Abstract
Platelets are essential cellular effectors of hemostasis and contribute to disease as circulating effectors of pathologic thrombosis. These are their most widely known biologic activities. Nevertheless, recent observations demonstrate that platelets have a much more intricate repertoire beyond these traditional functions and that they are specialized for contributions to vascular barrier integrity, organ repair, antimicrobial host defense, inflammation, and activities across the immune continuum. Paradoxically, on the basis of clinical investigations and animal models of disease, some of these newly discovered activities of platelets appear to contribute to tissue injury. Studies in the last decade indicate unique interactions of platelets and their precursor, the megakaryocyte, in the lung and implicate platelets as essential effectors in experimental acute lung injury and clinical acute respiratory distress syndrome. Additional discoveries derived from evolving work will be required to precisely define the contributions of platelets to complex subphenotypes of acute lung injury and to determine if these remarkable and versatile blood cells are therapeutic targets in acute respiratory distress syndrome.
Keywords: platelets, inflammation, acute lung injury, acute respiratory distress syndrome
Platelets are anucleate blood cells of myeloid origin that are generated by a unique polyploid precursor, the megakaryocyte, in a complex process termed thrombopoiesis (1). Circulating platelets are chief effector cells in physiologic hemostasis and pathologic thrombosis (2, 3), and these are their best-known biologic functions. Management of bleeding, or its risk, owing to thrombocytopenia or platelet dysfunction remains an important clinical issue for pulmonary and critical care physicians (4, 5). Nevertheless, platelets are versatile and intricate, and they have multiple additional activities in host response to injury and in tissue repair besides hemostasis (6, 7). Earlier observations (8) and a more recent genetic analysis (9) suggest that platelets may have evolved from primitive multitasking defensive cells with the capability to recognize and contain microbes in addition to the ability to seal wounds. Although this evolutionary relationship has not been rigorously proved, a substantial body of observations indicates that modern platelets are inflammatory effector cells with cellular and molecular activities that span the immune spectrum and operate in parallel with hemostatic effector functions (7, 10, 11). In addition to receptors for classic hemostatic agonists, human platelets express functional Toll-like receptors (TLRs), “immunoreceptors” such as FcγRIIA, small C-type lectin receptors, and other molecular recognition systems specialized for infectious and immune signaling (12). Furthermore, platelets have unique capacities that link inflammation and hemostasis. The latter features have recently generated terms such as thromboinflammation and immunothrombosis (12).
Platelets transit the pulmonary circulation and have important influences on physiologic defense and pathologic responses of the lungs (13). There is evidence that platelets have an “amicus or adversary” relationship with the lungs, with the capacity to mediate protection or injury depending on the conditions and the context (14). The contrasting facets of platelets as both protective and injurious effector cells are particularly apparent in the setting of inflammation.
This review profiles observations that have emerged since the topic of platelets in lung inflammation and injury was last considered in comprehensive fashion in the American Journal of Respiratory Cell and Molecular Biology (14). Although platelets contribute to diverse immune responses and to a spectrum of inflammatory disorders in the lungs and other organs (12, 15, 16), in this review we emphasize studies of their activities in experimental and clinical acute lung injury (ALI) and in acute respiratory distress syndrome (ARDS). In the first section of the article, we summarize recent discoveries and new observations related to platelet and megakaryocyte biology and inflammatory activities of platelets that have emerged since the previous review (14), with occasional reference to studies published earlier. This section includes newly reported examples of inflammatory and immune effector activities of platelets (Table 1) relevant to ARDS and discussion of the complicated issue of platelet effects on vascular barrier integrity. Although we emphasize inflammatory capabilities, we also touch on some of the other newly recognized, “nontraditional” responses of platelets (12). We also briefly highlight new information on genetic determinants of platelet number and their impact on outcome in ARDS, and we summarize the sketchy information available regarding general features of platelet phenotype and function in ARDS. In the second section of the review, we discuss recent experimental and clinical studies of platelet biology in specific conditions that trigger ARDS and in aspects of its management, and we summarize information relevant to the complicated issue of antiplatelet therapy in ARDS.
Table 1.
