Abstract
Pulmonary arteriovenous malformations (PAVMs) are rare, abnormal low resistance vascular structures that connect a pulmonary artery to a pulmonary vein, thereby bypassing the normal pulmonary capillary bed and resulting in an intrapulmonary right-to-left shunt. The spectrum of PAVMs extends from microscopic lesions causing profound hypoxemia and ground glass appearance on computed tomography (CT) but with normal catheter angiographic findings to classic pulmonary aneurysmal connections that abnormally connect pulmonary veins and arteries. These malformations most commonly are seen in hereditary hemorrhagic telangiectasia (HHT). They are rarely due to secondary conditions such as post congenital heart disease surgery or hepatopulmonary syndrome (HPS). The main complications of PAVM result from intrapulmonary shunt and include stroke, brain abscess, and hypoxemia. Local pulmonary complications include PAVM rupture leading to life-threatening hemoptysis or hemothorax. The preferred screening test for PAVM is transthoracic contrast echocardiography (TTCE). CT has become the gold standard imaging test to establish the presence of PAVM. Endovascular occlusion of the feeding artery is the treatment of choice. Collateralization and recanalization of PAVM following treatment may occur, and hence long term clinical and imaging follow-up is required to assess PAVM enlargement and PAVM reperfusion.
Keywords: Pulmonary arteriovenous malformations (PAVMs), computed tomography (CT), pulmonary catheter angiogram, echocardiography, hereditary hemorrhagic telangiectasia (HHT), diagnosis
Introduction
Pulmonary arteriovenous malformations (PAVMs) are rare, low-resistance, high-flow abnormal vascular structures that connect a pulmonary artery to a pulmonary vein bypassing the normal pulmonary capillary bed and resulting in an intrapulmonary right-to-left shunt. Various other terms used for PAVMs include pulmonary arterio-venous aneurysms (PAVA), pulmonary arteriovenous fistulas, pulmonary angiomas, cavernous hemangiomas (1,2). Churton was the first to report PAVM on an autopsy study in 1897 (3). Predominantly, these lesions represent congenital malformations with exception of the extremely uncommon acquired examples (1,2). They may involve a large single sac or a plexiform mass of dilated vascular channels or consist of dilated and tortuous communications between a pulmonary artery branch and a venous tributary (2).
In this manuscript, we will review various types of PAVMs, role of various imaging modalities in their evaluation, imaging appearances and complications of various types of PAVMs. We also review the clinical manifestations, pathophysiology and screening protocol for PAVMs with brief mention of available treatment options.
PAVM anatomy, classification and pathophysiology
Most PAVMs are congenital with HHT being the predominant cause. Approximately 80% to 90% of patients presenting with PAVMs eventually manifest with HHT, whereas the remaining are sporadic cases (1,4). Other studies have reported HHT/Osler-Weber-Rendu syndrome (OWRS) to be present in 36% of patients with single PAVM and 51–88% of patients with multiple PAVMs (5,6). On the contrary, 15% to 50% of HHT patients have PAVMs (7,8). Generally, a higher incidence of PAVM is noted in HHT1 than in HHT2, determined by the type of genetic mutation (9).
Since PAVM are most commonly congenital, they commonly arise due to aberrant development of pulmonary arteries and veins from a common plexus and then undergo slow enlargement due to gradually increased intraluminal arterial blood flow related pressure effects. Sometimes, necrosis of the shunt wall can occur leading to increased shunting and pulmonary hemorrhage (10). The condition remains under-recognized until the second decade of life. It is suggested that the gradual enlargement of the PAVM occurs with age as a response to increasing flow culminating in necrosis of the vessel wall (10). Evidence is lacking on the rate of growth of PAVM and its determinants however studies have suggested that they grow during pregnancy and puberty (10).
Acquired causes of PAVM are rare and include chest surgery, trauma, actinomycosis, schistosomiasis, hepatic cirrhosis related hepatopulmonary syndrome (HPS) and metastatic carcinoma (11,12). Acquired PAVM have been reported in up to 25% of patients with post-repair for single cardiac ventricle using a Glenn shunt connecting the superior vena cava to the pulmonary artery. A potential etiology is the exclusion of admixture with hepatic venous blood thereby causing lack of hepatic factors responsible for the inhibition of PAVM development (13). Few pathological studies had shown that all PAVMs begin as plexus-like connection which at later stage for unknown etiology are converted into aneurysmal dilation (14). Catheter angiography studies, however, do not support this theory and instead suggest that many small PAVMs are ultimately responsible in future for formation of larger simple type PAVM (15).
Hereditary hemorrhagic telangiectasia (HHT)
HHT is an autosomal dominant disorders characterized by abnormalities of vascular structures involving multiple organs. Most cases of HHT are due to mutations in the ENG gene and activin type-II-like receptor kinase 1 (ACVRL1) gene causing respectively HHT type 1 and HHT type 2 diseases (16). Pathology is comprised of dilatation of post-capillary venules, telangiectasia, and arteriovenous malformations (AVMs) in increasing order of severity.
The 2011 Guidelines Working Group Consensus criteria recommend the use of the Curaçao criteria in diagnosing HHT. These clinical criteria provide probability score for HHT based on the presence of recurrent and spontaneous epistaxis, mucocutaneous telangiectasia at typical sites (lips, oral cavity, fingers, nose), visceral AVMs (pulmonary, cerebral, hepatic), spinal or gastrointestinal telangiectasia (with or without bleeding) and a family history, specifically a first-degree relative with HHT (or genetic mutation) (17). Presence of three or more of these criteria confirms the diagnosis.
Pulmonary manifestations represent the most common serious complications in HHT and consist of PAVM, pulmonary hypertension, hemoptysis, hemothorax and pulmonary embolism (9). PAVM represent the most common pattern of the pulmonary involvement to be encountered in HHT patients. Compared to isolated forms, HHT related PAVMs are often multiple, bilateral and have a slight preference for the lung bases. Paradoxical embolism in HHT either originates from de novo in situ thrombus in PAVM or septic thrombus or air embolus originating upstream of the PAVMs (18). The resulting cerebral embolic events can be catastrophic. Patients with HHT can also rarely experience spontaneous PAVM rupture than can result in massive hemoptysis or hemothorax. In HHT patients with hemoptysis and absence of PAVM, other causes such as bronchial telangiectasias best assessed with bronchoscopy, and pulmonary embolic disease best assessed with CT pulmonary angiography should be considered (19).
