Abstract
We measured the changes in the cerebrospinal fluid (CSF) flow dynamics after compression of the bilateral jugular veins using phase contrast-magnetic resonance imaging (PC-MRI). PC-MRI was performed in 10 healthy male volunteers using a 3T clinical scanner with a two-dimensional gradient echo sequence. We successfully measured the changes in CSF flow velocity using PC-MRI with and without compression of the bilateral jugular veins. The relative velocity range decreased by about 30% when the bilateral jugular veins were compressed.
Keywords: magnetic resonance imaging, phase contrast, cerebrospinal fluid, jugular vein
Introduction
Phase contrast-magnetic resonance imaging (PC-MRI) has been used to measure cerebrospinal fluid (CSF) flow dynamics.1,2 Further, several researchers have successfully measured CSF flow at the cerebral aqueduct using cine PC-MRI.3–5 When both jugular veins are compressed, which leads to a rapid increase in intracranial pressure. The increased intracranial pressure raises the pressure in the spinal canal in normal anatomical structures. The CSF pressure is increased by compression of the bilateral jugular veins. A change in the CSF pressure may change the CSF flow dynamics. Hence, we hypothesized that the changes of CSF flow velocity in the aqueducts by bilateral jugular compression (or increased CSF pressure) are measurable with PC-MRI. Multi-compartment model consisting of the brain, artery, vein, subarachnoid space and the spinal canal has been published and compared to experimental results obtained from PC-MRI measurements.6,7 Currently, little is known about the effects that mechanical change of venous system would have influence on CSF flow dynamics. Hence, the purpose of this study was to measure the changes in CSF flow velocity using PC-MRI by compressing the bilateral jugular veins.
Materials and Methods
Volunteers
This study was approved by the institutional review board, and written informed consent was obtained from all the volunteers. Ten healthy volunteers (all men) with no known stenosis in the spine, aged 24 to 56 years (mean, 35.4 years), body weights ranging from 54 to 78 kg (mean, 64.4 kg), and body mass indices ranging from 17.4 to 25.1 (mean, 21.1) were recruited.
PC-MRI techniques
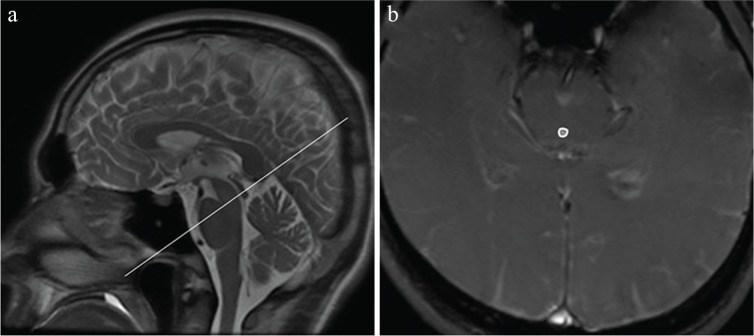
PC-MRI was performed using a 3T MRI system (Discovery 750; GE Medical Systems, Waukesha, WI, USA) with a 16-channel phased-array coil (head neck spine array coil). A midline sagittal T2 weighted scout image was obtained (Fig. 1a). The CSF velocity was defined as the peak systolic velocity in the transverse plane perpendicular to the midcollicular level (Fig. 1b) of the aqueduct, measured using the AW Volumeshare 5 software (GE Medical Systems). The acquisition parameters were: repetition time/echo time = 13.7/6.0 ms, flip angle = 20°, slice thickness = 5 mm, field of view = 20 × 20 cm with a matrix size of 256 × 198, number of slices = 1, velocity encoding parallel to slice direction = 10 cm/s, number of excitations = 1, and band width = ±15.63 kHz. Scan time depended on the volunteer’s heart rate (average scan time = 30 seconds).
Fig. 1.
Sample anatomic and velocity-encoded images. (a) Midline sagittal T2 weighted magnetic resonance scout image; the line indicates the location of the plane through the midcollicular level at the aqueduct, defining the plane of measurement for the cerebrospinal fluid flow velocity. (b) Portions of the velocity-encoded image with a region of interest placed in the aqueduct of the midbrain.
