Abstract
In the current study, we investigate the neuronal correlates of the Attention Training Technique (ATT), a psychotherapeutic intervention used in metacognitive therapy to enhance flexible cognitive control and ameliorate rumination.
We adapted the ATT in a neuroscientific attention paradigm in order to investigate the effects of its components: selective attention, attention switching and divided attention in comparison to a control task. Functional near-infrared spectroscopy was used to measure changes in blood oxygenation of fronto-lateral and parietal cortical areas. Furthermore, subjects rated their task performance, effort and attention drifts in each task condition.
We observed increased blood oxygenation in the right inferior frontal gyrus, right dorsolateral prefrontal cortex and superior parietal lobule during the ATT conditions in comparison to the control condition. Additionally, subjective effort was associated with blood oxygenation in the right inferior prefrontal cortex.
Our results are consistent with the theoretical underpinnings of the ATT suggesting that the ATT's mechanism of change lies in the training of areas of the cognitive control network and dorsal attention network. Aberrant functioning of both networks has been shown to be related to depression and rumination.
Keywords: Attention training technique, Selective attention, Divided attention, Attention switching, Cognitive control network, Dorsal attention network, Inferior frontal gyrus, Dorsolateral prefrontal cortex, Superior parietal lobule, Functional near-infrared spectroscopy (fNIRS)
Highlights
-
•
The neurophysiological underpinnings of the Attention Training Technique (ATT) were investigated in an experimental design.
-
•
The ATT requires activation of brain areas in the cognitive control network (CCN) and dorsal attention network (DAN).
-
•
We observed increased blood oxygenation in the right IFG, dlPFC and superior parietal lobule during the ATT.
-
•
Subjective effort during the ATT was associated with blood oxygenation in the IFG.
-
•
The ATT might influence depressive rumination through the training of the CCN and DAN.
1. Introduction
The Attention Training Technique (ATT) was developed based on the self-regulatory executive function model (S-REF) of psychological disorder (Wells, 1990; Wells and Matthews, 1996). The basic premise of this model is that emotional disorders are the result of a ‘cognitive attentional syndrome’ comprising perseverative or repetive thinking (worry and rumination) and threat-focused attention coping strategies that are difficult for the individual to bring under control. The ATT was developed to increase executive control of the CAS and to enhance corresponding metacognitive knowledge of control.
There is a large body of research supporting the contention that perseverative thinking such as worry and rumination is linked to negative emotional outcomes (Capobianco et al., 2018; Ehring and Watkins, 2008; Nolen-Hoeksema, 1991; Watkins, 2008). For example, rumination is indicated as a reliable predictor for relapse of depression (Michalak et al., 2011; Broderick and Korteland, 2004), severity of the disease (McLaughlin et al., 2007; Smith and Alloy, 2009) and treatment duration (Robinson and Alloy, 2003). In the same way, worry is associated with higher levels of anxiety as a main symptom of generalized anxiety disorder (Dilling et al., 2016) and is linked to the development of PTSD and intrusive thoughts following stress exposure (Holeva et al., 2001; Wells and Papageorgiou, 1998).
During perseverative thinking, subjects tend to focus on themselves and inward cognitive processes which results in prolonged cognitive representations of stressors (Brosschot et al., 2006; Ottaviani et al., 2016). Accompanied by this, subject with high tendencies for perseverative thinking usually use less adaptive emotion regulation (Aldao et al., 2010). The ATT aims to dissolve inflexible, self-focused and repetitive thinking (Knowles et al., 2016) by increasing metacognitive awareness of rumination (through the focus on attention allocation) and enhancing metacognitive (top-down) control of processing (Nassif and Wells, 2014; Wells, 2007). The ATT consists of an auditory attention task in which attention is focused on different auditory stimuli, while other sounds have to be ignored. Subjects are instructed to focus their attention on selected sounds (selective attention), switch between sounds (attentional switching) and focus on all sounds simultaneously (divided attention).
There is initial evidence that the ATT may be beneficial for the treatment of different psychiatric disorders including depression and anxiety disorders (Fergus and Bardeen, 2016; Knowles et al., 2016). Furthermore, the first neuroimaging data suggests that the ATT affects subcortical affective and cortical cognitive brain areas. In an investigation by Siegle et al. (2007), patients that were treated with a treatment package including the ATT showed enhanced activity within the dorsolateral prefrontal cortex (dlPFC) during a cognitive task and reduced amygdala activity to negative emotional material in an affective task after treatment (Siegle et al., 2007). Although initial evidence for the effectiveness of the ATT exists, to the best of our knowledge, the underlying neuronal mechanisms of the ATT components are unexplored.
