Abstract
Aims:
Prior investigation has found that mechanical ventilation with lower tidal volumes (Vt) following out-of-hospital cardiac arrest is associated with better neurologic outcomes. The relationship between Vt and neurologic outcome following in-hospital cardiac arrest (IHCA) has not previously been explored. In the present study, we investigate the association between Vt and neurologic outcome following IHCA.
Methods:
This was an observational study using a prospectively collected database of IHCA patients at a tertiary care hospital in the United States. The relationship between time-weighted average Vt per predicted body weight (PBW) over the first 6- and 48 h after cardiac arrest and neurologic outcome were assessed using propensity-score adjusted logistic regression.
Measurements and main results:
Of 185 IHCA patients who received invasive mechanical ventilation within 6 h of return of spontaneous circulation (ROSC), the average Vt over the first 6 h was 7.7 ±2.0 ml/kg and 68 (36.8%) patients received an average Vt >8.0 ml/kg. Of 121 patients who received mechanical ventilation for at least 48 h post-ROSC, the average Vt was 7.6 ±1.5 ml/kg and 46 (38.0%) patients received an average Vt > 8.0 ml/kg. There was no relationship between Vt/PBW over the first 6- or 48 h post-ROSC and neurologic outcome (OR 0.99; 95%CI 0.84–1.16; p = 0.89; OR 1.03; 95%CI 0.78–1.37; p = 0.83 respectively).
Conclusions:
This study did not identify a relationship between Vt and neurologic outcome following IHCA. This contrasts with results in OHCA, where higher Vt has been associated with worse neurologic outcome. Additional investigation is needed with respect to other potential benefits of low-Vt post IHCA.
Keywords: Mechanical ventilation
Introduction
In-hospital cardiac arrest (IHCA) is a common and devastating event impacting approximately 200,000 hospitalized patients in the United States annually.[1] Although survival and neurologic outcomes following IHCA have improved over the past decade, mortality remains high—as of 2009 just 22% of patients survive to hospital discharge [2]. Further, even amongst IHCA survivors, neurologic morbidity rates are notable with 28% experiencing significant neurologic injury at the time of discharge [2]. To date, there remains no known therapy to improve outcomes following IHCA and recent studies of targeted temperature management in this population have not reliably shown benefit [3].
Mechanical ventilation is a standard component of post-IHCA critical care following return-of-spontaneous circulation (ROSC). Nevertheless, the published literature offers little guidance with regards to target tidal volumes (Vt) for initial survivors of IHCA events. In general, ventilator management strategies are extrapolated from those used in other conditions. In the acute respiratory distress syndrome (ARDS), for instance, a low-Vt ventilation strategy has been shown to reduce time on the ventilator, lead to a more rapid fall in interleukin-6 levels, and improve mortality [4].
In a recent study of patients receiving mechanical ventilation following out-of-hospital cardiac arrest (OHCA), investigators found that targeting lower Vt over the first 48 h post-ROSC resulted in more favorable neurologic outcomes [5]. While the underlying pathophysiologic mechanisms of this finding have not yet been fully elucidated, the authors hypothesized that low-Vt ventilation may improve ‘lung-brain crosstalk,’ attenuate systemic inflammation, and/or lead to favorable changes in blood oxygen or carbon dioxide tension [5].
Patients who experience IHCA are more likely to have a witnessed arrest, have a non-shockable initial rhythm, and tend to be older with more medical comorbidities than patients who experience OHCA [2,6]. In the present study we sought to investigate the hypothesis that low-Vt ventilation is associated with better neurologic outcome in patients requiring mechanical ventilation following IHCA.