Pulmonary AVM clinical features and complications
Physiologic consequences depend on the degree of right-to-left shunt. These patients can be asymptomatic, but become symptomatic with increasing degree of mixing of deoxygenated blood through the PAVM causing hypoxemia, fatigue, dyspnea, and cyanosis. Pulmonary emboli (Figure 1) can lead to paradoxical systemic embolism due to right to left shunting through the PAVM. Complications of paradoxical systemic embolization include stroke and brain abscess (4). Strong association has been reported between a single PAVM with feeding artery (FA) diameter of >3 mm and various neurologic manifestations like infarction, abscess and seizure. The prevalence is greater for patients with multiple PAVM, suggesting increased predisposition for paradoxical embolization with a greater number of malformations (4).
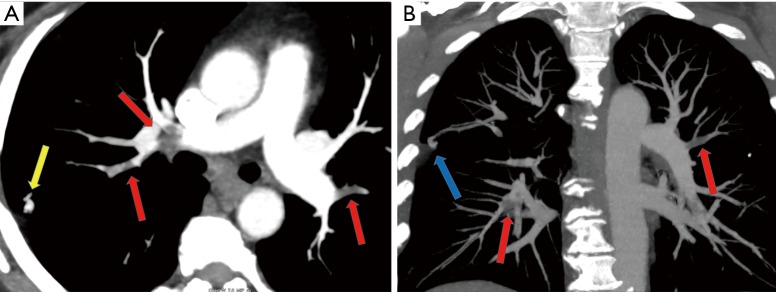
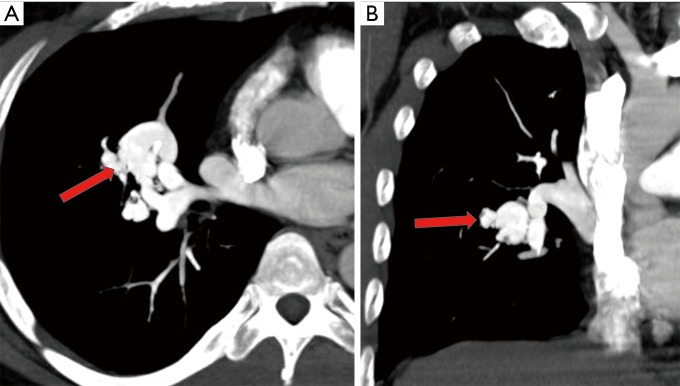
Figure 1.
Axial (A), coronal (B) MIP images demonstrate a small simple right upper lobe segmental pulmonary arteriovenous malformation (PAVM) (yellow and blue arrows) in a 58-year-old man with gastric cancer. Note multiple acute pulmonary emboli (red arrows) involving bilateral upper lobe pulmonary arteries which are predisposing factor for systemic embolism in the presence of PAVM.
A higher percentage of patients (70%) with diffuse PAVM have a history of brain ischemia or abscess, suggesting that the prevalence of paradoxical embolization may depend on the total surface area of all the venous–arterial channels that are patent for emboli to traverse (20). Overall, 30% of patients with pulmonary AVMs reported history of stroke, 10% of brain abscess, and 8–10% of pulmonary hemorrhage or hemothorax (21). Pulmonary arterial hypertension (PAH) is seen with higher frequency in families with HHT owing to a genetic mutation in the same family of proteins. Coexistence of PAVM in patients with PAH could have protective effect on the PAH by providing a low resistance bed, and thereby reducing right ventricular afterload. However, progression of PAH can predispose for enlargement of PAVM and subsequent rupture (22). Spontaneous thrombosis of a PAVM is uncommon and can result in systemic embolism (18). Rupture of PAVM (Figure 2) can be seen in those AVM with thin walls and with pregnancy from hormonal changes in PAVM wall and increased circulating blood volume. Besides, PAVMs are commonly located subpleurally and thus can result in hemoptysis and hemothorax and coughing-aggravated paradoxical air embolism-related neurological complications (18).
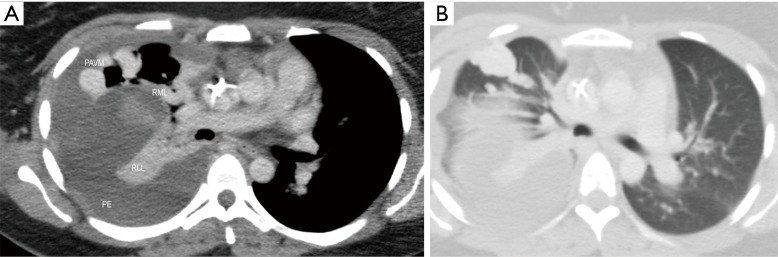
Figure 2.
A 31-year-old pregnant woman with acute dyspnea. Axial soft tissue (A) and lung window (B) images reveal peripheral sub-pleural right middle lobe pulmonary arteriovenous malformation (PAVM) with small site of rupture. Note large hemorrhagic right pleural effusion (PE) and associated complete right lower lobe (RLL) and partial right middle lobe (RML) collapse.
Work up for screening and diagnosis in patient with suspicion for PAVM
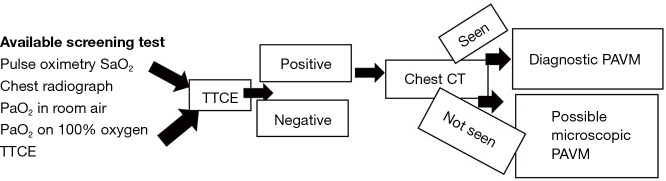
Transthoracic contrast echocardiography (TTCE) is the screening test of choice for PAVM with sensitivity up to 98.6% (9). Study comparing sensitivity and specificity of various screening tests revealed the following sensitivity and specificity: 53% and 90%, respectively, for pulse oximetry arterial oxygen saturation (SaO2); 60% and 100%, respectively, for chest radiograph; 73% and 80%, respectively, for abnormally low arterial oxygen measurement (PaO2) at room air; 100% and 40%, respectively, for PaO2 while breathing 100% oxygen; and 64% and 80%, respectively, for shunt measurement at catheter pulmonary angiography (23) (Figure 3). Hence although, SaO2 is overall easy test, it is not recommended as the screening tool of choice due to poor reproducibility and low sensitivity.
Figure 3.
Flow chart for screening of pulmonary arteriovenous malformation. SaO2, arterial oxygen saturation; PaO2, arterial oxygen measurement; TTCE, transthoracic contrast echocardiography; Positive, positive for PAVM; Negative, negative for PAVM.
PAVM in the pregnant population need additional care particularly during the third trimester due to increased risk for rupture and massive hemoptysis, hemothorax and even death due to decreased vascular wall stability and increased cardiac output. TTCE based screening should be considered after the first trimester in all pregnant HHT patients who have not already been screened for PAVMs (24).
