Abstract
Acquired pulmonary vein stenosis (PVS) is an uncommon occurrence in adults, but one that carries significant morbidity/mortality. PVS can be secondary to neoplastic infiltration/extrinsic compression, non-neoplastic infiltration/extrinsic compression, or iatrogenic intervention. This article: (I) reviews the common causes of acquired PVS; (II) illustrates direct and indirect cross-sectional imaging findings in acquired PVS (in order to avoid misinterpretation of these imaging findings); and (III) details the role of imaging before and after the treatment of acquired PVS.
Keywords: Pulmonary vein stenosis (PVS), pulmonary vein isolation (PVI), pulmonary vein ablation, imaging, radiology, computed tomography (CT), magnetic resonance imaging (MRI)
Introduction
Pulmonary vein stenosis (PVS), though a relatively uncommon occurrence (1,2), is associated with significant morbidity and mortality if not recognized and treated promptly. In adults PVS is often secondary to infiltration/extrinsic compression by a mediastinal or lung process. More recently, the increased use of catheter-based ablation therapy for treatment of atrial fibrillation has led to a new cohort of patients with PVS. Radiologists play an important role in the identification and evaluation of PVS, however this requires an understanding of the patient population at risk, the direct and indirect imaging findings of PVS on cross-sectional imaging, as well as relevant information in the treatment of this condition.
This article: (I) reviews the common causes of secondary PVS in adults; (II) illustrates direct and indirect cross-sectional imaging findings in secondary PVS; and (III) details the role of imaging before and after the treatment of secondary PVS in adults.
Embryology and anatomy
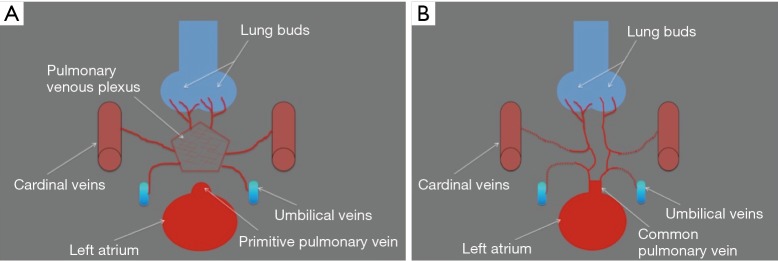
In early gestation, the veins draining the primitive lung buds do not connect to the primitive left atrium, but rather connect to various systemic venous systems (e.g., cardinal venous system, umbilical venous system) through the pulmonary venous plexus. An outpouching of the primordial left atrium develops and connects to the pulmonary venous plexus at around 30 days of gestation. At the same time, in normal development, the connections between the pulmonary venous plexus and the systemic venous systems atrophy, and the primitive pulmonary vein starts draining oxygenated blood from the lungs into the left atrium through a common confluence of the pulmonary veins. This common confluence of the pulmonary veins then integrates with the primordial left atrium, forming the posterior wall of the completed left atrium and generating the usual pattern of four separate ostia for the pulmonary veins (2,3) (Figure 1A,B).
Figure 1.
Embryologic development of the pulmonary veins. (A) Illustration shows that in early gestation, the veins draining the primitive lung buds do not connect to the primitive left atrium, but rather connect to various systemic venous systems (e.g., cardinal venous system, umbilical venous system) through the pulmonary venous plexus; the primitive pulmonary vein appears as an outpouching of the left atrium; (B) illustration shows that after 30 days of gestation the primitive pulmonary vein enlarges and connects to the pulmonary venous plexus, establishing for the first time venous flow from the lung buds to the left atrium; the connections of the pulmonary plexus with the systemic veins atrophy.
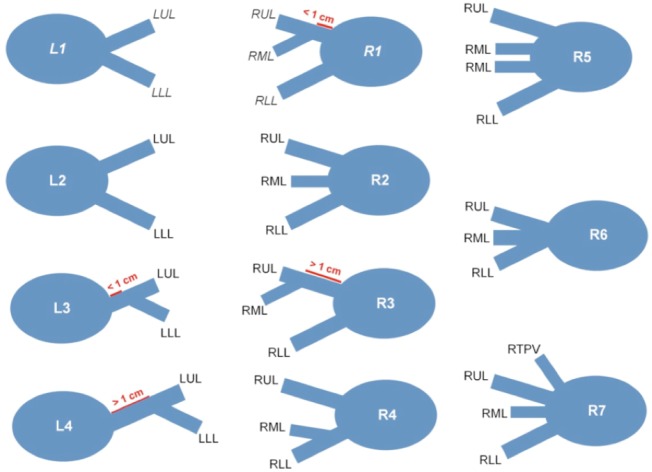
Although the pulmonary veins usually drain into four separate ostia, many different anatomic variants have been described (4). The most common pattern is that of two separate ostia on the left, one superiorly, draining the left upper lobe, and one inferiorly, draining the left lower lobe; and two separate ostia on the right, one superiorly, draining the right upper and middle lobes, and one inferiorly, draining the right lower lobe (Figure 2). Radiologists should be familiar with the other anatomic variants so that they can differentiate between a naturally occurring reduced number of ostia and a pulmonary vein occlusion.
Figure 2.
Diagram demonstrating the normal anatomic variants of the pulmonary veins. L1, two separate ostia for the left pulmonary veins that are not separated by atrial wall; L2, two separate ostia for the left pulmonary veins that are separated by atrial wall; L3, the left lower PV drains into the left upper PV with a short (<1 cm) common segment; L4, the left lower PV drains into the left upper PV with a long (>1 cm) common segment; R1, two separate ostia for the right pulmonary veins, with the vein draining the RML joining the right upper PV and forming a short (<1 cm) common segment; R2, three separate ostia for the right-sided pulmonary veins; R3, two separate ostia for the right pulmonary veins, with the vein draining the RML joining the right upper PV and forming a long (>1 cm) common segment; R4, two separate ostia for the right pulmonary veins, with the vein draining the RML joining the right lower PV; R5, four separate ostia for the right-sided pulmonary veins, with two veins draining the RML; R6, common ostium for the right-sided pulmonary veins; R7, four separate ostia for the right-sided pulmonary veins, with a separate ostium for the vein draining the RML and an accessory right top PV that drains the apical segment of the RUL. The most common anatomic pattern is L1/R1, with a total of four pulmonary veins draining into the left atrium. LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; RTPV, right top pulmonary vein; PV, pulmonary vein.