Methods
Data source & patient population
This study was performed through a query of a prospectively collected database combined with clinical variables retrospectively abstracted from the electronic medical record of hospitalized patients who experienced a cardiac arrest while being cared for at a tertiary care hospital in the United States between January 2008 and December 2015. Patients were included if they obtained ROSC (defined as ≥20 min of restored pulse) and received mechanical ventilation within 6 h of ROSC. Events that occurred in the emergency department, hospital wards, intensive care units (ICUs), and procedural areas (including operating rooms) were included. Patients transferred to the tertiary care center following IHCA at an outside hospital, non-index events, patients who were not intubated or who were intubated for <6 h, patients missing data regarding height, and patients who received extracorporeal membrane oxygenation (ECMO) were excluded. See Fig. 1.
Fig 1.

Cohort Selection.
Data collection
Demographic data, including patient height, were abstracted from the electronic medical record (EMR). Exhaled Vt is automatically recorded in the ICU EMR for patients receiving mechanical ventilation and recorded values are verified by the bedside nurse. The average Vt over 6 h was obtained by determining a time-weighted average of recorded Vt over the first 6 h. In cases where only one Vt was recorded over the first 6 h, it was assumed that the recorded Vt was used throughout the first 6 h. Average Vt over the first 48 h post-ROSC was obtained through the assessment of the area-under-the-curve of the Vt vs. time plot. For each patient, the site of cardiac arrest (i.e. ED vs. ICU vs. Ward vs. Procedural Area), timing of intubation, and reasons for death were determined via hand review of the clinical chart. Reasons for death were defined using a previously described classification system (Supplementary Table S1 in the online version at DOI:10.1016/j.resuscitation.2017.12.031) [7].
Exposures & outcomes
The average exhaled Vt over the first 6 h following ROSC was the primary exposure of interest. The average Vt over the first 48 h following ROSC (for those patients receiving mechanical ventilation for at least 48 h) was included as a secondary exposure of interest to align this study with that of Beitler et. al [5]. and to allow for the assessment of the relationship between Vt and neurologic outcome over time. A sensitivity analysis was performed using set Vt for all patients ventilated using a control mode of ventilation in which a Vt was set.
The primary outcome was discharge with favorable neurologic outcome as defined by a Cerebral Performance Category (CPC) Score of 1 or 2. CPC scores range from 1 (indicating normal function or minor neurocognitive deficit) to 5 (indicating death or brain death) [8]. Secondary outcomes included ventilator-free days in the 10 days following ROSC and in-hospital mortality.
As sensitivity analyses, CPC scores were also evaluated both as ordinal variables and as a dichotomized variable where a CPC score of 1 was considered a good neurologic outcome and a CPC score of 2–5 was considered a poor neurologic outcome. An additional sensitivity analysis was performed excluding patients who were not ventilated using a control mode of ventilation.
Statistical analysis
Descriptive statistics are reported with means with standard deviations or medians with interquartile ranges. Two-way comparisons were made with t-tests, Wilcoxon rank sum tests, chi-square tests, or Fishers exact tests as appropriate. The relationship between Vt and dichotomized CPC score was performed using propensity score covariate adjustment. The propensity score was determined through the use of logistic regression to estimate the probability of receiving high (>8 ml/kg) vs. low (≤8 ml/kg) Vt. Vt was used as a continuous variable and adjusted for predicted body weight (PBW) using the NHLBI ARDS Network equation [4]. Covariates in the propensity score included age, gender, total downtime, initial rhythm (shockable vs. non-shockable), arrest location, receipt of vasopressors (receipt of any vasopressor for at least 1 h in the first 6 h post-ROSC or 48 h post-ROSC depending on the exposure), and use of targeted temperature management (TTM). Multivariable logistic regression and inverse probability weighting were used to assess the relationship between the average Vt/PBW and dichotomized CPC score as alternative methods of covariate adjustment. In addition, ordinal logistic regression was used to assess the relationship between Vt and ordinal CPC score.
The relationship between Vt/PBW and in-hospital mortality was compared in univariate and after adjustment for the propensity score. The association between ventilator-free days over the first 10 days post-ROSC and Vt/PBW were likewise assessed in univariate and then following negative binomial regression adjusting for the propensity score. As a sensitivity analysis, patients who died within 10 days post-ROSC were assigned zero ventilator-free days to avoid the assignment of ventilator-free days to subjects who were ventilator free as a result of death. Data was missing regarding downtime for 5 patients and these patients were dropped from the multivariable analyses. All statistics were performed using STATA, version 14 (College Station, TX, StataCorp LP, USA).