Platelets as Effector Cells with Inflammatory and Immune Activities
| • Link hemostasis and inflammation: cellular mediators of “thromboinflammation,” “immunothrombosis” |
| • Influence endothelial barrier function, vascular permeability, and lymphatic vessel integrity |
| • Sense and respond to microbes and pathogens (bacteria, viruses, plasmodia) |
| • Release stored inflammatory mediators and immunomodulators |
| • Synthesize reactive oxygen species, inflammatory lipids, and in some cases inflammatory peptides and proteins |
| • Circulate with a complex transcriptome and proteome that can be dynamically altered in some inflammatory, infectious, and thrombotic syndromes |
| • Release microparticles (microvesicles) with inflammatory and immune activities |
| • Interact with and signal endothelial cells |
| • Interact with and signal leukocytes (polymorphonuclear leukocytes, eosinophils, and other granulocytes; monocytes; lymphocytes; dendritic cells; macrophages) |
| • Induce formation of neutrophil extracellular traps |
| • Orchestrate innate and adaptive responses across the immune continuum |
Megakaryocytes and Thrombopoiesis in the Lung: New Observations
Megakaryocyte number, distribution, and function and the process of thrombopoiesis may be altered in ARDS (14). Substantial gaps in knowledge in this area remain, and new findings in the field suggest previously unrecognized complexity in megakaryocyte biology in relationship to the lungs.
Many studies in humans and laboratory animals demonstrate that megakaryocytes and platelets are present in vessels in the mammalian lung under basal conditions (1, 13). Megakaryocytes released into venous blood from the bone marrow have an impact in pulmonary microvessels, resulting in an intravascular population of cells with the potential to spawn platelets (13) (Figure 1). Infusion of mature megakaryocytes into mice resulted in accumulation of the cells in lung vessels, where they shed platelets with characteristic phenotypic features and near-normal half-lives (17). Nevertheless, the contribution of the lung megakaryocyte pool to platelet formation in physiologic and pathologic conditions has been a source of controversy and ongoing study (1, 13). A recent report provided new insights regarding this issue, as well as additional unexpected and provocative findings. Lefrançais and coinvestigators examined the murine lung microcirculation and observed large numbers of genetically labeled intravascular megakaryocytes that dynamically released platelets (18). In their experiments, they used state-of-the-art intravital microscopy (19). Real-time imaging revealed events in the lung (18) (Figure 1) that are remarkably similar to proplatelet and preplatelet formation in vitro (1, 20, 21). Biogenesis of platelets from lung megakaryocytes had not been formally demonstrated before this, although it was implied by earlier studies (13). Calculations indicated that, under the conditions of the observations, intravascular lung megakaryocytes contributed approximately 50% of total platelet production (18). This is of importance because the magnitude of platelet biogenesis in the lungs has been a topic of debate in the field (1, 13). Together with these findings, however, the investigators unexpectedly identified immature and mature megakaryocytes in extravascular compartments of the lung, in addition to populations of hematopoietic progenitor cells. Using a lung transplant model and mice with genetic thrombocytopenia, they also observed that these progenitors can translocate from the lungs to the bone marrow and reconstitute platelet numbers under deficiency conditions (18). Intravascular translocation of bone marrow–derived megakaryocytes to lung microvessels was previously known, as noted above, but movement of megakaryocytes from lungs to marrow was unanticipated. Furthermore, detection of megakaryocytes and hematopoietic precursor cells in extravascular compartments of the lung was unexpected. Together, the observations by Lefrançais and colleagues yield new evidence that the lung is a major site of platelet production and that lung megakaryocytes have unexpected relationships with megakaryocytes in other anatomic locations, and they suggest that lung megakaryocytes may have additional previously undiscovered biologic features (18). Further examination of these issues in the mouse and in human lungs, particularly in the settings of inflammation and injury, will be interesting and important.
Figure 1.