The region of interest was placed in the aqueduct of the midbrain by one radiologist (S.I.) with 8 years of experience in radiology to measure the velocity in a caudal direction (Vpeak1) and a cranial direction (Vpeak2) as absolute values.
Procedure
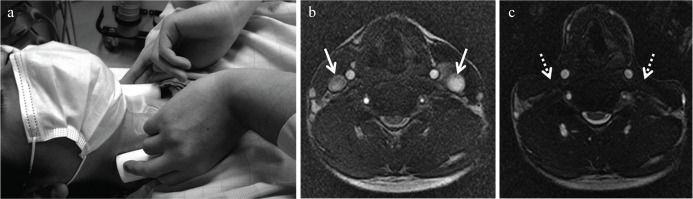
Velocity measurement of the CSF flow in the aqueduct (VMCA) was performed using the fixation devices. The devices were placed anterior to the bilateral sternocleidomastoid muscles. The bilateral jugular veins were compressed by the study volunteers themselves with the fixation devices (Fig. 2a). Before starting the MR scans, all volunteers practiced the compression technique to ensure that sufficient compression was achieved. During the practice, color Doppler echo was used to confirm that the bloodstream of the jugular vein was blocked off. We also confirmed that there were no changes in the heart rate during compression of the jugular veins.
Fig. 2.
Compression of the jugular veins. (a) Fixation devices were placed anterior to the bilateral sternocleidomastoid muscles and the jugular veins were compressed by the study volunteers themselves. (b and c) Before the acquisition of phase contrast-magnetic resonance imaging with compression, a steady-state free precession sequence (FIESTA) was acquired to confirm the absence of signals in the bilateral jugular veins. Signals in the bilateral jugular veins were noted at release (arrows); and the signals disappeared during compression (broken arrows).
We calculated the velocity range (VR) using the absolute values of peak velocity in the two directions:
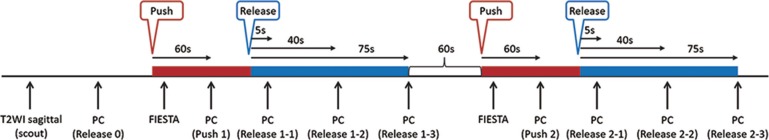
PC-MRI was repeatedly used to measure VR with and without compression of the bilateral jugular veins (VRpush and VRrelease). Before the acquisition of PC-MRI scans with compression, a steady-state free precession sequence (FIESTA) was acquired at the level of the fixation devices to confirm that signals in the bilateral jugular veins disappeared (Fig. 2b and c). The protocol of the procedures is shown in Fig. 3. Briefly, a T2 weighted image was obtained to detect the cerebral aqueduct. Next, PC-MRI was performed at rest (release) to measure VRrelease0. Volunteers then compressed their jugular vein and the absence of signals in the jugular vein was confirmed with an steady-state free precession (SSFP) sequence. Compression was maintained for more than 60 seconds, and then PC-MRI was performed again (VRpush1). The pressure was then released, and PC-MRI scans were performed at 5, 40, and 75 seconds after release (VRrelease1-1, VRrelease1-2, VRrelease1-3). After a 60-second interval, the procedure was repeated to obtain a second set of measurements (VRpush2, VRrelease2-1, VRrelease2-2, VRrelease2-3). The average VR at rest (VRrelease0, VRrelease1-3, and VRrelease2-3) was calculated for each volunteer and used to standardize the VR of each measurement, i.e., relative VR (rVR), using the following equation:
Fig. 3.
The scanning protocol. A T2 weighted image (T2WI) was obtained to detect the cerebral aqueduct. Next, phase contrast-magnetic resonance imaging (PC-MRI) was performed at rest (release) to measure VRrelease0. The absence of signals in the jugular veins after compression was confirmed with the acquisition of phase contrast-magnetic resonance imaging with compression, a steady-state free precession sequence (FIESTA). After 60 seconds of compression, a PC-MRI scan was performed (push 1). The compression was then released and PC-MRI images were obtained at 5, 40, and 75 seconds after the release (release 1-1, release 1-2, and release 1-3). After a 60-second, the procedure was repeated to obtain a second set of measurements (push 2, release 2-1, release 2-2, and release 2-3).
