Abstract
Purpose
Auditory hallucinations (AH), typically hearing voices, are a core symptom in schizophrenia. They may result from deficits in dynamic functional connectivity (FC) between cortical regions supporting speech production and language perception that interfere with the ability to recognize self-generated speech as not coming from external sources. We tested this hypothesis by investigating dynamic connectivity between the frontal cortex region related to language production and the temporal cortex region related to auditory processing.
Methods
Resting-state fMRI scans were acquired from 18 schizophrenia patients with AH (AH+), 17 schizophrenia patients without AH (AH-) and 22 healthy controls. A multiband sequence with TR = 427 ms was adopted to provide relatively high temporal resolution data for characterizing dynamic FC. Analysis focused on connectivity between speech production and language comprehension areas, eloquent language cortex in the left hemisphere. Two frequency bands of brain oscillatory activity were evaluated (0.01–0.027 Hz, 0.027–0.08 Hz) in which differential alterations that have been previously linked to schizophrenia. Conventional static FC maps of these seeds were also calculated.
Results
Dynamic connectivity analysis indicated that AH+ patients showed not only less temporal variability but transient lower strength in connectivity between speech and auditory areas than healthy controls, while AH- patients not. These findings were restricted to 0.027–0.08 Hz activity. In static connectivity analysis, no significant differences were observed in connectivity between speech production and language comprehension areas in either frequency band.
Conclusions
Reduced temporal variability and connectivity strength between key regions of eloquent language cortex may represent a mechanism for AH in schizophrenia.
Keywords: Schizophrenia, Auditory hallucinations, Dynamic, Functional connectivity, Language areas, Multiband
Abbreviations: AH, Auditory hallucinations; AH+, schizophrenia patients with AH; AH-, schizophrenia patients without AH; ANCOVA, analysis of covariance; DARTEL, Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra; FC, functional connectivity; MNI, Montreal Neurological Institute; PANSS, Positive and Negative Syndrome Scale; ROI, regions of interest; SCID, Structured Interview for DSM-IV
Highlights
-
•
Abnormal dynamic functional connectivity in schizophrenia with auditory hallucinations.
-
•
The dynamic connectivity goes wrong between expressive and receptive language regions.
-
•
The abnormality was restricted to the left hemisphere.
-
•
This abnormal dynamic connectivity was limited to a specific frequency band.
1. Introduction
Auditory hallucinations (AH) are a common and distressing symptom of schizophrenia affecting 60–90% of patients at some point during their illness course (Adams et al., 2013). Hallucinations are proposed to be caused by disturbed brain mechanisms that ineffectively distinguish self-generated mental experiences from those stimulated by external sensation (Feinberg, 2011). Neuroimaging and neurophysiological studies have indicated that abnormal connectivity between frontal speech production and temporal auditory perception cortex of the left hemisphere, representing key eloquent language-related regions, may represent a mechanism for AH in schizophrenia (Alderson-Day et al., 2015; Allen et al., 2012; Curcic-Blake et al., 2013; Ford et al., 2012; Hare et al., 2017; Hoffman and Hampson, 2011; Javitt and Sweet, 2015; Johnsen et al., 2013).
Prior neuroimaging studies of AH examined static functional connectivity(FC), which averages brain connectivity over an entire acquisition period, thus may not effectively capture functionally important features of the dynamic coherence of brain oscillations between regions. Recent EEG research has shown that FC in language-related cortex varies dynamically during the generation and processing of self-generated speech in healthy individuals (Wang et al., 2014). This observation of an important role for dynamic connectivity alterations is not specific to the language system, as multiple studies have shown dynamic FC alterations in schizophrenia that are more robust than those seen with static connectivity assessments (Allen et al., 2014; Damaraju et al., 2014; Du et al., 2017a; Du et al., 2017b; Du et al., 2016). While dynamic changes related to AH have been investigated with EEG (Wang et al., 2014), with its high temporal and low spatial resolution, to date there has not yet been an assessment of dynamic brain connectivity changes in FC between speech production and auditory perception areas in schizophrenia patients with AH.
In addition to the importance of assessing dynamic aspects of FC, connectivity changes may occur in specific frequency bands. Coherent oscillatory brain activity in the brain language network at specific low frequencies is also found important for the ability to differentiate self-generated and externally generated speech using EEG (Wang et al., 2014). Although the frequency bands of brain activity revealed by EEG are quite different from those of fMRI, the functional importance of coherent oscillations at specific frequency band recognized in electrophysiological research across brain regions (Buzsaki and Draguhn, 2004) has recently begun to be noted and actively investigated in fMRI studies (Meda et al., 2015; Zuo et al., 2010). Increasing evidence suggested that FC between brain regions arises from a band-limited slow rhythmic mechanism (Li et al., 2015; Xue et al., 2014), and more importantly that both dynamic changes of amplitude of low-frequency fluctuations (Hare et al., 2017; Yu et al., 2014) and FC pattern (Wang et al., 2017) of brain function in schizophrenia are found with frequency-dependent characteristics within frequency bands in fMRI data. Thus, whether there are specific frequency band-related characteristics of dynamic FC in schizophrenia that are associated with AH remains to be investigated.
