Abstract
Background
Little is known about the achievement of low density lipoprotein cholesterol (LDL-C) targets in patients at cardiovascular risk receiving stable lipid-lowering therapy (LLT) in countries outside Western Europe.
Methods
This cross-sectional observational study was conducted in 452 centres (August 2015−August 2016) in 18 countries in Eastern Europe, Asia, Africa, the Middle East and Latin America. Patients (n = 9049) treated for ≥3 months with any LLT and in whom an LDL-C measurement on stable LLT was available within the previous 12 months were included.
Results
The mean±SD age was 60.2 ± 11.7 years, 55.0% of patients were men and the mean ± SD LDL-C value on LLT was 2.6 ± 1.3 mmol/L (101.0 ± 49.2 mg/dL). At enrolment, 97.9% of patients were receiving a statin (25.3% on high intensity treatment). Only 32.1% of the very high risk patients versus 51.9% of the high risk and 55.7% of the moderate risk patients achieved their LDL-C goals. On multivariable analysis, factors independently associated with not achieving LDL-C goals were no (versus lower dose) statin therapy, a higher (versus lower) dose of statin, statin intolerance, overweight and obesity, female sex, neurocognitive disorders, level of cardiovascular risk, LDL-C value unknown at diagnosis, high blood pressure and current smoking. Diabetes was associated with a lower risk of not achieving LDL-C goals.
Conclusions
These observational data suggest that the achievement of LDL-C goals is suboptimal in selected countries outside Western Europe. Efforts are needed to improve the management of patients using combination therapy and/or more intensive LLTs.
Keywords: Cholesterol, statins, lipids, observational study, guidelines
Introduction
Increased levels of atherogenic lipoproteins containing apolipoprotein B, mainly low density lipoproteins, are a major causal factor in coronary heart disease. Lowering the concentration of low density lipoprotein cholesterol (LDL-C) reduces the risk of cardiovascular morbidity and mortality, with a direct correlation between the degree of absolute LDL-C lowering and the extent of event reduction.1
Statins are the preferred option for lowering LDL-C.2–4 Despite widespread recommendations on the use of intensive statin therapy, a large proportion of patients at high risk of cardiovascular events do not achieve the recommended LDL-C levels.5,6 Reasons for this include the presence of underlying genetic conditions, such as familial hypercholesterolaemia, suboptimal dosing (secondary to physician choice or because of the patient’s intolerance of the optimum dose of statins), poor adherence and an inadequate treatment response. Limited access to drug therapy, clinical inertia, negative media reports and concern about side-effects may contribute to the low rate of LCL-C goal attainment. Non-statin lipid-lowering therapies (LLTs; e.g. ezetimibe, niacin or fibrates) provide limited efficacy in lowering LDL-C.7 More effective non-statin LLTs have recently been approved, such as the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors alirocumab and evolocumab,8,9 but their use was not widespread at the time this study was conducted.
Large-scale studies10–14 have provided insights into the achievement of LDL-C targets, but largely concerned Western Europe and North America, with only limited data from outside these regions. The primary objective of this multinational observational study was to investigate the achievement of LDL-C targets as well their determinants, according to the 2011 ESC/EAS guidelines,7 which were operative at the time of the study, in everyday clinical practice in countries outside Western Europe.
Methods
This multinational, cross-sectional, observational study was conducted in 452 centres in 18 countries in Africa (Algeria, South Africa), Asia (Bangladesh, India, South Korea), Eastern Europe (Russia, Ukraine), Latin America (Argentina, Brazil, Colombia, Mexico) and the Middle East (Israel, Kuwait, Oman, Lebanon, Saudi Arabia, Turkey, United Arab Emirates) (Supplementary Table 1, available online). The first patient was enrolled in August 2015 and the last patient was enrolled in August 2016.
The study was conducted according to the Declaration of Helsinki principles, guidelines for Good Epidemiology Practice and local regulations. Where required, approval was obtained from local or regional institutional review boards and/or ethics committees. Patients provided written informed consent.
Study population
Patients (≥18 years) who had been receiving a stable dose and type of LLT for ≥3 months before enrolment and had their LDL-C value measured while receiving stable LLT in the previous 12 months were eligible. Patients participating in a clinical trial or who had received a PCSK9 inhibitor in the previous six months were excluded.