Pulmonary AVM imaging features
The diagnostic criteria for PAVM requires the presence of both a feeding artery and a draining vein coursing to and from the abnormal communication. The enhancing focus intervening between the feeding artery and draining vein could sometimes be an area of ground glass opacity in which case this likely constitutes a microscopic telangiectasia. PAVMs are divided into simple and complex types. The simple types are more common and account for 80% of these lesions. The feeding artery for most of the PAVMs arises from the pulmonary arteries but rarely systemic arteries, such as the intercostal, phrenic, bronchial and internal mammary arteries, can provide feeders (15). The feeding artery arises from the segmental pulmonary artery branches, and these segmental arteries often give rise to branches to adjacent normal lung parenchyma (15). The venous drainage of the PAVMs is typically through a pulmonary vein, but rarely through the inferior vena cava and the left atrium. PAVMs are more commonly located in the lower lobe, middle lobe and lingula thus explaining worsening hypoxia in the upright position (orthodeoxia) (2,15,18,21).
Simple PAVMs
Simple PAVMs are defined as lesions comprising of a single feeding artery, connected through a bulbous, aneurysmal, non-septated communication with one or more draining veins. The draining veins are generally 1–2 mm larger than the feeding arteries (2,18,21). On imaging, these simple PAVMs appear as a well-defined rounded or lobulated peripheral nodule (Figure 4). The diameter of the feeding artery is measured 1–2 cm proximal to the aneurysm sac of the PAVM (21). The diagnosis of a PAVM is confirmed only if the nodule has connection with both the artery and the vein. Mural thrombus within venous sacs is uncommon, and is seen if the sac is large (2,15,18,21).
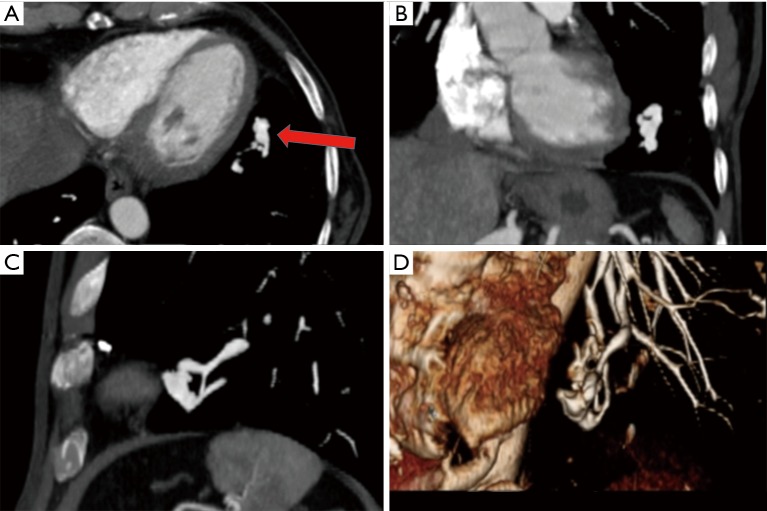
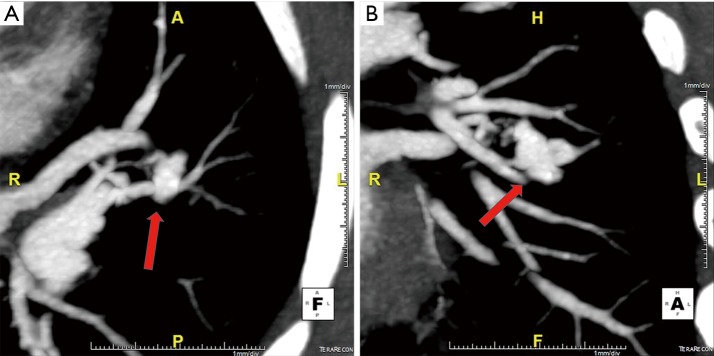
Figure 4.
Axial (A), coronal (B) and sagittal (C) maximum intensity projection (MIP) and volume rendered (D) images illustrate a simple lingular lobe PAVM (arrow) connecting single segmental lingular lobe pulmonary artery to vein via abnormal nexus of vascular connections.
Complex PAVMs
Complex PAVMs are defined as lesions supplied by two or more feeding arteries connecting via a septated aneurysmal sac to two or more draining veins (Figures 5,6). The connection in the complex types could be cirsoid or divided into multiple small interconnecting vessels. Complex PAVMs account for approximately 20% of PAVMs (15). The aneurysmal connection is due to the direct transmission of arterial pressure through the connection to the low pressure venous system (15). Aneurysms in simple PAVM are cavernous and non-septated while those in complex PAVM are septated (2,15,18,21). In the presence of patency of aneurysmal portion of PAVM, excellent enhancement is noted, however it lacks enhancement in the presence of a thrombus within the aneurysm (25). PAVM can appear as a nodule due to the aneurysmal portion or as tubular arcuate areas of non-dichotomous attenuation from dilated feeding arteries or draining veins particularly in the peripheral lung location (25). Characterization of the septate or non-septate nature of the aneurysmal connection on CT is limited due to the thinness of the septate (25).
Figure 5.
Axial (A), coronal (B) MIP images show a large complex right middle lobe PAVM (arrow) connecting multiple segmental right middle lobe pulmonary artery to veins via abnormal aneurysmal vascular connections.
Figure 6.
Axial (A), coronal (B) MIP images show a large complex lingular lobe PAVM (arrow) connecting multiple segmental lingular lobe pulmonary artery to veins via abnormal aneurysmal vascular connections.
Rare types of complex AVMs
Diffuse PAVMs and telangiectatic PAVMs are rare types of complex AVM. Multiple and diffuse PAVMs are more commonly seen in HHT and in patients with SVC to pulmonary artery related Glenn shunts.
Diffuse PAVMs
Diffuse PAVMs are defined as AVMs involving every subsegmental or segmental or both types of arteries of at least one lung lobe and are more extensive than multiple PAVMs (20) (Figure 7). Patients with diffuse pulmonary AVMs are at increased risk of hypoxemia, hemoptysis, and neurologic complications. Transcatheter embolotherapy may reduce the risk of neurologic complication but does not significantly improve the profound hypoxia. Antibiotic prophylaxis is recommended for bacteremic procedures to prevent brain abscess. Patients with diffuse pulmonary AVMs have a fairly good prognosis and can lead productive lives and hence lung transplantation is not recommended because survival with the disease is difficult to predict (20).