In the present study, we tested the hypothesis of abnormal dynamic FC between speech production and auditory perception cortex and its potential frequency-dependent characteristics in schizophrenia patients with AH relative to those without AH and also to healthy controls. In secondary exploratory analyses, static FC analyses between brain regions of interest (ROIs) were performed. Whole brain analyses are presented in Supplementary Materials.
2. Materials and methods
2.1. Participants
The ethics committee of West China Hospital of Sichuan University approved the study, and all participants provided written informed consent. This study included 35 schizophrenia patients and 22 healthy controls, recruited from the hospital's outpatient clinics. Clinical evaluations were conducted by two experienced psychiatrists, with over 5 years of clinical work as attending physicians, to confirm clinical diagnosis using the Structured Interview for DSM-IV (SCID) Axis I Disorders. Psychopathology ratings were obtained using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Eighteen patients reported experiencing hallucinations within the past 6 weeks (AH+), most within the past week, while the other 17 patients reported no AH in their lifetime (AH-). The scores of hallucination item (P3) of PANSS were significantly higher in the AH+ patients than those of AH- patients (3.56 ± 0.92 vs 1.0 ± 0.00, p < 0.01). The antipsychotic medication dosage (in chlorpromazine equivalents, mg/d) was somewhat higher in AH+ patients, but there was no significant difference between patient subgroups (320.77 ± 171.40 vs 253.57 ± 71.67, p = 0.19). Patients had not experienced a significant change in psychosis severity or change in psychopharmacological treatment during the preceding month.
Healthy comparison subjects were recruited from same local areas through poster advertisements to match patients in age, sex and educational backgrounds (Table 1). The non-patient version of the SCID was used to establish the lifetime absence of psychiatric illness. We excluded potential controls reporting history of major psychiatric illness in their first-degree relatives. All participants were right-handed and of Chinese Han ancestry. The following exclusion criteria applied to both groups: 1) age younger than 18 years or older than 60 years, 2) history of substance abuse or dependence, 3) pregnancy, 4) significant systemic or neurologic illness as assessed by clinical evaluations and medical records, and 5) comorbid affective illness or schizoaffective disorder.
Table 1.
Demographic and clinical characteristics of schizophrenia patients with and without auditory hallucinations and healthy controls.
| Demographic and clinical characteristics | Mean (SD) |
||||
|---|---|---|---|---|---|
| AH+ (N = 18) | AH- (N = 17) | HC (N = 22) | Statistics | p | |
| Age (years) | 35.22 (13.03) | 30.00 (10.13) | 34.91 (13.34) | F = 1.00 | 0.38 |
| Education (years) | 12.33 (3.46) | 13.18 (2.65) | 13.32 (5.15) | F = 0.33 | 0.72 |
| Duration of illness (years) | 5.87 (6.9) | 4.00 (3.47) | t = 1.02 | 0.32 | |
| Sex (number) | |||||
| Male | 9 | 12 | 9 | χ2 = 3.46 | 0.18 |
| Female | 9 | 5 | 13 | ||
| PANSS scores | p | ||||
| Total | 58.50 (13.8) | 45.10 (10.00) | t = 2.55 | 0.02 | |
| Negative | 14.92 (3.80) | 13.7 (5.75) | t = 0.59 | 0.56 | |
| Positive | 13.08 (5.55) | 8.00 (1.33) | t = 2.82 | 0.01 | |
| General | 30.50 (7.49) | 23.40 (4.86) | t = 2.57 | 0.02 | |
| Medication information (for antipsychotics) | |||||
| % on 1st generation | 0% (0/18) | 6.0% (1/17) | |||
| % on 2nd generation | 88.9% (16/18) | 82.4% (14/17) | |||
| % on both | 11.1% (2/18) | 11.8% (2/17) | |||
| CPZ dose (mg/day) | 320.77 (171.40) | 253.57 (71.67) | t = 1.35 | 0.19 | |
Abbreviations: AH+, schizophrenia patients with auditory hallucination; AH-, schizophrenia patients without auditory hallucination; CPZ-chlorpromazine equivalent antipsychotic dosage; HC, healthy comparisons; PANSS, positive and negative syndrome scale; SD, standard deviation
2.2. Data acquisition
MRI data was collected on a whole-body 3.0 T MR scanner (Siemens Trio, Erlangen, Germany) with a 32 channel phase array head coil. Resting-state fMRI data were acquired using a prototype simultaneous multi-slice multiband echo planar imaging sequence. This parallel imaging technique could simultaneously excite multiple slices with a multiband excitation pulse and read out images in an echo train. This approach can provide enhanced temporal information about BOLD signals closer to real time connectivity in brain, without significantly sacrificing spatial resolution or signal/noise ratio (Feinberg et al., 2013; Moeller et al., 2010). Protocol details: TR = 427 ms; TE = 30 ms; flip angle = 45°; multiband accelerate factor = 8; matrix = 64 × 64; field of view = 192 × 192 mm2; 48 slices with no gap, voxel size = 3 × 3 × 3 mm. Scan duration is ~7 min and each functional run contained 1000 volumes. A high-resolution T1-weighted anatomical image (TR/TE = 1900/2.26 ms, flip angle = 9°, 176 sagittal slices with thickness = 1 mm, FOV = 256 × 256 mm2 and data matrix = 256 × 256) was acquired for normalization and coregistration of functional data.
