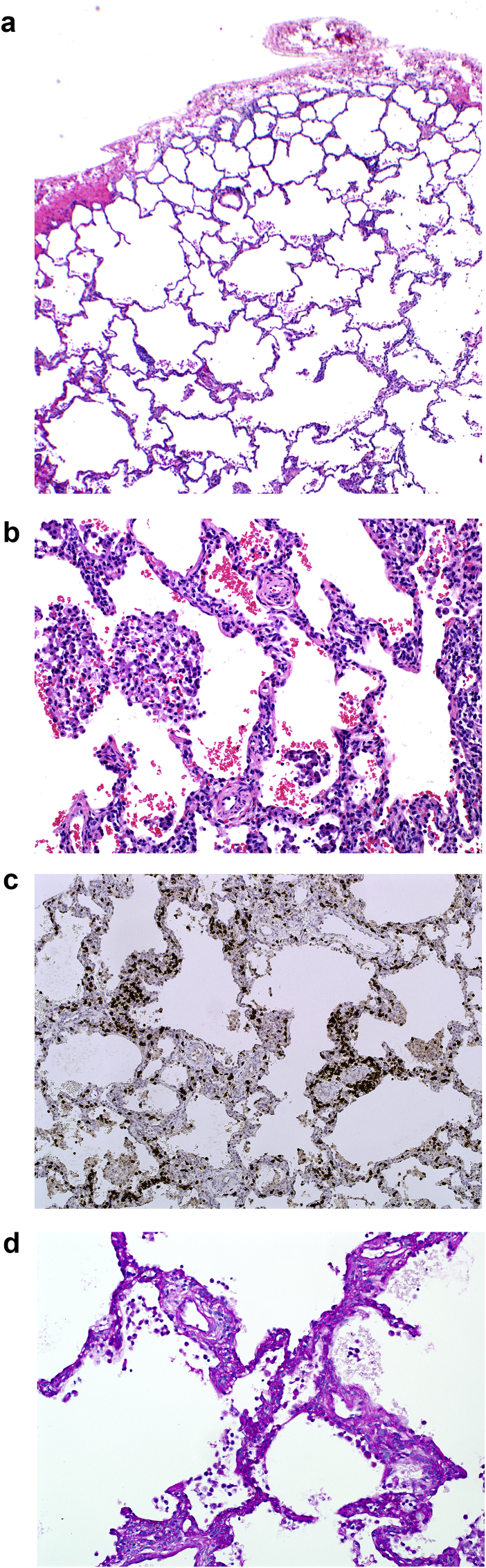
A wedge lung biopsy was done via video-assisted thoracoscopic surgery. Pathologic examination showed moderate alveolar simplification and hyperinflation (Fig. 2a), moderate-severe muscularization of intralobular arterioles (Fig. 2b), mild interstitial lymphocytic pneumonitis (Fig. 2c) and mild patchy pulmonary interstitial glycogenosis (Fig. 2d).
Fig. 2.
Wedge lung biopsy (a) Alveolar simplification. (b) Moderate-severe muscularization of intralobular arterioles. (c) Immunostain for CD3 showing interstitial lymphocytic pneumonitis. (d) PAS stain positive for pulmonary interstitial glycogenosis.
Since pathology results were suggestive of secondary changes in the lungs, the differential diagnosis at that point included primary lung disease vs. lung involvement secondary to CHD. Following stabilization of her respiratory and cardiovascular status, partial closure of the PDA was done to reduce the risk of acute increase in afterload. During the procedure, the surgeon visually identified microbullous disease.
Definitive diagnosis was made based on microarray-based comparative genomic hybridization, which revealed a frameshift mutation in the FLMNA gene, encoding the filamin A protein. The heterozygous mutation, c.2020_2021delAG (p. Arg6741GlyfsX41), resulted in a deletion of 2 bases (A and G) at codon 2020_2021 causing the reading frame to shift at exon 13 in one of the X chromosomes.