Megakaryocytes are present in the lungs and release platelets under experimental conditions. Megakaryocytes are present in microvessels of the lungs of humans and other mammals, and their numbers may be altered in lung injury, inflammation, and thrombosis (reviewed in [13]). Recent intravital microscopic observations of the lungs of ventilated mice demonstrated proplatelet formation and platelet release by intravascular megakaryocytes (18) in a fashion similar to that depicted in this cartoon. Thrombopoiesis by lung megakaryocytes in clinical acute lung injury and acute respiratory distress syndrome and other conditions remains to be examined. Lung megakaryocytes may have other functions besides thrombopoiesis, including inflammatory effector activities and alveolar repair. See text for additional details. Reprinted by permission from Reference 13.
Dysregulated intrapulmonary platelet production may contribute to lung disease and systemic vasculopathies (22), although this possibility remains largely unexplored (14). A second unexplored possibility is that lung megakaryocytes have activities that contribute to pulmonary inflammation, injury, and repair independent of thrombopoiesis (13). Earlier experiments with megakaryocytes isolated from human bone marrow and CD34+ hematopoietic progenitor cell–derived megakaryocytes demonstrated that these megakaryocytes synthesize cytokines and lipid mediators and respond to inflammatory stimulation (23, 24), suggesting functions beyond platelet biogenesis. Transcript analysis of megakaryocytes isolated from murine lungs also supports this possibility (18). A very recent report suggested that megakaryocytes contribute to systemic inflammation and experimental arthritis by releasing soluble and microparticle-associated IL-1 (25), although it is unknown if lung megakaryocytes have this capacity.
Platelets Are Inflammatory and Immune Effector Cells in the Lungs and Other Organs
There is evidence that platelets can mediate each of the cardinal features of acute and chronic inflammation: rubor (redness), tumor (swelling), calor (heat), and dolor (pain). Platelets also have the biologic potential to influence multiple additional inflammatory and immune processes (12). As an explanatory note, we consider the inflammatory and immune system to be a continuum that extends from acute, rapidly induced inflammation to chronic, complex immune responses and that mediates both homeostatic host defense and maladaptive and dysregulated tissue and organ injury. On the basis of this formulation, we use inflammation and immune responses interchangeably and in a complementary fashion. Platelets have activities across the immune continuum (7, 10, 11). Furthermore, they can modify or induce inflammation across the topography of the pulmonary system: in the blood, airway, and alveolar and pleural compartments.
Inflammatory and immune effector activities of platelets have been cataloged and examined in detail in recent reviews (7, 10, 15, 26–29). These effector activities are extensive (Table 1), and in some cases they represent “new biology”—that is, newly discovered biologic capacities that were not previously known to be part of the functional repertoire of platelets (30). We provide examples of effector activities of platelets relevant to ALI and ARDS in this and later sections of this article. The examples profiled are illustrative and are not comprehensive or exhaustive. Readers are encouraged to examine the literature for others.
Cell–cell interactions with myeloid leukocytes and endothelial cells are common and important effector functions of platelets (7). Biologic consequences of these interactions include localization of leukocytes and platelets at sites of inflammation and inflammatory injury, as well as complex intercellular signaling. Interactions of platelets with neutrophils (polymorphonuclear leukocytes [PMNs]) have been of prime interest because both cell types accumulate in the lungs in experimental and clinical ALI (Figure 2) and because both contribute to alveolar defense and dysfunction (14). Furthermore, PMNs are dominant leukocytes in most etiologies of ARDS (31).
Figure 2.
Cell–cell interactions of activated platelets mediate acute lung inflammation. Activated platelets interact with other platelets, endothelial cells, and leukocytes of a variety of types (polymorphonuclear leukocytes [PMNs], granulocytes of other classes, monocytes, and lymphocytes) in physiologic and pathologic inflammation of the lungs and other organs. Platelet–PMN interactions are particularly important in many models of experimental acute lung injury (ALI) and may be pivotal in clinical acute respiratory distress syndrome (ARDS) caused by common triggers, including microbial lung infection, nonpulmonary sepsis, aspiration, and trauma. Although inflammatory and hemostatic activities and cellular interactions of activated platelets are traditionally believed to be restricted to the vasculature, platelets and platelet–leukocyte aggregates have recently been detected in the alveolar spaces of mice with ALI on the basis of staining for CD41 (integrin subunit α2b; glycoprotein IIb), a platelet-specific marker, and counting of platelets in BAL samples (see text; reviewed in [12]), suggesting that platelets function in extravascular alveolar compartments in lung injury. Platelets have occasionally been reported in extravascular sites in autopsy samples from lungs of humans with ARDS, and platelet markers have been detected in BAL samples from subjects with ARDS (reviewed in [14]). Platelets can also influence extravascular cells and events by releasing microparticles and soluble mediators with signaling activities. See text and reference 12 for details.