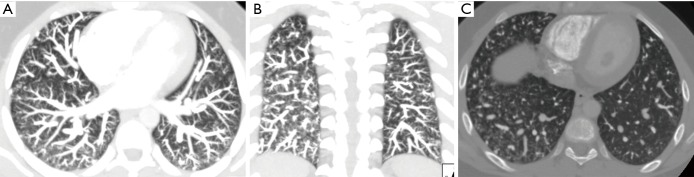
Figure 7.
Axial (A), coronal (B) MIP and axial bone window (C) images show diffuse multiple PAVM connecting segmental and subsegmental pulmonary arteries connecting to pulmonary veins. Note multiple additional small ground glass opacities in both lungs in figure C which are related to diffuse telangiectatic type of PAVM.
Telangiectatic PAVMs
Telangiectatic PAVMs are typically seen in children and more common in patients with HHT. On CT, they appear as a focal ground-glass opacity, and rarely with a small nodularity within (Figure 8). These lesions can have associated feeding and draining vessels. These lesions coexist with additional more classical appearing PAVM in other portion of lung (2,15,21,26).
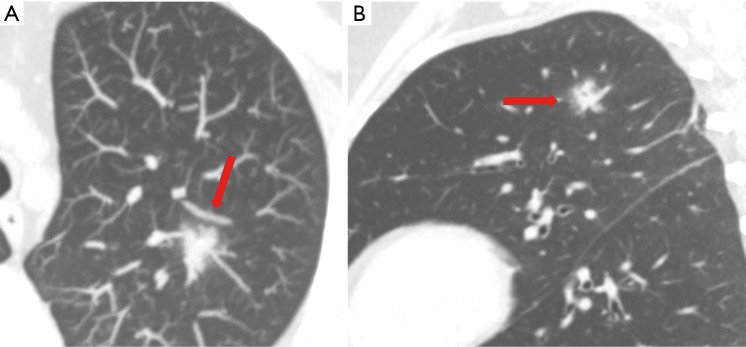
Figure 8.
Axial (A) and sagittal (B) MIP images in a 40-year-old woman showing a 17 mm left upper lobe pulmonary telangiectatic type of PAVM (arrow) appearing as ill-defined peribronchovascular mixed density with predominant ground glass component. Patient also had left upper posterior pleural hemangioma (not shown).
Reporting format for PAVMs on MDCT
Our reporting format for both types of PAVMs on MDCT includes comprehensive information about the number, morphological locations of PAVMs, along with number, and diameter of the feeding arteries. Generally, the diameter of the feeding artery is measured beyond the segmental portion of the vessel and beyond any parenchymal branches, typically 2–3 cm proximal to the arteriovenous communication. This is the critical component of reporting with MDCT since the FA size of 3 mm or more are typical targets for transcatheter embolization (TCE) procedure using coils or endovascular plugs or other devices. The diameter of the feeding artery is generally measured on lung parenchymal window settings. The size of the aneurysmal connection is often included so that it can be followed. We also always include the upper abdomen during CT pulmonary angiography to assesses for AVMs in the liver, kidney, gastrointestinal tract, and pancreas since most patients with pulmonary AVMs and HHT have visceral AVMs (12).
TTCE
Agitated saline contrast transthoracic echocardiography (TTCE) (bubble echocardiogram) is a useful noninvasive screening test for PAVMs. This involves injecting 10 mL of agitated saline into a peripheral vein and observing its course through the cardiac chambers with transthoracic echocardiography. The test is considered positive for presence of PAVM when the injected bubbles appear in the left cardiac chamber after three to eight cardiac cycles (27) (Figure 9). Visualization of microbubbles within one to two cardiac cycles imply an intracardiac shunt, such as patent foramen ovale or atrial septal defect, rather than an intrapulmonary shunt. It is to be noted that in the absence of intra-cardiac or intrapulmonary shunt, the pulmonary capillaries filter the microbubbles, and thus lack of appearance of bubbles in the left cardiac chambers (17,27). TTCE is a simple, safe, noninvasive, widely available, highly reproducible test, along with low false-negative rate compared with conventional screening methods, making it an excellent screening test for PAVM (27). Studies have shown a sensitivity of 95–100% for TTCE (23). It should be noted that a positive TTCE in the presence of a normal thoracic CT is diagnostic of a microscopic PAVM (27). TTCE can provide prediction of cerebrovascular events and brain abscess based on grading with pulmonary shunt grade 1 (<30 microbubbles) being not associated with an increased prevalence of CNS events, while grades 2 (30–100 microbubbles) and 3 (>100 microbubbles) pulmonary shunt being independent predictors of cerebrovascular events and brain abscess (28).
Figure 9.
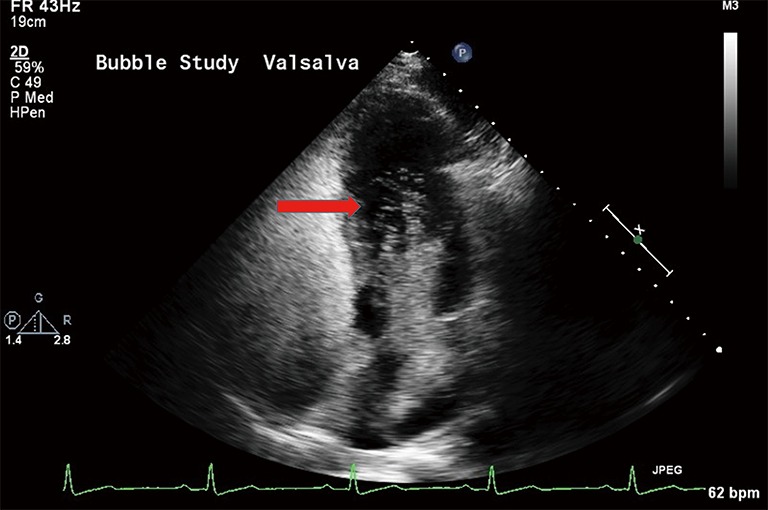
Four-chamber transthoracic echocardiogram image showing markedly positive bubble study with agitated saline bubble echoes within left cardiac chambers (arrow, left ventricle) within 4–8 cardiac cycles suggesting PAVM.
Chest radiography
Chest radiography with frontal and lateral projections is a rapid, easily available, and relatively low radiation, and inexpensive first line imaging technique for screening of a PAVM. However, it has low sensitivity for the detection of small sized PAVMs (18). Typical PAVMs on radiograph appear as rounded well-defined nodular lesion of varying size typically of 1–2 cm diameter with branching afferent feeding and dilated efferent draining vessels (Figure 10). However, more commonly, the radiographic appearances are quite subtle with less obvious feeding and draining vessels. Moreover, the radiographic finding of lung nodule is not specific and various differential diagnosis of lung nodules needs to be excluded. Complex PAVMs are generally ill-defined, except for appreciation of nodular branching pattern related to the dilated feeding and draining vessels. Diffuse complex AVM which involve entire pulmonary segments appear radiographically as an area of generalized increased opacity along with marked vascular prominence and absence of discrete lung nodules (2).