Magnetic resonance imaging (MRI) of the brain was performed after the diagnosis of Filamin A protein deficiency was made. Brain MRI (Fig. 1d) showed nodular thickening with signal intensity of gray matter along the lateral margins of the lateral ventricles, confirming the suspected subependymal gray matter heterotopia. Head magnetic resonance angiography (MRA) demonstrated no definite aneurysm or stenosis of the major intracranial arteries and a neck MRA demonstrated patent major cervical arteries. An MRI of the lumbar spine was normal without evidence of spinal dysraphism.
She was not able to take adequate oral intake and due to poor weight gain, she underwent gastrostomy tube placement at 4 months of age. She was sent home with gastrostomy tube feedings and supplemental oxygen via nasal cannula. Her medications included sildenafil, captopril, ipratropium nebulizations and pantoprazole. One month after discharge she was readmitted with increasing respiratory distress and hypercarbia, and was treated with intravenous methylprednisolone 10mg/kg/dose for 3 days. She responded quickly to the therapy with clinical improvement in her tachypnea and respiratory distress, decreased oxygen requirements and improvement in blood gases. Her pCO2 on capillary blood gases was 57 mmHg before the treatment and decreased to 45 mmHg after the treatment.
The patient is currently 12 months old and has been treated with supplemental oxygen at home, 0.25–0.5 lpm via nasal cannula, as well as sildenafil, captopril, chlorothiazide, and nebulized ipratropium. Due to her response to the systemic steroids, she was started on high dose budesonide 1 mg twice daily empirically. She has been receiving monthly high-dose intravenous steroids and prednisolone 3 mg daily. She is taking some purees by mouth and continues her gastrostomy tube feedings and has improved with her developmental milestones.
3. Discussion
We present the clinical, radiological and histopathological findings of a female infant presenting with hypoxemia, respiratory distress and FTT in early infancy. She was initially thought to have scimitar syndrome based on CXR findings. A more detailed evaluation revealed moderate to severe pulmonary arterial hypertension, a large PDA, and an abnormal chest HRCT. Pathologic examination of a wedge lung biopsy showed alveolar simplification and other changes initially attributed to congenital heart disease, but in retrospect, compatible with effects of filamin A deficiency. The definitive diagnosis of filamin A deficiency was made by genetic testing.
We believe that there are multiple interesting aspects of this case: 1) Initial evaluation with CXR mimicked scimitar syndrome, 2) Cardiac evaluation and lung biopsy findings appeared to be consistent with primary CHD, 3) Chest CT played an important role in suspecting and testing for filamin A deficiency, 4) Genetic studies played an indispensable role in diagnosing filamin A deficiency in this case, and 5) There may be a group of patients with Filamin A deficiency with lung involvement who respond to systemic glucocorticoid treatment.
Scimitar syndrome is a variant of partial anomalous pulmonary venous connection in which part or the entire right lung is drained by right pulmonary veins that connect anomalously to the inferior vena cava or portal veins [2]. The affected lung and its associated airways, which are drained by the scimitar vein, are often hypoplastic or have unusual bronchial or vascular distribution patterns. Chest radiography may show an asymmetrically small right lung and a curvilinear density representing these veins as they course below the diaphragm, giving the characteristic “scimitar” appearance [3]. At the time of diagnosis, most affected infants have pulmonary hypertension [4]. Our patient had respiratory distress, progressive hypoxemia, pulmonary hypertension, and a density at the medial right lung base on CXR similar to scimitar syndrome. As noted above, the possibility of scimitar syndrome was eliminated by visualization of normal pulmonary venous drainage on cardiac catheterization.