Causes of acquired PVS
Causes of PVS in adults can be grouped in three categories: neoplastic infiltration/extrinsic compression, non-neoplastic infiltration/extrinsic compression, and iatrogenic intervention.
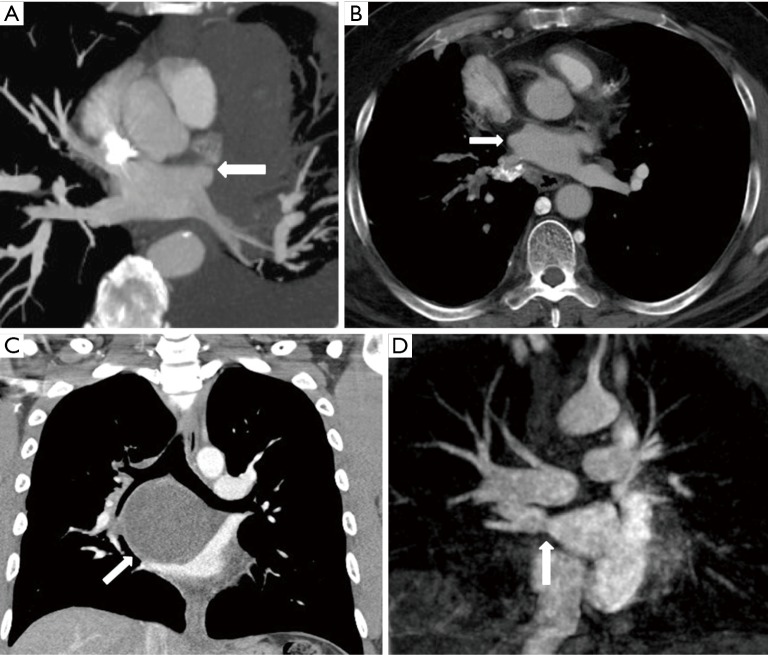
Any neoplastic disease occurring in the mediastinum and lungs can cause PVS by either infiltration or extrinsic compression of a pulmonary vein. Common neoplastic diseases that can cause PVS include primary lung cancer (5-9) and lymphoma (10) (Figure 3A). Non-thoracic primary neoplasms can also metastasize to the mediastinum and lungs and cause PVS (11). Non-neoplastic causes of PVS include fibrosing mediastinitis (12-16) (Figure 3B), sarcoidosis, and mediastinal cysts (17-19) (Figure 3C).
Figure 3.
Acquired pulmonary vein stenosis in adults secondary to lymphoma, fibrosing mediastinitis, bronchogenic cyst, and percutaneous radiofrequency catheter ablation for atrial fibrillation. (A) Axial reconstructed maximal intensity projection CT image shows lymphoma demonstrating mass effect on the left superior pulmonary vein with resultant stenosis (arrow); (B) axial reconstructed CT image shows fibrosing mediastinitis demonstrating mass effect on the right superior pulmonary vein with resultant stenosis (arrow); (C) coronal CT image shows bronchogenic cyst demonstrating mass effect on the right superior pulmonary vein with resultant stenosis (arrow); (D) coronal reconstructed maximal intensity projection MR image shows right inferior pulmonary vein stenosis after percutaneous radiofrequency catheter ablation for atrial fibrillation (arrow).
Iatrogenic causes of PVS include complications of percutaneous radiofrequency catheter ablation for atrial fibrillation, known as pulmonary vein isolation (PVI), and cardiothoracic surgery for repair of congenital heart disease. Even though the incidence of complications after PVI is low, the increasing use of this procedure makes it currently the most common iatrogenic cause of PVS in adults (2) (Figure 3D).
Imaging evaluation of the pulmonary veins
The pulmonary veins are typically well depicted by noninvasive imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), even when aforementioned imaging modalities are performed for other reasons. Although transthoracic echocardiography can be successfully used to visualize the pulmonary veins in children (2), its use in adults is not as sensitive as the other available noninvasive imaging modalities (20).
Although a routine chest CT scan with contrast typically depicts the pulmonary veins, more detailed anatomic depiction is achieved with the use of electrocardiogram (ECG)-gating for patients in normal sinus rhythm. CT has the advantage of providing good spatial resolution and a three-dimensional (3D) data set within a short scanning time. However, this imaging modality is associated with ionizing radiation, which can be a concern, especially in young patients (2).
MRI can be used to image the pulmonary veins (21,22). Many different techniques have been used, including traditional contrast-enhanced MRI (23) and, more recently, time-resolved magnetic resonance venography (MRV) (24). MRI, like CT, can provide a 3D data set, but has the advantage of not using ionizing radiation. However, its spatial resolution is slightly inferior to that of CT, it requires a longer scanning time, and it may be contraindicated in patients with metal implants (2).
For assessment of the pulmonary veins, CT and MR are typically performed with an intravenous contrast material, which should not be used in patients with significantly decreased renal function. The iodine-based contrast material used for CT is potentially nephrotoxic (25), whereas the gadolinium-based contrast material used for MRI is a risk factor for nephrogenic systemic fibrosis in patients with a very low glomerular filtration rate (26). Noncontrast MRV techniques have been used to visualize the pulmonary veins (27,28), but they are not regularly employed in the clinical setting, and our own experience has shown contrast techniques to be, on the whole, more reliable in depiction of the pulmonary vein anatomy.