Interestingly, although there is no direct data on the neuronal effects of the ATT, research on the cortical mechanisms of auditory selective attention implies that posterior parietal cortex (PPC) and ventrolateral prefrontal cortex (VLPFC) are activated when switching attention between auditory signals and behavioral inhibition (Hanlon et al., 2017; Huang et al., 2012; Lee et al., 2014). For example, Huang et al. (2012) found activation during voluntary attention shifting in a fronto-cingular attention network comprising the IFG, medial frontal cortex and anterior insula (Huang et al., 2012). Further, Hanlon et al. (2017) reported correlation between VLPFC activity and response to auditory stimuli in high conflict conditions (Hanlon et al., 2017). As the ATT addresses the functions of selective attention, attention switching and dividing attention, and is intended to enhance executive control (e.g. attention flexibility to disengage rumination), it is plausible that the neuronal mechanism of the technique lies in the facilitation of activation in related brain areas in the CCN.
In the study at hand, we developed an ATT-based experimental paradigm that incorporates the different training aspects or components of the ATT in an experimental procedure. As this is one of the first studies to investigate the neurophysiological mechanisms of the ATT, we examined the effects of the paradigm in a non-clinical group of healthy subjects. The investigation of trainable psychotherapeutic techniques as the ATT in non-clinical samples in Phase I Studies has some important advantages. For example, the investigated mechanism is not disturbed by medication or psychopathological effects. From an ethical standpoint, this procedure further reduces possible unnecessary testing of impaired subjects in the case of non-useful paradigms during first stage investigations. Instead, by using non-clinical samples, it is possible to propose the general psychophysiological mechanism that can be tested with respect to psychopathological alterations in the clinical model.
Hemodynamic changes in the CCN were measured with functional near-infrared spectroscopy (fNIRS). We compared three experimental conditions (selective attention vs. attention switching vs. divided attention) with a passive listening control condition. Furthermore, we assessed subjective ratings on the performance of the ATT, effort and attention drifts during the task. We hypothesized that hemodynamic responses would be strongest in the high effort ATT conditions and lowest in the passive control condition (attention switching > divided attention > selective attention > passive listening). In the same way, we expected that subjects would rate the ATT conditions as more effortful than the control condition (attention switching > divided attention > selective attention > passive listening) and their performance in the converse direction (passive listening > selective attention > divided attention > attention switching). With respect to attention drifts, we expected that conditions with longer times of sustained attention would lead to more attention drifts than those with shorter duration of maintained attention (selective attention = divided attention = passive listening > attention switching). Finally, we explored associations between subjective ratings and blood oxygenation during the task.
2. Material and methods
2.1. Participants
46 subjects participated in this study. The study was approved by the ethics committee at the University Hospital and the University of Tübingen. All subjects gave their written informed consent. Inclusion criteria of the study were the following: No history of or acute psychiatric disorder or neurological disorder, no chronic or acute diseases that affect the blood flow such as diabetes or kidney failure. All subjects were healthy as assessed by a structured clinical interview for DSM-IV by a clinical psychologist. Further, no subject reported state rumination as assessed with a resting-state questionnaire (as used in (Rosenbaum et al., 2018, Rosenbaum et al., 2017)) after the resting-state measurements. Of note, state rumination was not further analyzed due to too low variability of the data.
60% of the sample were female subjects. The mean age was 24 years (SD = 5.2); the average years of education were 16 (SD = 3.1). Mean depression scores as assessed with Beck's Depression Inventory II (BDI-II) were 4.5 (SD = 4.2), which indicated no depressive symptomatology (Beck et al., 1996).
2.2. Procedures
Subjects were recruited via mailing-lists of the University Hospital (staff) and University of Tübingen (students and staff). On the day of the study, subjects completed an interview in which demographic data was assessed and questionnaires on their depressive symptomatology (Beck's Depression Inventory). Further, attentional resources were assessed with the d2 Test of Attention (Daseking and Putz, 2015). Afterwards, the fNIRS measurement was performed, comprising a 5-minute, eyes-open resting-state measurement, followed by the attention paradigm and another resting-state measurement. During the attention paradigm, subjects were instructed to focus their attention on specific sounds according to the instructions of the ATT (Nassif and Wells, 2014; Teismann, 2012; Wells, 1990). None of the subjects were trained in the ATT before the measurement as our aim was to evaluate the immediate individual effect of the ATT components and not the effect of repeated training. Four conditions were assessed during the paradigm: (1) focus on a single sound, (2) switching the attention focus between certain sounds, (3) focus on all sounds at the same time during a block and – as a control condition – (4) focus passively on white noise. We chose a noise sound for the passive control condition instead of the ATT sounds to prevent subjects from performing the ATT instructions during the control condition involuntarily. During ATT conditions one to three, a mixture of the following six different sounds was presented: flowing water, birds' twittering, traffic noise, cricket's chirring, church bells ringing and the ticking of a clock. The sounds for the ATT are available online at http://www.metakognitivetherapie.de. All sounds in all conditions were presented with closed headphones at approximately 58–60 dB. Each condition was presented 7 times during the experiment in 40 s blocks. During each block, the instruction “focus on…sound x/ all sounds/ passive” was presented visually via a computer screen for 6 s before the focus changed. During the switching conditions, the focus changed every 6 s, while in the other three conditions the same focus was presented every 6 s. Each block consisted of 6 changes and repetitions of focus instructions. After each block, subjects were asked to rate on a 9-point Likert-scale how well they had performed the task (performance rating), how much effort they had put into the task (effort rating) and how often their attention had faded away from the task (rating of attentional drifts). A 15 to 25 s jittered rest followed the ratings before the next block began. Conditions were presented in a pseudorandomized way in which each set of the four conditions was randomized and presented with seven repetition-cycles.