The primary abnormality on lung biopsy in our patient was diffuse alveolar enlargement and simplification, which is indicative of poor intrauterine or postnatal lung growth [5]. This non-specific finding may be associated with prematurity, pulmonary hypoplasia, congenital heart disease (including PDA), and chromosomal syndromes. The factors that result in alveolar simplification in chromosomal syndromes and/or CHD are not completely understood, but it is recognized that some children with these disorders have enlarged alveoli despite term gestation and lack of risk factors for pulmonary hypoplasia [5].
The pulmonary arteriopathy found in lung biopsy specimens correlates with PAH, and is characterized by prominent muscularization of intralobular arterioles [6]. PAH can be diagnosed at any age [7]. It is characterized by progressive proliferation and remodeling of the pulmonary vasculature, causing increased pulmonary vascular resistance that ultimately leads to right ventricular failure [8]. Pediatric PAH is associated with a variety of underlying diseases and causes. Overall, about half the children with confirmed pulmonary hypertension have a history of CHD [9]. Based on the findings in our patient's biopsy, it was thought that these changes may be secondary to both alveolar simplification and pulmonary over circulation from the left to right shunt caused by the PDA. Primary PAH was another possible diagnosis.
ChILD represents a heterogeneous group of respiratory disorders that are mostly chronic and associated with high morbidity and mortality [10]. Typical features of ILD include the presence of diffuse opacities on chest radiography, and abnormal pulmonary function tests with evidence of a restrictive ventilatory defect (in older children) and/or impaired gas exchange [11]. A recent guideline, preferred the term diffuse lung disease (DLD) since in childhood, the term “interstitial” lung disease may be misleading, as some diseases are considered ILD based upon similarities in the clinical presentation and diagnostic evaluation, even though the primary pathology may occur outside of the interstitium [12]. Changes in nomenclature and classification in the last decade in this group of early childhood lung diseases reflect rapid progression in our understanding of these conditions with development of new diagnostic tools from CXR to chest HRCT, tissue diagnosis and more recently more frequent use of genetic studies in clinical practice. In our patient, chest HRCT was the most helpful diagnostic tool pointing towards a primary lung disease and specifically Filamin A deficiency [13].
Pulmonary interstitial glycogenosis (PIG) was first described by Canakis et al. in 2002 [14]. Based on radiological findings, PIG may occur as an isolated condition (diffuse PIG), or associated with an underlying lung growth disorder (patchy PIG) [10]. PIG is well described in the setting of CHD [15,16]. Patchy PIG is a common secondary reactive process associated with alveolar simplification and pulmonary hypertension. Patients with PIG typically have a favorable outcome and have responded to systemic glucocorticoid treatment. Because glucocorticoids are felt to accelerate alveolar remodeling and surfactant production in the early neonatal period, the anecdotal benefit of short-term steroid administration in PIG may be attributable to this mechanism [15]. We can speculate that our patient's response to systemic steroids may be related to coexistent PIG, although it was a relatively minor component of disease histologically. A beneficial effect specifically related to Filamin A deficiency cannot be excluded.
The FLNA gene encodes the actin cross-linking protein filamin A. Filamin A gene mutations are the most common cause of isolated periventricular nodular heterotopia [17], characterized by nodules of neurons ectopically placed along the walls of the lateral ventricles [18]. Periventricular heterotopia is usually diagnosed when patients present with a seizure disorder and neuroimaging studies demonstrate heterotopic gray matter in a periventricular distribution.
