Abstract
During aging, the cellular milieu of the brain exhibits tell-tale signs of compromised bioenergetics, impaired adaptive neuroplasticity and resilience, aberrant neuronal network activity, dysregulation of neuronal Ca2+ homeostasis, the accrual of oxidatively modified molecules and organelles, and inflammation. These alterations render the aging brain vulnerable to Alzheimer’s and Parkinson’s diseases and stroke. Emerging findings are revealing mechanisms by which sedentary overindulgent lifestyles accelerate brain aging, whereas lifestyles that include intermittent bioenergetic challenges (exercise, fasting, and intellectual challenges) foster healthy brain aging. Here we provide an overview of the cellular and molecular biology of brain aging, how those processes interface with disease-specific neurodegenerative pathways, and how metabolic states influence brain health.
Introduction
As with other organ systems, the functional capabilities of the brain decline progressively during aging, which manifests as decrements in learning and memory, attention, decision-making speed, sensory perception (vision, hearing, touch, smell, and taste), and motor coordination (Alexander et al., 2012; Dykiert et al., 2012; Levin et al., 2014). As we age, cognitive performance generally declines in multiple domains including executive function, working memory (particularly task switching), and episodic memory (Alexander et al., 2012). Older adults often have difficulty understanding rapid speech as a result of cognitive slowing and hearing loss, and they also have reduced comprehension of syntactically complex sentences and impaired word retrieval ability (Alexander et al., 2012). The time course of the age-related decline in brain performance roughly parallels the reduced performance of other organ systems with notable acceleration beyond 50 years of age (Mendonca et al., 2017).
As individuals traverse their sixth, seventh, and eighth decades, they become increasingly prone to the development of a neurodegenerative disorder, with Alzheimer’s disease (AD) and Parkinson’s disease (PD) being the most common (Mattson, 2004; Kalia and Lang, 2015; Scheltens et al., 2016; Aarsland et al., 2017). Aging is also the major risk factor for stroke (Krishnamurthi et al., 2013). Most industrialized countries are experiencing a rapid increase in the proportion of the population over the age of 65, an age range that can be considered the “danger zone” for AD, PD, and stroke. Within the next 30 years, the number of Americans living with diagnosed AD will more than double from the current number of 5 million to more than 12 million (Alzheimer’s Association, 2016). Between the years 2000 and 2013, the number of deaths from heart disease, cancer, and stroke decreased by more than 10%, whereas the number of deaths attributable to AD increased by 70%. In the United States, there are approximately 1 million individuals living with diagnosed PD. Globally, approximately 12 million people suffer a stroke each year with nearly 3 million of them dying (Bennett et al., 2014).
The human brain shrinks during normal aging, with reductions in both gray and white matter and an associated enlargement of the cerebral ventricles (Drayer, 1988). Longitudinal magnetic resonance imaging (MRI) studies have shown that age-related reductions in gray matter are most prominent in the temporal and frontal lobes (Jack et al., 1997). The rate of brain atrophy during aging can predict whether or not someone develops cognitive impairment and dementia (Jack et al., 2005). Cross-sectional histological analyses suggest that the atrophy results from a combination of dendritic regression and neuronal death (Dumitriu et al., 2010). While there is inter-individual variability in the rate of brain atrophy during aging, it has been suggested that brain imaging data can be used to establish a “biological age” of one’s brain (Cole and Franke, 2017). Environmental factors can influence the rate of brain structural changes during aging. For example, aerobic exercise increases hippocampal volume (Erickson et al., 2011), whereas excessive energy intake and obesity accelerate hippocampal atrophy (Cherbuin et al., 2015). Caloric restriction (CR) and intermittent fasting (IF) retard structural and functional decline during aging in laboratory rodents and monkeys (Duan et al., 2003; Willette et al., 2010).
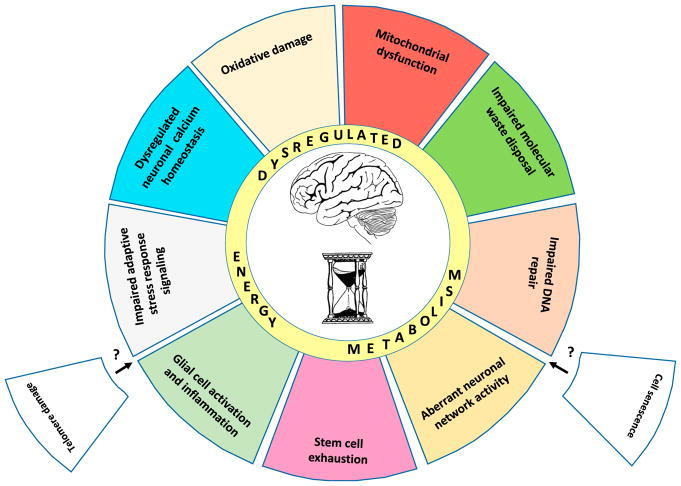
Interrogation of the brain at the cellular and molecular levels reveals many of the hallmarks of aging evident in other tissues (López-Otín et al., 2013). As detailed below, these hallmarks include (1) mitochondrial dysfunction; (2) the intracellular accumulation of oxidatively damaged proteins, nucleic acids, and lipids; (3) dysregulated energy metabolism; (4) impaired cellular “waste disposal” mechanisms (autophagylysosome and proteasome functionality); (5) impaired adaptive stress response signaling; (6) compromised DNA repair; (7) aberrant neuronal network activity; (8) dysregulated neuronal Ca2+ handling; (9) stem cell exhaustion; and (10) inflammation (Figure 1). Cellular senescence and telomere attrition, two hallmarks of aging in proliferative peripheral tissues (López-Otín et al., 2013), may occur in some types of glial cells in the brain, but this remains to be established.
Figure 1. Hallmarks of Brain Aging.
There are ten established hallmarks of brain aging. The illustration depicts nine of the hallmarks as colored slices of pie interacting prominently with “dysregulated energy metabolism,” which is shown as an inner ring of the aging wheel. Also shown are two slices (telomere damage and cell senescence) that are considered hallmarks of aging in proliferative peripheral tissues, but have not yet been established as hallmarks of brain aging.
Environmental factors experienced during early and midlife can affect the risk for poor brain function and neurodegenerative disease late in life. For example, similar to joint injuries and the risk of osteoarthritis later in life, traumatic brain injury (TBI) and emotional trauma during early or midlife can increase the risk for late life cognitive impairment and AD and PD (Blennow et al., 2016). In addition, considerable evidence has accumulated demonstrating that the amount, type, and frequency of dietary energy intake, and energy expenditure (exercise), are major determinants of brain health throughout the life course (Mattson et al., 2018). Here we review the current understanding of mechanisms of brain aging with a focus on energy metabolism and its interactions with pathways involved in cellular stress resistance, repair, and growth. We then consider how usual aging processes impact both upstream and downstream of the disease-defining proteopathic lesions in AD (amyloid plaques and Tau tangles) and PD (α-synuclein inclusions). We describe mechanisms by which a chronic positive energy balance accelerates brain aging, and how intermittent metabolic challenges (intermittent energy restriction and exercise) and drugs that target neuronal bioenergetics can protect the brain against age-related dysfunction and disease.
Cellular and Molecular Hallmarks of Brain Aging
Mitochondrial Dysfunction
Mitochondria are distributed throughout the dendrites and axons of neurons, where they generate the ATP required to support electrochemical neurotransmission, and cell maintenance and repair (Mattson et al., 2008). Mitochondria can grow in size and divide (mitochondrial biogenesis) and can be removed by degradation in lysosomes (mitophagy). In addition to their fundamental role in cellular energy metabolism, mitochondria play critical roles in cellular Ca2+ homeostasis and as a source of signals that regulate nuclear gene transcription (Hou et al., 2012; Yun and Finkel, 2014; Raefsky and Mattson, 2017). Moreover, the formation of mitochondrial membrane permeability transition pores (mPTPs) is a pivotal event in apoptosis, a form of programmed cell death that occurs normally during brain development and pathologically in a range of neurodegenerative conditions (Mattson, 2000).
Several technical approaches have been used to determine if and how aging impacts mitochondria in brain cells. They include analysis of mitochondria-specific proteins, functional interrogation of isolated mitochondria or mitochondria in synaptosome preparations, and manipulation of genes that encode mitochondrial proteins (Grimm and Eckert, 2017). Comparisons of mitochondria isolated from brain tissue of animals reveal numerous age-related alterations, including mitochondrial enlargement or fragmentation (Stahon et al., 2016; Morozov et al., 2017), increased oxidative damage to mitochondrial DNA (Kim and Chan, 2001; Santos et al., 2013), impaired function of the electron transport chain (ETC) (Yao et al., 2010; Pandya et al., 2015, 2016; Pollard et al., 2016), increased numbers of mitochondria with depolarized membranes (Lores-Arnaiz et al., 2016), impaired Ca2+ handling (Leslie et al., 1985; Pandya et al., 2015), and a reduced threshold for triggering mPTP formation (Brown et al., 2004). The decrement in mitochondrial function during brain aging involves a decline in cellular NAD+ levels and the NAD:NADH ratio (Braidy et al., 2014), which would be expected to compromise the activities of NAD+-dependent enzymes critical for neuronal function and viability, including protein deacetylases of the sirtuin family (Fang et al., 2017). Most cell types in the brain likely experience the accumulation of dysfunctional mitochondria during aging as suggested from studies of neurons and astrocytes established from brains of young and old mice, or allowed to “age” in culture (Lin et al., 2007; Ghosh et al., 2012).
CR reduces multiple readouts of brain mitochondrial aging including oxidative damage, ETC function, membrane destabilization, Ca2+ handling, and susceptibility to apoptotic triggers (Gabbita et al., 1997; Sanz et al., 2005; Lin et al., 2014; Amigo et al., 2017). Studies of muscle and liver cells have shown that mitochondrial biogenesis declines during aging and that both CR and exercise can stimulate mitochondrial biogenesis (Martin-Montalvo and de Cabo, 2013; Hood et al., 2016). Emerging evidence suggests that neural signaling pathways activated by exercise and CR can also stimulate mitochondrial biogenesis in neurons in the brain (Cheng et al., 2012; Raefsky and Mattson, 2017; Mattson et al., 2018). Roles of such mitochondrial adaptations to CR and exercise in structural and functional manifestations of brain aging remain to be determined.
Accumulation of Oxidatively Damaged Molecules
During aging, neurons tend to accumulate dysfunctional and aggregated proteins and mitochondria as a result of an oxidative imbalance: increased production of reactive oxygen species (ROS) and/or reduced antioxidant defenses. The major ROS produced in neurons are superoxide anion radical generated during mitochondrial respiration and by various oxidases, hydroxyl radical produced by the reaction of hydrogen peroxide with Fe2+ or Cu+, and nitric oxide (NO) generated in response to elevated intracellular Ca2+ levels (Halliwell, 2001). Diminished olfaction is a common feature of aging, and studies of mice show that levels of carbonylated proteins in neurons and astrocytes, and nitrated proteins in blood vessels, are increased in the olfactory bulb during aging. Aberrant NO-mediated oxidative damage is also implicated in vascular dysfunction in the aging cerebral cortex (Park et al., 2007). Peroxynitrite (formed when superoxide interacts with NO) and hydroxyl radical are highly reactive and can initiate the autocatalytic process of membrane lipid peroxidation (Mattson, 2009). The brains of very old dogs exhibit accumulations of the lipid peroxidation product 4-hydroxynonenal (HNE), associated with amyloid deposits and neurofibrillary tangles (Papaioannou et al., 2001). Modification of proteins on cysteine, lysine, and histidine residues by HNE, and on tyrosine residues by NO, impairs the function of numerous membrane proteins critical for cell metabolism and survival including glucose transporters, neurotrophic factor receptors, and ion-motive ATPases (Guix et al., 2005; Mattson, 2009; Perluigi et al., 2014).
Evidence that diminished antioxidant defenses and impaired ability to remove oxidatively damaged molecules are sufficient to accelerate aging comes from studies showing that genetic reduction in SOD2 levels in mice and Drosophila results in accelerated onset of neurological phenotypes associated with aging, including motor dysfunction, neuronal DNA damage, and neurodegeneration (Melov et al., 1998; Paul et al., 2007). In C. elegans, oxidative damage increases markedly in the postreproductive period, and interestingly, in long-lived insulin signaling pathway mutants there occurs a burst of oxidative stress in young adults and a subsequent maintenance of low levels of oxidative damage (Labuschagne et al., 2013). Oxidatively modified proteins are often targeted for proteasomal degradation by ubiquitination, while oxidatively damaged membranes and mitochondria are targeted to lysosomes in the process of autophagy (Nixon, 2013). However, excessive oxidative stress can impair of the function of proteasomes and lysosomes (Keller et al., 2002; Butler and Bahr, 2006; Zhang et al., 2017).
Impaired Lysosome and Proteasome Function
Because they are post-mitotic and must maintain their complex structural and function integration into neuronal networks throughout the lifespan of the organism, the ability to remove damaged and dysfunctional molecules and organelles is particularly important for neurons. This is accomplished by molecular machineries that identify damaged cellular constituents and move them to the lysosomes (autophagy) or proteasomes where they are degraded. The current understanding of the molecular mechanisms of autophagy and proteasomal degradation of proteins is reviewed in detail elsewhere (Galluzzi et al., 2017; Ver-Plank and Goldberg, 2017). In autophagy, cargo is enclosed within a membranous phagophore that then fuses with a lysosome and releases its contents into the acidic lysosomal lumen wherein hydrolases degrade the contents of the phagophore. Proteins are targeted for proteasomal degradation by ubiquitination, a process that involves three enzymes (E1, E2, and E3) with the E3 being a ligase that transfers the ubiquitin to a lysine residue of the target protein. Multiple other ubiquitins are then conjugated to each other to form a ubiquitin chain that is recognized by the 19S regulatory subunit of the proteasome, is unfolded, and then enters the barrel of the 20S subunit, where it is degraded. The importance of proteasomal degradation in brain aging is highlighted by the fact that mutations in the E3 ubiquitin ligase Parkin, or overexpression of the Parkin substrate α-synuclein, are sufficient to cause early-onset PD (Shimura et al., 2001).