As reference, the following is an example of a MRI specific protocol to evaluate the pulmonary veins: axial HASTE (half-Fourier acquisition single-shot turbo spin echo) and SSFP (steady state free precession) images/injection of gadolinium followed by 3D contrast enhanced MR angiography (MRA) in the coronal plane timed to the left atrium/in patients referred for evaluation of the pulmonary veins post ablation, fat suppressed delayed gadolinium enhancement images are obtained in the axial plane approximately 20 minutes post injection of gadolinium to evaluate for pulmonary vein ostial and left atrial scar.
Direct cross-sectional imaging findings in secondary PVS
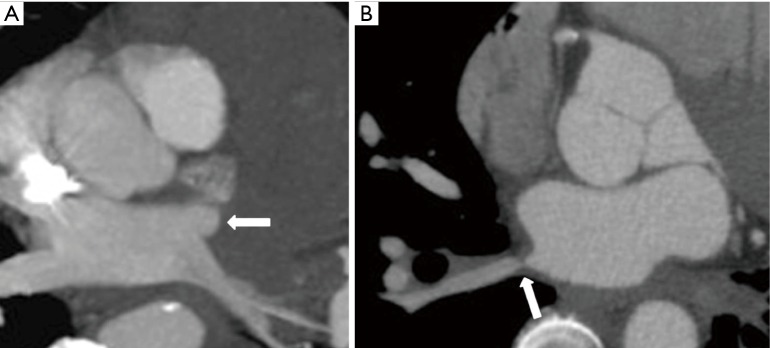
Direct cross-sectional imaging findings in patients with PVS include absence, abrupt cut off, and narrowing of a pulmonary vein (29,30) (Figure 4A,B). It is important to note that sometimes the left inferior pulmonary vein can occasionally become physiologically compressed between the left atrium and the descending thoracic aorta, mimicking a true stenosis (30). Because of the many described variations in pulmonary vein anatomy, comparison with old studies is important to confirm PVS.
Figure 4.
Direct cross-sectional imaging findings of acquired pulmonary vein stenosis in an adult. (A) Axial reconstructed maximal intensity projection CT image shows abrupt cut-off of the left superior pulmonary vein (arrow); (B) axial reconstructed CT image shows focal narrowing of the right inferior pulmonary vein (arrow).
Indirect cross-sectional imaging findings and imaging pitfalls in secondary PVS
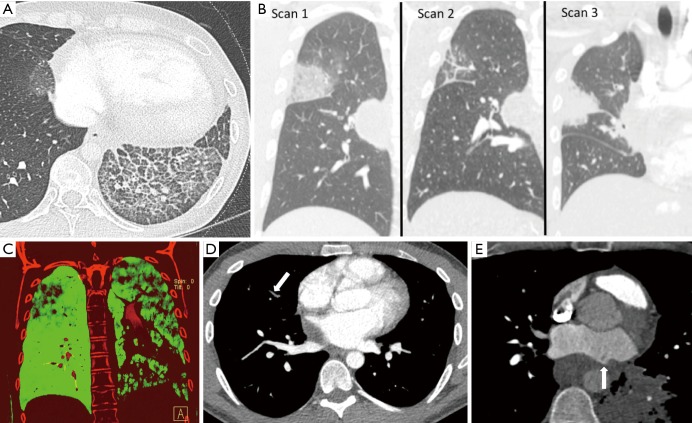
Indirect cross-sectional imaging findings in patients with PVS include regional lung edema, lung opacities that wax and wane, lung infarct, lung fibrosis, decreased lung perfusion, and longer blood/contrast transit time in the lung that is drained by the stenosed pulmonary vein. All of aforementioned indirect cross-sectional imaging findings may be mistaken for other conditions.
PVS causes an increase in the pulmonary venous pressure, which may lead to lung edema in the lung that is drained by the stenosed vein. CT imaging findings in lung edema include bronchial wall thickening, interlobular septal thickening, intralobular interstitial thickening, ground-glass opacity, and consolidative opacity (Figure 5A).
Figure 5.
Indirect cross-sectional imaging findings of acquired pulmonary vein stenosis in an adult: lung edema, waxing and waning lung opacities, decreased perfusion to the lung that is drained by the stenosed vein, non-opacified peripheral pulmonary veins, and mixing artifact at the interface between the stenosed pulmonary vein and the left atrium. (A) Axial CT shows interlobular septal thickening, intralobular interstitial thickening, and ground-glass opacity in the left lower lobe; lung edema secondary to left inferior pulmonary vein stenosis (can be mistaken for lung infection or lung hemorrhage); (B) coronal CT images from serial CT scans demonstrate waxing and waning lung opacities in the right upper and right middle lobes secondary to right superior pulmonary vein stenosis (can be mistaken for lung infection or lung infarction from pulmonary embolism); (C) coronal dual-energy CT image in demonstrates decreased lung perfusion to the left upper, left lower, and right upper lobes secondary to left superior pulmonary vein stenosis, left inferior pulmonary vein stenosis, right superior pulmonary vein stenosis; (D) axial CT image shows nonopacification of the right upper lobe pulmonary veins secondary to right superior pulmonary vein stenosis (can be mistaken for pulmonary emboli); (E) axial CT image shows mixing artifact at the interface between the left superior pulmonary vein and the left atrium (arrow) secondary to left superior pulmonary vein stenosis (can be mistaken for thrombus).
Indirect CT imaging findings can wax and wane depending on factors that transiently alter pulmonary venous pressures (Figure 5B). Because indirect cross-sectional imaging findings are often constrained to one or two lobes, they may be mistaken for entities such as lung infection (leading to inappropriate use of antibiotic therapy), lung infarction from pulmonary embolism (leading to inappropriate use of anti-coagulation therapy), lung fibrosis (leading to inappropriate lung biopsy), or lung cancer (leading to inappropriate lung biopsy) depending on the acuteness or chronicity of the cross-sectional imaging findings.