Figure 10.
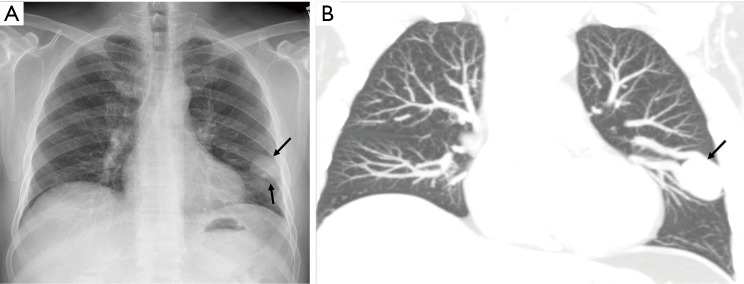
Frontal radiograph of the chest (A) in a 61-year-old man with HHT showing round soft tissue non-calcified nodule (arrows) in the left mid zone. Corresponding coronal CT image (B) shows 3.5 cm aneurysmal connection along with feeding artery and draining vein in lingula lobe (arrow) related to pulmonary arteriovenous malformation (PAVM).
CT protocol for PAVM
At our institution, we use CT with a modified pulmonary angiography protocol given its ability to allow for superior pulmonary vascular assessment of the feeding and draining vessels, arteriovenous communications and associated aneurysms, extra-pulmonary AVMs in the upper abdomen, and for treatment planning of the AVMs. In the pre-dual energy CT scan era, we did obtain a low-dose non-contrast CT to differentiate mimickers such as tiny calcified granulomas. This differentiation is important since these granulomas are located around enhancing feeding pulmonary artery branch. However, with the availability of dual energy CT scanners at our institution, we have stopped obtaining true non-contrast CT and are instead using virtual non-contrast CT reconstruction from the dual energy data, thereby reducing the radiation dose. We do not use IV microfilters since they prevent use of high-rate IV contrast material injections using power injectors but we do follow wet-to-wet hookup technique of tubing to prevent the risk of air embolization. Thin soft tissue reconstructions are subsequently transferred to dedicated 3D workstation to generate additional multi-planar 5 mm slab of thin maximum intensity projection (MIP) images on lung window settings, multiplanar thin soft tissue and lung window and 3D volume rendered reconstructions for optimal detection and treatment planning of PAVM.
Computed tomography (CT) appearance of evolving and classic PAVM
Currently, thin section chest CT scan is the gold standard for confirmation of PAVM and treatment planning (9). Digital subtraction pulmonary angiography has been replaced with Multidetector CT (MDCT) for evaluation of PAVM due to its higher sensitivity (70% vs. 83%) and non-invasive nature. Diagnosis of PAVM using non-contrast MDCT with 1-mm reconstructions is generally sufficient due to high spatial resolution and high contrast difference between bright pulmonary vessels and low attenuating pulmonary parenchyma (12). Study based on volumetric spiral CT showed CT has high sensitivity of 98% in identification of PAVMs compared with 60% sensitivity for catheter angiography and CT allowed confident segmental location in 86% of PAVMs. Although CT was reliable in the analysis of angioarchitecture of PAVMs (26%), it was inferior to catheter angiography (60%) for this purpose (25).
Evolving PAVMs on CT
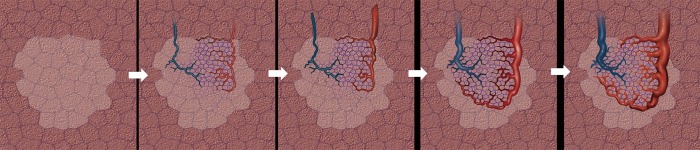
PAVM evolution occurs through three stages and can be visualized on CT scan with first stage consisting of a ground glass nodule/opacity related to early stage prominence of the post-capillary venules and inflammation centered around pulmonary artery branch followed in second stage by visibility of vessels within the ground glass lesion due to venous branching and connection between the precapillary pulmonary artery and the postcapillary venules and finally definite AVM formation which is comprised of an aneurysmal connection between the dilated draining vein and the feeding pulmonary artery with concomitant disappearance of the ground glass lesion (Figure 11) (16). Rarely, PAVMs will evolve to a giant lesion.
Figure 11.
Illustrations demonstrating evolution hypothesis of pulmonary arteriovenous malformation (PAVM) on CT scan. First image shows isolated nodular or ill-defined ground-glass attenuation. It corresponds to the initial venous telangiectatic stage which is followed by the pulmonary venous enlargement, and corresponds to microscopic arteriovenous connections. In next stages, vascular branching is depicted on CT: the feeding artery (blue color vessel) lies within the ground-glass nodule, while draining veins (red color vessel) are visible in the periphery of the nodule. Final image demonstrates the development of a true PAVM.
Classic PAVM on CT
A typical simple PAVM appears as a well-defined peripheral nodule, which can be rounded or multilobulated related to aneurysmal connection, into which one feeding artery, and from which one or more draining veins, can be seen (2). The draining veins are typically 1–2 mm larger than the feeding arteries. It cannot be overemphasized that the presence of both the artery and vein branch related to the nodule is required to make a diagnosis of PAVM and if only an artery or a vein is present in relation to the lung nodule, then a diagnosis of PAVM can be excluded. Following intravenous injection of contrast material, pulmonary arterial phase CT reveals enhancement of the PAVM sac along with its feeding and draining vessels. Uncommonly, the sac can undergo mural thrombosis and appears as non-enhancing peripheral hypodense nodule in the region of PAVM sac. On CT, diffuse segmental and less common lobar type PAVMs appear as smooth dilatation of branch pulmonary arteries and veins, with or without tortuosity. These diffuse type has absence of or less prominence of focal aneurysmal dilation on venous side of the PAVM while these are typical of classic simple and complex PAVMs.
Some studies recommend using CT scan of the chest in all patients with possible HHT and a grade 2 or 3 right to left shunt on TTCE. However, they suggest postponing CT in most patients with a grade 1 shunt or less, unless the shunt worsens in the future given the fact that PAVM is seen in 2.1% of patients with a grade 1 shunt on TTCE (9).
Repeat CT every 5–10 years is performed in younger population with an initial negative screen, and those with treated or non-treated PAVM (17).