Evidence that autophagic and proteasomal degradation is impaired in neurons during aging comes from studies showing age-related intracellular accumulation of autophagosomes with undegraded cargos, dysfunctional mitochondria, and polyubi-quitinated proteins (Nixon, 2013; Graham and Liu, 2017; Kerr et al., 2017). The ability of neuronal lysosomes to maintain a low luminal pH occurs during aging as a consequence of oxidative impairment of membrane vesicular ATPases (Colacurcio and Nixon, 2016). Indeed, HNE can impair lysosome function in cerebral cortical neurons, resulting in the accumulation of undegraded cargos and subsequent cell death (Zhang et al., 2017). Lipids also accumulate in autophagic vesicles (lipofuscin) or lipid-laden vesicles (Sulzer et al., 2008; Shimabukuro et al., 2016) in neurons during aging. Proteasome dysfunction and overload also occur during brain aging, which manifests as accumulations of polyubiquitinated proteins in neurons (Graham and Liu, 2017). Measurements of proteasome activity in different brain regions of aging rats revealed significant decreases in some brain regions (hippocampus and cerebral cortex), but not others (cerebellum and brainstem), suggesting differential vulnerability of neuronal populations to proteasome dysfunction during aging (Keller et al., 2000).
Interventions that stimulate autophagy, including dietary energy restriction (DER), protein restriction, and treatment with rapamycin, can extend lifespan in animal models (Madeo et al., 2015; Xilouri and Stefanis, 2016). Causal roles for impaired autophagy and proteasome function in brain aging come from studies in which autophagy is increased by either genetic or pharmacological manipulations. For example, genetic deletion of cystatin B, an inhibitor of lysosomal hydrolases, enhances autophagy and ameliorates learning and memory deficits in a mouse model of AD (Yang et al., 2011); overexpression of transcription factor EB, a key regulator of autophagy, rescues memory deficits in a mouse model of Tauopathy (Wang et al., 2016); and upregulation of autophagy ameliorates neurodegeneration in a zebrafish model (Lopez et al., 2017). Such findings reveal pivotal roles of lysosomes and autophagy in protecting neurons against the adversities of aging.
Dysregulation of Neuronal Calcium Homeostasis
The calcium ion (Ca2+) regulates neuronal function and structural adaptations of neuronal networks across timescales ranging from seconds to days, and even years in the case of long-term memory (Cohen et al., 2015). Upon release from presynaptic axon terminals, the excitatory neurotransmitter glutamate activates Na+-fluxing AMPA receptors on the postsynaptic dendrite, resulting in membrane depolarization and Ca2+ influx through the NMDA glutamate receptor channel and voltage-dependent Ca2+ channels. This results in a transient elevation of the cytoplasmic Ca2+ concentration as K+ channels and Na+ “pumps” are activated to restore the membrane potential and Ca2+ is removed via the activity of Ca2+ ATPases located in the plasma membrane and endoplasmic reticulum membrane. The transient Ca2+ elevation activates cytosolic kinases and phosphatases that alter the phosphorylation of various proteins in the dendrite including those involved in glutamate receptor trafficking to and from the membrane, cytoskeletal remodeling, and local protein synthesis. For example, synaptic activity-dependent Ca2+ influx stimulates the rapid insertion of AMPA glutamate receptors into the postsynaptic membrane while also inducing the translation of mRNA encoding the protein Arc, which mediates endocytosis of AMPA receptors (Kindler and Kreienkamp, 2012). Via activation of kinases, synaptic Ca2+ influx also activates transcription factors including cyclic AMP response element-binding protein (CREB) and PGC-1α (Cohen et al., 2015; Vaarmann et al., 2016), which then upregulate the expression of genes encoding various proteins involved in neuronal plasticity and cellular stress resistance (Mattson, 2012). Additional fine-tuning of subcellular Ca2+ dynamics is conferred by Ca2+ uptake and release mechanisms operative in the endoplasmic reticulum and mitochondria.
The ability of neurons to control Ca2+ dynamics within physiological limits is compromised during aging. Studies of hippocampal pyramidal neurons demonstrated that aging impairs Ca2+-induced after-hyperpolarizations, and thereby increases Ca2+ influx through L-type voltage-dependent Ca2+ channels and Ca2+ release from the endoplasmic reticulum (through ryanodine receptor channels), resulting in an aberrant elevation of cytoplasmic Ca2+ levels and consequent dysregulation of protein phosphorylation, cytoskeletal dynamics, and gene expression (Thibault et al., 2001; Toescu et al., 2004; Gant et al., 2006; Porte et al., 2008). The perturbed Ca2+ regulation is causally involved in age-related cognitive deficits because experimental manipulations that restore neuronal Ca2+ homeostasis also ameliorate cognitive deficits in aged rats (Deyo et al., 1989; Fukushima et al., 2008; Gant et al., 2015). Hippocampal neurons in old mice exhibit increased vulnerability to Ca2+-mediated excitotoxic degeneration and death (Camandola and Mattson, 2011). Likely contributing to such neuronal Ca2+ dysregulation during aging are compromised Ca2+ buffering resulting from reduced expression of Ca2+-binding proteins such as calbindin (Iacopino and Christakos, 1990; Mattson et al., 1991; de Jong et al., 1996) and impaired mitochondrial and endoplasmic reticulum Ca2+ handling (Mattson et al., 2008; Stutzmann and Mattson, 2011). The mechanisms by which sustained elevation of intracellular Ca2+ levels can damage and kill neurons include activation of Ca2+-dependent proteases (calpains) and triggering of caspase-mediated apoptosis and PARP1-mediated cell death (Mattson, 2000; Nixon, 2003; Fatokun et al., 2014).
Compromised Adaptive Cellular Stress Responses
Neurons are continually subjected to metabolic, ionic, and oxidative stress arising from their normal electrochemical activity, and from extrinsic factors imposed upon them by systemic bioenergetic challenges, and physical and psychological stress. Numerous signaling pathways have evolved to respond to cellular stresses adaptively so as to alleviate the immediate threat, alert other cells to the stressful situation, and bolster defenses against future stressors. Three major initiators of adaptive cellular stress responses are ATP consumption, Ca2+, and ROS. Action potentials and synaptic activity are mediated by Na+ and Ca2+ influx through plasma membrane channels, followed by their subsequent extrusion by membrane ion-motive ATPases (Na+ and Ca2+ “pumps”); the attendant consumption of ATP increases the AMP/ATP ratio, resulting in the activation of AMP-activated protein kinase (AMPK). AMPK then phosphorylates and thereby regulates the activities of proteins involved in energy metabolism (glucose transporters and the mTOR pathway), autophagy, and neuronal excitability (Weisová et al., 2009; Ikematsu et al., 2011; Lin and Hardie, 2018; Shah et al., 2017). Calcium is an important signal mediating cellular stress adaptation; Ca2+ binds to the protein calmodulin, resulting in the activation of kinases that promote the activation of transcription factors including CREB and nuclear factor κB (NF-κB) (Cohen et al., 2015; Saura and Cardinaux, 2017; Snow et al., 2014). One of the Ca2+-activated kinases, CamKII, also activates NO synthase. The NO then activates soluble guanylate cyclase to generate cyclic GMP, which engages downstream pathways that protect neurons against excitotoxic and metabolic stress (Knott et al., 2017). Ca2+ is also transported into mitochondria, where it engages mechanisms that increase oxidative phosphorylation and ROS production. Those ROS (superoxide and hydrogen peroxide) function as signaling molecules that can activate redox state-responsive transcription factors including NF-κB and nuclear regulatory factor 2 (NRF2) (Shih et al., 2005; Marosi et al., 2016). CREB, NF-κB, and NRF2 induce the expression of genes encoding proteins that mitigate cellular stress and eliminate or repair damaged molecules. For example, CREB induces the expression of the neurotrophic factor BDNF and the DNA repair enzyme APE1 (Yang et al., 2010, 2014); NF-κB upregulates the antioxidant enzyme SOD2, the Ca2+-binding protein calbindin, and the anti-apoptotic protein Bcl-2 (Sullivan et al., 1999; Mattson and Meffert, 2006); and NRF2 upregulates the antioxidant enzymes HO-1 and NQO1 (Son et al., 2010).
Adaptive stress response signaling pathways may become impaired during aging, thereby rendering neurons vulnerable to injury and neurodegenerative disorders (Stranahan and Mattson, 2012). For example, BDNF, NGF, and IGF-1 signaling is compromised during brain aging as a result of decreased expression of the neurotrophic factors and altered receptor expression or downstream signaling (Mattson et al., 2004; Tapia-Arancibia et al., 2008; Schliebs and Arendt, 2011; Neidl et al., 2016). Neurotrophic factor deficits likely contribute to impaired neuronal mitochondrial function, Ca2+ handling, and antioxidant defenses during aging (Mattson et al., 2004). Plasma membrane Ca2+-ATPase activity also declines in synaptic terminals in the aging brain (Zaidi et al., 1998), which may be caused by membrane lipid peroxidation (Mark et al., 1997). Dysregulation of Ca2+-CaMKII signaling adversely affects neuronal mitochondrial dynamics, which may contribute to neuronal dysfunction during aging (Jiang et al., 2015). Perturbed NO generation and downstream signaling are impaired in the hippocampus during normal aging, and restoration of NO metabolism and signaling can ameliorate age-related cognitive deficits in mice (Zhang et al., 2017). Finally, chronic uncontrolled stress (psychological or physical) impairs neuronal plasticity and can predispose neurons to degeneration by mechanisms involving hyperactivation of the hypothalamic—pituitary—adrenal axis and elevation of glucocorticoid levels (cortisol in humans and corticosterone in rodents). Sustained elevation of glucocorticoid levels inhibits the expression of BDNF and thereby impairs synaptic plasticity and increases neuronal vulnerability to degeneration (McEwen and Morrison, 2013).
Aberrant Neuronal Network Activity
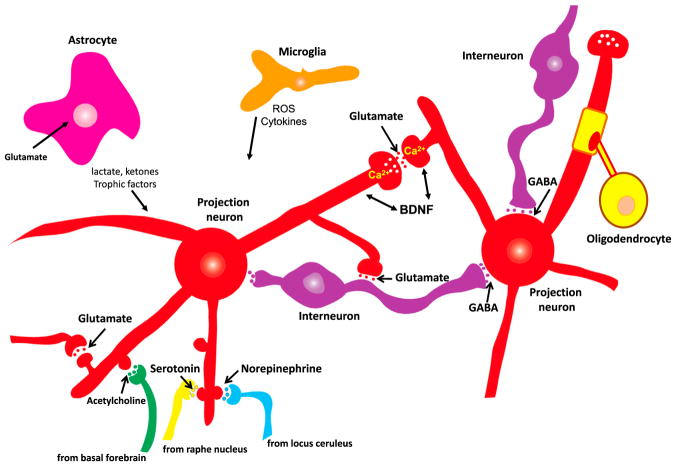
The general organization of neuronal circuits throughout the brain is based upon excitatory glutamatergic neurons with elaborate dendritic arbors and long axons that form synapses with other glutamatergic neurons and with inhibitory GABAergic interneurons (Figure 2). Synaptic inputs to the dendrites of excitatory neurons are arranged such that glutamatergic synapses are on distal dendrites, GABAergic synapses are on or adjacent to the cell body, and modulatory inputs from brainstem serotonergic and noradrenergic neurons, basal forebrain cholinergic neurons, and midbrain dopaminergic neurons are located proximal to the distal glutamatergic inputs. The structural integrity and proper integration of the synaptic activities of these different neurotransmitter systems are required for normal brain function. During brain aging, the fidelity of neuronal network activity within and between brain regions is perturbed, in some individuals relatively subtly and in others pathologically. Excitatory imbalances occur in the aging brain as a result of impaired GABAergic signaling, particularly reduced signaling via GABA-A receptors (Heise et al., 2013; Richardson et al., 2013; McQuail et al., 2015; Porges et al., 2017). Several hallmarks of brain aging can render neuronal circuits vulnerable to hyperexcitability and excitotoxic damage, including oxidative stress, mitochondrial dysfunction, impaired adaptive stress responses, and inflammation (Camandola and Mattson, 2011). Degeneration and dysfunction of G protein-coupled serotonergic, noradrenergic, dopaminergic, and cholinergic neurons that occur during normal aging may predispose to one or more age-related neurodegenerative disorders (Richter-Levin and Segal, 1996; Collier et al., 2011; Schliebs and Arendt, 2011; Mather and Harley, 2016). The latter neurotransmitter systems play key roles in learning and memory, decision making, and the regulation of mood. Their dysregulation can therefore contribute to cognitive impairment and depression in the elderly (Eyre et al., 2015).
Figure 2. Core Neuronal Circuitry and Glial Cells of the Mammalian Brain.
Excitatory neurons deploy the neurotransmitter glutamate and typically elaborate long axons that project relatively long distances within and between brain regions. Ca2+ is the principal second messenger mediating both presynaptic and postsynaptic plasticity at excitatory synapses. The major inhibitory neurons within brain regions are GABAergic and function to constrain excitatory neuronal circuits within physiological limits. Glutamatergic neurons also receive inputs from neuromodulatory transmitters including norepinephrine, serotonin, and acetylcholine. Astrocytes are the most abundant type of glial cell in the brain; they remove glutamate from the extracellular milieu and produce neurotrophic factors, lactate, and ketones to support neuronal growth and bioenergetics. Oligo-dendrocytes are glia that myelinate axons to increase the speed of action potential propagation along the axon. Microglia are the major innate immune cell in the brain; they produce reactive oxygen species (ROS) and cytokines and remove apoptotic cells and extracellular debris.
Communication between different brain regions occurs primarily via myelinated axonal projections of glutamatergic neurons, with the axons located in white matter tracts for inter-hemispheric communication (corpus callosum) and intra-hemispheric communication (e.g., superior and inferior longitudinal fasciculi, and uncinate fasciculus). White matter integrity is compromised during normal human brain aging, and this is exacerbated in subjects with cognitive impairment (Bennett and Madden, 2014; Liu et al., 2017). The mechanism underlying such demyelination involves oxidative DNA damage to oligodendrocytes (Tse and Herrup, 2017).