Results
Cohort characteristics
The final study cohort included 185 patients who experienced IHCA and received mechanical ventilation within the first 6 h following ROSC (see Fig. 1). The mean age of the study population was 68.4 ± 15.2 years and 36.2% were female (see Table 1 for complete data regarding the study cohort). The majority of patients in the cohort (60.5%) were intubated during the cardiac arrest while a minority were intubated prior to the arrest (26.5%) or following the arrest (13.0%).
Table 1.
Cohort Characteristics Overall and Divided by High/Low Vt over the First 6 h post-ROSC.
| Total Population n = 185 | High Vt* (>8ml/kg) n = 68 | Low Vt (≤8ml/kg) N=117 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean, SD) | 68.4 (15.2) | 68.7 (15.4) | 68.3(15.2) | 0.96 |
| Sex (%F) | 36.2 | 60.3 | 22 | <0.001 |
| Race (%W) | 66.5 | 67.7 | 65.8 | 0.80 |
| Arrest Characteristics | ||||
| Location (%) | ||||
| EDǂ | 15.7 | 10.3 | 18.8 | 0.02 |
| Ward | 31.4 | 38.2 | 27.4 | |
| ICU§ | 32.4 | 39.7 | 28.2 | |
| Procedural Area/OR | 20.5 | 11.8 | 25.6 | |
| Initial Rhythm (%) | ||||
| Shockable | 22.2 | 22.1 | 22.2 | >0.99 |
| Non-Shockable | 75.1 | 75 | 75.2 | |
| Unknown | 2.7 | 2.9 | 2.6 | |
| Downtime (minutes; median, IQR) | 10.0 (5.0.17.5) | 11.0 (7.0,19.0) | 9.0 (4.0,17.0) | 0.02 |
| Post-Arrest Characteristics | ||||
| Vasopressors (%received) | 78.9 | 85.3 | 75.2 | 0.10 |
| TTM (%cooled) | 30.8 | 23.5 | 35.0 | 0.10 |
Vt = Tidal Volume;
ED = Emergency Department
= ICU = Intensive Care Unit.
Over the first 6 h following ROSC, the mean Vt was 7.7 ±2.0 ml/kg and 68 (36.8%) of patients received Vt > 8.0 ml/kg. A total of 29 (15.1%) patients received Vt of ≤6 ml/kg, 19 (10.3%) received Vt ≥ 10 ml/kg, and 6 (3.2%) received Vt ≥ 12 ml/kg. Females (60.3% vs. 22.0%, p < 0.001) and patients with longer downtimes (median 11.0 [IQR 7.0, 19.0] minutes vs. 9.0 [4.0, 17.0] minutes, p = 0.02) were more likely to be ventilated with higher Vt. Patients who suffered cardiac arrest in the operating room (p = 0.02) were less likely to be ventilated with higher Vt compared to patients arresting in other locations (see Table 1).
For those patients who received mechanical ventilation for at least 48 h (n = 121), the mean Vt was 7.6 ±1.5 ml/kg and 46 (38.0%) of patients received Vt > 8.0 ml/kg (see Supplementary Table S2 in the online version DOI: 10.1016/j.resuscitation.2017.12.031 for characteristics of patients ventilated for at least 48 h). Of the 46 patients who received Vt > 8.0 ml/kg over the first 48 h, 38 (77.6%) received Vt >8.0 ml/kg over the first 6 h. Of those patients who were ventilated for at least 6 h but less than 48 h, 27 (42.2%) died within the first 48 h post-ROSC and 37 (57.8%) were extubated.