Foam pads and earplugs were employed to limit head motion and noise during scanning. All participants were simply instructed to keep still with their eyes closed, remaining awake, but not thinking of anything in particular. Head motion was limited to translational and rotational parameters at the thresholds of ±1.5 mm and ± 1.5° respectively. All patients reported experiencing no hallucinations during their scans.
2.3. Data preprocessing
Resting-state fMRI data were preprocessed using SPM 12 (http://www.fil.ion.ucl.ac.uk/spm) and DPABI (Version 2.3, http://rfmri.org/dpabi). Volumes obtained in the first 10s (24 volumes) were discarded to achieve signal equilibrium. The remaining data were corrected for head motion, and individual 4D volumes were then spatially normalized to Montreal Neurological Institute (MNI) space, retaining voxel size 3 mm3, using Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) (Ashburner, 2007). Next, linear trends were removed to account for scanner drift, and temporal band-pass filtering (0.01–0.08 Hz) was performed to reduce the effects of low-frequency drift and high-frequency physiological noise (Lowe et al., 1998). Finally, multiple linear regression was performed on potential nuisance variables including six head motion parameters and cerebrospinal fluid, white matter and global signals (Fox et al., 2005). Because spatial smoothing prior to network construction artificially increases correlations among nearby voxels, we did not perform a smoothing step before the connectivity analysis.
2.4. Image analysis
We used a seed-based correlation approach to estimate FC among regions. The ROIs for speech production and auditory perception were made based on the Brodmann atlas as implemented previously (Curcic-Blake et al., 2013; Hoffman et al., 2007). The speech production area (including Broca's area) included Brodmann areas 44 and 45 in posterior ventrolateral frontal cortex, whereas the auditory perception area (including Wernicke's area) was defined as the posterior part (posterior to y = −30) of Brodmann area 22 and Brodmann area 40 in temporal cortex. Time series in each of these regions were averaged to obtain seeds for assessing dynamic connectivity, as well as secondary static FC analyses.
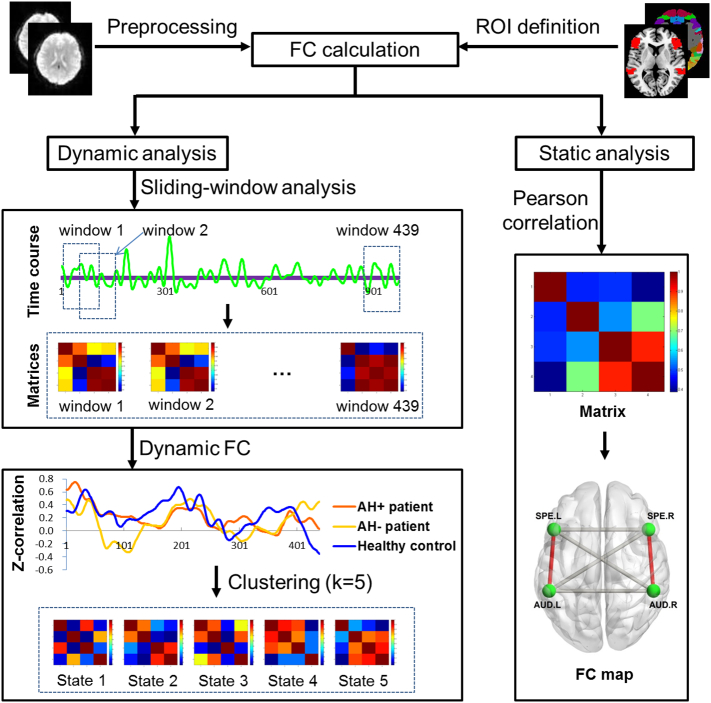
Two main analytic steps were performed: first, we calculated the dynamic FC between speech production and auditory perception areas. Second, conventional static FC maps were created using the selected ROIs as seeds. In both analyses, we calculated all possible connections between the 4 ROIs, with 6 connections in total, but our primary interest was in connectivity between speech and auditory areas of the left hemisphere which are the crucial eloquent cortical regions for language processing. To explore potential frequency dependent characteristics, all analyses were conducted separately in 0.01–0.027 Hz and 0.027–0.08 Hz frequency bands, as done previously (Hare et al., 2017; Yu et al., 2014). These analyses were also calculated in the full 0.01–0.08 Hz to provide findings comparable to previous results. The flowchart of analytic procedures is presented in Fig. 1.
Fig. 1.
Flowchart illustrating analytic procedures of present study.
2.5. Dynamic FC measurement
Dynamic FC was evaluated using DynamicBC toolbox (www.restfmri.net/forum/DynamicBC) (Liao et al., 2014) with a sliding window approach (Allen et al., 2014; Du et al., 2017b; Hutchison et al., 2013). We employed a window length of 100 TRs (42.7 s) as the window duration, comparable to previous studies (Allen et al., 2014; Damaraju et al., 2014), and slid the window forward in steps of 2 TRs (0.85 s). In each of the resulting 439 windows, FC was measured between ROIs. Fisher's z-transformed Pearson's correlation coefficients were computed for each estimated connectivity for statistical analyses. In the dynamic analysis, two measures were assessed: the temporal variability in connectivity strength and the most reoccurring transient FC strength. Temporal variability was defined as the variance of the connectivity strength across the all the windows.