FLNA mutations have been associated with severe diffuse lung disease, tracheobronchomalacia, and severe pulmonary hypertension [[19], [20], [21], [22], [23], [24]]. Masurel-Paulet first reported a male with periventricular nodular heterotopia and severe lung disease characterized by recurrent respiratory infections, bilateral lung atelectasis, lung cysts, tracheobronchomalacia, pulmonary arterial hypertension, asthma and long-term oxygen dependence starting at age of 3 months; histology of resected lung showed pan-pulmonary emphysema with marked reduction of bronchial cartilage [21]. De Wit reported the first affected female patient with periventricular nodular heterotopia, cardiovascular abnormalities (secundum atrial septal defect, coarctation of the aorta and mild aortic regurgitation), and pulmonary disease consisting of lobar emphysema of the right middle pulmonary lobe with severe malacia of the right sided bronchus intermedius. Lung pathology showed emphysema without inflammation [20]. Chest HRCT is characterized by marked hyperinflation involving multiple lobes of the lung, and a paucity of peripheral vascular structures similar to emphysema [13]. Our patient had typical HRCT findings, which facilitated the diagnosis of Filamin A deficiency. FLNA mutations are inherited in an X-linked dominant manner, with high perinatal mortality in affected male patients, whereas in female patients the prognosis depends on the phenotypic presentation and severity of the associated cardiovascular abnormalities [23]. At this time, case reports of Filamin A deficiency with lung involvement have involved different mutations and there has not been any phenotype-genotype associations reported. Patients with FLNA mutation may also have cardiovascular abnormalities including PDA, valvular disease, aortic root dilatation, aortic aneurysm, aortic coarctation, diffuse ectasia of aortic branch vessels, ventricular septal defect, atrial septal defect, and dilation of the great vessels [19,23]. Our patient had a large PDA with bidirectional shunting and secondary pulmonary hypertension, which complicated the diagnosis of primary lung disease. In mouse models, it was recently shown that a specific deletion of FLNA in smooth muscle cells of adult mice leads to significantly lowered blood pressure, together with a decrease in pulse pressure. However, both the aorta and carotid arteries showed a major outward hypertrophic remodeling, normally occurring in hypertensive conditions [25]. The role of Filamin A in development of pulmonary artery remodeling needs to be investigated.
Filamin A mutations are also associated with connective tissue manifestations: easy bruising, joint hypermobility and to a lesser extent, cutaneous anomalies including thin translucent skin and cutis laxa. Vascular dilatation (mainly the aorta), joint hypermobility and variable skin findings are also associated with X-linked periventricular nodular heterotopia, with seizures suggesting that this might represent a separate syndrome allelic to X-linked periventricular nodular heterotopia, termed Ehlers-Danlos syndrome-periventricular heterotopia variant [26]. Our patient has some evidence of joint hypermobility.
In reviewing the limited case reports, there is no specific treatment recommendation that has been shown to be beneficial for patients with Filamin A deficiency associated lung disease, including systemic steroids [[19], [20], [21]]. Some affected girls have eventually required lung transplantation, with clinical and morphologic features mimicking end-stage bronchopulmonary dysplasia with hyperinflation. Given that the primary problem in Filamin A deficiency is a structural abnormality without inflammation, similar to inherited disorders of connective tissue such as Ehlers-Danlos syndrome or Marfan syndrome, systemic steroids have not been part of the treatment of lung disease associated with Filamin A deficiency. Filamins are large, multi-domain actin cross-linking proteins with diverse functions. Besides regulating the actin cytoskeleton, they serve as important links between the extracellular matrix and the cytoskeleton by binding cell surface receptors, functioning as scaffolds for signaling proteins, and binding several other cytoskeletal proteins that regulate cell adhesion dynamics [27]. Filamin A is also required for T cell activation and plays a role in interleukin production and in inflammatory signaling [28]. The role of Filamin A in the immune response needs to be further investigated. An extensive immune evaluation in our patient did not reveal any abnormality. Our patient is the first report of a therapeutic response to high dose systemic steroids in respiratory involvement with Filamin A deficiency. We believe that this treatment may be beneficial in the future in patients with Filamin A deficiency and lung involvement, likely a consequence of the patchy PIG and mild inflammation observed in our patient. Given the severity and progression of her clinical status, we chose to use high dose steroids (including daily and inhaled steroids between pulse steroid doses).