Due to the increase in pulmonary venous pressure, PVS leads to a longer blood/contrast transit time in the lung that is drained by the stenosed vein resulting in decreased perfusion to the lung that is drained by the stenosed vein (Figure 5C), non-opacification of segmental and subsegmental veins in the lung lobe drained by the stenosed vein (Figure 5D), and mixing artifact in the left atrium adjacent to the pulmonary vein orifice (Figure 5E). Non-opacification of lobar, segmental, and subsegmental pulmonary veins can be mistaken for pulmonary arterial thromboembolic disease if the non-opacified veins are mistaken for the pulmonary arteries; and mixing artifact in the left atrium adjacent to the pulmonary vein orifice can be mistaken for thrombus.
Screening for PVS after PVI
Although the incidence of PVS after PVI is low, the consequences of this condition can be life threatening. Therefore, early detection of PVS is essential to prevent irreversible lung vasculature changes.
Symptoms caused by PVS commonly arise weeks to months after PVI (30), although there are reports of symptoms developing up to a year after PVI (20). Symptoms include shortness of breath, dry cough, and recurrent hemoptysis (2), and in some instances chest pain.
Every patient who presents with respiratory symptoms after having undergone an ablation procedure should be considered for PVS screening. At this time, there is no consensus on how to screen asymptomatic patients after PVI. At our institution, screening is performed at 3 months by tailored CT or MRI examination. If a significant stenosis is found, another imaging study is performed 3 months later (20).
Percutaneous intervention for PVS after PVI
Confirmation of occlusion
CT, the noninvasive exam with the best spatial resolution, has been shown in some instances to overestimate the severity of PVS after PVI. In many cases, what appears to be an occluded pulmonary vein is shown on angiography to be a pulmonary vein with a tiny opening through which a stent can be placed when appropriate (2).
CT assessment of severely stenosed veins may be limited by slow or incomplete opacification. For confirmation of the degree of stenosis, further interrogation is obtained with a pulmonary artery wedge angiogram (Figure 6). In this procedure, a pulmonary arterial catheter is advanced into a pulmonary arterial segment that drains into the pulmonary vein of interest. Subsequently, the pulmonary arterial segment is occluded by a balloon wedge catheter, and a small amount of nonionic contrast material is injected under fluoroscopic observation. Very tiny openings in the severely stenosed vein can be demonstrated with this technique (2).
Figure 6.
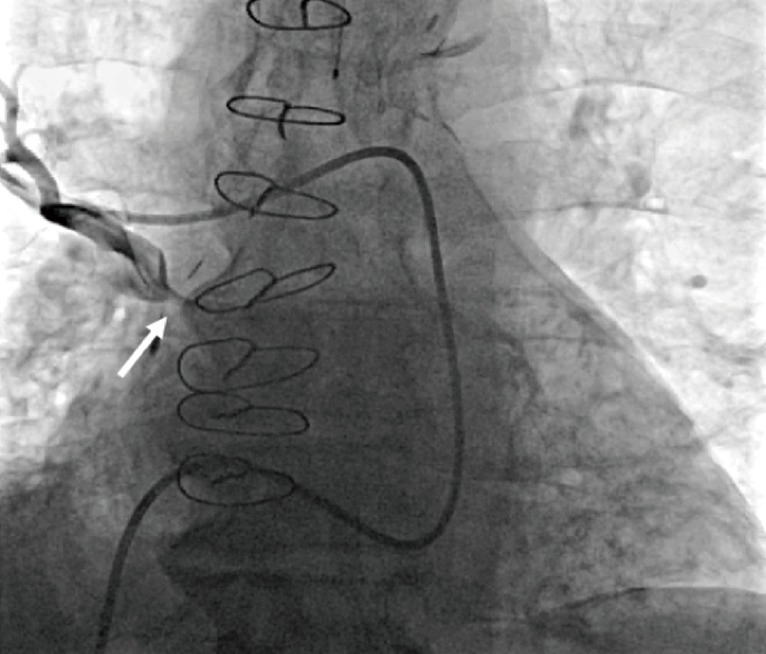
Pulmonary artery wedge angiography. Fluoroscopic angiographic image obtained with the catheter wedged in a right upper lobe pulmonary artery branch shows right superior pulmonary vein stenosis (arrow).
Decision to treat
PVS after PVI may be treated by percutaneous intervention. The decision to treat depends on the severity of stenosis and the presence of symptoms. Please note a discussion of treatment of PVS from extrinsic compression is limited to case reports.
Every symptomatic patient should be considered a potential candidate for percutaneous treatment. For symptoms to occur, at least 60% vessel diameter narrowing is usually necessary (20). As a normal pulmonary vein has an average diameter of 10 to 15 mm, a diameter of approximately 4 to 6 mm is usually necessary for symptoms to occur.
It is uncertain whether treatment is beneficial in asymptomatic patients with PVS. Most of this uncertainty arises from a lack of knowledge of the natural history of PVS. It is not known which patients will develop symptoms over time and which stenosis will progress to a complete occlusion, which could preclude the possibility of treatment. In our institution, the decision to treat asymptomatic patients is taken on a case-by-case basis in conjunction with the patient (20).
Treatment planning
There are two types of percutaneous procedures that can be used to treat PVS: ballooning and stenting. Ballooning, the first method used, has been associated with a rate of restenosis as high as 70% (31,32). Stenting is also associated with restenosis, but at a lower rate of around 10% for relatively large stents (20). Some studies have shown a decrease in the rate of restenosis when a stent with a diameter of at least 10 mm is used (31,33). However, not all stenotic veins can accommodate a large stent. In fact, some pulmonary veins demonstrate not just a focal stenosis but also diffuse hypoplasia. Placing a stent significantly larger than the vessel reference diameter (maximal diameter of the pulmonary vein just distal to the stenosis) can lead to a proliferative reaction at the edge of the stent that may cause restenosis at the edge with progression of the stenosis into the vessel (20). Therefore, at our institution, in the proper clinical setting, any pulmonary vein that can accommodate a stent of at least 8 mm in diameter is stented (20) (Figures 7,8); for pulmonary veins that can only accommodate stents smaller than 7 mm in diameter, we perform ballooning with the knowledge that the restenosis rate will be high (20). Some veins increase their reference diameter after this procedure, enabling the placement of a larger stent in a second procedure (20).