To identify transient FC strength, we used a k-means cluster algorithm on all the connectivity windows of all subjects to identify discrete structure of reoccurring connectivity patterns. The k-means algorithm clusters similar data into ‘n’ groups, minimizing the within-cluster sum of squares (Lloyd, 1982). The correlation distance metric was chosen as the similarity measure in clustering, which is sensitive to connectivity patterns irrespective of their magnitude (Damaraju et al., 2014). K-means clustering was performed in a search window of k ranging from 2 to 8 based on the whole sample, and the optimal number of clusters was estimated using the elbow criterion, calculated as the ratio of within-cluster distance to between-cluster distance (Allen et al., 2014). An optimal k of 5 was determined for both frequency bands. Thus, five most frequent transient states of FC strength were identified and compared between patient subgroups and healthy controls.
2.6. Static FC measurement
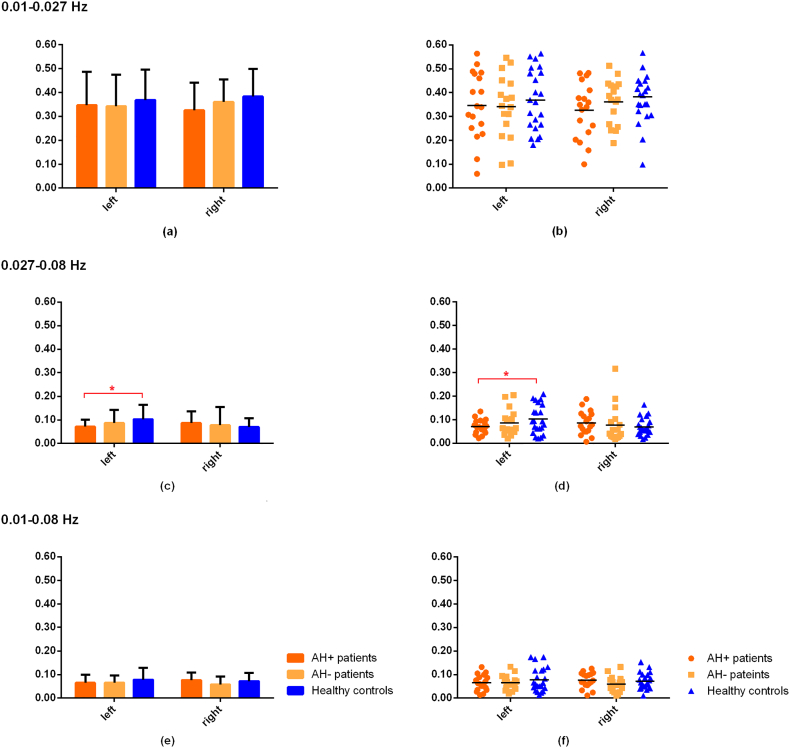
Static FC analysis, which measured average connectivity strength between ROIs, was also conducted. During the analysis, we also calculated the FC strength between ROIs (speech production and auditory perception areas bilaterally) within 0.01–0.027 Hz and 0.027–0.08 Hz separately, and the full 0.01–0.08 Hz. Detailed results of these analyses are presented in Supplemental materials.
2.7. Statistical analysis
Dynamic connectivity between ROIs at each frequency band, including temporal variability and transient connectivity strength were compared among patient subgroups and healthy controls using analysis of covariance (ANCOVA) with follow-up pairwise tests. Analyses were conducted controlling for age, sex and antipsychotic medication dosage. Correction for multiple comparisons was performed using the false discovery rate (FDR, p < 0.05). Parameters with significant inter-group differences were examined in relation to clinical symptom ratings using partial correlations with age, sex and medication dosage as covariates. As medication dose may be related to symptom severity, we recalculated these correlations without dosage correction.
3. Results
3.1. Demographic and clinical variables
Demographic and clinical characteristics for study participants are presented in Table 1. No significant inter-group differences were found in age, years of education or sex distribution (p > 0.05). As may be expected, the PANSS positive and total symptom ratings were higher in patients with AH than those without (see Table 1 for details).
3.2. Dynamic FC differences among groups
3.2.1. Frequency band: 0.027–0.08 Hz
AH+ patients showed decreased dynamic variability of FC between left frontal speech and left temporal auditory areas relative to healthy controls, consistent with our hypothesis. Group comparisons of transient FC strength demonstrated that in State 4, AH+ patients showed significantly lower connectivity strength between speech and auditory areas in the left hemisphere relative to healthy controls (Fig. 2, Table 2). This effect was not seen in AH- patents.
Fig. 2.
Temporal variability in connectivity between ipsilateral speech production and auditory perception areas in the left and right hemispheres at different frequency bands. The left column indicates group differences of dynamic variability in brain connectivity while the right column plots individual participant values.
Table 2.