Dual-energy computed tomography (DECT)
DECT uses differences in X-ray attenuation measured at two energy levels to estimate the density of different materials. Lower keV (40 to 60 KeV) virtual monochromatic images from DECT increase the image contrast, subpleural arteriole enhancement, enable analysis of peripheral pulmonary arteries and more confident evaluation of subsegmental pulmonary emboli which has a higher incidence in patients with HHT. The differentiation of a calcified lung nodule from an enhancing lung nodule is now possible with use of single-phase, post-contrast DECT by generating virtual unenhanced images and iodine-density images (29-31).
Magnetic resonance imaging (MRI)
Contrast-enhanced magnetic resonance angiography (MRA) is not currently used for PAVMs screening. MRA has been demonstrated to be an accurate method for detection of PAVM and recent studies have concluded that MRA is an adjunct to pre-embolization planning. Lack of ionizing radiation exposure is the major advantage of MRA. Four-dimensional (4D) time-resolved MRA is 3D radiofrequency-spoiled gradient-echo dynamic acquisition and consist of keyhole, partial Fourier, parallel imaging, and view-sharing technique to yield a high spatial and temporal resolution time-resolved 3D MRA sequence. Use of this MRA technique to assess PAVMs patency by analyzing pulmonary arterial and venous enhancement kinetics showed it to be a promising, feasible and an efficient tool for noninvasive assessment of PAVM patency (32).
Digital subtraction catheter pulmonary angiography (DSCPA)
Catheter angiography is rarely performed for diagnosis of a PAVM but is part of an embolization procedure. In addition to demonstrating the specific features of a PAVM such as the dilated feeding artery and draining pulmonary vein and the aneurysmal sac communication (Figure 12), DSPA helps for real time assessment of early venous return. The flow rate through the PAVMs depends on the size of the arteriovenous communications. DSPA accurately depicts the angioarchitecture of PAVMs (25). DSPA is commonly performed to assess PAVM patency, especially after endovascular occlusion given its ability to subtract the metallic devices and accurately demonstrate flow through the PAVM (33).
Figure 12.
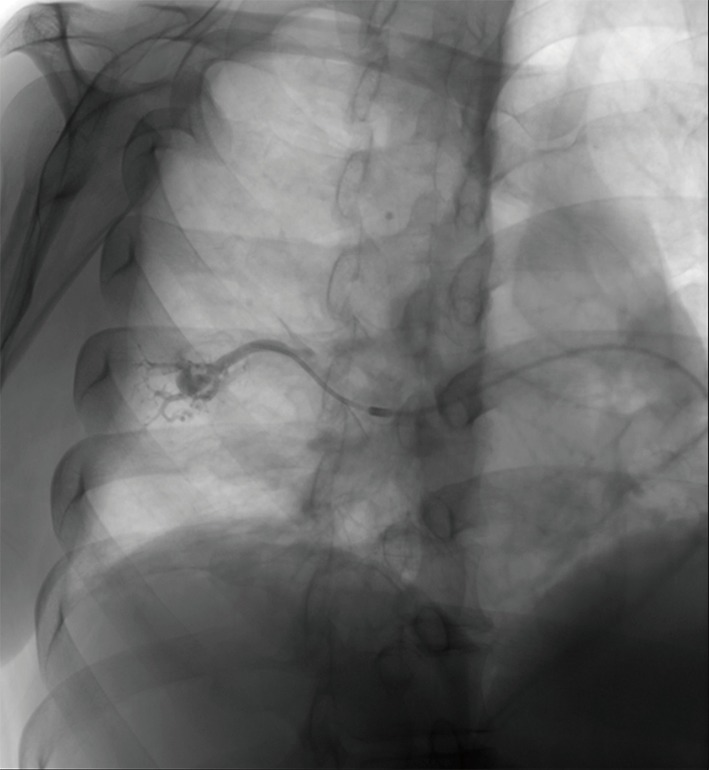
A right lower lobe pulmonary arteriovenous malformation is noted on pre-treatment pulmonary angiogram fluoroscopic image.
Follow up imaging of untreated PAVMs
Consensus guidelines from 2011 regarding imaging follow up for HHT patients with untreated <3 mm PAVMs and microscopic PAVMs, suggest follow-up frequency to be determined on a case-by-case basis with a follow-up CT interval of approximately 1–5 years (17). However, recent study based on imaging follow up of 13 patients of untreated small (<3 mm) and microscopic PAVMs showed that PAVM enlargement of these type of lesions was more infrequent than would be expected based on current guidelines and hence challenged the current surveillance imaging recommendation of a repeat thoracic CT every 5 years in these patients. This study showed that only one of 13 patients showed enlargement of these lesion at 10-year follow-up which was also successfully treated using transcatheter embolization (TCE) (26).
Treatment of PAVM
Studies have shown that definitive treatment should be provided in all cases, except for asymptomatic patients with small PAVMs and without evidence of HHT (1). Risk of stroke is independent of the size of PAVMs and hence embolization is recommended for all PAVMs amenable to the TCE. Studies have shown that patients with high-risk PAVMs, defined as a feeding artery size >3 mm, transcatheter embolization (TCE) using balloons, coils, or vascular plugs is a safe and effective procedure in preventing the occurrence of hemorrhagic and embolization-related complications (34).
Follow up guidelines for treated PAVM post embolization include contrast-enhanced chest CT at 6 months, and subsequently every 3–5 years to detect complications of failed embolization, reperfusion of the aneurismal sac, or the interval growth of new PAVMs (17).
Imaging differentials of pulmonary AVM
Pulmonary artery aneurysm
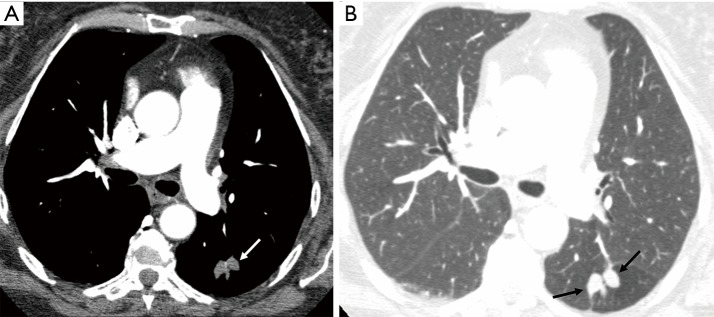
True aneurysm of pulmonary artery is rare and typically occurs at bifurcations of branch pulmonary artery with absence of a draining vein (Figure 13). They can be confused with PAVM if peripherally located and without any underlying cause (2).
Figure 13.