Physician and patient selection
To ensure that the results adequately reflect the management of dyslipidaemic patients in real-life practice, a national expert was consulted in each country to define the approximate contribution each medical specialty made in the management of such patients. A country-specific feasibility study was then conducted. Based on the information gained from these two activities, the profiles of suitable investigators and the appropriate proportion of each specialty were established. To limit bias in study site selection, participating centres/physicians were independently and randomly selected from these pre-established lists, aiming to ensure a balanced representation of each specialty. To limit patient selection bias, sites were instructed to recruit eligible patients consecutively (a minimum of five patients recruited per site). A predefined two-week interval was used during which all consecutive consenting patients, who attended their physician for any reason, were enrolled at any one site. As not all sites could start recruitment at the same time, a timeframe of three−six months (depending on the total number of sites/patients) was given to each country to recruit the targeted number of patients across all sites.
Data collection and management
Physicians completed a questionnaire that collected demographic data, medical specialty, years of practice, type of practice, location of practice, main workplace, mean number of patients consulted per day, choice of and adherence to practice guidelines for lipid disorders (i.e. ESC/EAS,7 ACC/AHA,15 other international/local/national guidelines) and the definition of statin intolerance used (i.e. intolerance to 1, 2, or ≥3 statins).
A case-report form was completed for each patient during a single visit. The data collected included: demographic information; the results of physical examinations; cardiovascular risk factors; medical history; type of hypercholesterolaemia (primary or familial – heterozygous, homozygous or unknown) (no ICD code for familial hypercholesterolaemia was available at the time the study was conducted); LDL-C values (calculated or measured directly; on current treatment and untreated if available) and other lipid variables; current LLTs and antithrombotic drugs; socioeconomic profile; and the investigator’s assessment of the patient’s cardiovascular risk level.
Data quality control was performed by trained personnel at ≥10% of sites chosen at random in each country.
Statistical analysis
The sample size calculation is presented in the Supplementary Material (available online). Baseline characteristics are presented as descriptive statistics with mean±SD or median (interquartile range) values for continuous variables and as counts (percentages) for categorical data.
The primary outcome was the proportion of patients taking LLT who did not achieve their LDL-C targets as defined by the 2011 ESC/EAS guidelines: <1.8 mmol/L (70 mg/dL) for very high risk, <2.5 mmol/L (100 mg/dL) for high risk and <3.0 mmol/L (115 mg/dL) for moderate risk patients.7 The Systematic Coronary Risk Estimation (SCORE) chart7 for high risk countries was used to retrospectively risk stratify patients in whom the relevant data were available. The high risk chart was selected following the guidelines for European countries7 due to the increasing rate of cardiovascular disease in non-European countries (see Supplementary Material, available online).
A multivariable logistic regression model was developed to test the relationship between the non-achievement of LDL-C targets and demographic, clinical and treatment characteristics (see Supplementary Material, available online). All analyses were conducted using SAS version 9.2.
Results
The characteristics of the 452 physicians (mean ± SD age 49.0 ± 9.3 years, 71.5% men) are detailed in Supplementary Table 2 (available online).
Of the 9886 patients assessed, 837 were ineligible for enrolment (Supplementary Figure 1, available online). The study population therefore consisted of 9049 patients, 39.2% from Asia, 20.8% from Latin America, 9.3% from Eastern Europe, 20.9% from the Middle East and 9.7% from Africa (Supplementary Table 1, available online). A total of 7224 (79.8%) patients were from urban areas, 6571 (72.7%) had completed secondary education or higher and 4240/9043 (46.9%) had health insurance that included drug reimbursement. The mean ± SD age of the population was 60.2 ± 11.7 years, 55.0% were men, hypertension was present in 71.5% and diabetes mellitus in 54.3% (Supplementary Table 3, available online). Overall, 36.7% patients had documented coronary artery disease, defined as a previous acute coronary syndrome (2249/3319, 67.8%), previous percutaneous coronary intervention (1788/3319, 53.9%) or previous coronary artery bypass graft (674/3319, 20.3%) and 6.5% had familial hypercholesterolaemia. The median (interquartile range) time since a diagnosis of dyslipidaemia was 4.0 (2.0−8.0) years.