Dynamic functional connectivity strength in AH+ and AH- schizophrenia patients and healthy controls at different transient states within the 0.027–0.08 Hz frequency band.
| All functional connectivity between ROIs | ANCOVA |
Post-hoc analysis directionality and p values |
||||
|---|---|---|---|---|---|---|
| F | p | p′a | AH+ vs HC | AH- vs HC | AH+ vs AH- | |
| State 4 | ||||||
| SPE. L-AUD. L | 6.04 | 0.004 | 0.026 | ↓0.001 | 0.177 | 0.162 |
| SPE. R-AUD. R | 0.64 | 0.529 | 0.530 | |||
| SPE. L-AUD. R | 0.67 | 0.517 | 0.530 | |||
| SPE. R-AUD. L | 1.72 | 0.189 | 0.283 | |||
| SPE. L-SPE. R | 2.15 | 0.127 | 0.253 | |||
| AUD. L-AUD. R | 2.42 | 0.099 | 0.253 | |||
| State 1, 2, 3, 5 | No significant findings | |||||
Abbreviations: ANCOVA, analysis of covariance; AH+, schizophrenia patients with auditory hallucination; AH-, schizophrenia patients without auditory hallucination; HC, healthy comparisons; AUD, auditory perception area; SPE, speech production area; ROI, region of interest; R, right; L, left
indicates that p′ has been corrected by FDR and the number of states (n = 5).
With regard to other connectivity patterns between ROIs in the right hemisphere and interhemispheric connections, there was no significant difference in the temporal variability or transient connectivity strength of any other connectivity between AH- patients and healthy controls, or between patient subgroups. Thus, connectivity alterations related to AH were specific to eloquent language cortex.
3.2.2. Frequency band: 0.01–0.027 Hz
There was no significant difference of temporal variability or transient FC strength in the connectivity between left hemisphere speech production and auditory perception areas in this band. No other connectivity parameter in this band significantly differed across groups.
3.2.3. Frequency band: 0.01–0.08 Hz
In this band, there were no significant inter-group differences in temporal variability, or in any of the five main transient connectivity strength states.
3.3. Static FC differences among groups
No significant findings were observed in any connectivity measures between ROIs at any frequency band after FDR correction.
3.4. Correlation with clinical ratings
No significant correlations were observed between the temporal variability or transient connectivity strength in FC between left hemisphere speech production and auditory perception areas and clinical ratings as revealed by PANSS composite scores, or in the PANSS item rating current hallucination severity. These findings did not change when medication dosage was not corrected.
4. Discussion
Recently, the concept that AH may result from abnormalities in time-varying neural connectivity within eloquent language cortex has received growing support (Jones, 2010; Lefebvre et al., 2016). As a result, identifying alterations in spatiotemporal brain dynamics associated with hallucinations has become an important research goal (Alderson-Day et al., 2016). While studies have supported the view of disrupted FC between left hemisphere speech production and auditory perception brain regions in schizophrenia patients with AH, clarifying the importance and characteristics of dynamic connectivity alterations and potential frequency-dependent mechanisms can advance understanding of this symptom in schizophrenia. The findings of the current study contribute toward this goal by demonstrating that altered dynamic connectivity between regions supporting speech production and language perception at a specific oscillation frequency are related to the presence of AH. These findings were not observed using static FC analysis.
While previous work has implied a mismatching synchronization of neural activities (Hoffman et al., 2011) and an abnormal casual model of information processing (Curcic-Blake et al., 2013) between speech production and auditory perception areas in schizophrenia with AH, our study provides novel evidence demonstrating that abnormality in this connectivity also involves a frequency specific pattern of altered neural dynamics. These findings support the theory that connectivity alterations in eloquent cortex of the left hemisphere are related to AH in schizophrenia, and suggest a neuroradiological procedure for identifying this abnormality in Psychoradiology, an evolving subspecialty of radiology focusing on psychiatric disorders (Kressel, 2017; Lui et al., 2016).
Three specific observations from the present study contributed to the understanding of brain alterations related to AH. First, reduced temporal variability in FC was found within left hemisphere eloquent language cortex in AH+ patients. This novel finding is consistent with the model that reduced time-varying connectivity between these regions that is crucial for differentiating self-generated speech is related to AH. Together with compromised connectivity strength, this pattern represents a reduced synchronization of neural activity in this circuitry. Speech production areas support language processing and send predictive signals for self-initiated speech to auditory cortex as an efferent copy in advance of speaking, which is important for discriminating internally and externally generated auditory information (Wang et al., 2014). Impaired information flow between these two regions could result in difficulty separating self-generated and external sounds, and a compensatory increase in internal speech production to make up for the information feedback loss leading to hallucinations, as has been previously proposed (Ford et al., 2013; Grossberg, 2000). Our observation in language-related brain circuitry is consistent with other reports indicating that brain circuitry in schizophrenia patients tends to linger in a state of weak and relatively rigid connectivity (Damaraju et al., 2012; Hutchison et al., 2013).