Functional MRI (fMRI) analyses of brain neuronal network activities in human subjects have revealed alterations associated with normal aging and age-related cognitive impairment. When a person closes their eyes and ceases interactions with their environment, activity is increased in a network that includes the precuneus, posterior cingulate cortex, medial prefrontal cortex, and angular gyrus. This “default mode network” (DMN) is believed to play important roles in remembering the past and thinking about the future and “mind wandering.” Reduced functional connectivity of the DMN occurs during normal aging and is considerably exacerbated in cognitively impaired elderly subjects (Leal and Yassa, 2013; Sala-Llonch et al., 2015). Whereas young adults engage fronto-parietal and salience networks in the resting state, older adults do not; these fMRI differences are associated with reduced gray matter volume and white matter integrity (Marstaller et al., 2015). During a memory task in older adults, the functional connectivity of the DMN and hippocampus is correlated with memory ability (Li et al., 2015). A DMN has also been described in rodents that exhibit disrupted functional connectivity in cognitively impaired compared to unimpaired aged animals (Ash et al., 2016). Future studies in which the effects of genetic and pharmacological manipulations on DMN activity are determined may reveal the cellular and molecular mechanisms underlying age-related disruption of functional connectivity of neuronal networks.
Impaired DNA Repair
DNA in the mitochondria and nucleus is regularly damaged by ROS during the normal course of cellular function and aging, and in neurons such DNA damage is increased following excitatory synaptic activity (Chow and Herrup, 2015; Yang et al., 2010). In healthy, young cells, damaged DNA bases are rapidly removed and replaced with undamaged bases by the coordinated activities of proteins in DNA repair pathways that include homologous recombination, mismatch repair, nucleotide excision repair, and base excision repair (BER). In neurons, BER is critical for the repair of oxidative DNA damage; the BER process involves a glycosylase (OGG1, UDG, and NEIL1) that recognize the damaged base, an endonuclease (APE1) that excises the damaged base, and a polymerase (Polβ) that integrates a new undamaged base into the DNA strand (Leandro et al., 2015). Analyses of brain tissue samples from humans and rodents have documented increases in the amount of damaged nuclear and mitochondrial DNA, and reductions in the expression and/or enzymatic activities of some DNA repair proteins, during aging. During human brain aging, certain regions of nuclear DNA are prone to the accumulation of oxidative damage, with promoters of genes involved in synaptic plasticity and mitochondrial function being particularly affected (Lu et al., 2004). Mitochondrial OGG1 and UDG activities (but not their expression levels) are reduced in hippocampus, frontal cortex, brainstem, and cerebellum of old mice compared to young mice (Imam et al., 2006). The expression of Polβ is decreased in the aging brain and this decrease is prevented by DER (Cabelof et al., 2003). While BER is generally considered particularly important for neurons, which are post-mitotic, there is also evidence that nucleotide excision repair and transcription-coupled repair are impaired during brain aging (Jaarsma et al., 2011). These changes may occur primarily in neural stem cells and glial cells, although this remains to be determined.
Impaired DNA repair is sufficient to cause accelerated aging phenotypes. People with Cockayne syndrome, Werner syndrome, and ataxia telangiectasia exhibit the rapid progression of multiple aging phenotypes beginning when they are young (Scheibye-Knudsen, 2016). All three premature aging syndromes are caused by mutations in proteins involved in DNA repair (CSB, Werner, and ATM) and affected individuals exhibit neurological deficits consistent with accelerated brain aging. Cockayne patients exhibit degeneration of cerebellar Purkinje cells and cochlear and retinal neurons (Weidenheim et al., 2009), and CSB-deficient mice exhibit early hearing loss and mitochondrial dysfunction in hippocampal and cortical neurons at an early age (Nagtegaal et al., 2015; Thomsen et al., 2018). Werner syndrome is characterized by AD-like neuropathology and cognitive deficits (Leverenz et al., 1998; Rekik et al., 2017). ATM deficiency results in progressive death of cerebellar Purkinje and granule neurons and consequent impaired control of body movements (Rothblum-Oviatt et al., 2016). Interestingly, DER significantly reduces neurodegeneration and neurological deficits, and increases the lifespan of DNA excision repair-deficient mice (an animal model of accelerated aging) by attenuating the accrual of oxidative DNA lesions (Vermeij et al., 2016). Presumably, by retarding other hallmarks of brain aging, DER can compensate for a genetic defect that causes a dramatic accelerated aging phenotype.
Inflammation
Similar to other organ systems, local inflammation is a common feature of brain aging. Glial cells, particularly microglia, often exhibit an activated state in the aged brain that is characterized by the acquisition of an ameboid morphology and the production of pro-inflammatory cytokines including interleukin 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) (Cribbs et al., 2012; Norden and Godbout, 2013). The complement cascade, a sequence of protein-protein interactions that “attacks” and damages cell membranes, may also be activated in the aging brain and is implicated in the pathogenesis of AD and ischemic stroke (Arumugam et al., 2009; Stephan et al., 2013; Hong et al., 2016). Genetic or pharmacological inhibition of the complement cascade can ameliorate synapse loss and neuronal death that occurs during normal aging and in mouse models of AD and stroke (Arumugam et al., 2007; Shi et al., 2015; Hong et al., 2016). In addition, activated microglia express an inducible form of NO synthase and produce large amounts of NO that can cause oxidative damage to neurons. Moreover, toll-like receptors (TLRs), best known for immune cell responses to invading pathogens, are increasingly implicated in neuroinflammation in age-related brain disorders (Okun et al., 2011). Experimental activation of microglial TLR4 receptors can exacerbate neuronal degeneration in models of age-related neurodegenerative conditions, whereas pharmacological inhibition of microglial activation is neuroprotective (Colonna and Butovsky, 2017). Aberrant activation of innate immune responses likely contributes to and results from other hallmarks of brain aging (Figure 1).
While aberrant activation of immune cells contributes to synaptic degeneration and functional impairment in brain aging and neurodegenerative conditions, when properly regulated the same pathways play important roles in neuroplasticity and neuronal stress resistance. For example, (1) TNF-α plays an important role in hippocampal synaptic plasticity and learning and memory (Snow et al., 2014); (2) complement proteins mediate adaptive synaptic remodeling in the healthy developing and adult brain (Schafer et al., 2012; Stevens et al., 2007); (3) TLRs are expressed in neurons, where they play roles in regulating developmental and adult neuroplasticity (Okun et al., 2011, 2012); (4) hippocampal neurons in TNF-α receptor-deficient mice exhibit increased vulnerability to dysfunction and degeneration in models of epileptic seizures, TBI, and AD (Bruce et al., 1996; Sullivan et al., 1999; Montgomery et al., 2011); and (5) TLRs 2 and 4 regulate food intake, the parasympathetic nervous system, and body weight (Okun et al., 2014). Astrocytes may also contribute to adaptive responses to age-related neuronal stress. They produce neurotrophic factors, remove glutamate from synapses, and bolster neuronal bioenergetics (Mattson and Rychlik, 1990; Camandola and Mattson, 2017; Rose et al., 2018). These functions of astrocytes may be compromised during aging, thereby exacerbating pathological neuroinflammatory processes.
Impaired Neurogenesis
While the vast majority of neurons in the adult mammalian brain are produced during embryonic or early postnatal development, new hippocampal dentate gyrus granule neurons and olfactory bulb interneurons are generated from neuronal stem cells in the adult brain (Ming and Song, 2011). Dentate granule neurons play critical roles in learning and memory in general, and spatial pattern separation (memory of spatial relationships between objects in the environment) in particular. Reductions in hippocampal and olfactory neurogenesis occur during normal aging and may contribute to cognitive and olfactory deficits (Lazarov et al., 2010). Several other hallmarks of aging may contribute to impaired neurogenesis during aging. Aging neural progenitor cells exhibit reduced mitochondrial oxidative metabolism (Stoll et al., 2011), and genetic compromise of mitochondrial ETC function in hippocampal neuronal progenitor cells in adult mice impairs neurogenesis in a manner similar to that seen in normal aging (Beckervordersandforth et al., 2017). Oxidative stress, impaired DNA repair, and inflammation may also contribute to age-related reductions in neurogenesis (Ekdahl et al., 2003; Kim et al., 2008; Regnell et al., 2012; L’Episcopo et al., 2013).
Cell Senescence and Telomere Attrition?
Mitotic cells throughout the body experience telomere shortening and senescence during aging. With successive rounds of cell division, the ends of chromosomes (telomeres), which are comprised of a repeating hexanucleotide DNA sequence (TTAGGG), can shorten, which can be prevented by the telomerase reverse transcriptase. During interphase, the telomeric DNA is protected by several telomere-associated proteins. Extensive telomere shortening and impaired protection of telomeric DNA can trigger a DNA damage response and either apoptosis or cell senescence (Zhang et al., 2016a). Because neurons are post-mitotic, their telomeres do not shorten (although they can be damaged by oxidative stress) and they do not undergo senescence. However, during the first few days of differentiation from stem cells, newly generated neurons are particularly sensitive to apoptosis triggered by telomere damage (Cheng et al., 2007). Hippocampal neurogenesis is reduced, and hippocampus-dependent spatial learning and memory are impaired, in telomerase-deficient mice (Rolyan et al., 2011). Reactivation of telomerase in late-generation telomerase-deficient mice restores olfactory neurogenesis to normal levels with a consequent amelioration of an olfactory deficit (Jaskelioff et al., 2011).
When cells undergo senescence, they cease dividing and grow in size, express the proteins p21 and p16Ink4a, are resistant to apoptosis, and produce pro-inflammatory cytokines (Childs et al., 2014). Senescence may be the fate of some neural progenitor cells and glial cells during aging as indicated by increased expression of p16Ink4a associated with reduced numbers of proliferating progenitors in the subventricular zone (Molofsky et al., 2006). Moreover, when human neural progenitors are maintained in culture they exhibit a limited number of population doublings and then undergo senescence (Wright et al., 2006). Future studies in which senescent cells are selectively removed from the brain in animal models of aging and age-related neurodegenerative disorders should clarify whether cell senescence is a bona fide hallmark of brain aging.
Dysregulated Energy Metabolism
During aging, the metabolism of glucose and lipids is impaired in cells in peripheral tissues and in the brain. Circulating glucose concentrations generally increase during aging as a result of a compromised ability of cells to increase glucose transport in response to insulin. Insulin resistance is characterized by elevated fasting blood insulin and glucose levels, and is a major risk factor for diabetes, cardiovascular disease, and stroke. Peripheral insulin resistance is also associated with poorer cognitive function during aging (Thambisetty et al., 2013) and may be a risk factor for AD (Neth and Craft, 2017). Neurons may exhibit insulin resistance and impaired glucose transport in aging as suggested by progressive reductions in glucose utilization demonstrated by PET imaging of radiolabeled glucose uptake; this impaired glucose metabolism is particularly prominent in the temporal, parietal, and frontal lobes, and motor cortex (Goyal et al., 2017). Reduced glucose utilization in the temporal and parietal lobes is much more profound in elderly subjects with mild cognitive impairment and AD compared to age-matched neurologically normal subjects (Kato et al., 2016).
When insulin binds to its membrane receptor, the cytoplasmic tyrosine kinase domain of the receptor is activated, resulting in phosphorylation of the insulin receptor-interacting protein IRS-1. Insulin resistance is characterized by reduced tyrosine phosphorylation of IRS-1 and increased phosphorylation on serine 312 of IRS-1. Analyses of postmortem brain tissue from elderly subjects demonstrate increased serine 312 IRS-1 phosphorylation, suggesting neuronal insulin resistance (Moloney et al., 2010; Yarchoan et al., 2014). Circulating extra-cellular vesicles (EVs) believed to be released from neurons also exhibit an insulin resistance phosphorylation profile in cognitively impaired elderly subjects (Kapogiannis et al., 2015). In addition to insulin resistance, the neuronal glucose transporter GLUT3 is vulnerable to impairment by oxidative stress and HNE (Mark et al., 1997; Mattson, 2009). However, while neuronal glucose metabolism is compromised during aging, the ability of neurons to acquire and utilize the ketone bodies β-hydroxybutyrate (BHB) and acetoacetate is maintained, apparently even in neurons in patients with AD (Cunnane et al., 2016). As described below in Metabolic Factors Can Accelerate or Decelerate Brain Aging, interventions that bolster neuronal bioenergetics have considerable potential to mitigate multiple hallmarks of brain aging and may thereby forestall neurodegenerative disorders.
Dyslipidemia (elevated blood concentrations of low-density lipoproteins and triglycerides) is a risk factor for stroke and vascular dementia, and may also increase one’s risk for AD (Appleton et al., 2017). Within the brain, the metabolism of multiple lipid species is altered during aging as indicated by the accumulation of long-chain ceramides (Cutler et al., 2004) and lipid-laden cells (Shimabukuro et al., 2016), and a decline in brain tissue levels of omega-3 fatty acids (Denis et al., 2013). Genetic evidence also links lipid metabolism to poor brain outcomes during aging. Thus, the strongest genetic risk factor for late-onset sporadic AD is having an ε4 allele of the gene encoding apolipoprotein E, a protein that transports cholesterol and lipoproteins (Lane-Donovan et al., 2014). Despite considerable investigation, however, the mechanism by which the ε4 APOE isoform endangers neurons is unclear and may not be the result of altered cholesterol metabolism. Two possibilities are that APOE ε4 lacks the antioxidant (HNE-scavenging) ability of APOE ε2 and ε3 (Pedersen et al., 2000), and that APOE ε4 alters trafficking of proteins (including APP) through the endosomal pathway (Lane-Donovan et al., 2014).
Perspective on How Mechanisms of Aging Impact Neurological Disorders
Previous review articles provide comprehensive descriptions of alterations in brain cellular metabolism that occur in aging and their interface with neurodegenerative disorders (Kapogiannis and Mattson, 2011; Ryan et al., 2015; Yin et al., 2016; Swerdlow, 2016; Camandola and Mattson, 2017). PET imaging studies reveal impaired cerebral glucose utilization occurring very early in AD pathogenesis, even before overt clinical symptoms are evident (Friedland et al., 1989; Ceravolo et al., 2008). Findings from postmortem analyses reveal reductions in levels of glucose transporters and decrements in ETC complex activities and glycolytic flux in vulnerable brain regions of AD patients (Simpson et al., 1994; Valla et al., 2001; An et al., 2018). PD patients exhibit reduced glucose utilization in brain regions involved in motor control, and deep brain stimulation of the globus pallidus (which alleviates motor symptoms) increases glucose utilization in those brain regions (Fukuda et al., 2001). Neurons affected in PD exhibit reduced mitochondrial respiratory chain function (Bose and Beal, 2016; Grünewald et al., 2016). In stroke, neuronal energy failure results from catastrophic oxygen and glucose deprivation caused by occlusion of a cerebral artery, usually as the result of clot formation at the site of an atherosclerotic lesion in the vessel (Moskowitz et al., 2010). The extent of brain damage is greater and functional recovery poorer in older compared to younger stroke patients (Umarova, 2017).