Association between Vt and neurologic outcome at discharge
In unadjusted analysis, there was no difference in average Vt over the first 6 h post-ROSC in patients with good vs. poor neurologic outcome (7.64 ± 2.10 ml/kg vs. 7.72 ±1.95 ml/kg, p = 0.80). After adjusting for the propensity score, there remained no relationship between average Vt and neurologic outcome (OR 0.98;95%CI 0.84–1.16; p = 0.89). A similar result was seen using inverse probability weighting (0.99, 95%CI 0.84–1.17, p = 0.92). When using multivariable logistic regression and including the pre-specified covariates directly in the regression model, there was no relationship between Vt and dichotomized CPC score (OR 0.98; 95%CI 0.83–1.15; p = 0.77).
Among patients ventilated for at least 48 h following ROSC, there was no difference in average Vt over the first 48 h post-ROSC in patients with good vs. poor neurologic outcome (7.70 ± 1.41 ml/kg vs. 7.59 ±1.62 ml/kg, p = 0.71). In the propensity score adjusted logistic regression, there was no significant relationship between Vt and dichotomized CPC score (OR 1.03; 95%CI 0.78–1.37; p = 0.83, see Fig. 2). Adjusting for covariates through multiple logistic regression likewise did not reveal a significant relationship between Vt and neurologic outcome at discharge (OR 1.00; 95%CI 0.74–1.37; p = 0.97).
Fig. 2.
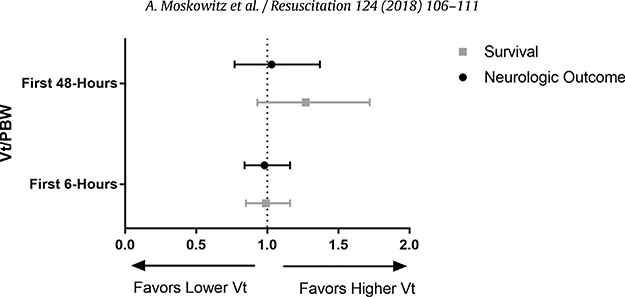
Odds Ratio (95%CI) for Favorable Neurologic Outcome or Survival per 1 ml/kg increase in Tidal Volume.
When CPC score was considered as an ordinal variable, there remained no association between Vt over the first 6 h (OR 1.02; 95%CI 0.88–1.18; p = 0.81) or Vt over the first 48 h (OR 0.93;95%CI 0.73–1.18; p = 0.54) and neurologic outcome after propensity adjustment. Similarly, when CPC was dichotomized with a CPC score of 1 indicating good neurologic outcome, there was no association between Vt over the first 6 h (OR 0.95, 95%CI 0.80–1.13, p = 0.59) or Vt over the first 48 h (OR 0.92, 95%CI 0.67–1.28, p = 0.63) and neurologic outcome after propensity adjustment.
Most patients were ventilated with a control-mode of ventilation (n = 171, 92.4%). There were 14 (7.6%) of patients who were ventilated using a non-control method of ventilation (i.e. pressure support ventilation). In a sensitivity analysis excluding these latter patients, there was no relationship identified between Vt at 6-h (OR 1.01, 95%CI 0.85–1.20, p = 0.90) or 48-h (OR 1.03, 95%CI 0.77–1.39, p = 0.83) and neurologic outcome. There was also no association when using set Vt instead of exhaled Vt (for 6-h OR 0.98, 95%CI 0.74–1.31, p = 0.91 and for 48-h OR 1.03, 95%CI 0.69–1.53, p = 0.90)
Association between Vt and in-Hospital mortality
A total of 87 (47.0%) of patients in the cohort died during their hospital stay. Of those, the most common reasons-for-death were ‘co-morbid withdrawal of care” (54.0%) and “neurologic withdrawal of care” (24.1%). No patients in the cohort expired due to “refractory respiratory failure.”
In propensity-score adjusted logistic regression, there was no relationship between Vt over the first 6-h (OR 0.99; 95%CI 0.85–1.16; p = 0.94) or Vt over the first 48-h (OR 01.27; 95%CI 0.93–1.72; p = 0.13)and survival.