Notably, our analysis did not reveal significant static connectivity abnormalities in AH+ patients. Averaging connectivity in this approach may fail to detect variable connectivity alterations. Although previous work has identified static FC abnormalities associated with language-related regions (Chang et al., 2017; Hoffman and Hampson, 2011), some still failed to identify the specific disconnectivity between frontal speech production and temporal auditory perception areas (Chang et al., 2017), whilst patients in the other study were experiencing hallucination during scanning (Hoffman and Hampson, 2011), which might make the abnormal connectivity stand out. Other possibilities including the heterogeneity in the patients and methodology could also account for the inconsistency. However, by studying the static and dynamic FC in the same participant sample, our findings highlight the importance of dynamic rather than static connectivity disturbances for this prominent neuropsychiatric symptom.
Future studies could investigate variability of circuitry alterations over time in relation to the frequency of hallucination experiences, and determine whether this abnormality is related to the state of actively having hallucinations or to an enduring neural system alteration that creates a vulnerability to experiencing AH. Although the impaired temporal variability of FC was not correlated to clinical symptoms, further research is needed to investigate this relationship in longitudinal studies tracking patients through acute episodes of illness during which AH are more frequent.
Second, impaired time-varying FC between left hemisphere language areas in AH+ patients was only found at our higher frequency band of resting-state brain activity (0.027–0.08 Hz). This observation suggests frequency band specificity of functionally relevant oscillatory activity in eloquent language cortex in relation to AH. This type of effect has long been observed in electrophysiological data, and our findings suggest that with higher temporal resolution, such frequency-specific effects can be detected with fMRI. Although the origins and physiological mechanisms of activity in different frequency bands remain to be fully understood in fMRI data, previous neuroimaging studies have indicated that functional abnormalities in schizophrenia are frequency band specific and more robust in the higher frequency band within the frequency range of brain oscillations typically examined in resting-state studies (Hoptman et al., 2010; Wang et al., 2017; Yu et al., 2013).
Finally, abnormal connectivity between frontal speech and temporal auditory areas in AH+ patients was restricted to the left hemisphere. This is consistent with our hypothesis since all patients were right-handed with a typical left hemisphere lateralization in language processing. Observing effects specific to eloquent left hemisphere circuitry in relation to auditory/verbal hallucinations adds confidence regarding their validity. This observation is also consistent with prior reports of left lateralized abnormalities in brain function (Alderson-Day et al., 2016; Curcic-Blake et al., 2013; Jardri et al., 2011) and anatomical studies of cortical folding abnormalities of left language areas in schizophrenia (Cachia et al., 2008; Palaniyappan et al., 2013). Thus, our findings are consistent with relevant prior reports, and add confidence to the model that dynamic connectivity between language production and comprehension areas in the left hemisphere are a crucial component of the pathophysiology of AH in schizophrenia.
It is noteworthy that there were no significant differences in dynamic FC between AH+ and AH- patients at any frequency band. In the higher frequency band which may be more sensitive to functional abnormalities in schizophrenia (Wang et al., 2017), we observed a nonsignificant trend for lower temporal variability in hallucinating patients than AH- patients in the connectivity between left hemisphere language areas. The small sample size of our patient sample may contribute to this outcome, as this circuitry may be impaired, but more modestly so, in schizophrenia patients without hallucinations.
Several limitations in our study are important to consider. First, all patients included in this study were being treated with antipsychotic treatment. While drug dose was not related to our MRI measures, it may be advantageous to confirm our findings in untreated patients. Second, PANSS total scores of hallucinating patients were greater than AH- patients. Even though this was in part caused by ratings of hallucination symptoms increasing total scores, effects of overall symptom severity should be taken into consideration when interpreting our findings. Third, our sample size is not large. Thus, while our study had sufficient power to detect clinically relevant effects, replication in a larger sample to establish generalizability of our findings is important.
Our findings, in more general terms, suggest that ongoing refinements in image acquisition and analysis techniques may be useful for investigating brain system function in psychiatric disorders and potentially in their clinical evaluation. However, there are also potential limitations associated with our image acquisition and analysis methodology. We adopted a dynamic sliding window step of 2 TR (0.85 s), briefer than the 2 s period used in most prior studies in this area. While this represents an advance in temporal resolution, brain neurophysiology occurs at a faster time interval. While hemodynamic BOLD signals have a longer relative time course, full understanding of the neurobiological implications of our findings will require a more advanced understanding of the relation of neural and hemodynamic measures. Second, a faster sampling rate for investigating dynamics of brain activity by the use of multiband acquisition provides advantages for modeling brain oscillations but may have certain limitations. For example, it has been suggested that false-positive activation of BOLD signals could arise when a higher multiband accelerator factor over 4 was adopted (Todd et al., 2016). Finally, we did not employ a simultaneous EEG or other measuring for the participants during the MRI scanning which might be done in future studies. Last, different states of transient FC strength were determined with cluster analysis, and the biological and psychological substrate of each state need to be further elucidated.
In conclusion, investigation of dynamic FC in schizophrenia patients provides evidence for impaired temporal variability and connectivity strength between brain regions known to be important for speech production and comprehension as a potential neurophysiological substrate for AH. These effects were specific to eloquent cortex in the left hemisphere and to a higher frequency component of brain oscillations typically examined in resting-state fMRI studies. These findings offer new insights into the neurobiological processes associated with AH in schizophrenia.