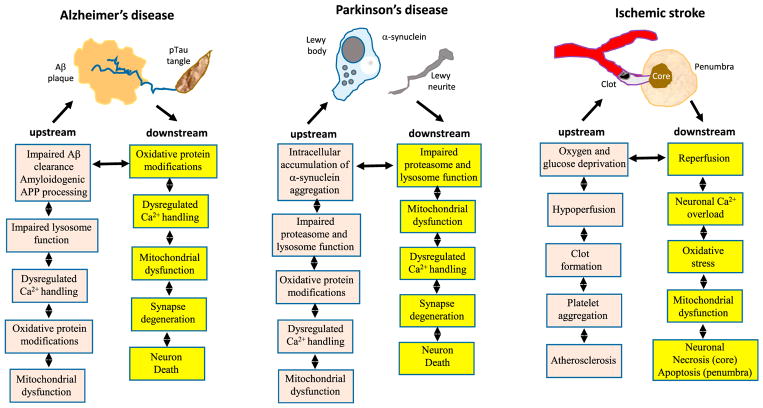
Aging is the major risk factor for AD, PD, and stroke, but major advances in the genetics of AD and PD have not yet led to effective disease-modifying treatments. In this section we present one view of how hallmarks of brain aging impact and are impacted by specific disease processes in AD, PD, and stroke (Figure 3), recognizing the fact that specific sequences of events remain to be established.
Figure 3. Examples of Roles for Aging Processes, Acting Both Upstream and Downstream of Disease-Defining Molecular Lesions, in the Pathogenesis of AD, PD, and Stroke.
There is considerable evidence that the indicated hallmarks of brain aging act upstream of the disease-defining Aβ plaques and pTau neurofibrillary tangles. On the other hand, aggregating Aβ and pTau can cause oxidative stress, dysregulation of Ca2+ homeostasis, mitochondrial dysfunction, and other hallmarks of aging in neurons. In PD, core aging processes result in the intracellular accumulation of neurotoxic forms of α-synuclein, and conversely, the accumulation of α-synuclein exacerbates aging processes resulting in neuronal dysfunction and death. Aging renders the brain vulnerable to stroke by promoting atherosclerosis and by compromising the ability of neurons to withstand and recover from the ischemic stress.
Alzheimer’s Disease and Related Dementias
The diagnosis of probable AD is ascribed to individuals that exhibit age-related cognitive decline that first manifests as mild short-term memory impairment and then progresses inexorably to severe deficits in essentially all cognitive domains. The final diagnosis of AD is established upon histological examination of the brain at autopsy for cases in which levels of Aβ plaques and Tau neurofibrillary tangles exceed diagnostic levels (Geddes et al., 1997). The Aβ precursor protein (APP) has been a major focus of AD research because mutations in APP or the presenilin-1 enzyme (γ-secretase) that cleaves APP to generate Aβ cause rare cases of inherited early-onset AD. APP is processed by three enzyme activities: β- and γ-secretases cleave at the N terminus and C terminus of Aβ to generate amyloidogenic Aβ, while α-secretase cleaves in the middle of the Aβ sequence and so prevents Aβ production. The α-secretase cleavage releases a secreted form of APP (sAPPα) that activates signaling pathways in neurons that enhance synaptic plasticity and cellular stress resistance (Mattson et al., 1993; Nigam et al., 2017). While AD is the most common form of dementia, Tau mutations can cause fronto-temporal dementia, which manifests abundant neurofibrillary tangles with negligible Aβ pathology (Rademakers et al., 2012; Arendt et al., 2016). Moreover, in approximately 25% of patients diagnosed with probable AD, the levels of Aβ plaques and Tau tangles do not reach the AD threshold; instead, these patients often exhibit extensive loss of hippocampal pyramidal neurons with moderate numbers of neurons bearing Tau, α-synuclein, and/or TDP43 aggregates (Mattson, 2015a). In addition, many individuals who live into their eighth and ninth decades with minimal decline in cognitive function exhibit extensive Aβ plaque accumulation at autopsy, but have minimal neuronal loss. The mechanisms by which neurons are able to resist the neurotoxic effects of Aβ in some elderly individuals have not been established, but experimental data suggest potential roles for neurotrophic factor signaling and robust adaptive cellular stress response pathways (Mattson, 2012).
Cell culture and animal models in which one or more of the gene mutations that cause inherited AD are expressed, together with studies in which neurons are exposed directly to Aβ, have provided valuable information as to the molecular and cellular mechanisms of AD pathogenesis both upstream and downstream of the Aβ and Tau pathologies (Figure 3). The gist of the cumulative data is that (1) hallmarks of aging promote amyloidogenic APP processing and Tau pathology, (2) Aβ and Tau accumulations accelerate hallmarks of aging, and (3) there is no common linear pathway to synaptic dysfunction and neuronal death in AD. For indepth information on the roles of different hallmarks of brain aging in AD and related dementias, we refer the reader to the following review articles: oxidative stress (Texel and Mattson, 2011), mitochondrial dysfunction (Mattson et al., 2008; DuBoff et al., 2013), impaired autophagy (Nixon, 2013; Kerr et al., 2017), impaired DNA repair (Madabhushi et al., 2014; Leandro et al., 2015), aberrant neuronal network excitability (Palop and Mucke, 2016; Vossel et al., 2017), impaired adaptive stress response signaling (Stranahan and Mattson, 2012), dysregulated neuronal Ca2+ homeostasis (Bezprozvanny and Mattson, 2008; Stutzmann and Mattson, 2011), impaired energy metabolism (Dauncey, 2014), neuroinflammation (Heppner et al., 2015), and stem cell deficits (Lazarov et al., 2010). Due to space limitations, in this section we describe one specific example of how oxidative damage, impaired autophagy, Ca2+ dyshomeostasis, and aberrant neuronal network activity can interact reciprocally with Aβ pathology to cause synaptic dysfunction and neuronal death in AD.
Proteins modified by the lipid peroxidation product HNE accumulate in the brain during aging and HNE increases the production a particularly neurotoxic form of Aβ (Aβ42) by increasing γ-secretase cleavage of APP; this occurs as a result of covalent modification of the γ-secretase substrate receptor nicastrin by HNE (Gwon et al., 2012). Administration of an HNE-scavenging histidine analog reduces the production of Aβ42 in the brains of 3xTgAD mice, suggesting the possibility of developing therapeutic interventions that suppress HNE accumulation. As Aβ self-aggregates and accumulates on the membrane of neurons, ROS are generated, membrane lipid peroxidation occurs, and HNE is produced (Keller et al., 1997). Data suggest that HNE contributes to the neurotoxicity of Aβ by impairing the function of membrane ion-motive ATPases and glucose and glutamate transporters, resulting in neuronal hyperexcitability and disruption of neuronal Ca2+ homeostasis, thereby rendering neurons vulnerable to excitotoxicity and metabolic failure (Mattson et al., 1992; Mark et al., 1997; Mattson, 2009). Aberrant and sustained elevation of intracellular Ca2+ levels can further exacerbate oxidative stress and lysosome dysfunction. HNE also directly impairs lysosome function, and lysosome dysfunction results in the accumulation of Aβ in neurons, which is then expelled from the neurons in EVs. EVs are small vesicles (50–150 nm diameter) released from multivesicular bodies upon their fusion with the plasma membrane or from budding of the plasma membrane (Budnik et al., 2016). EVs may propagate Aβ pathology between, within, and across neuronal networks as AD progresses (Eitan et al., 2016; Zhang et al., 2017). Even moderate deficits in DNA repair, neurotrophic factor signaling, and mitochondrial function can render neurons vulnerable to death when they are concomitantly exposed to Aβ (Sykora et al., 2015; Camandola and Mattson, 2017).
Parkinson’s Disease
The diagnosis of PD is based on the symptoms of postural instability, rigidity, bradykinesia, and tremor resulting in part from the dysfunction and degeneration of dopaminergic neurons in the substantia nigra (SN) that innervate the striatum (Rodriguez-Oroz et al., 2009). Dopaminergic neurons in PD often manifest “Lewy bodies,” which are large accumulations of α-synuclein in their cytoplasm, an abnormality akin to Tau tangles in neurons affected in AD. While the clinical diagnosis of PD is based on motor symptoms, non-motor symptoms associated with α-synuclein pathology in the autonomic and enteric nervous systems are also evident and may precede SN-striatal pathology (Klingelhoefer and Reichmann, 2015).
Compelling evidence suggests that mitochondrial dysfunction and the accumulation of α-synuclein aggregates in neurons are mechanistically linked pivotal events in the pathogenesis of PD. A historical summary of the evidence includes (1) the discovery that chemicals that selectively inhibit mitochondrial ETC complex I (MPP+, paraquat, 6-hydroxydopamine, and rotenone) can cause dopaminergic neuron degeneration, with clinical symptoms indistinguishable from PD (Langston, 1996); (2) mitochondria in brain cells of PD patients exhibit reduced complex I activity and excessive DNA damage (Di Monte, 1991); and (3) the identification of the genetic abnormalities that cause early-onset familial PD converge on mitochondria and autophagy. The mutations include autosomal dominant mutations in α-synuclein that result in aberrant accumulation of α-synuclein aggregates, which can overload the autophagy pathway and cause mitochondrial dysfunction (Wong and Krainc, 2017), and autosomal recessive mutations in Parkin and PINK1, which impair the ability of neurons to recognize and remove (via autophagy) dysfunctional mitochondria (Pickrell and Youle, 2015), although other studies suggest that mitophagy may not be impaired in some cases of familial PD (Lee et al., 2018). Mutations in the gene encoding LRRK2 that cause early-onset PD impair vesicle trafficking to lysosomes and may thereby promote the accumulation of α-synuclein and dysfunctional mitochondria (Cookson, 2016). LRRK2 mutations may also promote neuronal degeneration by causing aberrant protein synthesis (Martin et al., 2014).
PD provides a remarkable example of a disease underlain by a molecular pathogenesis that involves an exacerbation of hallmarks of aging. Oxidative damage, dysregulated neuronal Ca2+ homeostasis, impaired DNA repair, impaired adaptive cellular stress responses, aberrant neuronal network activity, and neuroinflammation all occur in affected brain regions during the course of PD, and each of these age-related alterations increases the vulnerability of neurons to α-synuclein pathology, and mitochondrial and autophagy dysfunction (Olanow et al., 2015; Ransohoff, 2016; Sepe et al., 2016; Jahanshahi and Rothwell, 2017; Menzies et al., 2017; Surmeier et al., 2017a). Conversely, aggregating α-synuclein and mitochondrial dysfunction exacerbate oxidative stress and DNA damage, destabilize neuronal Ca2+ homeostasis, and promote neuroinflammation (Schapira et al., 2014). Support for the latter two statements comes from studies of cultured neurons and transgenic mice that overexpress mutant or wild-type human α-synuclein. For example, exposure of neurons to agents that induce oxidative stress, inhibit mitochondrial complex I, or impair lysosome function can trigger the accumulation of α-synuclein aggregates (Schildknecht et al., 2013). Such accumulation is sufficient to trigger neuronal degeneration as demonstrated in a study in which suppression of α-synuclein production using RNAi technology prevented degeneration of dopaminergic neurons in a rat rotenone model of PD (Zharikov et al., 2015). On the other hand, overexpression of mutant or wild-type α-synuclein is sufficient to trigger the appearance of hallmarks of aging in young mice, including the accumulation of oxidatively modified proteins, DNA damage, mitochondrial dysfunction, microglial activation, and impaired autophagy (Martin et al., 2006; Lin et al., 2012).
Finally, mechanisms that may explain how α-synuclein pathology is propagated trans-neuronally in a retrograde manner within the CNS are emerging. Similar to prions, aggregating forms of α-synuclein can “seed” the aggregation of monomeric α-synuclein. For example, injection of pathogenic “strains” of α-synuclein into one brain region will trigger the spread of α-synuclein to other brain regions (Brundin and Melki, 2017). However, because α-synuclein is a cytoplasmic protein and pathogenic α-synuclein aggregates accumulate intracellularly, the prion-like mechanism does not readily explain how α-synuclein pathology is transferred from one neuron to adjacent neurons (Surmeier et al., 2017b). One potential mechanism involves extracellular vesicles (EVs). Lysosome dysfunction and oxidative stress can trigger the release of EVs containing aggregating α-synuclein, and those EVs can then be internalized and trigger the accumulation of toxic α-synuclein aggregates in previously healthy neurons (Emmanouilidou et al., 2010; Poehler et al., 2014; Zhang et al., 2018). Another potential mechanism for spreading of α-synuclein pathology is suggested by a study showing that misfolded preformed fibrils bind to LAG3 (lymphocyte-activation gene 3) and that LAG3 initiates α-synuclein preformed fibril endocytosis, transmission, and cytotoxicity (Mao et al., 2016).
Stroke
A stroke occurs when blood flow in a cerebral artery is interrupted by the formation of a clot, which is usually followed by dissolution of the clot and reperfusion of the brain tissue supplied by the artery. Depending upon which cerebral vessel is affected, stroke symptoms may include sudden weakness, facial numbness, slurred speech, impaired vision, and paralysis on the side of the body contralateral to the stroke (Yew and Cheng, 2015). Cerebral ischemia results in the development of a core region of brain damage wherein cells die rapidly by necrosis and a surrounding penumbra in which cells experience partial ischemia and neurons may undergo delayed apoptotic cell death (Moskowitz et al., 2010). The cellular and molecular mechanisms responsible for the death of neurons in stroke involve many of the same alterations that occur during normal brain aging (Figure 3) including energy deprivation and mitochondrial dysfunction, excitotoxicity, cellular Ca2+overload, mitochondrial dysfunction, oxidative stress, DNA damage, and inflammation (Weinstein et al., 2004).