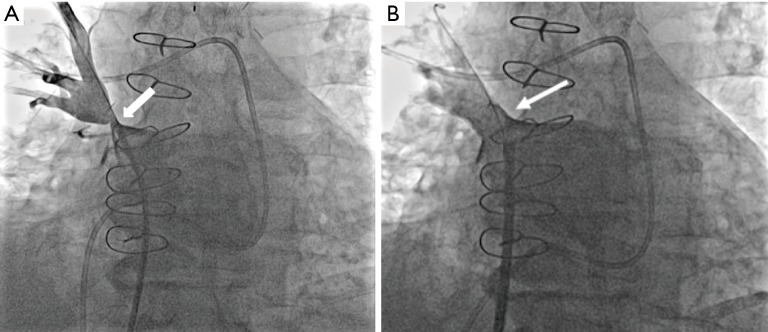
Figure 7.
Direct pulmonary venography. (A) Fluoroscopic angiographic image obtained during a direct pulmonary venography before stenting shows right superior pulmonary vein stenosis (arrow); (B) fluoroscopic angiographic image obtained during a direct pulmonary venography after stenting shows elimination of the right superior pulmonary vein stenosis (arrow).
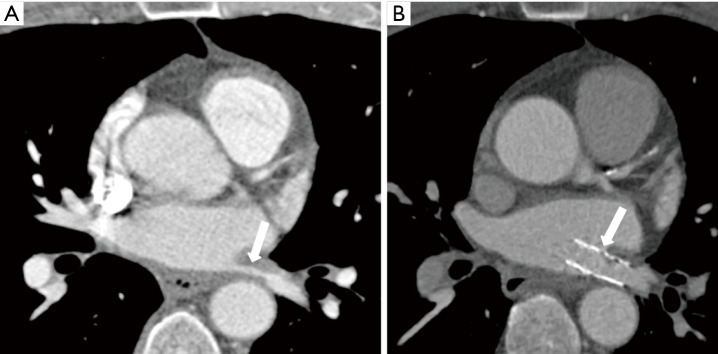
Figure 8.
Percutaneous intervention for acquired pulmonary vein stenosis in an adult secondary to percutaneous radiofrequency catheter ablation for atrial fibrillation. (A) Axial reconstructed CT image shows left superior pulmonary vein stenosis before percutaneous intervention (arrow); (B) axial reconstructed CT image shows increased pulmonary vein diameter after percutaneous stent placement (arrow).
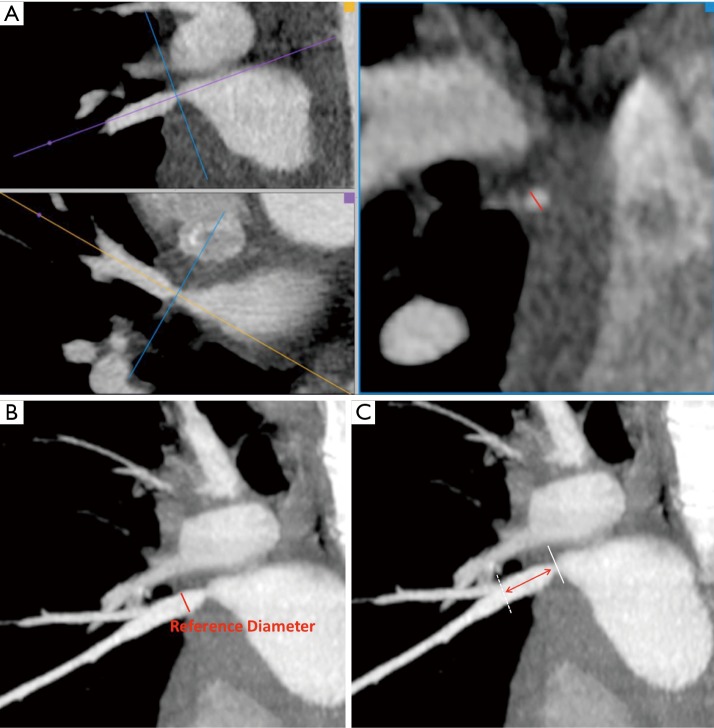
To plan the stenting procedure, the interventionalist should know the minimal diameter at the stenosis, the maximal diameter of the pulmonary vein just distal to the stenosis (also called the reference diameter), and the distance from the stenosis site to the first distal branching point. The reference diameter is the theoretical normal diameter of the pulmonary vein and represents the diameter to which the stenosis can potentially be dilated. The distance from the stenosis site to the first branch is used to assess whether there is enough length to accommodate the stent in the central pulmonary vein proximal to the branching point. Although a distal branch can be crossed as long as a conventional, not covered, stent is often used, this typically means the stent will be dilated to a smaller diameter, namely that of the branch it is extending into. These measurements are derived from multiplanar reconstruction images, usually from a CT scan, although an MRI scan can also be used (Figure 9A,B,C).
Figure 9.
Planning a stenting procedure for pulmonary vein stenosis treatment by using contrast-enhanced CT multiplanar reconstruction. (A) Measurement of the minimum diameter at the stenosis in a true perpendicular plane (red line); (B) measurement of the reference diameter, the maximum diameter of the pulmonary vein distal to the stenosis (red line); (C) measurement of the distance from the stenosis site to the first distal branching point (red double arrow).