2.3. fNIRS
To measure changes of oxygenated (O2HB) and deoxygenated haemoglobin (HHB), the optical imaging method of functional near-infrared spectroscopy (fNIRS) was used. fNIRS is a reliable, easy to use imaging method with relatively high time resolution (Haeussinger et al., 2011, Haeussinger et al., 2014). We used a continuous wave, multichannel NIRS system (ETG-4000 Optical Topography System; Hitachi Medical Co., Japan) with a temporal resolution of 10 Hz. We used three probesets to measure parts of the CCN and the dorsal attention network (DAN): Two frontal probesets (reference points F3 and F4 according to the international 10–20 System (Jasper, 1958)) with 9 optodes each, and one parietal probeset (reference point Pz) with 15 optodes, resulting in a total of 46 channels (see Table 1). Corresponding brain areas of each channel were extrapolated from reference points based on the Colin 27 template (Cutini et al., 2011; Tsuzuki and Dan, 2014).
Table 1.
fNIRS channels for the different probesets and corresponding brain areas.
| Brain area | Left frontal probeset | Right frontal probeset |
|---|---|---|
| Pars opercularis (part of inferior prefrontal gyrus) | 6 | 19 |
| Pars triangularis (part of inferior prefrontal gyrus) | 4, 7, 9 | 18, 21 |
| Dorsolateral prefrontal cortex | 5, 10, 11, 12 | 15, 20, 23, 24 |
| Retrosubicular area | 1 | 14, 16 |
| Temporopolar area | 2 | 13 |
| Subcentral area | 3 | 17 |
| Pre-motor and supplementary motor cortex | 8 | 22 |
| Parietal probeset | ||
| Somatosensory association cortex | 25, 26, 27, 28, 30, 31, 32, 34, 35, 36, 37 | |
| Angular gyrus | 42 | |
| Supramarginal gyrus | 29, 33 | |
| V3 | 38, 39, 40, 41, 43, 44, 45, 46 | |
After data collection, the following preprocessing was done with MATLAB R2017a (MathWorks Inc., Natick, USA): Data were bandpass filtered (0.001–0.1 Hz) and processed with the correlation based signal improvement algorithm of Cui et al. (2010) to reduce movement artefacts (Cui et al., 2010). As the algorithm of Cui et al. (2010) offsets the data of HHB and O2HB, in the following, only the corrected oxygenated signal was further processed. Afterwards, single channels strongly contaminated by different types of artefacts were interpolated, followed by an ICA-based reduction of clenching artefacts. In a final step, a further bandpass filter (0.01 to 0.1 Hz) was used and data was z-transformed to allow for better comparisons between subjects. For the z-transformation, each data-point was divided by the standard deviation of the concatenated signal of all channels (the average activity over all time points of the concatenated signal is approximately zero due to detrending). The blocks for each condition were averaged with a 15 s baseline correction and a linear detrending. Data was analyzed for 5 regions of interest (ROI): bilateral dorsolateral prefrontal cortex (dlPFC), bilateral inferior frontal gyrus (IFG) and somatosensory association cortex (SAC).
2.4. Data analysis
Data was further analyzed with IBM SPSS Statistics Version 24. For fNIRS data, repeated measurement ANOVAs with the within-subject factor condition (focus single vs. focus switch vs. focus all vs. focus passive) were performed for five ROIs. Results were corrected for multiple comparisons by the procedure of Armitage-Parmar (Sankoh et al., 1997).
We hypothesized an increase in O2HB concentration from the passive control condition to the other three experimental conditions. Further, we assumed that the conditions with the highest mental effort (focus switching and focus all sounds) would show higher O2HB values/concentration than the less demanding condition (focus on a single sound) in the five ROIs (passive < single < switching = all).