Of the 7944 (87.8%) patients in whom the SCORE cardiovascular risk could be calculated or with classifying associated risk factors, 4842 (60.9%) were at very high risk, 2621 (33.0%) were at high risk, 411 (5.2%) were at moderate risk and 70 (0.9%) were at low risk. Physician-estimated risk correlated poorly with calculated risk: over half of the patients at high/very high calculated risk were estimated by physicians to be at a lower risk level (Supplementary Figure 2, available online). Conversely, 52.2% of the calculated low risk patients were estimated by physicians to be at a higher level of risk.
The LDL-C value before starting LLT (at the time of first diagnosis) was available in 3249 (35.9%) patients. The mean ± SD value was 3.9 ± 1.4 mmol/L (149.5 ± 54.3 mg/dL) (Supplementary Table 4, available online); 2166/3249 (66.7%) of patients had an LDL-C value >3.4 mmol/L (130 mg/dL) (Supplementary Figure 3, available online).
LLT and achievement of LDL-C goals
At study enrolment (when all patients were receiving stable LLT), 97.9% were receiving a statin (85.9% on statin monotherapy, 7.0% statin+fibrate and 3.0% statin+cholesterol absorption inhibitor; Table 1). Other cardiovascular drugs are detailed in Supplementary Table 5 (available online). Twenty-five per cent of statin-treated patients were receiving high intensity statin therapy (atorvastatin 40/80 mg or rosuvastatin 20/40 mg) and 23.4% were on the highest dose regimen available in their country.
Table 1.
Lipid-lowering therapies, overall and by cardiovascular risk level.
| Variable | No. of patients (n = 9049) | Risk level |
||||
|---|---|---|---|---|---|---|
| Low (n = 70) | Moderate (n = 411) | High (n = 2621) | Very high (n = 4842) | Not assesseda (n = 1105) | ||
| LLT | ||||||
| Any statin | 8863 (97.9) | 64 (91.4) | 394 (95.9) | 2550 (97.3) | 4782 (98.8) | 1073 (97.1) |
| High intensity statin (in statin-treated patients)b | 2238/8863 (25.3) | 14/64 (21.9) | 88/394 (22.3) | 415/2550 (16.3) | 1554/4782 (32.5) | 167/1073 (15.6) |
| On highest dose (in statin-treated patients)c | 2072/8852 (23.4) | 13/64 (20.3) | 83/394 (21.1) | 441/2547 (17.3) | 1322/4776 (27.7) | 213/1071 (19.9) |
| Statin monotherapy | 7770 (85.9) | 50 (71.4) | 316 (76.9) | 2232 (85.2) | 4225 (87.3) | 947 (85.7) |
| Statin+fibrate±other LLT | 630 (7.0) | 13 (18.6) | 38 (9.2) | 219 (8.4) | 284 (5.9) | 76 (6.9) |
| Statin+cholesterol absorption inhibitor±other LLT | 272 (3.0) | 0 | 26 (6.3) | 68 (2.6) | 154 (3.2) | 24 (2.2) |
Data are presented as n (%) or n/n (%) values.
LLT: lipid-lowering therapy.
Patients without a serious pathology classifying them as very high or high cardiovascular risk, and in whom the SCORE could not be calculated due to missing data.
Atorvastatin 40/80 mg or rosuvastatin 20/40 mg.
Marketed in the country.
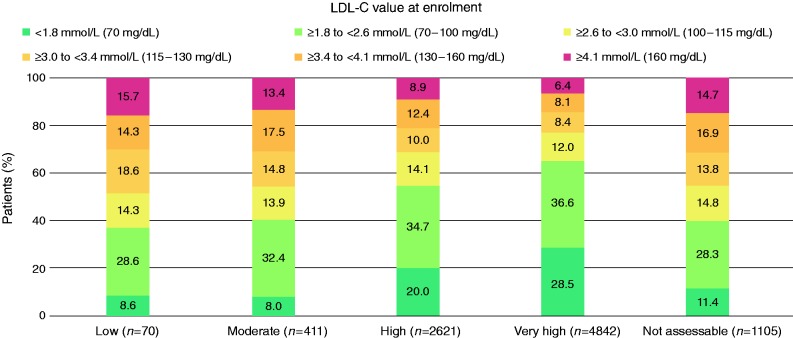
The mean ± SD LDL-C value at enrolment was 2.6 ± 1.3 mmol/L (101.0 ± 49.2 mg/dL) (Supplementary Table 3, available online). Achievement of LDL-C values is illustrated in Figure 1. The changes in lipid values after starting LLT are shown in Supplementary Table 6 (available online). The proportion of patients who achieved the LDL-C targets was greatest in the moderate risk group and lowest in the very high risk group (32.1% for very high risk, versus 51.9% for high risk and 55.7% for moderate risk), regardless of whether risk was calculated or physician-estimated (Supplementary Figure 4, available online).