When PVS progresses to complete occlusion, it is sometimes possible to cross the occluded segment and recanalize the vein, followed by either dilation or stenting depending on the reference diameter (34). However, if there is total obliteration of the vessel, percutaneous treatment is no longer possible. Chronic and complete obliteration of a pulmonary vein can result in lung infarction, which may manifest with cough, hemoptysis, wheeze, and pleuritic pain (35). If the patient’s quality of life is significantly impaired, an open thoracotomy and lobectomy may be necessary to alleviate the symptoms (35,36).
Postprocedure complications
Bleeding complications are the most frequent and serious complications post venoplasty and stenting of PV. Usually, these are acute in presentation in a perioperative setting. Several other early and delayed complications have been discussed in multiple case series; these include pulmonary vein perforation, stent dislodgment, hemoptysis, pulmonary hemorrhage, ST-elevation and transient neurologic deficits. The complication rates range from 0% to 25%, but need to be kept in mind before considering intervention (37). In a series of 98 patients requiring 145 catheterizations, only two vein perforations were reported (20). In this same series, only one stent dislodgement was reported. Transient hemoptysis is a self-limited condition, usually resolving within 24 to 72 hours after the procedure (20).
A more delayed complication is in-stent restenosis (Figure 10). The risk of restenosis depends on the diameter of the stent that was placed, the reference diameter of the pulmonary vein, and the time interval between the PVI and the intervention for PVS (20).
Figure 10.
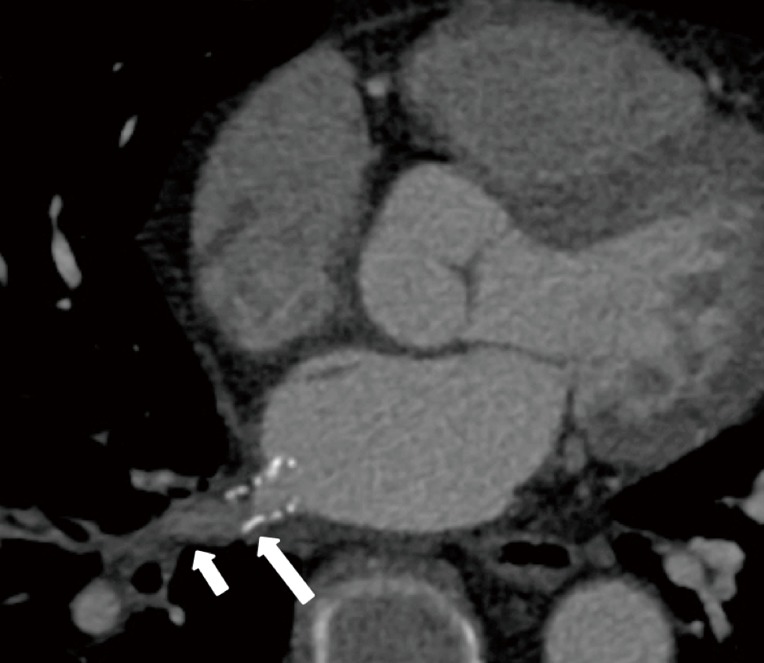
In-stent restenosis after percutaneous stenting of a right inferior pulmonary vein stenosis secondary to fibrosing mediastinitis. Axial reconstructed CT image demonstrates hypoattenuating material in the distal half of the stent (long arrow), suggestive of intimal proliferation, and poor contrast opacification of the pulmonary vein segment distal to the stent (short arrow), suggesting in-stent restenosis.
A useful test for following up with patients who have undergone a percutaneous procedure for the treatment of PVS is radionuclide quantitative lung perfusion imaging. In quantitative flow imaging, regions of interest are drawn around each lung, and the relative blood flow is calculated (Figure 11).
Figure 11.
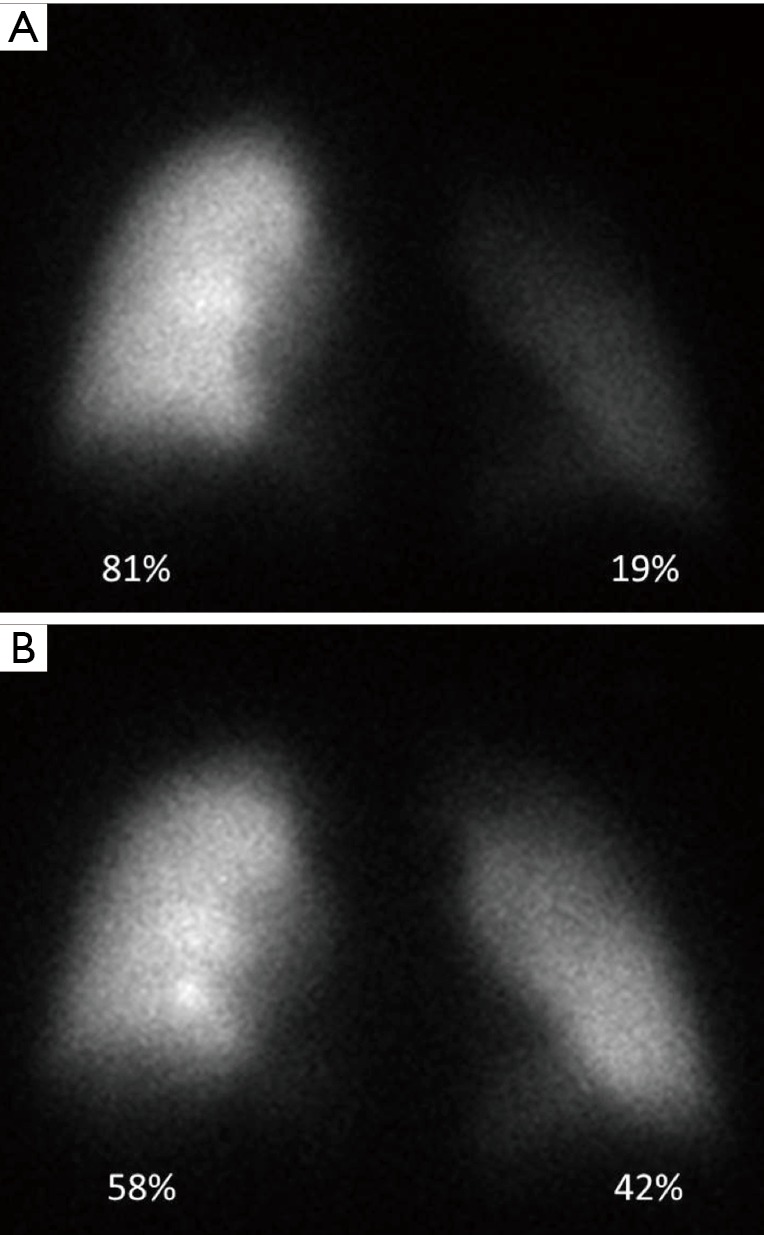
Role of quantitative lung perfusion scintigraphy before and after percutaneous stenting of a left inferior pulmonary vein stenosis in an adult secondary to percutaneous radiofrequency catheter ablation for atrial fibrillation. (A) Pre-treatment quantitative lung perfusion scintigraphy demonstrates decreased perfusion of the left lung as compared to the right lung; (B) post stenting quantitative lung perfusion scintigraphy demonstrates improved perfusion to the left lung when compared to the pre-treatment study.