Further, in an exploratory analysis, we checked the data for covariations between subjective ratings on performance, effort and attention drifts and blood oxygenation during the paradigm with mixed multi-level models. To do so, we z-standardized ratings per subject to display within subject variations due to the experimental procedure and regressed the NIRS data on the ratings. In the mixed models, subjects were used as nesting variables. Therefore, the regression coefficients display the experimentally induced covariation of blood-oxygenation and subjective performance, effort and attention drifts.
3. Results
3.1. Subject ratings
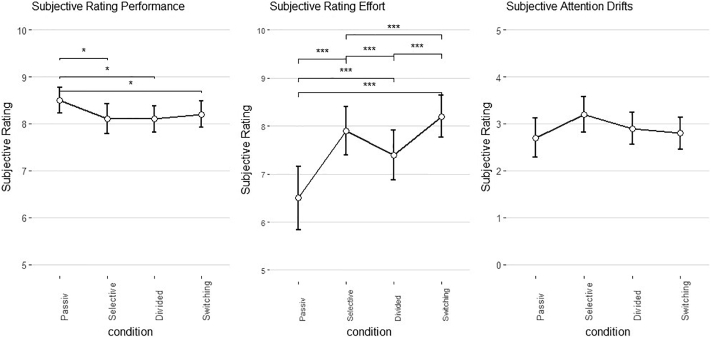
Subjective ratings during the paradigm suggested significant differences between the experimental conditions in terms of performance (F(3,135) = 3.957, p < .05, η2 = 0.084), and effort (F(3,135) = 23.705, p < .001, η2 = 0.355), but not for attentional drifting. Post-hoc tests revealed that subjects performed subjectively better in the passive control condition than in the selective attention (t(45) = 3.12, p < .01, d = 0.46) and divided attention (t(45) = 3.45, p < .001, d = 0.51) conditions.
With regards to subjectively rated effort, the control condition was rated as most effortless (passive vs. divided attention: t(45) = 3.845, p < .001, d = 0.57); passive vs. selective attention: t(45) = 5.018, p < .001, d = 0.73; passive vs. attention switching: t(45) = 6.104, p < .001, d = 0.90). Furthermore, all ATT conditions differed significantly from each other: the highest rated effort occurred in the attention switching condition (switching vs. divided: t(45) = 5.559, p < .001, d = 0.81; switching vs. selective attention: t(45) = 3.474, p < .001, d = 0.51), followed by the selective attention and the divided attention conditions (selective vs. divided attention: t(45) = 3.616, p < .001, d = 0.53) (see Fig. 1).
Fig. 1.
Subjective ratings on performance (left), effort (middle) and attention drifts (right) during the different experimental conditions. *p < .05, ** p < .01, ***p < .001.
Attentional drifting showed only marginally significant differences for the experimental condition (F(3,135) = 2.403, p < .1, η2 = 0.05) with a trend toward highest attentional drifts during the selective attention condition. On average, subjects drifted away between 2.8 (SD =1.4) and 3.2 (SD = 1.3) times during 40 s task performance as rated by subjective impression.
3.2. fNIRS brain activity
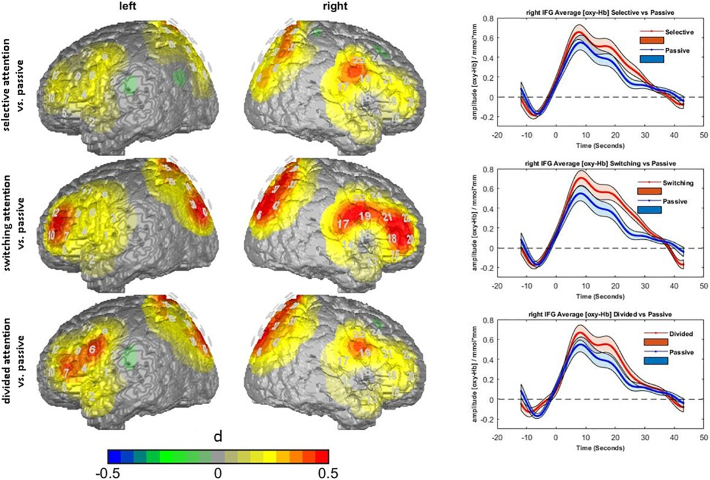
Hemodynamic responses were observed in all conditions and all ROIs that were significantly different from zero (t(45) = 5.139 to 12.228, all p < .001). As indicated by repeated measurement ANOVAs, we observed significant differences between the experimental conditions in the right IFG (F(3,135) = 4.305, p < .01, η2 = 0.087), right dlPFC (F(3,135) = 2.898, p < .05, η2 = 0.060) and SAC (F(3,135) = 3.817, p < .05, η2 = 0.078). Furthermore, marginally significant trends were observed in the left dlPFC (F(3,135) = 2.494, p < .1, η2 = 0.053), and left IFG (F(3,135) = 2.385, p < .1, η2 = 0.05) (see Fig. 2).