Finally, in our patient, chest HRCT findings were more helpful in the diagnosis than was tissue sampling. Clinically, filamin A deficiency should be considered in any young female with unexplained heterogeneous pulmonary over inflation on CT, especially when associated with cardiovascular anomalies or seizure disorder. Similarly, pathologists evaluating pediatric lung biopsies should consider the diagnosis of filamin A deficiency in any young female with alveolar simplification and distension (“emphysematous” changes) in the setting of patent ductus arteriosus or other cardiac anomalies. Correlation of the pathology with chest CT is essential to recognize the characteristic imaging features and to suggest the correct diagnosis. Presumptive diagnosis can be established by further demonstration of nodular periventricular heterotopias on brain imaging, but definitive diagnosis must be established by sequencing the FLNA gene. Although many patients with ChILD require tissue sampling, in those patients with radiologic suspicion of filamin A deficiency, particularly with periventricular nodular heterotopia on brain imaging, genetic testing may obviate the need for lung biopsy.
Financial disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Funding
No external funding.
Acknowledgements
The authors thank Drs. Mark Luquette, Faqian Li and Emilian Racila from Department of Laboratory Medicine and Pathology at the University of Minnesota for their support in preparation and interpretation of pathology slides.
References
- 1.Healy F., Hanna B.D., Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13(1):10–15. doi: 10.1016/j.prrv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Bo I., Carvalho J.S., Cheasty E., Rubens M., Rigby M.L. Variants of the scimitar syndrome. Cardiol Young. 2016;26(5):941–947. doi: 10.1017/S1047951115001651. [DOI] [PubMed] [Google Scholar]
- 3.Woodring J.H., Howard T.A., Kanga J.F. Congenital pulmonary venolobar syndrome revisited. Radiographics. 1994;14(2):349–369. doi: 10.1148/radiographics.14.2.8190958. [DOI] [PubMed] [Google Scholar]
- 4.Najm H.K., Williams W.G., Coles J.G., Rebeyka I.M., Freedom R.M. Scimitar syndrome: twenty years' experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161–1168. doi: 10.1016/S0022-5223(96)70129-0. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 5.Dishop M.K. New entities and diagnostic challenges in pediatric lung disease. Surg Pathol Clin. 2010;3(3):495–513. doi: 10.1016/j.path.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Tuder R.M., Marecki J.C., Richter A., Fijalkowska I., Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28(1):23–42. doi: 10.1016/j.ccm.2006.11.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beghetti M., Berger R.M. The challenges in paediatric pulmonary arterial hypertension. Eur Respir Rev. 2014;23(134):498–504. doi: 10.1183/09059180.00007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert M., Morrell N.W., Archer S.L., Stenmark K.R., MacLean M.R., Lang I.M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Berger R.M., Beghetti M., Humpl T., Raskob G.E., Ivy D.D., Jing Z.C. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379(9815):537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spagnolo P., Bush A. Interstitial lung disease in children younger than 2 years. Pediatrics. 2016;137(6) doi: 10.1542/peds.2015-2725. [DOI] [PubMed] [Google Scholar]
- 11.Clement A., Nathan N., Epaud R., Fauroux B., Corvol H. Interstitial lung diseases in children. Orphanet J Rare Dis. 2010;5:22. doi: 10.1186/1750-1172-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurland G., Deterding R.R., Hagood J.S., Young L.R., Brody A.S., Castile R.G. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188(3):376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semple T., Owens C.M. The radiology of diffuse interstitial pulmonary disease in children: pearls, pitfalls and new kids on the block in 2015. Radiol Med. 2016;121(5):352–361. doi: 10.1007/s11547-015-0599-9. [DOI] [PubMed] [Google Scholar]