Depending on the model and the organ, both the platelet and the leukocyte have been reported to attract or capture the other cell type (12). In a model of transfusion-related acute lung injury (TRALI), sequestration of platelets in the lungs was dependent on neutrophils (32). In a more recent study using several murine models, polarized neutrophils projected uropods that were believed to “scan” for platelets in the bloodstream; platelet–neutrophil contact then appeared to initiate additional critical inflammatory events, including neutrophil migration (33). Transient depletion of platelets or neutrophils, or blocking P-selectin glycoprotein ligand (PSGL)-1, reduced lung edema and mortality in TRALI, using the model mentioned above. In additional observations clusters of PSGL-1 on the neutrophil uropod interacting with P-selectin on activated, adherent platelets mediated signaling of neutrophils on the basis of blocking studies and experiments with genetically altered mice. The latter findings are consistent with the established importance of intercellular adhesion and signaling mediated by P-selectin and PSGL-1 in platelet–leukocyte interactions demonstrated by extensive previous observations (reviewed in [7]). Together, the experiments in this report suggested the primacy of neutrophils in interactions with platelets in inflammation and thromboinflammatory injury (33).
In other experimental conditions, however, platelets appear to provide localization signals for neutrophils. In a mouse model of focal injury to mesenteric endothelium, platelet protease-activated receptor 4 cleavage by thrombin promoted leukocyte recruitment in a mechanism involving fibrin deposition and platelet surface proteins glycoprotein Ibα (GPIbα) and P-selectin (34). In a second example, chemokine stimulation was reported to induce murine platelets to adhere to distinct sites in systemic venules and to “capture” neutrophils and a subset of monocytes via CD40-CD40L/CD154 interaction (35). Mechanistic experiments indicated that platelet–leukocyte signaling via platelet P-selectin and neutrophil PSGL-1 triggered mitogen-activated protein kinase–dependent activation of integrins on the leukocytes and their extravasation.
Additional recent observations indicate that platelet activity is important for LPS-induced neutrophil recruitment into the lungs (36–38), adding to earlier reports of platelet-facilitated leukocyte accumulation in a variety of models (12). Expanding on the theme of platelet–neutrophil interaction, another group reported intricate reciprocal signaling between the two cell types. Platelet depletion reduced lung microvascular platelet–neutrophil aggregate formation, neutrophils in BAL samples, and survival in a mouse model of Escherichia coli pneumonia (39). Activated platelets adhered to neutrophils via P-selectin interacting with PSGL-1; this facilitated the formation of neutrophil microparticles (microvesicles) in a platelet GPIbα-dependent fashion. Microparticles are released by platelets (12) (Table 1), neutrophils (40), and other cell types, and they may have important roles in intercellular communication in lung inflammation and ARDS (40). In the murine E. coli model, neutrophil microparticles transferred arachidonic acid to the platelets, leading to enhanced synthesis of thromboxane A2 (TXA2), which secondarily induced endothelial activation and neutrophil intravascular “crawling” and transmigration (39). This sort of daedal cellular cross-talk is consistent with the complexity of platelet interactions with leukocytes and endothelium that has been either demonstrated or suggested by many other investigations (7, 12) (Figure 2). “Heterotypic” interactions between platelets and neutrophils may create signaling “microdomains” that contribute to lung injury and systemic vascular damage on the basis of murine models of TRALI and sickle cell vasculopathy (41). Evidence for localized intracellular signaling between intimately interacting human leukocytes, platelets, and endothelial cells has been reviewed previously (42).