Complete cessation of brain tissue perfusion results in rapid catastrophic ATP depletion in neurons in the ischemic core, resulting in sustained membrane depolarization, Na+ influx, cell swelling, and membrane rupture (necrosis). Neurons in the ischemic penumbra experience an incomplete ischemia that may result in an acute membrane depolarization from which they initially recover. However, during reperfusion there occurs dysregulation of Ca2+ homeostasis that can trigger apoptosis mediated by formation of mitochondrial permeability transition pores and activation of cysteine proteases called caspases (Mattson, 2000; Broughton et al., 2009). Caspase 3 cleaves multiple substrate proteins that execute the cell death process in a manner that enables the neuron to die without membrane rupture, enabling microglia to recognize and remove the dead cell. In addition, some neurons in the ischemic penumbra may die by another recently described type of death called pyroptosis, which involves caspase 1 activation, formation of pores in the plasma membrane, and release of IL-1β (Fann et al., 2013; Adamczak et al., 2014). Inflammation plays a particularly prominent role in stroke pathogenesis. Release of damage-associated pattern molecules (DAMPs) such as high-mobility group box 1 (HMGB1) protein and interleukin 1α (IL-1 α) from the ischemic core region activates inflammatory cascades in cells in the penumbra (Fann et al., 2013, 2018). DAMPs activate the receptor for advanced glycation end products, TLRs, the C-type lectin mincle, and Notch, all of which contribute to neuronal death in the penumbra (Tang et al., 2007, 2013; Arumugam et al., 2017; Fann et al., 2018). In addition, the complement cascade arm of the innate immune system and the infiltration of circulating leukocytes into the cerebral parenchyma contribute to neuronal degeneration in stroke (Arumugam et al., 2007; Gelderblom et al., 2009).
Interventions that target core mechanisms of aging (metabolic impairment, hyperexcitability, Ca2+ dysregulation, oxidative stress, impaired adaptive stress response signaling, and inflammation) and apoptosis can reduce brain damage and improve functional outcome in animal models of stroke. Examples include glutamate receptor antagonists (Simon and Shiraishi, 1990), K+ channel openers (Liu et al., 2002), neurotrophic factors, and complement inhibitors (Arumugam et al., 2007; Chen et al., 2018). However, clinical trials of such neuroprotective agents have not yet demonstrated efficacy in human stroke patients. Likely contributing to this lack of success in stroke patients are the facts that most preclinical stroke studies are in young animals, whereas stroke patients are mostly elderly; treatment is often initiated prior to the onset of ischemia in animal studies, whereas there is typically a delay of at least several hours in human stroke patients; and stroke site and ischemia duration are uniform in animal studies, but highly variable in human stroke patients (Fisher et al., 2009), making it difficult to discern modest beneficial effects in clinical trials.
Metabolic Factors Can Accelerate or Decelerate Brain Aging
An important feature of brain aging is that there is considerable inter-individual variability. Some individuals in their 90s are “sharp as a tack,” while others exhibit cognitive decline before the age of 60. While there are undoubtedly genetic factors that influence the rate of brain aging, there is also a major environmental component. Indeed, even in inbred strains of mice and rats, some individuals exhibit impaired learning and memory capacity in old age whereas others do not (Gallagher et al., 2006). In this section, we summarize evidence that energy intake and expenditure during the life course have a major impact on the rate of brain aging and the risk for AD, PD, and stroke.
Chronic Metabolic Morbidity Accelerates Brain Aging
There are strong positive associations between metabolic morbidities (obesity, dyslipidemia, and insulin resistance) and the risk of all major age-related diseases including diabetes, cardiovascular and cerebrovascular diseases, and many types of cancer. In animal models of the latter disorders, the disease processes are accelerated by feeding diets high in saturated fat and simple sugars (Hariri and Thibault, 2010; Koopmans and Schuurman, 2015). Individuals who are sedentary and overindulgent are also prone to impaired brain function and neurodegenerative disorders as they age. On average, the cognitive performance of individuals who are metabolically morbid is poorer than their age-matched healthy counterparts (Kullmann et al., 2016; Stillman et al., 2017). Brain imaging studies have documented reduced gray matter volumes and white matter integrity in multiple brain regions, and reduced functional connectivity between brain regions in obese individuals, particularly those with abdominal obesity and insulin resistance (Debette et al., 2014; Janowitz et al., 2015; Kullmann et al., 2015). Generally similar abnormalities in brain structure and neuronal network connectivity occur in individuals with type 2 diabetes (Macpherson et al., 2017). Even among individuals who are not obese, higher body mass index is associated with perturbed resting state connectivity in the DMN and sensory-motor networks (Beyer et al., 2017; Doucet et al., 2018). In addition, body mass index is inversely associated with glucose utilization in the prefrontal cortex, a brain region that plays critical roles in executive function, attention, memory, and insight (Volkow et al., 2009). Brain function and structure are also adversely affected by obesity and diabetes in animal models. For example, when rats are fed a diet high in saturated fats and sugar, they perform worse on a place recognition task and exhibit elevated markers of oxidative stress and inflammation in their hippocampus (Beilharz et al., 2014). High-fat feeding impairs cognitive flexibility in a delayed matching to position task in rats (McNeilly et al., 2011). Insulin resistance and obesity induced by diet or genetic mutation of the leptin receptor in rats or mice result in a reduction in synaptic spine density on dendrites of hippocampal dentate granule neurons, and impaired hippocampal synaptic plasticity and neurogenesis (Stranahan et al., 2008a, 2008b). Thus, both correlational data from human studies and controlled trials in animals demonstrate that a chronic positive energy balance adversely affects brain structure and function.
Data suggest that metabolic morbidity accelerates most, if not all, of the hallmarks of brain aging. Impaired cerebral glucose utilization in the prefrontal cortex is inversely associated with body mass index in human subjects (Volkow et al., 2009), suggesting an adverse effect of a chronic positive energy balance on neuronal bioenergetics. Rats or mice maintained on high-fat and/or high-sugar diets, and diabetic animals, exhibit many of the cellular and molecular hallmarks of brain aging including oxidative damage (Elahi et al., 2016), neuroinflammation (Jayaraman et al., 2014), impaired neuronal Ca2+ homeostasis (Thibault et al., 2013), impaired autophagy (Li et al., 2017), and dysregulation of neuronal network activity (Margineanu et al., 1998). Insulin-resistant mice exhibit impaired hippocampal neurogenesis, which is associated with oxidative stress, inflammation, and impaired hippocampus-dependent spatial learning and memory (Lindqvist et al., 2006; Stranahan et al., 2008a; Gurung et al., 2016). Activation of inflammatory microglia may contribute to the loss of synapses on hippocampal dentate granule neuron dendrites caused by obesity and diabetes (Stranahan et al., 2008b; Hao et al., 2016). The ability of neurons to respond adaptively to bioenergetic and oxidative stress is compromised by excessive energy intake as indicated by reduced expression of BDNF (Stranahan et al., 2008b), PGC-1α (Morselli et al., 2014), and SIRT1 (Heyward et al., 2016). Obese and diabetic rodents exhibit elevated glucocorticoid levels, which can suppress BDNF expression and impair hippocampal synaptic plasticity and neurogenesis (Stranahan et al., 2008a; Wosiski-Kuhn et al., 2014). In this regard, the mechanisms by which chronic uncontrolled stress and metabolic morbidity compromise the ability of neurons to respond to stress are similar and involve an acceleration of aging processes (Sapolsky, 1999).
Sedentary and overindulgent lifestyles accelerate pathological processes underlying age-related brain diseases by mechanisms involving hallmarks of brain aging. This is established in the case of ischemic stroke (Lucke-Wold et al., 2012). Findings from both cross-sectional and longitudinal epidemiological studies suggest that obesity, diabetes, and insulin resistance are also risk factors for AD and PD (Luchsinger and Gustafson, 2009). In transgenic mouse models of AD, diet-induced obesity and diabetes exacerbate cognitive deficits by mechanisms involving or in addition to Aβ and Tau pathologies (Takeda et al., 2010; Walker et al., 2017). Obesogenic diets accelerate the development of motor and non-motor phenotypes and associated α-synuclein pathology in transgenic PD mouse models (Griffioen et al., 2013). Such findings suggest that interventions that improve one’s metabolic status may counteract brain aging. Evidence summarized in the next section supports this possibility.
Physiological Bioenergetic Challenges Retard Brain Aging
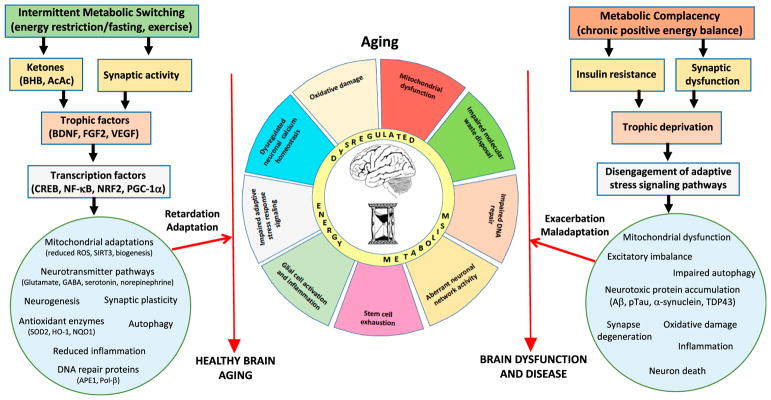
The evolutionarily pressure to compete successfully for limited food sources resulted in selection for individuals whose brains and bodies functioned well (perhaps optimally) when in a food-deprived (fasted) state (Mattson, 2015b). Fast forward to the present day and the common pattern of meal consumption of three meals plus snacks spaced throughout waking hours. The latter eating pattern is abnormal when viewed in the light of our ancestors prior to the agricultural revolution who ate less frequently. A compelling scientific literature shows that, compared to animals fed ad libitum, those fed intermittently (alternate day fasting or daily time-restricted feeding) exhibit improvements in many health indicators and, as reviewed elsewhere, their lifespan is extended and hallmarks of aging are diminished (Longo and Mattson, 2014; López-Otín et al., 2016; Mattison et al., 2017). Intermittent energy restriction improves cognitive and motor performance, and can protect neurons against dysfunction and degeneration in animal models of epilepsy, stroke, PD, and AD (Bruce-Keller et al., 1999; Duan and Mattson, 1999; Yu and Mattson, 1999; Halagappa et al., 2007; Alirezaei et al., 2010; Fann et al., 2014; Parikh et al., 2016; Prehn et al., 2017). Regular aerobic exercise, which is necessary for the survival of many animals, can also enhance brain health throughout the life course; exercise reduces anxiety and improves cognition in laboratory animals and human subjects (Intlekofer and Cotman, 2013; Boraxbekk et al., 2016; Castellano et al., 2017; Chirles et al., 2017; Raichlen and Alexander, 2017). While fasting and vigorous exercise are different challenges to the body and brain, emerging findings are revealing that they each elicit similar adaptive cellular responses that can enhance neuroplasticity and stress resistance. As reviewed elsewhere (Mattson, 2012; Mattson et al., 2018), these include upregulation of neurotrophic factor signaling, autophagy, and DNA repair; suppression of oxidative stress and inflammation; stabilization of neuronal calcium homeostasis and neuronal network activity; and stimulation of mitochondrial biogenesis and neurogenesis (Figure 4).
Figure 4. Working Model for How Intermittent Metabolic Challenges Bolster Brain Health during Aging, Whereas a Chronic Positive Energy Balance Hastens Brain Aging and Associated Brain Diseases.
Left: eating and lifestyle patterns that result in intermittent depletion of liver glycogen stores and mobilization of fatty acids to generate ketones (fasting and exercise) also typically increase neuronal network activity in many brain regions. Signaling pathways are activated in brain cells that upregulate the expression of trophic factors and activate transcription factors that induce the expression of genes encoding proteins that enhance neural plasticity and resilience during aging. These adaptations to intermittent metabolic switching include mitochondrial biogenesis and stress resistance; adaptive modifications of neurotransmitter signaling pathways; upregulation of autophagy, antioxidant defenses, and DNA repair; stimulation of neurogenesis; and suppression of inflammation. In these ways intermittent metabolic switching counteracts core brain aging mechanisms, thereby slowing age-related declines in neurological function and reducing the risk of AD, PD, and stroke. Right: sedentary overindulgent lifestyles accelerate brain aging and increase the risk of AD, PD, and stroke. A chronic positive energy balance results in metabolic morbidity (insulin resistance and dyslipidemia) and reduced activation of signaling pathways that promote synaptic plasticity and cellular stress resistance. As a consequence, neurons suffer: impaired mitochondrial function, autophagy, and DNA repair; excessive oxidative stress; dysregulated neuronal network activity and Ca2+ homeostasis; the accumulation of potentially toxic protein aggregates; and inflammation. In these ways, metabolic complacency accelerates age-related decrements in brain function and increases the risk of AD, PD, and stroke.
Emerging findings suggest that depletion of liver glycogen stores and mobilization of fatty acids from adipose cells are important metabolic responses to fasting and extended exercise with regard to brain health and neuroprotection (Mattson et al., 2018). The fatty acids are metabolized to the ketone bodies β-hydroxybutyrate (BHB) and acetoacetate (AcAc) in the liver. Neurons utilize the ketones to generate acetyl-coenzyme A and thence ATP in their mitochondria. But BHB also functions as a signaling molecule that stimulates Bdnf gene expression by modifying histone acetylation and by activating the transcription factor NF-κB (Marosi et al., 2016; Sleiman et al., 2016). Generally similar to the physiological responses of muscle cells to exercise, it appears that neurons respond to intermittent energy restriction and exercise by enhancing their tolerance of metabolic stress and by growing in size. A picture is emerging in which pathways activated during the metabolic challenges of exercise and fasting prepare the cells to grow during the recovery period (rest, feeding, and sleep) (Mattson et al., 2018). In the case of neurons, such intermittent metabolic switching may stimulate mitochondrial biogenesis to enable neurite outgrowth and the formation of new synapses (Cheng et al., 2012; Vaarmann et al., 2016). In addition, recent findings suggest that intermittent metabolic switching enhances the function, stress resistance, and quality control of mitochondria, in part by inducing the expression of the mitochondrial protein deacetylase SIRT3. The activities of ETC proteins and the mitochondrial antioxidant enzyme SOD2 are enhanced when deacetylated by SIRT3, while deacetylation of cyclophilin D can inhibit formation of mPTPs, thereby preventing neuronal excitotoxicity and apoptosis (Cheng et al., 2016; Shi et al., 2017).
While adaptive cellular stress response pathways are upregulated in brain cells during periods of energy restriction and exercise, a recovery period (eating, resting, and sleeping) is required to enable the structural and functional plasticity of neuronal circuits that are believed to sustain optimal brain function and resilience throughout the life course (Mattson et al., 2018). In this view, the metabolic challenge suppresses mTOR and overall protein synthesis, and autophagy and gene expression pathways involved in cellular stress resistance are upregulated. During the recovery period, mTOR is activated and protein synthesis is increased to provide the new proteins required for cell growth (neurite outgrowth, synapse formation, and neurogenesis) and the increased bioenergetics (mitochondrial biogenesis) required to support the cell growth (Figure 4; Camandola and Mattson, 2017; Mattson et al., 2018).