Figure 1.
LDL-C value at enrolment (on lipid-lowering treatment) according to calculated cardiovascular risk level (calculated using SCORE16). Blue shaded rectangles indicate patients who have achieved the LDL-C target for their risk group. LDL-C: low density lipoprotein cholesterol; SCORE: Systemic Coronary Risk Estimation.
The percentage of patients who achieved the relevant target goals was 51.4% (3929/7639) when estimated by physicians versus 39.9% (3140/7874) when based on the ESC/EAS7 recommendations (P < 0.001). Among the patients in whom the SCORE risk was not assessable, the mean ± SD LDL-C value decreased with increasing number of risk factors (smoker, hypertension, diabetes, family history of premature cardiovascular disease): 3.2 ± 1.1 mmol/L for zero risk factors, 3.0 ± 1.5 mmol/L for one risk factor, 2.9 ± 1.1 for two risk factors and 2.9 ± 1.0 mmol/L for three risk factors.
Factors independently associated with the non-achievement of LDL-C goals
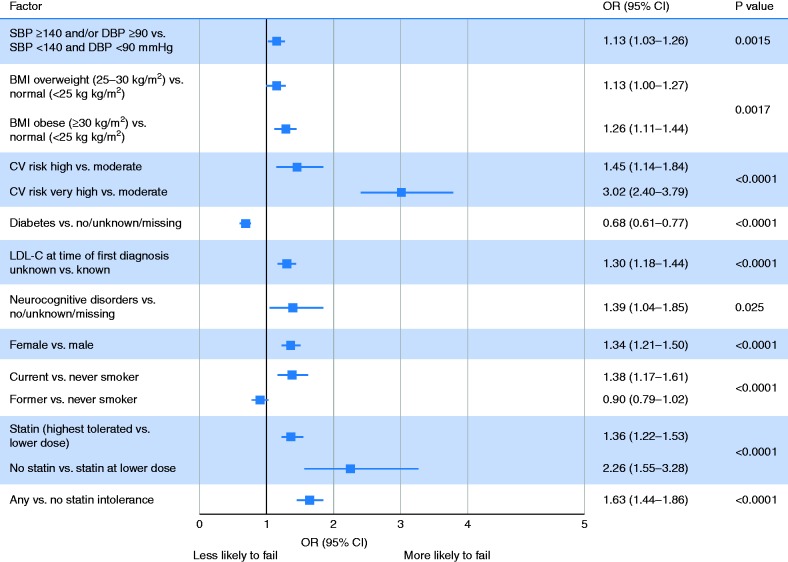
On multivariable analysis, patients at high or very high cardiovascular risk were, respectively, 1.5-fold and 3.0-fold less likely to achieve their LDL-C goals compared with moderate risk patients (Figure 2). No statin therapy, versus a lower dose statin, was associated with 2.3-fold greater likelihood of not achieving the goal, and a higher versus lower dose of statin was associated with a 1.4-fold greater likelihood of not achieving the goal. Overweight and obesity, high blood pressure, female sex, neurocognitive disorders, statin intolerance, unknown LDL-C value at diagnosis and current smoking were also associated with not achieving targets, whereas diabetes was associated with a lower risk of failure.
Figure 2.
Clinical and demographic factors independently associated with failure to reach LDL-C goal. BMI: body mass index; CI: confidence interval; CV: cardiovascular; DBP: diastolic blood pressure; LDL-C: low-density lipoprotein cholesterol; OR: odds ratio; SBP: systolic blood pressure.