Intravital microscopy demonstrates that platelet–neutrophil interactions occur dynamically in the mouse lung under basal and injury conditions (43). Platelet–neutrophil aggregates formed in lung microvessels (Figure 2) under direct examination, and dramatically increased when ALI was induced with intratracheal E. coli LPS. Increased platelet–neutrophil aggregate formation was accompanied by activation and intravascular and intraalveolar accumulation of platelets (Figure 2). The latter finding is one of a number of observations indicating that platelets can gain access to extravascular spaces of the lung in parenchymal and airway inflammation (12). Aspirin (ASA) and 15-epi-lipoxin A4, an ASA-triggered endogenous antiinflammatory lipid, reduced platelet activation, intrapulmonary platelet sequestration, and ALI in this model, suggesting a rationale for therapeutic interruption of thromboinflammation in clinical ALI (43). (Also see Antiplatelet Agents in Prevention and Therapy of ARDS section below.)
Formation of neutrophil extracellular traps (NETs) is a specific consequence of platelet–neutrophil interaction that is of considerable topical interest (44, 45). NETs are extracellular lattices of chromatin, histones, and granule constituents that are released by neutrophils in a process commonly termed NETosis (Figure 3). NETs capture and, under some conditions, kill microbes and may be important in host defense, although this question remains controversial (46, 47). Evolving experimental evidence has generated a general conclusion that inappropriate, or unregulated, NET formation is a mechanism of inflammatory vasculopathy and tissue injury (44, 45, 47–50). Platelets induce NET formation (51), although other pharmacologic and physiologically relevant agonists, including bacteria, viruses, LPS, platelet-activating factor, chemokines, and complement fragments, also induce or amplify NET formation by human PMNs in vitro (45, 48). Heme, a danger-associated molecular pattern (DAMP) that mediates tissue injury (52) and modifies experimental sepsis (53), induces NET deployment by human and murine PMNs (54, 55). NETs mediate damage to lung cells in vitro and contribute to lung injury and dysfunction in vivo in experimental models (12, 44). Histones, which are key components of NET lattices (Figure 3), mediated experimental alveolar injury induced by LPS, C5a, and immune complexes (56).
Figure 3.
Activated platelets trigger formation of neutrophil extracellular traps (NETs) by polymorphonuclear leukocytes. Activated platelets, chemokines and other host factors, and pathogens induce NETosis and deployment of NET lattices by human and murine polymorphonuclear leukocytes. Formation of NETs may mediate capture and extracellular killing of bacteria and other pathogens and therefore may be a mechanism of antimicrobial protection of the lungs and other organs, although this concept is controversial (46, 48, 51). Conversely, experimental and limited clinical observations indicate that NETs form in a dysregulated fashion and are key effectors of inflammatory tissue damage in a variety of conditions, including ARDS (reviewed in references 44, 45, 47–50). On the basis of experimental findings, NET-associated factors, including elastase, other granule enzymes, and histones, can injure endothelial and alveolar epithelial cells. NETs are also components of thrombi and may mediate alveolar capillary and pulmonary vascular thrombosis. See text for details. Reprinted by permission from Reference 12.
In murine models of primary lung transplant dysfunction involving ischemia–reperfusion injury, NETs were observed in transplanted lungs by immunofluorescence microscopy (57). Activation and intrapulmonary accumulation of platelets were also detected, and ASA administration before orthotopic lung transplant after cold ischemia reduced platelet sequestration and lung injury assessed by BAL markers and oxygenation. Disruption of NETs by intrabronchial administration of DNase also improved oxygenation and reduced lung injury. In a translational facet of this study, NET components were detected in BAL samples from human subjects collected after lung transplant (57). Platelet-induced NET formation is also reported to contribute to TRALI (32, 58, 59) and ventilator-induced lung injury (VILI) (60) (see later sections of this review). Together, these observations indicate that platelet-triggered NET formation contributes to some types of experimental ALI and suggest that NETosis may occur in related clinical conditions. In addition to TRALI, VILI, and primary graft dysfunction in the transplanted lung, substantial evidence indicates that platelet-induced NET formation is important in experimental sepsis and may contribute to septic ALI (see Platelets in Sepsis and Septic ALI section below).