Fig. 2.
Left: Brain maps of the contrasts of the three ATT conditions vs. the passive control task. Differences are plotted as effect sizes (d). Warm colors indicate higher activation in the ATT condition than in the control condition. Right: hemodynamic responses in the right IFG ROI during the 40 s task performance in the three ATT conditions (red) and the passive control condition (blue). Contrasts are, from top to bottom: selective vs. passive, switching vs. passive and divided vs. passive.
Post-hoc tests revealed a significantly higher hemodynamic response in the right IFG and SAC in the selective attention condition (rIFG: t(45) = 2.213, pcorr < 0.05, d = 0.33; SAC: t(45) = 2.15, pcorr < 0.05, d = 0.32), divided attention condition (rIFG: t(45) = 2.228, pcorr < 0.05, d = 0.33; SAC: t(45) = 2.89, pcorr < 0.05, d = 0.43) and attention switching condition (rIFG: t(45) = 3.36, pcorr < 0.01, d = 0.50; SAC: t(45) = 3.05, pcorr < 0.01, d = 0.45) as compared to the control condition. However, no differences between the three attention conditions were found. With respect to the right dlPFC, only the contrast between the attention switching condition and the control condition showed significance (t(45) = 2.689, pcorr < 0.05, d = 0.40).
In our exploratory analysis, we found a positive covariation of blood-oxygenation and subjectively rated effort during the experiment in the right IFG (t(137) = 2.496, p < .05), but not in any other ROI. Neither the subjective performance nor attentional drifts were significantly associated with O2HB concentration.
4. Discussion
The aim of this study was to investigate the neural underpinnings of the Attention Training Technique, a psychotherapeutic technique that is normally used to reduce self-focused perseverative thinking as part of metacognitive therapy. To this end, we adapted the three phases of the ATT to measure the effects of selective attention, divided attention and attention switching on the cognitive control network and dorsal attention network. As a control comparison, a white noise was presented to the subjects with the instruction to passively listen to this noise.
In line with our hypotheses, we observed elevated blood oxygenation in the right IFG, right dlPFC and SAC during the experimental ATT conditions in comparison to the control condition. However, we did not observe any differences between the ATT conditions although effect sizes showed a tendency to be the highest in the attention switching condition. In line with this, in the right dlPFC, only the attention switching condition was significantly different from the passive control condition. Subjects rated their performance to be better in the control condition than in the experimental ATT conditions. Fittingly, the subjective effort was highest in the attention switching condition, followed by the selective attention condition, the divided attention condition and, rated as least effortful, the passive control condition. However, associations between O2HB and subjective ratings were only found in the right IFG.
From the data of this study, we suggest that the underlying neuronal mechanism of the ATT lies within the recruitment and training of attention-related areas in the CCN and DAN, including the dlPFC, IFG and superior parietal lobule. Interestingly, in one of our previous studies, subjects with high habitual tendencies to ruminate showed reduced blood oxygenation in the right IFG during stress exposure, which was accompanied by increased momentary ruminations following stress (Rosenbaum et al., 2018). Indeed, a large body of evidence suggests reduced frontal cortical activation in depressed subjects during cognitively demanding tasks (Groenewold et al., 2013; Rosenbaum et al., 2016; Snyder, 2013; Zhang et al., 2015), which might explain deficits in inhibition and emotion regulation in depressed subjects. On a neuronal level, the ATT mechanism of change might lie in influencing these deficits in functional brain area recruitment in depressed and high ruminating subjects. On a psychological level, this might reflect the hypothesized enhancement of voluntary inhibitory control in depression and high ruminating subjects such that they can disengage perseverative thinking as implicated in the S-REF model (Wells and Matthews, 1996). In line with this, in a primary study of Siegle et al. (2007), depressed patients that were trained in a combined treatment program including the ATT showed increased activity in the dlPFC during a digit sorting task and reduced amygdala reactivity to negative emotional material after treatment (Siegle et al., 2007). The proposed mechanism is further supported by cognitive-behavioral emotion regulation models, in which attention refocusing is – besides the modification of the situation, cognitive reappraisal and behavioral emotion regulation – a basic way to influence the emotionality of a stimulus (Gross, 1998; Quoidbach et al., 2015). Interestingly, initial evidence from Jacobs et al. (2016) suggests that Rumination Focused Cognitive Behavioral Therapy in adolescents also affects the right IFG in terms of connectivity of this brain region with the left posterior cingulate cortex (PCC). In this study, decreases in FC between the right IFG and left PCC further correlated with decreases in depression severity and rumination (Jacobs et al., 2016). However, it is important to keep in mind that the current study investigated the neurophysiological underpinnings of the ATT components in a non-clinical group of subjects, which only gives evidence for a general physiological mechanism. The relation of this mechanism to the reduction of symptoms has to be investigated in future studies with clinical subjects.