Discussion
The results from this observational study in patients receiving stable LLT from countries outside Western Europe suggest that regardless of how cardiovascular risk is assessed (calculated using SCORE16 or physician-estimated), about one-third of very high risk patients and half of high risk patients achieve their risk-based target goals, whereas over half to two-thirds of moderate risk patients achieve their goal. Physician-estimated risk correlated poorly with calculated risk, with an underestimation of higher risk patients and an overestimation of low risk patients. These findings suggest that currently used LLTs are insufficient to achieve LDL-C goals and/or a gap exists between guideline recommendations on the use of such treatments and physician practice in these countries.
The Lipid Treatment Assessment Project (L-TAP) provided insights into the evolution of LDL-C control. In the first study, conducted in primary care settings in the USA (1996−1997), LDL-C goal attainment overall was 38%, decreasing to 18% among patients with established heart disease.10 L-TAP 2 (2006−2007),11 conducted about one decade after L-TAP,10 showed an improvement in the achievement of national guideline levels ranging from 47% (in Spain) to 84% (in Korea) across nine countries. The overall 73% success rate for LDL-C goal achievement was largely dependent on risk level, with a lower success rate for the highest risk patients (74% in moderate risk, 67% in high risk and 30% in very high risk patients).11 These data suggest that statins may be insufficient to achieve target goals in a sizeable percentage of higher risk patients, but that differences in guidelines, physician practices (e.g. not using high intensity statin therapy), patient characteristics and healthcare systems probably contributed to this variation.11 The centralized pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS) found that 55.3% of patients achieved their LDL-C target.6 Normal body mass index, not smoking, not having metabolic syndrome, being on statin therapy and good treatment adherence were independently associated with LDL-C goal achievement. The pan-Middle East CEPHEUS survey found that the LDL-C goal was attained in 52.7% of high risk and 32.0% of very high risk patients. Goal attainment was directly related to female sex, age <40 years, diabetes and a family history of cardiovascular disease.17 Similar findings were reported in the pan-Asian CEPHEUS study.18
More recent data show improvements in the management of dyslipidaemia, but LDL-C goal achievement remains low. The EUROASPIRE cross-sectional surveys (1999−2013) showed increases in the achievement of target LDL-C levels in patients with coronary disease and in the prescribing of LLT over time, with a greater use of high intensity statins; despite these changes, 75−81% of patients still had LDL-C values ≥1.8 mmol/L.12,19 The survey also identified increases in obesity and diabetes and no reduction in rates of smoking. Similar findings for LDL-C goal achievement were reported for the multinational DYSIS study, conducted in 57,885 outpatients treated with statins;20 the median (interquartile range) LDL-C value was 2.5 (2.0−3.3) mmol/L, similar to that in this study – 2.4 (1.9−3.1) mmol/L – and only 26.8% of patients achieved their risk-based target LDL-C level. Of the 76% of patients classified as at very high risk, 21.7% attained their LDL-C goal, which is notably lower than the rate of 32.1% reported in our study. A Japanese observational study in high cardiovascular risk patients21 showed that 56% of the coronary heart disease population met the targets for LDL-C (<2.5 mmol/L). Goal achievement was higher in patients with acute coronary syndrome than in patients with coronary heart disease (68 versus 55%). Among the patients with acute coronary syndrome, 59% were currently being treated with LLT, but only 3% were receiving a high intensity statin. These figures were slightly lower for the population with coronary heart disease (51 and 2%, respectively).
In our study, the estimation of risk by physicians correlated poorly with the calculated risk. This finding, which may be related to the use of SCORE in countries outside Western Europe, or a dissociation between SCORE and other risk evaluation tools, or a failure to use any risk scoring system, raises concern, given that intensity of preventive actions is dependent on the patient’s total cardiovascular risk.7 A sizeable percentage of higher risk patients were estimated to be at a lower level of risk; as such, the optimum LDL-C targets are less likely to be achieved, with the potential to miscommunicate the level of risk to patients and not to prescribe high intensity LLT. Furthermore, 52.2% of low risk patients were classified at a higher level of risk, resulting in over-treatment and unnecessary costs. Our study also indicated that the presence of risk factors (excluding diabetes), statin intolerance or lack of statin therapy, and female sex were associated with not achieving LDL-C targets. The identification of female sex as a predictor of goal failure is of interest and contrasts with the DYSIS survey, in which it was predictive of target achievement,20 and suggests that women may be undertreated in our population. Neurocognitive disorders were associated with not achieving LDL-C targets, whereas diabetes was associated with an increased likelihood of achieving the LDL-C goal and may reflect the more intensive management these patients receive and/or better adherence to prescribed drugs.