Acknowledgments
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grants 81371527, 81671664, 81621003). Dr. Lui would also like to acknowledge the support from Chang Jiang Scholars (Award No. Q2015154) of China and National Program for Support of Top-notch Young Professionals (National Program for Special Support of Eminent Professionals, Organization Department of the Communist Party of China Central Committee, Award No. W02070140).
Declaration of interest
The authors declare no conflict of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.06.018.
Appendix A. Supplementary data
Supplementary material
References
- Adams R.A., Stephan K.E., Brown H.R., Frith C.D., Friston K.J. 2013. The Computational Anatomy of Psychosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., McCarthy-Jones S., Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav. Rev. 2015;55:78–87. doi: 10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., Diederen K., Fernyhough C., Ford J.M., Horga G., Margulies D.S., McCarthy-Jones S., Northoff G., Shine J.M., Turner J., van de Ven V., van Lutterveld R., Waters F., Jardri R. Auditory hallucinations and the Brain's resting-state networks: findings and methodological observations. Schizophr. Bull. 2016;42(5):1110–1123. doi: 10.1093/schbul/sbw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P., Modinos G., Hubl D., Shields G., Cachia A., Jardri R., Thomas P., Woodward T., Shotbolt P., Plaze M., Hoffman R. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr. Bull. 2012;38(4):695–703. doi: 10.1093/schbul/sbs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex. 2014;24(3):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cachia A., Paillere-Martinot M.L., Galinowski A., Januel D., de Beaurepaire R., Bellivier F., Artiges E., Andoh J., Bartres-Faz D., Duchesnay E., Riviere D., Plaze M., Mangin J.F., Martinot J.L. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. NeuroImage. 2008;39(3):927–935. doi: 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Chang X., Collin G., Xi Y., Cui L., Scholtens L.H., Sommer I.E., Wang H., Yin H., Kahn R.S., van den Heuvel M.P. Resting-state functional connectivity in medication-naive schizophrenia patients with and without auditory verbal hallucinations: a preliminary report. Schizophr. Res. 2017;188:75–81. doi: 10.1016/j.schres.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Curcic-Blake B., Liemburg E., Vercammen A., Swart M., Knegtering H., Bruggeman R., Aleman A. When Broca goes uninformed: reduced information flow to Broca's area in schizophrenia patients with auditory hallucinations. Schizophr. Bull. 2013;39(5):1087–1095. doi: 10.1093/schbul/sbs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E., Turner J., Preda A., Van Erp T., Mathalon D., Ford J., Potkin S., Calhoun V. American College of Neuropsychopharmacology; 2012. Static and Dynamic Functional Network Connectivity during Resting State in Schizophrenia. [Google Scholar]
- Damaraju E., Allen E.A., Belger A., Ford J.M., McEwen S., Mathalon D.H., Mueller B.A., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., Vaidya J.G., van Erp T.G., Calhoun V.D. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pearlson G.D., Yu Q., He H., Lin D., Sui J., Wu L., Calhoun V.D. Interaction among subsystems within default mode network diminished in schizophrenia patients: a dynamic connectivity approach. Schizophr. Res. 2016;170(1):55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fryer S.L., Fu Z., Lin D., Sui J., Chen J., Damaraju E., Mennigen E., Stuart B., Mathalon D.H., Calhoun V.D. Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.10.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pearlson G.D., Lin D., Sui J., Chen J., Salman M., Tamminga C.A., Ivleva E.I., Sweeney J.A., Keshavan M.S., Clementz B.A., Bustillo J., Calhoun V.D. Identifying dynamic functional connectivity biomarkers using GIG-ICA: application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum. Brain Mapp. 2017;38(5):2683–2708. doi: 10.1002/hbm.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Corollary discharge, hallucinations, and dreaming. Schizophr. Bull. 2011;37(1):1–3. doi: 10.1093/schbul/sbq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg D.A., Beckett A., Chen L. Arterial spin labeling with simultaneous multi-slice echo planar imaging. Magn. Reson. Med. 2013;70(6):1500–1506. doi: 10.1002/mrm.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.M., Dierks T., Fisher D.J., Herrmann C.S., Hubl D., Kindler J., Koenig T., Mathalon D.H., Spencer K.M., Strik W., van Lutterveld R. Neurophysiological studies of auditory verbal hallucinations. Schizophr. Bull. 2012;38(4):715–723. doi: 10.1093/schbul/sbs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.M., Mathalon D.H., Roach B.J., Keedy S.K., Reilly J.L., Gershon E.S., Sweeney J.A. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr. Bull. 2013;39(6):1272–1280. doi: 10.1093/schbul/sbs129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. How hallucinations may arise from brain mechanisms of learning, attention, and volition. J. Int. Neuropsychol. Soc. 2000;6(5):583–592. doi: 10.1017/s135561770065508x. [DOI] [PubMed] [Google Scholar]