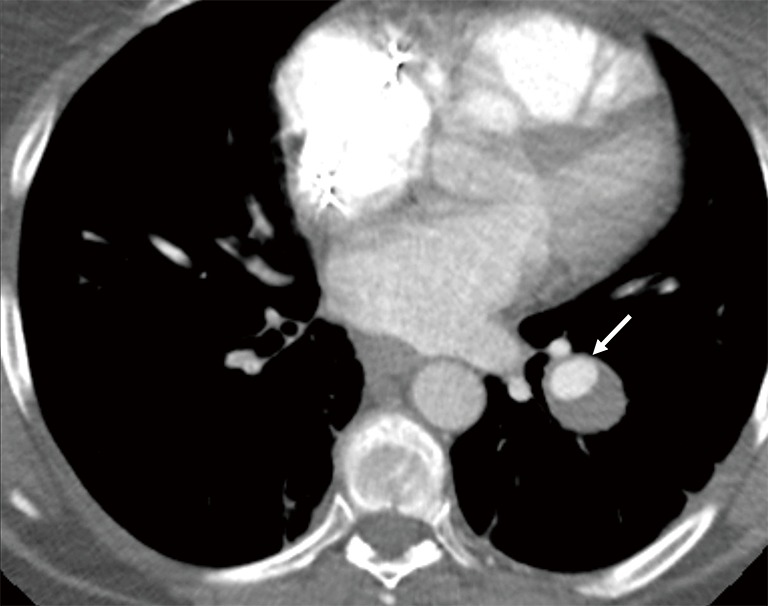
Axial contrast enhanced CT image in a 50-year-old man with left lower lobe pulmonary artery aneurysm with partial thrombosis (arrow) connecting with branch pulmonary artery. Note lack of connecting draining vein which is typical feature of PAVM.
Pulmonary vein varix
These can be congenital (more common) or acquired (rare) and can be easily mistaken for PAVM. However, accurate assessment of this lesion can reveal lack of arterial connection, but communication with only pulmonary vein branch (Figure 14). It is typically located in the atretic vein segment of a lung (35).
Figure 14.
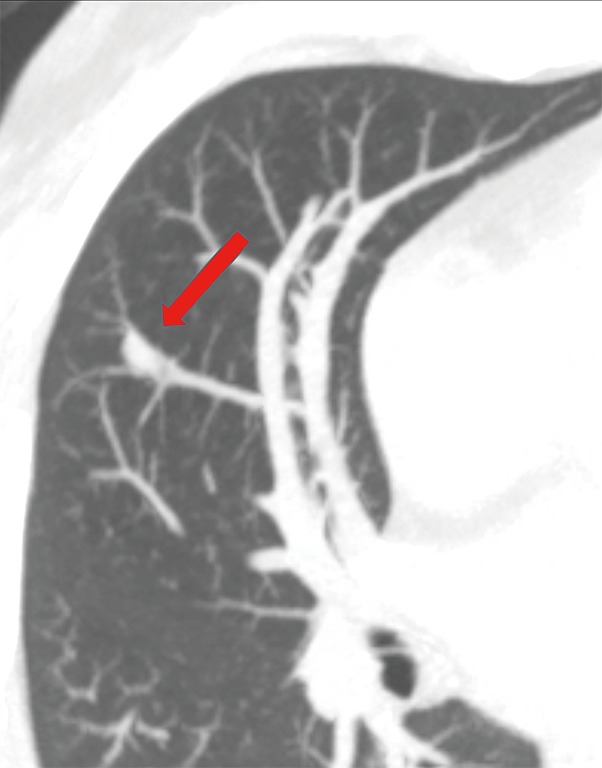
Axial MIP image in a 48-year-old woman shows a small 7 mm right middle lobe pulmonary vein varix (arrow) given its connection to vein and lack of connection to the pulmonary artery.
Calcified granuloma
Please see discussion within the PAVM section.
Bronchocele
Bronchoceles are dilated mucous-filled bronchi. If a bronchocele with high attenuating contents related to allergic bronchopulmonary aspergillosis is imaged only on post-contrast CT, and if close attenuation is not paid to their location within the bronchus, it can be mistaken for a PAVM. However, the appearance of a branching bronchial structure associated with an area of peripheral hyperlucency due to air-trapping in the adjacent lung are quite characteristic of a bronchocele (Figure 15) (36).
Figure 15.
Bronchocele. Axial contrast enhanced CT image in soft tissue (A) and lung window (B) in a 65-year-old man shows non-enhancing nodular branching soft tissue low attenuation lesion in left lower lobe superior segment related to bronchocele (arrows) due to localized bronchial atresia and hyperlucency in adjacent lung. Note lack of feeding artery and draining vein which are typical feature of PAVM.
Conclusions
PAVMs are abnormal vascular structures that most often connect a pulmonary artery to a pulmonary vein, thus bypassing the normal pulmonary capillary bed and resulting in an intrapulmonary right-to-left shunt. The spectrum of PAVMs extends from microscopic lesions causing profound hypoxemia with ground-glass appearance on CT but normal catheter angiographic findings to single or multiple large abnormal pulmonary artery to pulmonary venous connections. They are commonly associated with HHT. The main complications of PAVMs result from intrapulmonary shunt and include stroke, brain abscess, and hypoxemia. The TTCE is the preferred screening test while diagnostic confirmation is best performed using CT scan. Endovascular occlusion of the feeding artery is the treatment of choice. Collateralization and recanalization of PAVM following treatment may occur, and hence long term clinical and imaging follow-up is required to assess PAVM enlargement and PAVM reperfusion.