Combined, the results from these studies call for the optimization of existing treatment strategies and the use of more effective LLT, including combination therapy, alongside a collaborative effort to improve adherence to guidelines and combat therapeutic inertia. IMPROVE-IT22 was the first study to show the incremental benefit of a non-statin (ezetimibe) in addition to standard statin therapy in reducing cardiovascular events in acute coronary syndrome. In the FOURIER trial in patients with atherosclerotic cardiovascular disease and LDL-C ≥70 mg/dL (1.8 mmol/L) and who were receiving statin treatment, the fully human PCSK9 inhibitor evolocumab reduced LDL-C levels by 59% versus a placebo and gave a 15% reduction in cardiovascular events.8 The ODYSSEY OUTCOMES study reported that alirocumab reduced the rate of major adverse cardiovascular events by 15% in almost 19,000 patients with recent acute coronary syndrome and elevated levels of atherogenic lipoproteins, despite intensive or maximum tolerated statin therapy, and was associated with a lower risk of all-cause death.23 Post hoc data with alirocumab from the ODYSSEY LONG TERM study also suggest cardiovascular benefits.24 These findings suggest a real therapeutic benefit of non-statin therapies in addition to maximally tolerated LLT in the prevention of cardiovascular events and that lowering LDL-C to levels far below current recommended targets confers additional clinical benefits.
Limitations
This observational study offers insights into the achievement of lipid goals in patients treated in everyday clinical practice. It is, however, subject to limitations, including missing data, primarily the absence of LDL-C values before first diagnosis, genetic/family history data for diagnosing familial hypercholesterolaemia, and apolipoprotein A1 or B data. Due to the study design, LLTs and doses were not pre-specified and varied according to site/physician preference. In addition, observational studies cannot show causal associations. Although there was potential for selection bias, processes of random selection and consecutive recruitment were followed to minimize such bias, with eligible patients being identified from those attending their physician for any reason. As our population primarily consisted of educated urban residents with health insurance, it is not fully representative of all patients treated with LLT in each country and our results probably overestimate LDL-C goal achievement in the countries as a whole. We did not assess patient adherence to LLTs. SCORE was used in non-European populations in whom its validity has not been verified. All countries were considered at high cardiovascular risk, according to SCORE; a sensitivity analysis, in which countries with lower mortality rates were reclassified as at low risk, did not change our overall findings (Supplementary Table 7, available online). The recommended maximum waist circumference varies in different regions. As the numbers of patients in the low and moderate risk groups were small, the findings may not be representative of these groups.
Conclusions
These observational data suggest that achievement of LDL-C goals is suboptimal in some countries outside Western Europe. Only 32.1% of very high risk patients (according to SCORE) versus 55.7% of high risk and 51.9% of moderate risk patients achieved their LDL-C goals. Non-achievement of goals was more common in women and when other risk factors, excluding diabetes, were present or were poorly controlled. The proportion of patients receiving high intensity statin therapy or on the highest dose available was low. Even when statins were given at the highest tolerated dose, this was insufficient to achieve LDL-C goals in many instances. There is a need to improve the management of these patients using combination and/or more intensive LLTs, together with patient education and support to improve adherence.
Supplemental Material
Supplemental material, Appendix for Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice Study (ICLPS) by Nicolas Danchin, Wael Almahmeed, Khalid Al-Rasadi, Joseph Azuri, Abdelkrim Berrah, Carlos Alberto Cuneo, Yuri Karpov, Upendra Kaul, Meral Kayıkçıoğlu, Olena Mitchenko, Alvaro J Ruiz, Carlos A Aguilar Salinas, Raul D Santos, Florence Mercier, Dirk Blom and for the ICLPS Investigators in European Journal of Preventive Cardiology
Acknowledgements
We thank the patients and ICLPS investigators for their contributions. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications) provided medical writing assistance, funded by the sponsors.
Author contribution
ND contributed to the conception or design of the work. ND, WA, KAR, JA, AB, CAC, YK, UK, MK, OM, AJR, CAAS, RDS and DB contributed to the acquisition or interpretation of data for the work. ND drafted the paper. FM performed the analyses. All authors critically revised the manuscript and gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: sponsored by Sanofi.