Association between Vt and days receiving mechanical ventilation
Overall, patients in the cohort had a median of 6.0 (IQR 3.0–8.0) ventilator-free days over the first 10 days following ROSC. There was no difference in median ventilator-free days between those patients who received a Vt of >8 ml/kg and those who received≤8 ml/kg PBW in the first 6 h post-ROSC (5.0 [IQR 3.0–8.0] vs. 6.0 [IQR 2.0–8.0], p = 0.54) or in the first 48 h post-ROSC (4.0 [IQR 0–6.0] vs. 4.0 [IQR 0.0–6.0], p = 0.70). There was likewise no significant (β=−0.02, p = 0.42 for first 6 h, β =−0.10, p = 0.14 for first 48 h). When those patients who expired in the first 10 days post-ROSC were assigned “0” ventilator free days, patients who received higher vs. lower Vt in both the first 6 h post-ROSC (0.0 [IQR 0.0–5.0] vs. 0.0 [IQR 0.0–7.0], p = 0.90) and the first 48 h post-ROSC (0.0 [IQR 0–4.0] vs. 4.0 [IQR 0.0–4.0], p = 0.88) had similar numbers of ventilator free days. This difference did not reach statistical significance after propensity score adjustment (β=−0.03, p = 0.74 for first 6 h, β= −0.06, p = 0.61 for first 48 h). See Table 2 and Supplementary Table S3 in the online version DOI: 10.1016/j.resuscitation.2017.12.031 for outcomes stratified by high/low Vt.
Table 2.
Outcomes by Vt over the First 6 h post-ROSC.
| Total Population n = 185 | High Vt (>8ml/kg) n = 68 | Low Vt (≤8ml/kg) N = 117 | p-Value | |
|---|---|---|---|---|
| CPC* 1 or 2 (n, %) | 87 (47.0) | 32(47.5) | 55 (47.0) | 0.99 |
| Ventilator-Free Days (median, IQR) | 6.0 (3.0–8.0) | 5.0 (3.0–8.0) | 6.0 (2.0–8.0) | 0.54 |
| Mortality (n, %died) | 87 (47.0) | 33 (48.5) | 54 (46.s) | 0.76 |
| Reason for Death | ||||
| Sudden Cardiac Arrest | 8 (9.2) | 3(9.1) | 5 (9.3) | 0.98 |
| Refractory Shock | 11 (12.6) | 4(12.1) | 7(13.0) | |
| Refractory Hypoxemia | 0 (0.0) | 0.0 | 0.0 | |
| Neurologic Withdrawal of Care | 21 (24.1) | 7 (21.2) | 14(25.9) | |
| Co-Morbid Withdrawal of Care | 47 (54.0) | 19(57.6) | 28 (51.6) |
CPC = Cerebral Performance Category.
Discussion
In the present study, we did not find any association between either Vt in the immediate post-arrest period or Vt over the was likewise no relationship between Vt and in-hospital mortality. These findings were consistent in sensitivity analyses using dichotomized or ordinal CPC outcome variables and when using different methodologies of covariate adjustment. There was no identified relationship between tidal volume and ventilator-free days.
Low-tidal volume ventilation in patients with ARDS has been associated with a decreased duration of mechanical ventilation, a more rapid fall in inflammatory biomarkers, and decreased mortality [4]. These findings may potentially be extended to the population of critically ill patients without ARDS and to patients undergoing abdominal surgery [9,10]. The pathophysiologic underpinning for improved outcomes in patients receiving low-Vt ventilation remain incompletely understood, but likely relates to a combination of preventing excessive stretch (especially at the boundary between aerated and atelectatic lung), limiting barotrauma, and reducing inflammation.
To date, few studies have explored the use of low-Vt ventilation in the post-cardiac arrest period. In one prospective, multi-national observational study, Sutherasan et. al. found that Vt decreased overtime from 8.9 ml/kg in 1998–6.5 ml/kg in 2010 in patients receiving mechanical ventilation after cardiac arrest. Higher Vt in this post-arrest population was associated with an increased occurrence of ARDS [11]. Among patients with either cardiac arrest (n = 25) or congestive heart failure (n = 26) in a cardiac intensive care unit, higher tidal volumes were associated with an increased risk of mortality [12].