Contrary to our hypothesis, we did not observe a significant difference in O2HB concentration between the three experimental conditions, despite tendencies toward higher effect sizes in the attention switching condition. However, based on the observed effect sizes, we estimate the difference between the ATT conditions in the examined ROIs to be equal to or smaller than d = 0.3, which would result in high sample sizes for significance testing. It might also be the case that the recruited brain areas during the different phases of the ATT do not differ that much. For example, selective attention and attention switching might include the same areas for attention focus at the beginning of the process. During the process of selective attention training, the subject would have to maintain the attention focus over a longer period of time, which could result in habituation (and a reduced O2HB concentration), and re-focus their attention when attention drifts occur, which should recruit similar brain areas as attention switching. In the same way, the divided attention condition requires the subjects to expand their attentional focus and maintain this wide perceptual focus. Habituation might also occur here, followed by a self-paced refocusing that recruits the same areas as the attention-switching condition. Following this possible interpretation, the only difference between the three conditions might lie in the number of self-paced and instructed attentional shifts. In line with this interpretation, fMRI studies on divided and selective attention to auditory and visual sentence comprehension found that both selective attention – during single tasking – and divided attention – during dual tasking – recruited the same brain regions, but more strongly if more information had to be processed (e.g. during dual tasking) (Moisala et al., 2015).
Not surprisingly, subjects rated their performance in the passive listening control task higher than in the ATT task conditions, as the control condition was easier. However, performances on all three ATT conditions were rated as equal. In line with our hypothesis, subjects rated the invested effort according to the difficulty of the task conditions: The attention switching condition was rated as most effortful, followed by the selective attention condition, the divided attention condition and the passive listening control condition. In line with this finding, we expected that subjectively rated effort would covary with the O2HB concentration in the CCN. However, such a relation was only found in the right IFG, which is in line with the importance of this area for voluntary inhibitory control, but seems unsatisfactory in terms of reliability and validity. It might be the case that the nature of subjective ratings is too unreliable for the prediction of biological variables in small samples. As the ATT asks the subjects to focus their attention on sounds without any behavioral performance, it is difficult to design a reliable behavioral index for attention focusing. It will be up to future studies to fill this gap.
Interestingly, we also observed considerable activation in the frontal, lateral, and parietal ROIs during the passive control condition that was highly significant compared to baseline activation (see supplementary material Fig. S1). We chose to present a white noise sound during the control condition to prevent subjects from performing the ATT instruction during the passive condition. However, during passive listening, automatic attention processes such as orientation occur that recruit attention-related brain areas, which might explain why such high activations occurred during the passive listening condition.
This study had some important limitations. Firstly, we did not use the original ATT but an adapted neuroscientific paradigm. As we sought to randomize the ATT conditions, implement a control condition and leave the subjects blind to the original training, we implemented blocks of 40 s task performance. In the original ATT, no passive listening condition exists. Instead, the training starts with selective listening, followed by attention switching and at last divided attention. Furthermore, the three conditions have different durations in the original training, with the longest duration for selective attention followed by attention switching and divided attention. However, the neuronal processes in terms of brain areas recruited by the ATT are likely to be the same as in our investigation. As noted above, the current study leaves open the question of whether the proposed neurophysiological process will be accompanied by symptom reductions in clinical populations. The paradigm developed in the current study might be of use for such future investigations.
A further limitation concerns the fNIRS method. The investigated brain areas are limited to the placement of our probeset and the penetration depth of the near-infrared light, which is approximately 3 cm. Therefore, only the superficial parts of the cortex could be investigated. We chose the examined brain regions due to prior studies that highlighted the role of the CCN and DAN in selective and divided attention. Furthermore, by using fNIRS, it was possible to measure cortical oxygenation while subjects were sitting, as is usually the case during the ATT training. Therefore, the ecological validity of the fNIRS measurement is excellent. However, the current paradigm can be adjusted for fMRI measurements and future fMRI studies can therefore validate the results of this study.
To the knowledge of the authors, this is the first study that measured blood oxygenation during the ATT. We observed significant increases in areas of the CCN and DAN during the ATT conditions, suggesting that the ATT training will affect these brain regions. As depressed subjects have been shown to have reduced frontal functioning in the CCN and DAN during cognitive tasks and emotion regulation, the neuronal mechanism of the ATT might lie in the training of these cortical networks.