- 14.Canakis A.M., Cutz E., Manson D., O'Brodovich H. Pulmonary interstitial glycogenosis: a new variant of neonatal interstitial lung disease. Am J Respir Crit Care Med. 2002;165(11):1557–1565. doi: 10.1164/rccm.2105139. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch G.H., Young L.R. Histologic resolution of pulmonary interstitial glycogenosis. Pediatr Dev Pathol. 2009;12(6):475–480. doi: 10.2350/08-12-0575.1. [DOI] [PubMed] [Google Scholar]
- 16.Radman M.R., Goldhoff P., Jones K.D., Azakie A., Datar S., Adatia I. Pulmonary interstitial glycogenosis: an unrecognized etiology of persistent pulmonary hypertension of the newborn in congenital heart disease? Pediatr Cardiol. 2013;34(5):1254–1257. doi: 10.1007/s00246-012-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapham K.R., Yu T.W., Ganesh V.S., Barry B., Chan Y., Mei D. FLNA genomic rearrangements cause periventricular nodular heterotopia. Neurology. 2012;78(4):269–278. doi: 10.1212/WNL.0b013e31824365e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carabalona A., Beguin S., Pallesi-Pocachard E., Buhler E., Pellegrino C., Arnaud K. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A. Hum Mol Genet. 2012;21(5):1004–1017. doi: 10.1093/hmg/ddr531. [DOI] [PubMed] [Google Scholar]
- 19.Eltahir S., Ahmad K.S., Al-Balawi M.M., Bukhamsien H., Al-Mobaireek K., Alotaibi W. Lung disease associated with filamin A gene mutation: a case report. J Med Case Rep. 2016;10:97. doi: 10.1186/s13256-016-0871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wit M.C., Tiddens H.A., de Coo I.F., Mancini G.M. Lung disease in FLNA mutation: confirmatory report. Eur J Med Genet. 2011;54(3):299–300. doi: 10.1016/j.ejmg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Masurel-Paulet A., Haan E., Thompson E.M., Goizet C., Thauvin-Robinet C., Tai A. Lung disease associated with periventricular nodular heterotopia and an FLNA mutation. Eur J Med Genet. 2011;54(1):25–28. doi: 10.1016/j.ejmg.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Gerard-Blanluet M., Sheen V., Machinis K., Neal J., Apse K., Danan C. Bilateral periventricular heterotopias in an X-linked dominant transmission in a family with two affected males. Am J Med Genet A. 2006;140(10):1041–1046. doi: 10.1002/ajmg.a.31197. [DOI] [PubMed] [Google Scholar]
- 23.de Wit M.C., de Coo I.F., Lequin M.H., Halley D.J., Roos-Hesselink J.W., Mancini G.M. Combined cardiological and neurological abnormalities due to filamin A gene mutation. Clin Res Cardiol. 2011;100(1):45–50. doi: 10.1007/s00392-010-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord A., Shapiro A.J., Saint-Martin C., Claveau M., Melancon S., Wintermark P. Filamin A mutation may be associated with diffuse lung disease mimicking bronchopulmonary dysplasia in premature newborns. Respir Care. 2014;59(11):e171–e177. doi: 10.4187/respcare.02847. [DOI] [PubMed] [Google Scholar]
- 25.Retailleau K., Arhatte M., Demolombe S., Jodar M., Baudrie V., Offermanns S. Smooth muscle filamin A is a major determinant of conduit artery structure and function at the adult stage. Pflugers Arch. 2016;468(7):1151–1160. doi: 10.1007/s00424-016-1813-x. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein E., Frentz S., Morgan T., Garcia-Minaur S., Leventer R.J., McGillivray G. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in Filamin A. Eur J Hum Genet. 2013;21(5):494–502. doi: 10.1038/ejhg.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppala J., Tossavainen H., Rodic N., Permi P., Pentikainen U., Ylanne J. Flexible structure of peptide-bound filamin a mechanosensor domain pair 20-21. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi K., Altman A. Filamin A is required for T cell activation mediated by protein kinase C-theta. J Immunol. 2006;177(3):1721–1728. doi: 10.4049/jimmunol.177.3.1721. [DOI] [PubMed] [Google Scholar]