Acknowledgements
We thank Ms. Erin Moore, MA, Medical Illustrator for drawing some of the illustrations in this manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Burke CM, Safai C, Nelson DP, et al. Pulmonary arteriovenous malformations: a critical update. Am Rev Respir Dis 1986;134:334-9. [DOI] [PubMed] [Google Scholar]
- 2.Gill SS, Roddie ME, Shovlin CL, et al. Pulmonary arteriovenous malformations and their mimics. Clin Radiol 2015;70:96-110. 10.1016/j.crad.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Churton T. Multiple aneurysms of pulmonary artery. Br Med J 1897;1:1223. [Google Scholar]
- 4.Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology 2000;55:959-64. 10.1212/WNL.55.7.959 [DOI] [PubMed] [Google Scholar]
- 5.Bosher LH, Jr, Blake DA, Byrd BR. An analysis of the pathologic anatomy of pulmonary arteriovenous aneurysms with particular reference to the applicability of local excision. Surgery 1959;45:91-104. [PubMed] [Google Scholar]
- 6.Schwarzer D, Mader I, Petrovitch A, et al. Expectoration of embolization coils 15 years after embolization of pulmonary arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia. Pneumologie 2014;68:282-5. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson CH, Burchell HB, Good CA, et al. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous fistula: survey of a large family. N Engl J Med 1959;261:625-36. 10.1056/NEJM195909242611301 [DOI] [PubMed] [Google Scholar]
- 8.Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med 2004;169:994-1000. 10.1164/rccm.200310-1441OC [DOI] [PubMed] [Google Scholar]
- 9.Circo S, Gossage JR. Pulmonary vascular complications of hereditary haemorrhagic telangiectasia. Curr Opin Pulm Med 2014;20:421-8. 10.1097/MCP.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 10.Shumacker HB, Jr, Waldhausen JA. Pulmonary arteriovenous fistulas in children. Ann Surg 1963;158:713-20. 10.1097/00000658-196310000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prager RL, Laws KH, Bender HW., Jr Arteriovenous fistula of the lung. Ann Thorac Surg 1983;36:231-9. 10.1016/S0003-4975(10)60465-1 [DOI] [PubMed] [Google Scholar]
- 12.Cummings KW, Bhalla S. Pulmonary vascular diseases. Clin Chest Med 2015;36:235-48, viii. 10.1016/j.ccm.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg 2003;76:1759-66. 10.1016/S0003-4975(03)00450-8 [DOI] [PubMed] [Google Scholar]
- 14.Hales MR. Multiple small arteriovenous fistulae of lungs. Am J Pathol 1956;32:927. [PMC free article] [PubMed] [Google Scholar]
- 15.White RI, Jr, Mitchell SE, Barth KH, et al. Angioarchitecture of pulmonary arteriovenous malformations: an important consideration before embolotherapy. AJR Am J Roentgenol 1983;140:681-6. 10.2214/ajr.140.4.681 [DOI] [PubMed] [Google Scholar]
- 16.Lacout A, Marcy PY, Thariat J, et al. VEGF target in HHT lung patients: the role of bevacizumab as a possible alternative to embolization. Med Hypotheses 2012;78:689-90. 10.1016/j.mehy.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 17.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. 10.1136/jmg.2009.069013 [DOI] [PubMed] [Google Scholar]
- 18.Lacombe P, Lacout A, Marcy PY, et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: An overview. Diagn Interv Imaging 2013;94:835-48. 10.1016/j.diii.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 19.Lincoln MJ, Shigeoka JW. Pulmonary telangiectasia without hypoxemia. Chest 1988;93:1097-8. 10.1378/chest.93.5.1097 [DOI] [PubMed] [Google Scholar]
- 20.Faughnan ME, Lui YW, Wirth JA, et al. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest 2000;117:31-8. 10.1378/chest.117.1.31 [DOI] [PubMed] [Google Scholar]
- 21.White RI, Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 1988;169:663-9. 10.1148/radiology.169.3.3186989 [DOI] [PubMed] [Google Scholar]
- 22.Montani D, Price LC, Girerd B, et al. Fatal rupture of pulmonary arteriovenous malformation in hereditary haemorrhagic telangiectasis and severe PAH. Eur Respir Rev 2009;18:42-6. 10.1183/09059180.00011113 [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen AD, Oxhoj H, Andersen PE, et al. Pulmonary arteriovenous malformations: screening procedures and pulmonary angiography in patients with hereditary hemorrhagic telangiectasia. Chest 1999;116:432-9. 10.1378/chest.116.2.432 [DOI] [PubMed] [Google Scholar]
- 24.Ference BA, Shannon TM, White RI, Jr, et al. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest 1994;106:1387-90. 10.1378/chest.106.5.1387 [DOI] [PubMed] [Google Scholar]
- 25.Remy J, Remy-Jardin M, Wattinne L, et al. Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology 1992;182:809-16. 10.1148/radiology.182.3.1535899 [DOI] [PubMed] [Google Scholar]
- 26.Ryan DJ, O'Connor TM, Murphy MM, et al. Follow-up interval for small untreated pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Clin Radiol 2017;72:236-41. 10.1016/j.crad.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 27.Nanthakumar K, Graham AT, Robinson TI, et al. Contrast echocardiography for detection of pulmonary arteriovenous malformations. Am Heart J 2001;141:243-6. 10.1067/mhj.2001.112682 [DOI] [PubMed] [Google Scholar]
- 28.Velthuis S, Vorselaars VMM, van Gent MWF, et al. Role of transthoracic contrast echocardiography in the clinical diagnosis of hereditary hemorrhagic telangiectasia. Chest 2013;144:1876-82. 10.1378/chest.13-0716 [DOI] [PubMed] [Google Scholar]
- 29.Ohana M, Jeung MY, Labani A, et al. Thoracic dual energy CT: acquisition protocols, current applications and future developments. Diagn Interv Imaging 2014;95:1017-26. 10.1016/j.diii.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 30.Knoss N, Hoffmann B, Krauss B, et al. Dual energy computed tomography of lung nodules: differentiation of iodine and calcium in artificial pulmonary nodules in vitro. Eur J Radiol 2011;80:e516-9. 10.1016/j.ejrad.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Sudarski S, Hagelstein C, Weis M, et al. Dual-energy snap-shot perfusion CT in suspect pulmonary nodules and masses and for lung cancer staging. Eur J Radiol 2015;84:2393-400. 10.1016/j.ejrad.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 32.Boussel L, Cernicanu A, Geerts L, et al. 4D time-resolved magnetic resonance angiography for noninvasive assessment of pulmonary arteriovenous malformations patency. J Magn Reson Imaging 2010;32:1110-6. 10.1002/jmri.22384 [DOI] [PubMed] [Google Scholar]
- 33.Milic A, Chan RP, Cohen JH, et al. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol 2005;16:1675-83. 10.1097/01.RVI.0000182163.25493.BB [DOI] [PubMed] [Google Scholar]
- 34.Hart JL, Aldin Z, Braude P, et al. Embolization of pulmonary arteriovenous malformations using the Amplatzer vascular plug: successful treatment of 69 consecutive patients. Eur Radiol 2010;20:2663-70. 10.1007/s00330-010-1851-2 [DOI] [PubMed] [Google Scholar]
- 35.Gupta H, Mayo-Smith WW, Mainiero MB, et al. Helical CT of pulmonary vascular abnormalities. AJR Am J Roentgenol 2002;178:487-92. 10.2214/ajr.178.2.1780487 [DOI] [PubMed] [Google Scholar]
- 36.Logan PM, Muller NL. High-attenuation mucous plugging in allergic bronchopulmonary aspergillosis. Can Assoc Radiol J 1996;47:374-7. [PubMed] [Google Scholar]