At our institution, every patient undergoes quantitative lung perfusion imaging within a few days of the percutaneous procedure for the treatment of PVS to establish a baseline. For patients who have a stent placed that is smaller than 10 mm, the follow-up plan depends upon the immediate post-procedure lung perfusion scan. If this scan shows an increase in blood flow in the affected lung, another lung perfusion scan is performed after 3 to 4 months. If there is no significant increase in pulmonary flow after the procedure, a CT scan is performed after 3 to 4 months (20). For those patients who have stents placed that are 10 mm or larger, either a CT or a lung perfusion scan is performed yearly for the first 3 to 4 years. After 4 years of follow-up, if no restenosis has been identified, a CT scan or a lung perfusion scan is performed every few years. As longer-term follow-up is gathered on these patients we may stop recommending further imaging after 4–5 years if in-stent restenosis for larger stents is not detected beyond that point.
Conclusions
PVS, though uncommon, is a condition with important clinical ramifications. Radiologists can play a crucial role in the identification and evaluation of PVS, however this requires an understanding of the patient population at risk, the direct and indirect imaging findings of PVS on imaging, and what information is relevant in the treatment of this condition.
Acknowledgements
The authors thank Megan M. Griffiths, MA, for her editing contribution to the manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sun CC, Doyle T, Ringel RE. Pulmonary vein stenosis. Hum Pathol 1995;26:880-6. 10.1016/0046-8177(95)90011-X [DOI] [PubMed] [Google Scholar]
- 2.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007;115:103-8. 10.1161/CIRCULATIONAHA.106.646166 [DOI] [PubMed] [Google Scholar]
- 3.Neill CA. Development of the pulmonary veins with reference to the embryology of anomalies of pulmonary venous return. Pediatrics 1956;18:880-7. [PubMed] [Google Scholar]
- 4.Marom EM, Herndon JE, Kim YH, et al. Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology 2004;230:824-9. 10.1148/radiol.2303030315 [DOI] [PubMed] [Google Scholar]
- 5.Can MM. Severe pulmonary vein stenosis due to invasion of metastatic lung cancer. Anadolu Kardiyol Derg 2013;13:E17. [DOI] [PubMed] [Google Scholar]
- 6.Kazawa N, Kitaichi M, Hiraoka M, et al. Small cell lung carcinoma: Eight types of extension and spread on computed tomography. J Comput Assist Tomogr 2006;30:653-61. 10.1097/00004728-200607000-00017 [DOI] [PubMed] [Google Scholar]
- 7.Stinson JM, Goodwin RA., Jr Pulmonary vein obstruction by bronchogenic carcinoma. South Med J 1976;69:1482-3. 10.1097/00007611-197611000-00027 [DOI] [PubMed] [Google Scholar]
- 8.Williamson WA, Tronic BS, Levitan N, et al. Pulmonary venous infarction secondary to squamous cell carcinoma. Chest 1992;102:950-2. 10.1378/chest.102.3.950 [DOI] [PubMed] [Google Scholar]
- 9.Hamzeh I, Rashid A, Shaib F, et al. Pulmonary vein stenosis due to a compressive malignant tumor detected by transesophageal echocardiography. Circulation 2011;123:349-50. 10.1161/CIRCULATIONAHA.110.958082 [DOI] [PubMed] [Google Scholar]
- 10.Webb WR. Lymphoma and lymphoproliferative disease. In: Webb WR, Higgins CB. editors. Thoracic imaging: pulmonary and cardiovascular radiology. 2nd edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2011:131. [Google Scholar]
- 11.Chen CL, Tunick PA, Kronzon I. Pulmonary vein compression by tumor: an unusual Doppler flow pattern. Echocardiography 2005;22:746-7. 10.1111/j.1540-8175.2005.00114.x [DOI] [PubMed] [Google Scholar]
- 12.Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. Radiographics 2001;21:737-57. 10.1148/radiographics.21.3.g01ma17737 [DOI] [PubMed] [Google Scholar]
- 13.Bindelglass IL, Trubowitz S. Pulmonary vein obstruction: an uncommon sequel to chronic fibrous mediastinitis. Ann Intern Med 1958;48:876-91. 10.7326/0003-4819-48-4-876 [DOI] [PubMed] [Google Scholar]
- 14.Chang SH, Shih CW, Lei MH. Idiopathic mediastinal fibrosis with involvement of the pulmonary vessels and left main coronary artery. Catheter Cardiovasc Interv 2012;79:1019-22. 10.1002/ccd.23154 [DOI] [PubMed] [Google Scholar]
- 15.Leong DP, Dundon BK, Steele PM. Unilateral pulmonary vein stenosis secondary to idiopathic fibrosing mediastinitis. Heart 2008;94:776. 10.1136/hrt.2007.124404 [DOI] [PubMed] [Google Scholar]
- 16.Malagari K, Papiris S. Fibrosing mediastinitis causing rapidly progressive dyspnea, pulmonary edema and death in a 16 yr old male. Monaldi Arch Chest Dis 2004;61:124-7. 10.4081/monaldi.2004.711 [DOI] [PubMed] [Google Scholar]
- 17.Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics 2004;24:e17. 10.1148/rg.e17 [DOI] [PubMed] [Google Scholar]