Acknowledgments
Acknowledgments
The authors would like to thank Ramona Taeglich, Betti Schopp, Deborah Häuser and Hendrik Laicher for their excellent work and their valuable support with the measurements.
Financial disclosures
Ann-Christine Ehlis was partly supported by IZKF Tübingen (Junior Research Group 2115-0-0). David Rosenbaum was partly supported by STORZ MEDICAL AG (CIV 16-03-014924). No author reported conflicts of interest. Further, we acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Authors contributions
D. R., M.J.M. & J.H. contributed to the analysis and interpretation of the data for the work and did the primary drafting. A.W., A.J.F. and A.C.E. contributed to the design and acquisition of the work and revised it critically for important intellectual content.
All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.06.021.
Appendix A. Supplementary data
Supplementary material
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W.F. Comparison of Beck depression inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Broderick Patricia C., Korteland Constance. A prospective study of rumination and depression in early adolescence. Clin. Child Psychol. Psychiatry. 2004;9:383–394. [Google Scholar]
- Brosschot J.F., Gerin W., Thayer J.F. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J. Psychosom. Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Capobianco L., Morris J.A., Wells A. Worry and rumination: do they prolong physiological and affective recovery from stress? Anxiety Stress Coping. 2018;31:291–303. doi: 10.1080/10615806.2018.1438723. [DOI] [PubMed] [Google Scholar]
- Cui X., Bray S., Reiss A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage. 2010;49:3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini S., Scatturin P., Zorzi M. A new method based on ICBM152 head surface for probe placement in multichannel fNIRS. NeuroImage. 2011;54:919–927. doi: 10.1016/j.neuroimage.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Daseking M., Putz D. TBS-TK Rezension. Psychol. Rundsch. 2015;66:265–267. [Google Scholar]
- Dilling H., Freyberger H.J., Cooper J.E., Weltgesundheitsorganisation, editors. Taschenführer zur ICD-10-Klassifikation psychischer Störungen: mit Glossar und Diagnostischen Kriterien sowie Referenztabellen ICD-10 vs. ICD-9 und ICD-10 vs. DSM-IV-TR, 8., überarbeitete Auflage unter Berücksichtigung der Änderungen gemäss ICD-10-GM (German Modification) 2016 ed. Hogrefe; Bern: 2016. [Google Scholar]
- Ehring T., Watkins E.R. Repetitive negative thinking as a transdiagnostic process. Int. J. Cogn. Ther. 2008;1:192–205. [Google Scholar]
- Fergus T.A., Bardeen J.R. The attention training technique: a review of a neurobehavioral therapy for emotional disorders. Cogn. Behav. Pract. 2016;23:502–516. doi: 10.1016/j.cbpra.2015.11.001. ABCT's 50th Anniversary Special Series. [DOI] [Google Scholar]
- Groenewold N.A., Opmeer E.M., Jonge P., Aleman A., Costafreda S.G. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Gross J.J. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. New Direct. Res. Emotion. 1998;2:271–299. [Google Scholar]
- Haeussinger F.B., Heinzel S., Hahn T., Schecklmann M., Ehlis A.-C., Fallgatter A.J. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussinger F.B., Dresler T., Heinzel S., Schecklmann M., Fallgatter A.J., Ehlis A.-C. Reconstructing functional near-infrared spectroscopy (fNIRS) signals impaired by extra-cranial confounds: an easy-to-use filter method. NeuroImage. 2014;95:69–79. doi: 10.1016/j.neuroimage.2014.02.035. [DOI] [PubMed] [Google Scholar]
- Hanlon F.M., Dodd A.B., Ling J.M., Bustillo J.R., Abbott C.C., Mayer A.R. From behavioral facilitation to inhibition: the neuronal correlates of the orienting and reorienting of auditory attention. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holeva V., Tariier N., Wells A. Prevalnce and predcitors of acute stress disorder and PTSD follwing road traffic accidents: thought control strategies and social support. Behav. Ther. 2001;32:65–83. [Google Scholar]
- Huang S., Belliveau J.W., Tengshe C., Ahveninen J. Brain networks of novelty-driven involuntary and cued voluntary auditory attention shifting. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R.H., Watkins E.R., Peters A.T., Feldhaus C.G., Barba A., Carbray J., Langenecker S.A. Targeting ruminative thinking in adolescents at risk for depressive relapse: rumination-focused cognitive behavior therapy in a pilot randomized controlled trial with resting state fMRI. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958;10:370–375. [Google Scholar]
- Knowles M.M., Foden P., El-Deredy W., Wells A. A systematic review of efficacy of the attention training technique in clinical and nonclinical samples. J. Clin. Psychol. 2016;72:999–1025. doi: 10.1002/jclp.22312. [DOI] [PubMed] [Google Scholar]