Platelets Have Complex Effects on Pulmonary and Systemic Endothelial Permeability and Vascular Barrier Integrity
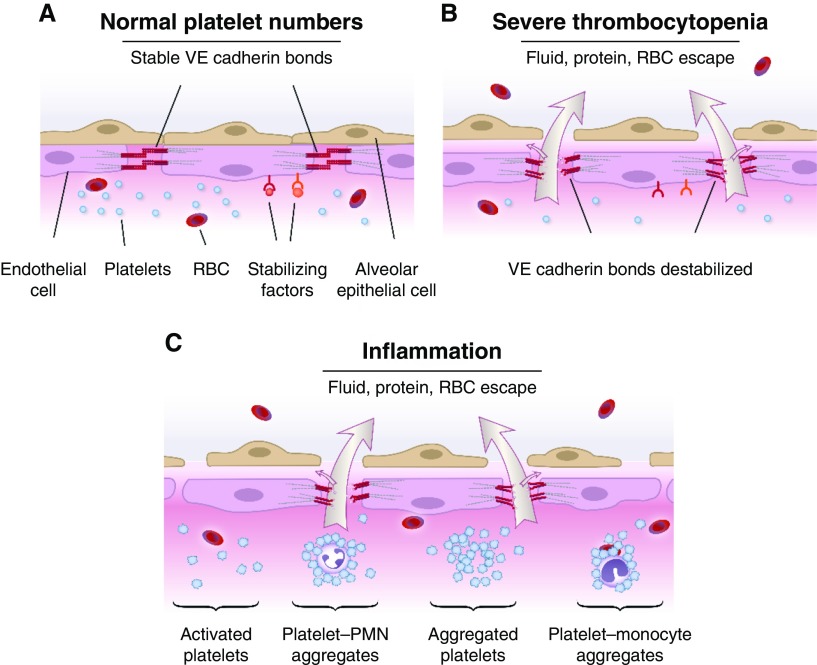
Increased alveolar–capillary membrane permeability with leak of protein-rich pulmonary edema fluid is a defining characteristic and central feature in the pathophysiology of ARDS (reviewed in [61, 62]). Platelets have complex influences on the permeability of pulmonary and systemic vessels (reviewed in [12, 13]) (Figure 4). Under basal conditions with normal or sufficient platelet numbers, semipermeable endothelial barrier integrity is maintained, whereas severe thrombocytopenia results in destabilized endothelial cell–cell interactions and increased endothelial permeability to water, proteins, and red blood cells (RBCs). Experiments with cultured pulmonary endothelial cell monolayers, isolated whole-lung preparations, and instrumented large animal models support these concepts (13). There is similar evidence that platelets are required for endothelial barrier integrity in the systemic circulation (13, 63, 64). Nevertheless, there is also evidence that activated platelets—paradoxically—have the converse effect and induce increased alveolar–capillary permeability and pulmonary edema (12, 13) (Figure 4). Evidence for platelet-mediated endothelial barrier destabilization has been generated in experiments with in vitro models and in in vivo small animal studies using a variety of methodologies to assess alveolar barrier function and leak (60, 65–69). The mechanisms involved in the “Janus-faced” activities of platelets in regulation of endothelial barrier integrity in the lung and other organs in physiologic and pathophysiologic inflammation—protective in some conditions, injurious in others—remain to be completely defined (reviewed in [12, 13]). Some platelet mediators, such as serotonin, appear to have both permeability-reducing and permeability-enhancing properties, depending on the physiological context (13, 70).
Figure 4.