Neuroprotection with Bioenergetic Challenge-Based Pharmacological Interventions
While brain health can be bolstered by intermittent metabolic switching, pharmacological approaches that tap some of the key adaptive cellular stress response pathways engaged by fasting and exercise are being pursued for the purposes of enhancing cognition and forestalling or treating age-related neurological disorders. A few such pharmacological agents with demonstrated efficacy in animal models of neurodegenerative disorders are listed in Table 1. One approach is to bolster mitochondrial function and SIRT3 activity by administration of ketone esters or the NAD+ precursor nicotinamide riboside, both of which have been demonstrated to be beneficial in AD models (Gong et al., 2013; Kashiwaya et al., 2013; Hou et al., 2018). Another approach is to induce mild intermittent bioenergetic cellular stress by administering mitochondrial uncoupling agents, mitochondrial ATP-dependent K+ channel openers, or 2-deoxyglucose (which impairs cellular glucose utilization). The mitochondrial uncoupler 2, 4-dinitrophenol protects neurons and improves functional outcome in animal models of TBI, AD, and PD (Pandya et al., 2007; Liu et al., 2015a; Geisler et al., 2017). The K+-ATP channel opener diazoxide is very effective in protecting neurons in stroke, PD, and AD models (Liu et al., 2002, 2010; Yang et al., 2005). When administered once daily (which induces a mild transient cellular metabolic stress), 2-deoxyglucose induces adaptive cellular stress responses in neurons and protects them against degeneration in stroke and PD models (Yu and Mattson, 1999; Duan and Mattson, 1999). Similar to what occurs in response to fasting and exercise, administration of rapamycin stimulates autophagy while reducing overall protein synthesis; this results from inhibition of the mTOR pathway. Such pharmacological treatments that impact cellular signaling pathways that are also activated by fasting and exercise can suppress the accrual of hallmarks of brain aging and, indeed, rapamycin and nicotinamide riboside treatment can increase the lifespan of mice (Harrison et al., 2009; Zhang et al., 2016b).
Table 1.
Examples of Adaptive Metabolic Cellular Stress Response-Based Pharmacological Strategies to Ameliorate Age-Related Neurological Deficits and Diseases
| Agent | Mechanism of Action | Preclinical Findings | References |
|---|---|---|---|
| Ketone (ester) | energy substrate | improves cognition and endurance | Murray et al., 2016; Kashiwaya et al., 2000, 2013; Marosi et al., 2016 |
| induces BDNF expression | beneficial in seizure, AD, and PD models | ||
| Nicotinamide riboside | bolsters bioenergetics and sirtuin activity | extends lifespan and beneficial in AD model | Gong et al., 2013; Zhang et al., 2016a; Hou et al., 2018 |
| 2,4-dinitrophenol | mild mitochondrial uncoupling | beneficial in TBI, AD, and PD models | Pandya et al., 2007; Geisler et al., 2017; Lee et al., 2017 |
| adaptive cellular stress responses | |||
| Diazoxide | K-ATP channel opener | beneficial in stroke, PD, and AD models | Liu et al., 2002, 2010; Yang et al., 2005 |
| 2-deoxyglucose | induces adaptive cellular stress responses | neuroprotective in stroke and PD models | Duan and Mattson, 1999; Yu and Mattson, 1999 |
| Rapamycin | mTOR inhibitor and autophagy inducer | beneficial in stroke, AD, and PD models | Buckley et al., 2014; Spilman et al., 2010; Siddiqui et al., 2015 |
| extends lifespan | |||
| Exendin-4 and liraglutide | GLP-1R agonist and insulin sensitizer | beneficial in stroke, AD, and PD models | Li et al., 2009, 2010 |
| induces BDNF expression | |||
| Metformin | inhibits liver glucose production, activates AMP, and inhibits mTOR | beneficial in PD and AD models | Martin-Montalvo et al., 2013; Bayliss et al., 2016; Niccoli et al., 2016 |
| extends lifespan |
Two final examples of therapeutic approaches that target fundamental mechanisms of brain aging focus on the insulin-sensitizing hormone glucagon-like peptide 1 (GLP1) and the small molecule drug metformin. GLP-1 receptors are coupled to cyclic AMP production and CREB activation. GLP-1 receptor agonists increase insulin sensitivity and are neuroprotective in animal models of AD, PD, and stroke (Li et al., 2009, 2010; Liu et al., 2015b). A recent clinical trial revealed a therapeutic benefit of the GLP-1 analog exanatide in PD patients (Athauda et al., 2017). Metformin, which has been prescribed for diabetes for decades, inhibits liver glucose production, but also activates multiple cellular pathways that counteract aging processes, including activation of AMPK and inhibition of mTOR and inflammatory pathways (Barzilai et al., 2016). Metformin treatment ameliorates neurodegenerative and behavioral phenotypes in animal models of PD and AD (Bayliss et al., 2016; Niccoli et al., 2016). Such findings support the potential application of pharmacological agents that target metabolism to counteract brain aging and associated neurodegenerative disorders.
Concluding Remarks
Research in the fields of aging and neuroscience has revealed multiple hallmarks of brain aging at the molecular, cellular, and systems levels. Because they involve highly interdependent and interactive signaling pathways and regulatory systems, none of the hallmarks of brain aging occur in isolation. Given this complexity of the aging process, one might conclude that developing approaches for promoting optimal brain function and disease resistance during aging is a daunting challenge. However, studies of animal models have shown that the amount and frequency of energy intake can have a major impact on brain health span and vulnerability to AD, PD, and stroke. Optimism that prescriptions for healthy brain aging are within our grasp is supported by an emerging understanding of the mechanisms by which energy restriction and exercise enhance neuronal neuroplasticity and resistance of the brain to stress and aging. Recent findings suggest that feeding and exercise regimens that result in intermittent metabolic switching from liver-derived glucose to fat-derived ketones facilitates brain neuroplasticity and resilience (Mattson et al., 2018). As summarized in the preceding section and in Figure 4, accumulating data suggest that regular intermittent metabolic switching can counteract all of the hallmarks of aging. When viewed in the light of evolution, the latter findings are not surprising because success in the competition for scarce and sporadic food availability has been the major determinant of survival and reproductive success during evolution of all mammals, including humans (Mattson, 2015b). Accordingly, brains and the bodies they control evolved to function optimally in the setting of intermittent food deprivation. The evidence now justifies implementation by the medical community of strategies for enhancing the brain health of their patients by the prescription and active facilitation of daily and weekly patterns of meal consumption and exercise that result in intermittent metabolic switching. We would argue that the failure of the pharmaceutical industry to develop drugs that impact outcomes in age-related brain disorders is because of their focus on disease-specific targets. A novel approach grounded in the neurobiology of aging is to develop pharmacological interventions that impose intermittent bioenergetic challenges that activate many of the same adaptive cellular responses engaged by intermittent energy restriction and exercise. Recent findings from studies of animal models of age-related neurological disorders support this approach (Table 1). A new mindset for the design of preclinical studies and clinical trials will be required in which the goal is to develop safe agents with a relatively short halflife and a dosing schedule that imposes a moderate metabolic challenge followed by a recovery period.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging (AG000312-15).
References
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, Dahl GP, Dietrich WD, Keane RW. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Amigo I, Menezes-Filho SL, Luévano-Martínez LA, Chausse B, Kowaltowski AJ. Caloric restriction increases brain mitochondrial calcium retention capacity and protects against excitotoxicity. Aging Cell. 2017;16:73–81. doi: 10.1111/acel.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, Chia CW, Egan JM, Ferrucci L, Troncoso J, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14:318–329. doi: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton JP, Scutt P, Sprigg N, Bath PM. Hypercholesterolaemia and vascular dementia. Clin Sci (Lond) 2017;131:1561–1578. doi: 10.1042/CS20160382. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126:238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Woodruff TM, Lathia JD, Selvaraj PK, Mattson MP, Taylor SM. Neuroprotection in stroke by complement inhibition and immunoglobulin therapy. Neuroscience. 2009;158:1074–1089. doi: 10.1016/j.neuroscience.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Manzanero S, Furtado M, Biggins PJ, Hsieh YH, Gelderblom M, MacDonald KP, Salimova E, Li YI, Korn O, et al. An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab. 2017;37:2098–2111. doi: 10.1177/0271678X16661201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash JA, Lu H, Taxier LR, Long JM, Yang Y, Stein EA, Rapp PR. Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats. Proc Natl Acad Sci USA. 2016;113:12286–12291. doi: 10.1073/pnas.1525309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Santos VV, Deo M, Davies JS, Kemp BE, Elsworth JD, Andrews ZB. Metformin prevents nigrostriatal dopamine degeneration independent of AMPK activation in dopamine neurons. PLoS One. 2016;11:e0159381. doi: 10.1371/journal.pone.0159381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Ebert B, Schäffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, et al. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93:560–573 e6. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Krishnamurthi RV, Barker-Collo S, Forouzanfar MH, Naghavi M, Connor M, Lawes CM, Moran AE, Anderson LM, Roth GA, et al. Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart. 2014;9:107–112. doi: 10.1016/j.gheart.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Luck T, Riedel-Heller SG, Loeffler M, Schroeter ML, Stumvoll M, Villringer A, Witte AV. Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum Brain Mapp. 2017;2017:11. doi: 10.1002/hbm.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Boraxbekk CJ, Salami A, Wåhlin A, Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network-A multimodal approach. Neuroimage. 2016;131:133–141. doi: 10.1016/j.neuroimage.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Guillemin GJ, Smythe G, Sachdev P. Mapping NAD(+) metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology. 2014;15:177–198. doi: 10.1007/s10522-013-9489-5. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Brown MR, Geddes JW, Sullivan PG. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Brundin P, Melki R. Prying into the prion hypothesis for Parkinson’s disease. J Neurosci. 2017;37:9808–9818. doi: 10.1523/JNEUROSCI.1788-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Hess DL, Sazonova IY, Periyasamy-Thandavan S, Barrett JR, Kirks R, Grace H, Kondrikova G, Johnson MH, Hess DC, et al. Rapamycin upregulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp Transl Stroke Med. 2014;6:8. doi: 10.1186/2040-7378-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D, Bahr BA. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid Redox Signal. 2006;8:185–196. doi: 10.1089/ars.2006.8.185. [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Yanamadala S, Raffoul JJ, Guo Z, Soofi A, Heydari AR. Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair (Amst) 2003;2:295–307. doi: 10.1016/s1568-7864(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochim Biophys Acta. 2011;1813:965–973. doi: 10.1016/j.bbamcr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017;36:1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano CA, Paquet N, Dionne IJ, Imbeault H, Langlois F, Croteau E, Tremblay S, Fortier M, Matte JJ, Lacombe G, et al. A 3-month aerobic training program improves brain energy metabolism in mild Alzheimer’s disease: preliminary results from a neuroimaging study. J Alzheimers Dis. 2017;56:1459–1468. doi: 10.3233/JAD-161163. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Borghetti D, Kiferle L, Tognoni G, Giorgetti A, Neglia D, Sassi N, Frosini D, Rossi C, Petrozzi L, et al. CSF phosporylated TAU protein levels correlate with cerebral glucose metabolism assessed with PET in Alzheimer’s disease. Brain Res Bull. 2008;76:80–84. doi: 10.1016/j.brainresbull.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Chen X, Arumugam TV, Cheng YL, Lee JH, Chigurupati S, Mattson MP, Basta M. Combination therapy with low-dose IVIG and a C1-esterase inhibitor ameliorates brain damage and functional deficits in experimental ischemic stroke. Neuromolecular Med. 2018;20:63–72. doi: 10.1007/s12017-017-8474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Shinya K, Wan R, Tang SC, Miura T, Tang H, Khatri R, Gleichman M, Ouyang X, Liu D, et al. Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. J Neurosci. 2007;27:3722–3733. doi: 10.1523/JNEUROSCI.0590-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Sargent-Cox K, Fraser M, Sachdev P, Anstey KJ. Being overweight is associated with hippocampal atrophy: the PATH Through Life Study. Int J Obes. 2015;39:1509–1514. doi: 10.1038/ijo.2015.106. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith JC. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57:845–856. doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Herrup K. Genomic integrity and the ageing brain. Nat Rev Neurosci. 2015;16:672–684. doi: 10.1038/nrn4020. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Li B, Tsien RW, Ma H. Evolutionary and functional perspectives on signaling from neuronal surface to nucleus. Biochem Biophys Res Commun. 2015;460:88–99. doi: 10.1016/j.bbrc.2015.02.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Franke K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci. 2017;40:681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. Cellular functions of LRRK2 implicate vesicular trafficking pathways in Parkinson’s disease. Biochem Soc Trans. 2016;44:1603–1610. doi: 10.1042/BST20160228. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci. 2016;9:53. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauncey MJ. Nutrition, the brain and cognitive decline: insights from epigenetics. Eur J Clin Nutr. 2014;68:1179–1185. doi: 10.1038/ejcn.2014.173. [DOI] [PubMed] [Google Scholar]
- de Jong GI, Naber PA, Van der Zee EA, Thompson LT, Disterhoft JF, Luiten PG. Age-related loss of calcium binding proteins in rabbit hippocampus. Neurobiol Aging. 1996;17:459–465. doi: 10.1016/0197-4580(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Debette S, Wolf C, Lambert JC, Crivello F, Soumaré A, Zhu YC, Schilling S, Dufouil C, Mazoyer B, Amouyel P, et al. Abdominal obesity and lower gray matter volume: a Mendelian randomization study. Neurobiol Aging. 2014;35:378–386. doi: 10.1016/j.neurobiolaging.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Denis I, Potier B, Vancassel S, Heberden C, Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms. Ageing Res Rev. 2013;12:579–594. doi: 10.1016/j.arr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. Mitochondrial DNA and Parkinson’s disease. Neurology. 1991;41(Suppl 2):38–42. doi: 10.1212/wnl.41.5_suppl_2.38. discussion 42–43. [DOI] [PubMed] [Google Scholar]
- Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb Cortex. 2018;28:988–997. doi: 10.1093/cercor/bhx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayer BP. Imaging of the aging brain. Part I Normal findings. Radiology. 1988;166:785–796. doi: 10.1148/radiology.166.3.3277247. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBoff B, Feany M, Götz J. Why size matters - balancing mitochondrial dynamics in Alzheimer’s disease. Trends Neurosci. 2013;36:325–335. doi: 10.1016/j.tins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykiert D, Der G, Starr JM, Deary IJ. Age differences in intra-individual variability in simple and choice reaction time: systematic review and meta-analysis. PLoS One. 2012;7:e45759. doi: 10.1371/journal.pone.0045759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM, Suire C, Maharana C, Jicha GA, Liu D, et al. Extracellular vesicle-associated Aβ mediates trans-neuronal bioenergetic and Ca2+-handling deficits in Alzheimer’s disease models. NPJ Aging Mech Dis. 2016;2:16019. doi: 10.1038/npjamd.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi M, Hasan Z, Motoi Y, Matsumoto SE, Ishiguro K, Hattori N. Region-specific vulnerability to oxidative stress, neuroinflammation, and tau hyperphosphorylation in experimental diabetes mellitus mice. J Alzheimers Dis. 2016;51:1209–1224. doi: 10.3233/JAD-150820. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre H, Baune B, Lavretsky H. Clinical advances in geriatric psychiatry: a focus on prevention of mood and cognitive disorders. Psychiatr Clin North Am. 2015;38:495–514. doi: 10.1016/j.psc.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol Med. 2017;23:899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 2013;12:941–966. doi: 10.1016/j.arr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM, Mattson MP, Arumugam TV. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol. 2014;257:114–119. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, et al. Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55:1082–1096. doi: 10.1007/s12035-017-0394-9. [DOI] [PubMed] [Google Scholar]
- Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171:2000–2016. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH STAIR Group. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland RP, Jagust WJ, Huesman RH, Koss E, Knittel B, Mathis CA, Ober BA, Mazoyer BM, Budinger TF. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurology. 1989;39:1427–1434. doi: 10.1212/wnl.39.11.1427. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, Lozano AM, Hammerstad J, Lyons K, Koller WC, et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: a PET study of resting-state glucose metabolism. Brain. 2001;124:1601–1609. doi: 10.1093/brain/124.8.1601. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, Wu LJ, Xu H, Zhao MG, Ueda K, et al. Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 2008;28:9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Butterfield DA, Hensley K, Shaw W, Carney JM. Aging and caloric restriction affect mitochondrial respiration and lipid membrane status: an electron paramagnetic resonance investigation. Free Radic Biol Med. 1997;23:191–201. doi: 10.1016/s0891-5849(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordr) 2006;28:221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Chen KC, Kadish I, Blalock EM, Thibault O, Porter NM, Landfield PW. Reversal of aging-related neuronal Ca2+ dysregulation and cognitive impairment by delivery of a transgene encoding FK506-binding protein 12.6/1b to the hippocampus. J Neurosci. 2015;35:10878–10887. doi: 10.1523/JNEUROSCI.1248-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JW, Tekirian TL, Soultanian NS, Ashford JW, Davis DG, Markesbery WR. Comparison of neuropathologic criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(Suppl):S99–S105. doi: 10.1016/s0197-4580(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Geisler JG, Marosi K, Halpern J, Mattson MP. DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement. 2017;13:582–591. doi: 10.1016/j.jalz.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26:353–360 e3. doi: 10.1016/j.cmet.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SH, Liu H. Life and death in the trash heap: the ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral ischemia. Ageing Res Rev. 2017;34:30–38. doi: 10.1016/j.arr.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen KJ, Rothman SM, Ladenheim B, Wan R, Vranis N, Hutchison E, Okun E, Cadet JL, Mattson MP. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol Aging. 2013;34:928–935. doi: 10.1016/j.neurobiolaging.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM. Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol. 2016;79:366–378. doi: 10.1002/ana.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gurung S, Agbaga MP, Myers DA. Cognitive differences between Sprague-Dawley rats selectively bred for sensitivity or resistance to diet induced obesity. Behav Brain Res. 2016;311:122–130. doi: 10.1016/j.bbr.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Gwon AR, Park JS, Arumugam TV, Kwon YK, Chan SL, Kim SH, Baik SH, Yang S, Yun YK, Choi Y, et al. Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer’s disease. Aging Cell. 2012;11:559–568. doi: 10.1111/j.1474-9726.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci. 2013;33:9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Heyward FD, Gilliam D, Coleman MA, Gavin CF, Wang J, Kaas G, Trieu R, Lewis J, Moulden J, Sweatt JD. Obesity weighs down memory through a mechanism involving the neuroepigenetic dysregulation of Sirt1. J Neurosci. 2016;36:1324–1335. doi: 10.1523/JNEUROSCI.1934-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DA, Tryon LD, Carter HN, Kim Y, Chen CC. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473:2295–2314. doi: 10.1042/BCJ20160009. [DOI] [PubMed] [Google Scholar]
- Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, Cheng A. Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells. 2012;30:2535–2547. doi: 10.1002/stem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA. 2018;115:E1876–E1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci USA. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikematsu N, Dallas ML, Ross FA, Lewis RW, Rafferty JN, David JA, Suman R, Peers C, Hardie DG, Evans AM. Phosphorylation of the voltage-gated potassium channel Kv2.1 by AMP-activated protein kinase regulates membrane excitability. Proc Natl Acad Sci USA. 2011;108:18132–18137. doi: 10.1073/pnas.1106201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol Aging. 2006;27:1129–1136. doi: 10.1016/j.neurobiolaging.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, van der Pluijm I, de Waard MC, Haasdijk ED, Brandt R, Vermeij M, Rijksen Y, Maas A, van Steeg H, Hoeijmakers JH, van der Horst GT. Age-related neuronal degeneration: complementary roles of nucleotide excision repair and transcription-coupled repair in preventing neuropathology. PLoS Genet. 2011;7:e1002405. doi: 10.1371/journal.pgen.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Rothwell JC. Inhibitory dysfunction contributes to some of the motor and non-motor symptoms of movement disorders and psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2017;372:1–10. doi: 10.1098/rstb.2016.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz D, Wittfeld K, Terock J, Freyberger HJ, Hegenscheid K, Völzke H, Habes M, Hosten N, Friedrich N, Nauck M, et al. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage. 2015;122:149–157. doi: 10.1016/j.neuroimage.2015.07.086. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation. 2014;11:162. doi: 10.1186/s12974-014-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HC, Hsu JM, Yen CP, Chao CC, Chen RH, Pan CL. Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons. Proc Natl Acad Sci USA. 2015;112:8768–8773. doi: 10.1073/pnas.1501831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Inui Y, Nakamura A, Ito K. Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res Rev. 2016;30:73–84. doi: 10.1016/j.arr.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing Res Rev. 2002;1:279–293. doi: 10.1016/s1568-1637(01)00006-x. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 2017;40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Chan PH. Oxidative stress and neuronal DNA fragmentation mediate age-dependent vulnerability to the mitochondrial toxin, 3-nitropropionic acid, in the mouse striatum. Neurobiol Dis. 2001;8:114–126. doi: 10.1006/nbdi.2000.0327. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S, Kreienkamp HJ. Dendritic mRNA targeting and translation. Adv Exp Med Biol. 2012;970:285–305. doi: 10.1007/978-3-7091-0932-8_13. [DOI] [PubMed] [Google Scholar]
- Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11:625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- Knott EP, Assi M, Rao SN, Ghosh M, Pearse DD. Phospho-diesterase inhibitors as a therapeutic approach to neuroprotection and repair. Int J Mol Sci. 2017;18:1–10. doi: 10.3390/ijms18040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans SJ, Schuurman T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur J Pharmacol. 2015;759:231–239. doi: 10.1016/j.ejphar.2015.03.044. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, et al. Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obes Rev. 2015;16:273–281. doi: 10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne CF, Stigter EC, Hendriks MM, Berger R, Rokach J, Korswagen HC, Brenkman AB. Quantification of in vivo oxidative damage in Caenorhabditis elegans during aging by endogenous F3-isoprostane measurement. Aging Cell. 2013;12:214–223. doi: 10.1111/acel.12043. [DOI] [PubMed] [Google Scholar]
- Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83:771–787. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW. The etiology of Parkinson’s disease with emphasis on the MPTP story. Neurology. 1996;47(Suppl 3):S153–S160. doi: 10.1212/wnl.47.6_suppl_3.153s. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Yassa MA. Perturbations of neural circuitry in aging, mild cognitive impairment, and Alzheimer’s disease. Ageing Res Rev. 2013;12:823–831. doi: 10.1016/j.arr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro GS, Sykora P, Bohr VA. The impact of base excision DNA repair in age-related neurodegenerative diseases. Mutat Res. 2015;776:31–39. doi: 10.1016/j.mrfmmm.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Heo G, Lee KM, Kim AH, Chung KW, Im E, Chung HY, Lee J. Neuroprotective effects of 2,4-dinitrophenol in an acute model of Parkinson’s disease. Brain Res. 2017;1663:184–193. doi: 10.1016/j.brainres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Sanchez-Martinez A, Zarate AM, Benincá C, Mayor U, Clague MJ, Whitworth AJ. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J Cell Biol. 2018;217:1613–1622. doi: 10.1083/jcb.201801044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie SW, Chandler LJ, Barr EM, Farrar RP. Reduced calcium uptake by rat brain mitochondria and synaptosomes in response to aging. Brain Res. 1985;329:177–183. doi: 10.1016/0006-8993(85)90523-2. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Yu CE, Schellenberg GD. Aging-associated neuropathology in Werner syndrome. Acta Neuropathol. 1998;96:421–424. doi: 10.1007/s004010050914. [DOI] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev. 2014;43:100–117. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kehoe EG, McGinnity TM, Coyle D, Bokde AL. Modulation of effective connectivity in the default mode network at rest and during a memory task. Brain Connect. 2015;5:60–67. doi: 10.1089/brain.2014.0249. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Wang L, Wang P, Xue Y, Li X, Qiao X, Zhang X, Xu T, Liu G, et al. Autophagy impairment mediated by S-nitrosation of ATG4B leads to neurotoxicity in response to hyperglycemia. Autophagy. 2017;13:1145–1160. doi: 10.1080/15548627.2017.1320467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Lin DT, Wu J, Holstein D, Upadhyay G, Rourk W, Muller E, Lechleiter JD. Ca2+ signaling, mitochondria and sensitivity to oxidative stress in aging astrocytes. Neurobiol Aging. 2007;28:99–111. doi: 10.1016/j.neurobiolaging.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, et al. Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Coman D, Jiang L, Rothman DL, Hyder F. Caloric restriction impedes age-related decline of mitochondrial function and neuronal activity. J Cereb Blood Flow Metab. 2014;34:1440–1443. doi: 10.1038/jcbfm.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H, Villarreal J, Cutler RG, et al. The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;22:443–457. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang Y, Gharavi R, Park HR, Lee J, Siddiqui S, Telljohann R, Nassar MR, Cutler RG, Becker KG, Mattson MP. The mitochondrial uncoupler DNP triggers brain cell mTOR signaling network reprogramming and CREB pathway upregulation. J Neurochem. 2015a;134:677–692. doi: 10.1111/jnc.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015b;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Lee SE, Wojta K, Ramos EM, Klein E, Chen J, Boxer AL, Gorno-Tempini ML, Geschwind DH, Schlotawa L, et al. Tauopathy Genetics Consortium. A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain. 2017;140:1128–1146. doi: 10.1093/brain/awx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Lombardi P, Karadayian AG, Orgambide F, Cicerchia D, Bustamante J. Brain cortex mitochondrial bioenergetics in synaptosomes and non-synaptic mitochondria during aging. Neurochem Res. 2016;41:353–363. doi: 10.1007/s11064-015-1817-5. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke-Wold BP, Turner RC, Lucke-Wold AN, Rosen CL, Huber JD. Age and the metabolic syndrome as risk factors for ischemic stroke: improving preclinical models of ischemic stroke. Yale J Biol Med. 2012;85:523–539. [PMC free article] [PubMed] [Google Scholar]
- Macpherson H, Formica M, Harris E, Daly RM. Brain functional alterations in Type 2 Diabetes - A systematic review of fMRI studies. Front Neuroendocrinol. 2017;47:34–46. doi: 10.1016/j.yfrne.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353:aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineanu DG, Niespodziany I, Wülfert E. Hippocampal slices from long-term streptozotocin-injected rats are prone to epileptiform responses. Neurosci Lett. 1998;252:183–186. doi: 10.1016/s0304-3940(98)00580-1. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139:769–781. doi: 10.1111/jnc.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller L, Williams M, Rich A, Savage G, Burianová H. Aging and large-scale functional networks: white matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience. 2015;290:369–378. doi: 10.1016/j.neuroscience.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Kim JW, Lee BD, Kang HC, Xu JC, Jia H, Stankowski J, Kim MS, Zhong J, Kumar M, et al. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell. 2014;157:472–485. doi: 10.1016/j.cell.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, de Cabo R. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal. 2013;19:310–320. doi: 10.1089/ars.2012.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Harley CW. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 2016;20:214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Late-onset dementia: a mosaic of prototypical pathologies modifiable by diet and lifestyle. NPJ Aging Mech Dis. 2015a;1:15003. doi: 10.1038/npjamd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015b;20:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Rychlik B. Glia protect hippocampal neurons against excitatory amino acid-induced degeneration: involvement of fibroblast growth factor. Int J Dev Neurosci. 1990;8:399–415. doi: 10.1016/0736-5748(90)90073-b. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res. 2011;217:134–141. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- Mendonca GV, Pezarat-Correia P, Vaz JR, Silva L, Heffernan KS. Impact of aging on endurance and neuromuscular physical performance: the role of vascular senescence. Sports Med. 2017;47:583–598. doi: 10.1007/s40279-016-0596-8. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Montgomery SL, Mastrangelo MA, Habib D, Narrow WC, Knowlden SA, Wright TW, Bowers WJ. Ablation of TNF-RI/RII expression in Alzheimer’s disease mice leads to an unexpected enhancement of pathology: implications for chronic pan-TNF-α suppressive therapeutic strategies in the brain. Am J Pathol. 2011;179:2053–2070. doi: 10.1016/j.ajpath.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Datta D, Paspalas CD, Arnsten AFT. Ultra-structural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol Aging. 2017;51:9–18. doi: 10.1016/j.neurobiolaging.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014;9:633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–4032. doi: 10.1096/fj.201600773R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagtegaal AP, Rainey RN, van der Pluijm I, Brandt RM, van der Horst GT, Borst JG, Segil N. Cockayne syndrome group B (Csb) and group a (Csa) deficiencies predispose to hearing loss and cochlear hair cell degeneration in mice. J Neurosci. 