- Lee A.K.C., Larson E., Maddox R.K., Shinn-Cunningham B.G. Using neuroimaging to understand the cortical mechanisms of auditory selective attention. Hear. Res. Human Auditory NeuroImaging. 2014;307:111–120. doi: 10.1016/j.heares.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Borkovec T.D., Sibrava N.J. The effects of worry and rumination on affect states and cognitive activity. Behav. Ther. 2007;38:23–38. doi: 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Michalak J., Hölz A., Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression: rumination as a predictor of relapse. Psychol. Psychother. Theory Res. Pract. 2011;84:230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- Moisala M., Salmela V., Salo E., Carlson S., Vuontela V., Salonen O., Alho K. Brain activity during divided and selective attention to auditory and visual sentence comprehension tasks. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif Y., Wells A. Attention training reduces intrusive thoughts cued by a narrative of stressful life events: a controlled study. J. Clin. Psychol. 2014;70:510–517. doi: 10.1002/jclp.22047. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J.F., Verkuil B., Lonigro A., Medea B., Couyoumdjian A., Brosschot J.F. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Quoidbach J., Mikolajczak M., Gross J.J. Positive interventions: an emotion regulation perspective. Psychol. Bull. 2015;141:655–693. doi: 10.1037/a0038648. [DOI] [PubMed] [Google Scholar]
- Robinson M.S., Alloy L.B. Negative cognitive styles and stress-reactive rumination interact to predict depression: a prospective study. Cogn. Ther. Res. 2003;27:275–291. [Google Scholar]
- Rosenbaum D., Hagen K., Deppermann S., Kroczek A.M., Haeussinger F.B., Heinzel S., Berg D., Fallgatter A.J., Metzger F.G., Ehlis A.-C. State-dependent altered connectivity in late-life depression: a functional near-infrared spectroscopy study. Neurobiol. Aging. 2016;39:57–68. doi: 10.1016/j.neurobiolaging.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D., Haipt A., Fuhr K., Haeussinger F.B., Metzger F.G., Nuerk H.-C., Fallgatter A.J., Batra A., Ehlis A.-C. Aberrant functional connectivity in depression as an index of state and trait rumination. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-02277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D., Thomas M., Hilsendegen P., Metzger F.G., Haeussinger F.B., Nuerk H.-C., Fallgatter A.J., Nieratschker V., Ehlis A.-C. Stress-related dysfunction of the right inferior frontal cortex in high ruminators: an fNIRS study. NeuroImage Clin. 2018;18:510–517. doi: 10.1016/j.nicl.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh A.J., Huque M.F., Dubey S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Ghinassi F., Thase M.E. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cogn. Ther. Res. 2007;31:235–262. [Google Scholar]
- Smith J.M., Alloy L.B. A roadmap to rumination: a review of the definition, assessment, and conceptualization of this multifaceted construct. Clin. Psychol. Rev. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann T. Springer, Berlin. York; New: 2012. Kognitive Verhaltenstherapie depressiven Grübelns. [Google Scholar]
- Tsuzuki D., Dan I. Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses. NeuroImage. 2014;85:92–103. doi: 10.1016/j.neuroimage.2013.07.025. Celebrating 20 Years of Functional Near Infrared Spectroscopy (fNIRS) [DOI] [PubMed] [Google Scholar]
- Watkins E.R. Constructive and unconstructive repetitive thought. Psychol. Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. Panic disorder in association with relaxation induced anxiety: an attentional training approach to treatment. Behav. Ther. 1990;21:273–280. [Google Scholar]
- Wells A. The attention training technique: theory, effects, and a metacognitive hypothesis on auditory hallucinations. Cogn. Behav. Pract. 2007;14:134–138. [Google Scholar]
- Wells A., Matthews G. Modelling cognition in emotional disorder: the S-REF model. Behav. Res. Ther. 1996;34:881–888. doi: 10.1016/s0005-7967(96)00050-2. [DOI] [PubMed] [Google Scholar]
- Wells A., Papageorgiou C. Worry and the incubation of intrusive images following stress. Behav. Res. Ther. 1998;33:579–583. doi: 10.1016/0005-7967(94)00087-z. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dong W., Dang W., Quan W., Tian J., Chen R., Zhan S., Yu X. Near-infrared spectroscopy for examination of prefrontal activation during cognitive tasks in patients with major depressive disorder: a meta-analysis of observational studies: NIRS examination of depression. Psychiatry Clin. Neurosci. 2015;69:22–33. doi: 10.1111/pcn.12209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material