Most recently, Beitler et. al. retrospectively analyzed 256 initial survivors of OHCA who received mechanical ventilation for at least 48 h post ROSC. In that population, 38% of patients received a time-weighted average Vt of over 8 ml/kg. Notably, lower-Vt ventilation was associated with more favorable neurologic outcomes in addition to more ventilator-free and shock-free days [5]. There was no significant association between Vt and survival to hospital discharge. The authors suggest that their findings with respect to neurologic outcome may reflect improved ‘lung-brain crosstalk,’ an attenuated systemic inflammatory response, and/or favorable changes in blood oxygen or carbon dioxide tension resulting from lower Vt ventilation [5].
As arterial carbon dioxide tension (PaCO2) affects cerebral blood flow, one potential mechanism through which selection of tidal volume might impact post-arrest outcome is with respect to its effects on PaCO2. In the one prior study of Vt after OHCA, there was no relationship identified between tidal volume and the PaCO2 over the first 48 h post-arrest [5]. Prior studies have evaluated the relationship between PaCO2 and outcomes with mixed results, but findings generally support avoiding hypocapnia and targeting normocapnia [13,14]. Given the many potentially contributing factors to PaCO2 post-arrest and lack of uniformity with respect to when and how frequently PaCO2 is measured in our post-IHCA patients, we did not assess the relationship between tidal volume and PaCO2 in this study. This is an important avenue of research for future investigation.
Selection of the Vt target and the relationship between Vt and outcomes have not been fully explored among initial survivors of IHCA. Similar to the findings by Beitler et. al. in the OHCA population, approximately 35–40% of patients in this study were ventilated with Vt > 8 ml/kg. In this cohort, however, higher Vts were not associated with worse neurologic outcomes. The reason for the discordance between this study and the study by Beitler et. al. is not entirely clear, but possibly reflects differences in patient populations and arrest characteristics between OHCA and IHCA. In this IHCA cohort, for instance, 75% of patients had an initial non-shockable rhythm as compared to 50% of the OHCA population in the study by Beitler et. al. Further, while the most common cause of death following OHCA is often cited as neurologic withdrawal of care [15], comorbid withdrawal of care was the most common reason for death in this cohort. No patients in this IHCA cohort expired due to refractory hypoxemia.
Female sex was a strong predictor of high-Vt ventilation in this IHCA cohort. Whereas 35.6% of the total cohort was female, 60.3% of those ventilated with Vt > 8 ml/kg were female. The finding that females are more likely to be ventilated with higher Vt/PBW than men has been demonstrated previously [16,17]. Although the reason for higher Vt/PBW in females is unclear, one potential hypothesis is that clinicians in the post-arrest period may be faced with a number of pressing and competing management tasks leading to the selection of Vt without first checking the patient height. While there was no association between Vt and CPC score in this study, there may be other benefits of low-Vt ventilation (e.g. reduced inflammatory response) that female patients may not be receiving as routinely.
This was a small, single center study which limits the study’s statistical power and generalizability of the results. Nevertheless, to our knowledge, this is the largest focused study of Vt following IHCA in the literature. The observational nature of the analysis leaves open the possibility of residual confounding. CPC score at hospital discharge, while commonly used in studies of cardiac arrest victims, may not possess adequate power to detect more subtle neurologic injury [18]. To this end, however, sensitivity analyses were performed classifying just a CPC score of 1 as a good neurologic outcome. In addition, data regarding pre-admission CPC score was not available and could not be controlled for in this study. Finally, this study was not designed to explore other potential benefits of low-Vt ventilation such as time to shock reversal.