- 18.Parambil JG, Gersh BJ, Knight MZ, et al. Bronchogenic cyst causing atrial fibrillation by impinging the right inferior pulmonary vein. Am J Med Sci 2006;331:336-8. 10.1097/00000441-200606000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Yildiz CE, Ercan S, Ergenoglu MU, et al. Surgical treatment of a bronchial cyst causing compression to the left atrium and pulmonary artery. Heart Surg Forum 2009;12:E297-9. 10.1532/HSF98.20081145 [DOI] [PubMed] [Google Scholar]
- 20.Prieto LR. The state of the art in pulmonary vein stenosis - diagnosis & treatment. J Atr Fibrillation 2010;2:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WS, Yeon KM, Kim I, et al. Radiological evaluation of pulmonary vein obstruction including two examinations by magnetic resonance imaging. Pediatr Radiol 1993;23:6-11. 10.1007/BF02020210 [DOI] [PubMed] [Google Scholar]
- 22.Hauser TH, Peters DC, Wylie JV, et al. Evaluating the left atrium by magnetic resonance imaging. Europace 2008;10 Suppl 3:iii22-7. 10.1093/europace/eun223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Voort PH, van den Bosch H, Post JC, et al. Determination of the spatial orientation and shape of pulmonary vein ostia by contrast-enhanced magnetic resonance angiography. Europace 2006;8:1-6. 10.1093/europace/euj006 [DOI] [PubMed] [Google Scholar]
- 24.Schonberger M, Usman A, Galizia M, et al. Time-resolved MR venography of the pulmonary veins precatheter-based ablation for atrial fibrillation. J Magn Reson Imaging 2013;37:127-37. 10.1002/jmri.23808 [DOI] [PubMed] [Google Scholar]
- 25.Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013;268:719-28. 10.1148/radiol.13122276 [DOI] [PubMed] [Google Scholar]
- 26.Martin DR. Nephrogenic systemic fibrosis. Pediatr Radiol 2008;38 Suppl 1:S125-9. 10.1007/s00247-007-0589-8 [DOI] [PubMed] [Google Scholar]
- 27.Krishnam MS, Tomasian A, Malik S, et al. Three-dimensional imaging of pulmonary veins by a novel steady-state free-precession magnetic resonance angiography technique without the use of intravenous contrast agent: initial experience. Invest Radiol 2009;44:447-53. 10.1097/RLI.0b013e3181a7c6cb [DOI] [PubMed] [Google Scholar]
- 28.Körperich H, Gieseke J, Esdorn H, et al. Ultrafast time-resolved contrast-enhanced 3D pulmonary venous cardiovascular magnetic resonance angiography using SENSE combined with CENTRA-keyhole. J Cardiovasc Magn Reson 2007;9:77-87. 10.1080/10976640600897351 [DOI] [PubMed] [Google Scholar]
- 29.Porres DV, Morenza OP, Pallisa E, et al. Learning from the pulmonary veins. Radiographics 2013;33:999-1022. 10.1148/rg.334125043 [DOI] [PubMed] [Google Scholar]
- 30.Shroff GS, Guirguis MS, Ferguson EC, et al. CT imaging of complications of catheter ablation for atrial fibrillation. Clin Radiol 2014;69:96-102. 10.1016/j.crad.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 31.Prieto LR, Schoenhagen P, Arruda MJ, et al. Comparison of stent versus balloon angioplasty for pulmonary vein stenosis complicating pulmonary vein isolation. J Cardiovasc Electrophysiol 2008;19:673-8. 10.1111/j.1540-8167.2008.01110.x [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AM, Prieto LR, Latson LA, et al. Transcatheter angioplasty for acquired pulmonary vein stenosis after radiofrequency ablation. Circulation 2003;108:1336-42. 10.1161/01.CIR.0000086322.21781.6A [DOI] [PubMed] [Google Scholar]
- 33.Neumann T, Kuniss M, Conradi G, et al. Pulmonary vein stenting for the treatment of acquired severe pulmonary vein stenosis after pulmonary vein isolation: clinical implications after long-term follow-up of 4 years. J Cardiovasc Electrophysiol 2009;20:251-7. 10.1111/j.1540-8167.2008.01316.x [DOI] [PubMed] [Google Scholar]
- 34.Hill J, Qureshi AM, Worley S, et al. Percutaneous recanalization of totally occluded pulmonary veins after pulmonary vein isolation – intermediate-term follow-up. Catheter Cardiovasc Interv 2013;82:585-91. [DOI] [PubMed] [Google Scholar]
- 35.Steliga MA, Ghouri M, Massumi A, et al. Lobectomy for pulmonary vein occlusion secondary to radiofrequency ablation. J Cardiovasc Electrophysiol 2010;21:1055-8. 10.1111/j.1540-8167.2010.01763.x [DOI] [PubMed] [Google Scholar]
- 36.Libretti L, Ciriaco P, Zannini P. Pulmonary vein stenosis requiring lobectomy after radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Surg (Torino) 2012;53:821-3. [PubMed] [Google Scholar]
- 37.Obeso A, Tilve A, Jimenez A, et al. Spontaneous massive hemothorax presenting as a late complication of stent implantation in a patient with pulmonary vein stenosis following radiofrequency ablation for atrial fibrillation. Interact Cardiovasc Thorac Surg 2018;26:869-72. 10.1093/icvts/ivx380 [DOI] [PubMed] [Google Scholar]