References
- 1.Cholesterol Treatment Trialists (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol 2014; 63: 2889–2934. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – executive summary. J Clin Lipidol 2014; 8: 473–488. [DOI] [PubMed] [Google Scholar]
- 4.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016; 23: NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 5.Banegas JR, Lopez-Garcia E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: The EURIKA study. Eur Heart J 2011; 32: 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans MP, Castro Cabezas M, Strandberg T, et al. Centralized Pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS): Overall findings from eight countries. Curr Med Res Opin 2010; 26: 445–454. [DOI] [PubMed] [Google Scholar]
- 7.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011; 32: 1769–1818. [DOI] [PubMed] [Google Scholar]
- 8.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 9.Roth EM. Alirocumab for hyperlipidemia: ODYSSEY Phase III clinical trial results and US FDA approval indications. Future Cardiol 2016; 12: 115–128. [DOI] [PubMed] [Google Scholar]
- 10.Pearson TA, Laurora I, Chu H, et al. The Lipid Treatment Assessment Project (L-TAP): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med 2000; 160: 459–467. [DOI] [PubMed] [Google Scholar]
- 11.Waters DD, Brotons C, Chiang CW, et al. Lipid Treatment Assessment Project 2: A multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation 2009; 120: 28–34. [DOI] [PubMed] [Google Scholar]
- 12.Kotseva K, De Bacquer D, Jennings C, et al. Time trends in lifestyle, risk factor control, and use of evidence-based medications in patients with coronary heart disease in Europe: Results from 3 EUROASPIRE surveys, 1999–2013. Glob Heart 2016; 12: 315–322. [DOI] [PubMed] [Google Scholar]
- 13.Reiner Z, De Backer G, Fras Z, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries—findings from the EUROASPIRE IV survey. Atherosclerosis 2016; 246: 243–250. [DOI] [PubMed] [Google Scholar]
- 14.Shioji K, Izuhara M, Mitsuoka H, et al. Achievement rates of Japan Atherosclerosis Society Guidelines 2007 LDL-cholesterol goals with rosuvastatin or atorvastatin in patients who had not achieved their goal with atorvastatin. Cardiovasc Ther 2014; 32: 97–104. [DOI] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S1–S45. [DOI] [PubMed] [Google Scholar]
- 16.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur Heart J 2003; 24: 987–1003. [DOI] [PubMed] [Google Scholar]
- 17.Arafah M, Al-Hinai AT, Al Mahmeed W, et al. Centralized pan-Middle East survey on the undertreatment of hypercholesterolemia: Results from the CEPHEUS study in Arabian Gulf countries. Angiology 2014; 65: 919–926. [DOI] [PubMed] [Google Scholar]
- 18.Park JE, Chiang CE, Munawar M, et al. Lipid-lowering treatment in hypercholesterolaemic patients: The CEPHEUS Pan-Asian survey. Eur J Prev Cardiol 2012; 19: 781–794. [DOI] [PubMed] [Google Scholar]
- 19.Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016; 23: 636–648. [DOI] [PubMed] [Google Scholar]
- 20.Gitt AK, Lautsch D, Ferrieres J, et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis 2016; 255: 200–209. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto T, Uno K, Miyoshi I, et al. Low-density lipoprotein cholesterol levels and lipid-modifying therapy prescription patterns in the real world: An analysis of more than 33,000 high cardiovascular risk patients in Japan. Atherosclerosis 2016; 251: 248–254. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GG, Szarek M, Bhatt DL, et al. Alirocumab in patients after acute coronary syndrome, Orlando, FL: American College of Cardiology, 2018. [Google Scholar]
- 24.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice Study (ICLPS) by Nicolas Danchin, Wael Almahmeed, Khalid Al-Rasadi, Joseph Azuri, Abdelkrim Berrah, Carlos Alberto Cuneo, Yuri Karpov, Upendra Kaul, Meral Kayıkçıoğlu, Olena Mitchenko, Alvaro J Ruiz, Carlos A Aguilar Salinas, Raul D Santos, Florence Mercier, Dirk Blom and for the ICLPS Investigators in European Journal of Preventive Cardiology