- Hare S.M., Ford J.M., Ahmadi A., Damaraju E., Belger A., Bustillo J., Lee H.J., Mathalon D.H., Mueller B.A., Preda A., van Erp T.G., Potkin S.G., Calhoun V.D., Turner J.A., Functional Imaging Biomedical Informatics Research N. Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr. Bull. 2017;43(2):389–396. doi: 10.1093/schbul/sbw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Hampson M. Functional connectivity studies of patients with auditory verbal hallucinations. Front. Hum. Neurosci. 2011;6:6. doi: 10.3389/fnhum.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Hampson M., Wu K., Anderson A.W., Gore J.C., Buchanan R.J., Constable R.T., Hawkins K.A., Sahay N., Krystal J.H. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb. Cortex. 2007;17(11):2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Fernandez T., Pittman B., Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol. Psychiatry. 2011;69(5):407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X.N., Butler P.D., Javitt D.C., D'Angelo D., Mauro C.J., Milham M.P. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Della Penna S., Duyn J.H., Glover G.H., Gonzalez-Castillo J., Handwerker D.A., Keilholz S., Kiviniemi V., Leopold D.A., de Pasquale F., Sporns O., Walter M., Chang C. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Javitt D.C., Sweet R.A. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nature reviews. Neuroscience. 2015;16(9):535–550. doi: 10.1038/nrn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen E., Hugdahl K., Fusar-Poli P., Kroken R.A., Kompus K. Neuropsychopharmacology of auditory hallucinations: insights from pharmacological functional MRI and perspectives for future research. Expert. Rev. Neurother. 2013;13(1):23–36. doi: 10.1586/ern.12.147. [DOI] [PubMed] [Google Scholar]
- Jones S.R. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr. Bull. 2010;36(3):566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kressel H.Y. Setting Sail: 2017. Radiology. 2017;282(1):4–6. doi: 10.1148/radiol.2016162471. [DOI] [PubMed] [Google Scholar]
- Lefebvre S., Demeulemeester M., Leroy A., Delmaire C., Lopes R., Pins D., Thomas P., Jardri R. Network dynamics during the different stages of hallucinations in schizophrenia. Hum. Brain Mapp. 2016;37(7):2571–2586. doi: 10.1002/hbm.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.M., Bentley W.J., Snyder L.H. Functional connectivity arises from a slow rhythmic mechanism. Proc. Natl. Acad. Sci. U. S. A. 2015;112(19):E2527–E2535. doi: 10.1073/pnas.1419837112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Wu G.R., Xu Q., Ji G.J., Zhang Z., Zang Y.F., Lu G. DynamicBC: a MATLAB toolbox for dynamic brain connectome analysis. Brain Connect. 2014;4(10):780–790. doi: 10.1089/brain.2014.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S.P. Least squares quantization in PCM. IEEE Trans. Inf. Theory. 1982;28(2):129–137. [Google Scholar]
- Lowe M.J., Mock B.J., Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lui S., Zhou X.J., Sweeney J.A., Gong Q. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281(2):357–372. doi: 10.1148/radiol.2016152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Wang Z., Ivleva E.I., Poudyal G., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Schretlen D.J., Calhoun V.D., Lui S., Damaraju E., Pearlson G.D. Frequency-specific neural signatures of spontaneous low-frequency resting state fluctuations in psychosis: evidence from bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) consortium. Schizophr. Bull. 2015;41(6):1336–1348. doi: 10.1093/schbul/sbv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S., Yacoub E., Olman C.A., Auerbach E., Strupp J., Harel N., Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010;63(5):1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L., Crow T.J., Hough M., Voets N.L., Liddle P.F., James S., Winmill L., James A.C. Gyrification of Broca's region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2 year follow-up. Schizophr. Res. 2013;147(1):39–45. doi: 10.1016/j.schres.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Todd N., Moeller S., Auerbach E.J., Yacoub E., Flandin G., Weiskopf N. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: sensitivity and slice leakage artifacts. Neuroimage. 2016;124(Pt A):32–42. doi: 10.1016/j.neuroimage.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Mathalon D.H., Roach B.J., Reilly J., Keedy S.K., Sweeney J.A., Ford J.M. Action planning and predictive coding when speaking. NeuroImage. 2014;91:91–98. doi: 10.1016/j.neuroimage.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang Y., Long Z., Zheng J., Zhang Y., Han S., Wang Y., Duan X., Yang M., Zhao J., Chen H. Frequency-specific alteration of functional connectivity density in antipsychotic-naive adolescents with early-onset schizophrenia. J. Psychiatr. Res. 2017;95:68–75. doi: 10.1016/j.jpsychires.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Xue S.W., Li D., Weng X.C., Northoff G., Li D.W. Different neural manifestations of two slow frequency bands in resting functional magnetic resonance imaging: a systemic survey at regional, interregional, and network levels. Brain Connect. 2014;4(4):242–255. doi: 10.1089/brain.2013.0182. [DOI] [PubMed] [Google Scholar]
- Yu R., Hsieh M.H., Wang H.L., Liu C.M., Liu C.C., Hwang T.J., Chien Y.L., Hwu H.G., Tseng W.Y. Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Chien Y.L., Wang H.L., Liu C.M., Liu C.C., Hwang T.J., Hsieh M.H., Hwu H.G., Tseng W.Y. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum. Brain Mapp. 2014;35(2):627–637. doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F., Castellanos F.X., Biswal B.B., Milham M.P. The oscillating brain: complex and reliable. NeuroImage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material