Statistical analysis
rVRpush1 was compared with rVRrelease1-1 and rVRrelease1-2, and rVRpush2 was compared with rVRrelease2-1 and rVRrelease2-2 using the Wilcoxon test. The JMP software (Ver. 10.0.2; SAS Institute, Cary, NC, USA) was used for the analyses. P values of < 0.05 were considered statistically significant.
Results
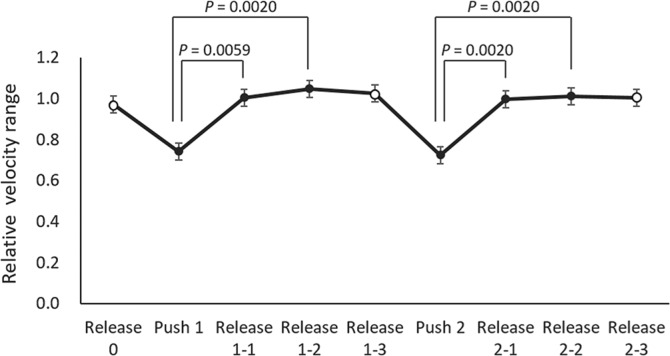
The average (± standard deviation) rVRpush1 and rVRpush2 in all volunteers was 0.74 (± 0.16) and 0.73 (± 0.17), respectively, whereas the average rVRrelease1-1, rVRrelease1-2, rVRrelease2-1, and rVRrelease2-2 was 1.00 (± 0.11), 1.05 (± 0.05), 1.00 (± 0.17), and 1.01 (± 0.11), respectively. The averages and standard errors of the relative velocity range are shown in Fig. 4. rVRpush1 was significantly lower than rVRrelease1-1 (P = 0.0059) and rVRrelease1-2 (P = 0.0020), and rVRpush2 was significantly lower than rVRrelease2-1 (P = 0.0020) and rVRrelease2-2 (P = 0.0020).
Fig. 4.
The averages and standard errors of the relative velocity range. There was no overlap between push and release. rVRpush1 was significantly lower than rVRrelease1-1 (P = 0.0059) and rVRrelease1-2 (P = 0.0020), and rVRpush2 was significantly lower than rVRrelease2-1 (P = 0.0020) and rVRrelease2-2 (P = 0.0020).
Discussion
In this study, we successfully measured the changes in CSF flow velocities after compression of the bilateral jugular veins using PC-MRI. In the 10 healthy volunteers, bilateral jugular vein compression decreased rVR by 30%.
CSF flow within the spinal canal and the cranial vault is regulated by the cardiac cycle. During systole, arterial blood flows into the cranial vault faster than venous blood exits it, yielding a net gain in intracranial and parenchymal blood volume. Complex interdependent brain motion and CSF flow accommodate this net increase in blood volume. During systole, CSF moves caudally from the ventricles and subarachnoid space into the spinal canal to accommodate the excess CSF. During CSF diastole, venous blood exits the cranial vault faster than arterial blood enters it, with a resultant net loss in intracranial blood volume and a reversal of CSF flow.2,8,9 The flow of CSF with each cardiac pulse into and out of the spinal subarachnoid space has been measured by PC-MRI.10,11 The pressure of the midbrain aqueduct may be changed by alterations of blood vessel capacity owing to heartbeats. Because the cranial capacity remains constant, a change in the CSF space capacity is caused by a change in blood vessel capacity. This volumetric change causes an increase in CSF space pressure, thus resulting in craniocaudal flow in the aqueduct of the midbrain.