2015;35:4280–4286. doi: 10.1523/JNEUROSCI.5063-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidl R, Schneider A, Bousiges O, Majchrzak M, Barbelivien A, de Vasconcelos AP, Dorgans K, Doussau F, Loeffler JP, Cassel JC, Boutillier AL. Late-life environmental enrichment induces acetylation events and nuclear factor κB-dependent regulations in the hippocampus of aged rats showing improved plasticity and learning. J Neurosci. 2016;36:4351–4361. doi: 10.1523/JNEUROSCI.3239-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth BJ, Craft S. Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front Aging Neurosci. 2017;9:345. doi: 10.3389/fnagi.2017.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Cabecinha M, Tillmann A, Kerr F, Wong CT, Cardenes D, Vincent AJ, Bettedi L, Li L, Grönke S, et al. Increased glucose transport into neurons rescues Aβ toxicity in Drosophila. Curr Biol. 2016;26:2291–2300. doi: 10.1016/j.cub.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SM, Xu S, Kritikou JS, Marosi K, Brodin L, Mattson MP. Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J Neurochem. 2017;142:286–296. doi: 10.1111/jnc.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Castro K, Mughal MR, Pita MA, Stranahan AM, et al. Evidence for a developmental role for TLR4 in learning and memory. PLoS One. 2012;7:e47522. doi: 10.1371/journal.pone.0047522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Rothman S, Wan R, Cong WN, De Cabo R, Martin-Montalvo A, Levette A, Maudsley S, Martin B, et al. Toll-like receptors 2 and 4 modulate autonomic control of heart rate and energy metabolism. Brain Behav Immun. 2014;36:90–100. doi: 10.1016/j.bbi.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Bartus RT, Volpicelli-Daley LA, Kordower JH. Trophic factors for Parkinson’s disease: To live or let die. Mov Disord. 2015;30:1715–1724. doi: 10.1002/mds.26426. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Grondin R, Yonutas HM, Haghnazar H, Gash DM, Zhang Z, Sullivan PG. Decreased mitochondrial bioenergetics and calcium buffering capacity in the basal ganglia correlates with motor deficits in a nonhuman primate model of aging. Neurobiol Aging. 2015;36:1903–1913. doi: 10.1016/j.neurobiolaging.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Royland JE, MacPhail RC, Sullivan PG, Kodavanti PR. Age- and brain region-specific differences in mitochondrial bioenergetics in Brown Norway rats. Neurobiol Aging. 2016;42:25–34. doi: 10.1016/j.neurobiolaging.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Papaioannou N, Tooten PC, van Ederen AM, Bohl JR, Rofina J, Tsangaris T, Gruys E. Immunohistochemical investigation of the brain of aged dogs. I Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AM, Lin AL. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany NY) 2016;8:2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Swomley AM, Butterfield DA. Redox proteomics and the dynamic molecular landscape of the aging brain. Ageing Res Rev. 2014;13:75–89. doi: 10.1016/j.arr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, Chutna O, Outeiro TF, Winkler J, Masliah E, Klucken J. Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy. 2014;10:2171–2192. doi: 10.4161/auto.36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard AK, Craig EL, Chakrabarti L. Mitochondrial complex 1 activity measured by spectrophotometry is reduced across all brain regions in ageing and more specifically in neurodegeneration. PLoS One. 2016;11:e0157405. doi: 10.1371/journal.pone.0157405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging. 2008;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela AM, Fabian S, Grittner U, Spranger J, Flöel A. Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27:1765–1778. doi: 10.1093/cercor/bhw008. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raefsky SM, Mattson MP. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic Biol Med. 2017;102:203–216. doi: 10.1016/j.freeradbiomed.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Alexander GE. Adaptive capacity: an evolutionary neuroscience model linking exercise, cognition, and brain health. Trends Neurosci. 2017;40:408–421. doi: 10.1016/j.tins.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Regnell CE, Hildrestrand GA, Sejersted Y, Medin T, Moldestad O, Rolseth V, Krokeide SZ, Suganthan R, Luna L, Bjørås M, Bergersen LH. Hippocampal adult neurogenesis is maintained by Neil3-dependent repair of oxidative DNA lesions in neural progenitor cells. Cell Rep. 2012;2:503–510. doi: 10.1016/j.celrep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Rekik K, Francés B, Valet P, Dray C, Florian C. Cognitive deficit in hippocampal-dependent tasks in Werner syndrome mouse model. Behav Brain Res. 2017;323:68–77. doi: 10.1016/j.bbr.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci. 2013;33:1218–1227a. doi: 10.1523/JNEUROSCI.3277-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Segal M. Serotonin, aging and cognitive functions of the hippocampus. Rev Neurosci. 1996;7:103–113. doi: 10.1515/revneuro.1996.7.2.103. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rolyan H, Scheffold A, Heinrich A, Begus-Nahrmann Y, Langkopf BH, Hölter SM, Vogt-Weisenhorn DM, Liss B, Wurst W, Lie DC, et al. Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain. 2011;134:2044–2056. doi: 10.1093/brain/awr133. [DOI] [PubMed] [Google Scholar]
- Rose CR, Felix L, Zeug A, Dietrich D, Reiner A, Henneberger C. Astroglial glutamate signaling and uptake in the hippocampus. Front Mol Neurosci. 2018;10:451. doi: 10.3389/fnmol.2017.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11:159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, Junqué C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 2015;6:663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RX, Correia SC, Zhu X, Smith MA, Moreira PI, Castellani RJ, Nunomura A, Perry G. Mitochondrial DNA oxidative damage and repair in aging and Alzheimer’s disease. Antioxid Redox Signal. 2013;18:2444–2457. doi: 10.1089/ars.2012.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ibañez J, Gómez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr. 2005;37:83–90. doi: 10.1007/s10863-005-4131-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Saura CA, Cardinaux JR. Emerging roles of CREB-regulated transcription coactivators in brain physiology and pathology. Trends Neurosci. 2017;40:720–733. doi: 10.1016/j.tins.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384:545–555. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M. Neurodegeneration in accelerated aging. Dan Med J. 2016;63:1–10. [PubMed] [Google Scholar]
- Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Gerding HR, Karreman C, Drescher M, Lashuel HA, Outeiro TF, Di Monte DA, Leist M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies. J Neurochem. 2013;125:491–511. doi: 10.1111/jnc.12226. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Sepe S, Milanese C, Gabriels S, Derks KW, Payan-Gomez C, van IJcken WF, Rijksen YM, Nigg AL, Moreno S, Cerri S, et al. Inefficient DNA repair is an aging-related modifier of Parkinson’s disease. Cell Rep. 2016;15:1866–1875. doi: 10.1016/j.celrep.2016.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SZA, Zhao D, Hussain T, Yang L. Role of the AMPK pathway in promoting autophagic flux via modulating mitochondrial dynamics in neurodegenerative diseases: Insight into prion diseases. Ageing Res Rev. 2017;40:51–63. doi: 10.1016/j.arr.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX, Li S, Dodart JC, et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet. 2017;26:1915–1926. doi: 10.1093/hmg/ddx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- Shimabukuro MK, Langhi LG, Cordeiro I, Brito JM, Batista CM, Mattson MP, Mello Coelho Vd. Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Sci Rep. 2016;6:23795. doi: 10.1038/srep23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Bhaumik D, Chinta SJ, Rane A, Rajagopalan S, Lieu CA, Lithgow GJ, Andersen JK. Mitochondrial quality control via the PGC1α-TFEB signaling pathway is compromised by parkin Q311X mutation but independently restored by rapamycin. J Neurosci. 2015;35:12833–12844. doi: 10.1523/JNEUROSCI.0109-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Shiraishi K. N-methyl-D-aspartate antagonist reduces stroke size and regional glucose metabolism. Ann Neurol. 1990;27:606–611. doi: 10.1002/ana.410270604. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife. 2016;5:e15092. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WM, Stoesz BM, Kelly DM, Albensi BC. Roles for NF-κB and gene targets of NF-κB in synaptic plasticity, memory, and navigation. Mol Neurobiol. 2014;49:757–770. doi: 10.1007/s12035-013-8555-y. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, Baltan S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci. 2016;36:9990–10001. doi: 10.1523/JNEUROSCI.1316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stillman CM, Weinstein AM, Marsland AL, Gianaros PJ, Erickson KI. Body-brain connections: the effects of obesity and behavioral interventions on neurocognitive aging. Front Aging Neurosci. 2017;9:115. doi: 10.3389/fnagi.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, Horner PJ. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem. 2011;286:38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008a;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008b;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem. 2008;106:24–36. doi: 10.1111/j.1471-4159.2008.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 2017a;483:1013–1019. doi: 10.1016/j.bbrc.2016.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM. Parkinson’s disease is not simply a prion disorder. J Neurosci. 2017b;37:9799–9807. doi: 10.1523/JNEUROSCI.1787-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Bioenergetics and metabolism: a bench to bedside perspective. J Neurochem. 2016;139(Suppl 2):126–135. doi: 10.1111/jnc.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong WN, Brenerman BM, et al. DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 2015;43:943–959. doi: 10.1093/nar/gku1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Wang YC, Li YI, Lin HC, Manzanero S, Hsieh YH, Phipps S, Hu CJ, Chiou HY, Huang YS, et al. Functional role of soluble receptor for advanced glycation end products in stroke. Arterioscler Thromb Vasc Biol. 2013;33:585–594. doi: 10.1161/ATVBAHA.112.300523. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Mattson MP. Impaired adaptive cellular responses to oxidative stress and the pathogenesis of Alzheimer’s disease. Antioxid Redox Signal. 2011;14:1519–1534. doi: 10.1089/ars.2010.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held LL, An Y, Kraut M, Metter J, Egan J, Ferrucci L, O’Brien R, Resnick SM. Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging. Neurobiol Aging. 2013;34:2271–2276. doi: 10.1016/j.neurobiolaging.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Anderson KL, DeMoll C, Brewer LD, Landfield PW, Porter NM. Hippocampal calcium dysregulation at the nexus of diabetes and brain aging. Eur J Pharmacol. 2013;719:34–43. doi: 10.1016/j.ejphar.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen K, Yokota T, Hasan-Olive MM, Sherazi N, Fakouri NB, Desler C, Regnell CE, Larsen S, Rasmussen LJ, Dela F, et al. Initial brain aging: heterogeneity of mitochondrial size is associated with decline in complex I-linked respiration in cortex and hippocampus. Neurobiol Aging. 2018;61:215–224. doi: 10.1016/j.neurobiolaging.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tse KH, Herrup K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech Ageing Dev. 2017;161(Pt A):37–50. doi: 10.1016/j.mad.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umarova RM. Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: Implications for understanding neglect. Cortex. 2017;97:327–338. doi: 10.1016/j.cortex.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Vaarmann A, Mandel M, Zeb A, Wareski P, Liiv J, Kuum M, Antsov E, Liiv M, Cagalinec M, Choubey V, Kaasik A. Mitochondrial biogenesis is required for axonal growth. Development. 2016;143:1981–1992. doi: 10.1242/dev.128926. [DOI] [PubMed] [Google Scholar]
- Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij WP, Dollé ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, Wu H, Roks AJ, Botter SM, van der Eerden BC, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537:427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VerPlank JJS, Goldberg AL. Regulating protein breakdown through proteasome phosphorylation. Biochem J. 2017;474:3355–3371. doi: 10.1042/BCJ20160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, Pradhan K. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 2017;16:311–322. doi: 10.1016/S1474-4422(17)30044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Dixit S, Saulsberry AC, May JM, Harrison FE. Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol Dis. 2017;100:87–98. doi: 10.1016/j.nbd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang R, Carrera I, Xu S, Lakshmana MK. TFEB overexpression in the P301S model of tauopathy mitigates increased PHF1 levels and lipofuscin puncta and rescues memory deficits. eNeuro. 2016;3 doi: 10.1523/ENEURO.0042-16.2016. ENEURO.0042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenheim KM, Dickson DW, Rapin I. Neuropathology of Cockayne syndrome: evidence for impaired development, premature aging, and neurodegeneration. Mech Ageing Dev. 2009;130:619–636. doi: 10.1016/j.mad.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Weinstein PR, Hong S, Sharp FR. Molecular identification of the ischemic penumbra. Stroke. 2004;35(Suppl 1):2666–2670. doi: 10.1161/01.STR.0000144052.10644.ed. [DOI] [PubMed] [Google Scholar]
- Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, McLaren DG, Canu E, Kastman EK, Kosmatka KJ, Xu G, Field AS, Alexander AL, Colman RJ, et al. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:987–994. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psycho-neuroendocrinology. 2014;42:165–177. doi: 10.1016/j.psyneuen.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Xilouri M, Stefanis L. Chaperone mediated autophagy in aging: starve to prosper. Ageing Res Rev. 2016;32:13–21. doi: 10.1016/j.arr.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY, Sun YH, Yao HH, Wang H, Wu J, Hu G. Systematic administration of iptakalim, an ATP-sensitive potassium channel opener, prevents rotenone-induced motor and neurochemical alterations in rats. J Neurosci Res. 2005;80:442–449. doi: 10.1002/jnr.20467. [DOI] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated upregulation of apurinic endonuclease 1. J Biol Chem. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY, Louneva N, Lee VM, Kim SF, Trojanowski JQ, Arnold SE. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128:679–689. doi: 10.1007/s00401-014-1328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528–536. [PubMed] [Google Scholar]
- Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A, Gao J, Squier TC, Michaelis ML. Age-related decrease in brain synaptic membrane Ca2+-ATPase in F344/BNF1 rats. Neurobiol Aging. 1998;19:487–495. doi: 10.1016/s0197-4580(98)00078-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, Lai MK, Kappei D, Kumar AP, Sethi G. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res Rev. 2016a;25:55–69. doi: 10.1016/j.arr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016b;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zhang S, Eitan E, Mattson MP. Early involvement of lysosome dysfunction in the degeneration of cerebral cortical neurons caused by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2017;140:941–954. doi: 10.1111/jnc.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Eitan E, Wu TY, Mattson MP. Intercellular transfer of pathogenic α-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4-hydroxynonenal. Neurobiol Aging. 2018;61:52–65. doi: 10.1016/j.neurobiolaging.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG, Greenamyre JT, Burton EA. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest. 2015;125:2721–2735. doi: 10.1172/JCI64502. [DOI] [PMC free article] [PubMed] [Google Scholar]