Conclusions
Patients who achieve ROSC and require mechanical ventilation following IHCA are frequently ventilated at Vt >8 ml/kg. Women, in particular, are at increased risk of receiving higher Vt/PBW. There was no association between higher Vt (at either 6 or 48 h) and either neurologic outcome or mortality. This contrasts with results in OHCA, where higher Vt has been associated with worse neurologic outcome. While our data do not suggest a relationship between low-Vt post-IHCA and neurologic outcome, additional investigation is needed with respect to other potential benefits of low-Vt ventilation in the post IHCA period.
Contributions
All authors contributed substantially to the design of the work, data acquisition, and interpretation of the results. AM, KB and MD conceived of the project. AM, SG, MC, and PP participated in chart review and data abstraction. AM and AG performed the statistical analyses. AM, and MD drafted the manuscript. All authors reviewed the manuscript and revised it for intellectual content. All approved the manuscript prior to submission.
Supplementary Material
Funding sources
Dr. Moskowitz, Dr. Berg, and Dr. Donnino are funded by grants from the National Institutes of Health (NIH). Dr. Donnino is funded by 5K24HL127101. Dr. Moskowitz is funded by 2T32HL007374–37. Dr. Berg is funded by K23HL128814–02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Cocchi is funded by the Scientist Development Grant from the American Heart Association (15SDG22420010).
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflicts.
A Spanish translated version of the abstract of this article appears as Appendixin the final online version at https://doi.org/10.1016/j.resuscitation.2017.12.031.
References
- [1].Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, et al. American Heart Association get with the Guidelines-Resuscitation investigators. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med 2011;39(11):2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. American Heart Association get with the Guidelines-Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012;367(20):1912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chan PS, Berg RA, Tang Y, Curtis LH, Spertus JA. American Heart Association’s get with the Guidelines-Resuscitation investigators. association between therapeutic hypothermia and survival after In-hospital cardiac arrest. JAMA 2016;316(13):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- [5].Beitler JR, Ghafouri TB,Jinadasa SP, Mueller A, Hsu L, Anderson RJ, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med 2017;195(9):1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buanes EA, Heltne JK. Comparison of in-hospital and out-of-hospital cardiac arrest outcomes in a Scandinavian community. Acta Anaesthesiol Scand 2014;58(3):316–22. [DOI] [PubMed] [Google Scholar]
- [7].Moskowitz A, Omar Y, Chase M, Lokhandwala S, Patel P, Andersen LW, et al. Reasons for death in patients with sepsis and septic shock.J Crit Care 2017;38: 284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Don-nino MW, et al. American heart association emergency cardiovascular care committee; council on cardiopulmonary, critical care, perioperative and resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation 2011;124(19): 2158–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308(16):1651–9. [DOI] [PubMed] [Google Scholar]
- [10].Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M,Neuschwander A, et al. IMPROVE Study Group:a trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369(5):428–37. [DOI] [PubMed] [Google Scholar]
- [11].Sutherasan Y, Peñuelas O, Muriel A, Vargas M, Frutos-Vivar F, Brunetti I, et al. VENTILA group. Management and outcome of mechanically ventilated patients after cardiac arrest. Crit Care 2015;19:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shorofsky M, Jayaraman D, Lellouche F, Husa R, Lipes J. Mechanical ventilation with high tidal volume and associated mortality in the cardiac intensive care unit. Acute Card Care 2014;16(1):9–14. [DOI] [PubMed] [Google Scholar]
- [13].Schneider AG, Eastwood GM, Bellomo R, Bailey M, Lipcsey M, Pilcher D, et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 2013;84(7):927–34. [DOI] [PubMed] [Google Scholar]
- [14].Roberts BW, Kilgannon JH, Chansky ME, Mittal N,Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation 2013;127(21):2107–13. [DOI] [PubMed] [Google Scholar]
- [15].Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004;30(11):2126–8. [DOI] [PubMed] [Google Scholar]
- [16].Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, Wilson JX. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radic Biol Med 2010;48(1):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32(9):1817–24. [DOI] [PubMed] [Google Scholar]
- [18].Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009;80(3):297–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