The collaterals are the preferred pathway of venous drainage. Therefore, jugular compression can lead the venous drainage into the collaterals and do not necessarily decrease the venous outflow from the intracranial space. However, the CSF pressure is still increased, which is shown in a classical physical examination called Queckensted test.12 Increased CSF pressure in turn reduce the peak flow in the aqueduct, because the effect of driving force of CSF flow (pressure change in the ventricles) can be diminished by the increased pressure in the spine. This may be the reason why aqueduct CSF flow responds to jugular compression. The Valsalva maneuver is strongly related to jugular compression. A recent study using showed that the CSF flow velocity between the head and the spine decreases during the Valsalva maneuver as we observed in our study.13
There are some limitations to this study. First, we could not fully understand the flow dynamics during jugular compression; e.g. the carotid arterial flow and the collaterals. In our study, however, we ensured during jugular compression that the shape of the carotid arteries was not compressed morphologically. However, the compression might have led to mild carotid compression and less arterial flow into the brain, thus influencing the aqueduct CSF velocities.
Conclusion
We successfully measured the changes in CSF flow dynamics using PC-MRI with and without compression of the bilateral jugular veins. The relative velocity range decreased by about 30% when the bilateral jugular veins were compressed.
Footnotes
Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1.Enzmann DR, Pelc NJ. Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology 1991; 178:467–474. [DOI] [PubMed] [Google Scholar]
- 2.Nitz WR, Bradley WG, Watanabe AS, et al. Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology 1992; 183:395–405. [DOI] [PubMed] [Google Scholar]
- 3.Sharma AK, Gaikwad S, Gupta V, Garg A, Mishra NK. Measurement of peak CSF flow velocity at cerebral aqueduct, before and after lumbar CSF drainage, by use of phase-contrast MRI: utility in the management of idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg 2008; 110:363–368. [DOI] [PubMed] [Google Scholar]
- 4.Luetmer PH, Huston J, Friedman JA, et al. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2002; 50:534–543; discussion 543–544. [DOI] [PubMed] [Google Scholar]
- 5.Chiang WW, Takoudis CG, Lee SH, Weis-McNulty A, Glick R, Alperin N. Relationship between ventricular morphology and aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus. Invest Radiol 2009; 44:192–199. [DOI] [PubMed] [Google Scholar]
- 6.Loth F, Yardimci MA, Alperin N. Hydrodynamic modeling of cerebrospinal fluid motion within the spinal cavity. J Biomech Eng 2001; 123:71–79. [DOI] [PubMed] [Google Scholar]
- 7.Linninger AA, Xenos M, Sweetman B, Ponkshe S, Guo X, Penn R. A mathematical model of blood, cerebrospinal fluid and brain dynamics. J Math Biol 2009; 59: 729–759. [DOI] [PubMed] [Google Scholar]
- 8.Enzmann DR, Pelc NJ. Brain motion: measurement with phase-contrast MR imaging. Radiology 1992; 185:653–660. [DOI] [PubMed] [Google Scholar]
- 9.Quencer RM, Post MJ, Hinks RS. Cine MR in the evaluation of normal and abnormal CSF flow: intracranial and intraspinal studies. Neuroradiology 1990; 32:371–391. [DOI] [PubMed] [Google Scholar]
- 10.Zhu DC, Xenos M, Linninger AA, Penn RD. Dynamics of lateral ventricle and cerebrospinal fluid in normal and hydrocephalic brains. J Magn Reson Imaging 2006; 24: 756–770. [DOI] [PubMed] [Google Scholar]
- 11.Raksin PB, Alperin N, Sivaramakrishnan A, Surapaneni S, Lichtor T. Noninvasive intracranial compliance and pressure based on dynamic magnetic resonance imaging of blood flow and cerebrospinal fluid flow: review of principles, implementation, and other noninvasive approaches. Neurosurg Focus 2003; 14:e4. [DOI] [PubMed] [Google Scholar]
- 12.Pearce JM. Queckenstedt’s manoeuvre. J Neurol Neurosurg Psychiatr 2006; 77:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhadelia RA, Madan N, Zhao Y, et al. Physiology-based MR imaging assessment of CSF flow at the foramen magnum with a valsalva maneuver. AJNR Am J Neuroradiol 2013; 34:1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]