Platelets contribute to maintenance of basal alveolar–capillary barrier integrity, but they also mediate vascular leak and increased alveolar–capillary permeability in acute lung injury. Experimental and limited clinical observations indicated that platelets are critical in maintaining physiologic, semipermeable alveolar, and systemic endothelial barrier integrity, but that activated platelets nevertheless contribute to disrupted vascular barrier function and increased permeability edema in inflammation and acute lung injury. (A) When platelet numbers are sufficient, vascular endothelial cadherin (VE cadherin) bonds are stable, and water and solute transfer out of vessels is restricted in the absence of injury or inflammation. Platelets may also contribute to lymphatic integrity on the basis of recent observations in mice. (B) When platelet numbers are severely reduced, basal endothelial barrier function is impaired, leading to leakage of water and protein from microvessels. Severe thrombocytopenia also contributes to nontraumatic extravasation of red blood cells (RBCs), particularly in inflammation. The mechanisms contributing to platelet-mediated endothelial barrier stabilization in lung and systemic vessels are incompletely defined and under active study (reviewed in [12, 13, 64, 75]). (C) Activated platelets mediate or amplify increased permeability of alveolar and systemic endothelial barriers in inflammation, in part by signaling PMNs and monocytes, triggering formation of neutrophil extracellular traps (see Figure 3), and releasing mediators that alter vascular barrier function. As an example of the latter mechanism, activated platelets can synthesize and release soluble and microparticle-associated IL-1β, a major agonist for increased endothelial permeability. Adapted by permission from Reference 13. See text and References 12 and 13 for details.
The complexity of platelet regulation of vascular barrier integrity is further illustrated by observations of hemorrhage in inflammation. Like intraalveolar and interstitial edema, alveolar hemorrhage and interstitial accumulation of RBCs are central features of early phases of ALI and its pathologic correlate, diffuse alveolar damage (DAD) (71–73). Platelets influence escape of RBCs from microvessels, in addition to regulating the transvascular flux of water and solutes (reviewed in [12, 64]). Experimental models demonstrate that thrombocytopenia contributes to nontraumatic hemorrhage in the skin, brain, and lungs under acute inflammatory conditions, and they indicate that this is independent of classic platelet hemostatic activities (12, 74, 75). For example, severe thrombocytopenia was associated with spontaneous alveolar hemorrhage in LPS-induced ALI (74) and bacterial pneumonia (76) in mice. Signaling by platelet glycoprotein VI (GPVI) and a member of the tyrosine-based activation motif immunoreceptor family, C-type lectin 2, was required to prevent inflammation-induced hemorrhage in the lungs and skin in mice (77, 78). Neutrophils are critical in thrombocytopenic hemorrhage in some models (79, 80). For example, individual platelets were reported to seal neutrophil-induced vascular defects via a GPVI-mediated mechanism in murine immune complex–induced skin inflammation (79). The mechanisms involved in thrombocytopenic inflammatory hemorrhage may depend on the experimental conditions and, perhaps, specific vascular beds. A very recent report suggested this: Platelet degranulation was required to prevent bleeding in ischemic brain inflammation in mice but not in LPS-induced ALI or immune complex–induced skin inflammation (81). These experimental studies of inflammatory hemorrhage in the lungs and other organs reveal intriguing activities of platelets that appear to differentially mediate vascular barrier integrity and are potentially clinically relevant. Nevertheless, they must be critically assessed in the context of human biology (12).
There is emerging evidence that platelets influence barrier function of lymphatic vessels in immune responses (Table 1) (82–84). This is a newly reported biologic activity of platelets observed in mouse models. Physiologic lymphatic function is critical for prevention of alveolar flooding and clearance of pulmonary edema in inflammation and injury (61). Thus, platelet influences on lymphatic integrity and barrier function may be important facets of interstitial and alveolar fluid accumulation in ALI that have not previously been considered. Platelets may also be involved in immune cell trafficking in lung lymphatic vessels (12), although this issue has not been examined extensively.
Platelet Numbers Are Altered in Critical Illness, Influence the Outcome of ARDS, and Are Regulated by Genetic Determinants in Clinical ALI
Thrombocytopenia is common in critically ill patients at risk for, or with, ARDS and is associated with negative outcomes, including death (4, 14, 85–90). In clinical observations and a model of gram-negative pneumonia with sepsis, thrombocytopenia was associated with dysregulated inflammation and impaired host defense (76, 91). Complex mechanisms contribute to thrombocytopenia in ICU patients, including altered thrombopoiesis and platelet consumption and sequestration (4, 5), each of which may occur in ARDS. The inflamed lung is a significant site of platelet sequestration (14). The lifespan and ultimate fate of platelets sequestered in microvessels and deposited in platelet-fibrin thrombi in the inflamed or injured lung are unknown. Furthermore, determinants of platelet production, sequestration, and destruction in ARDS have not been characterized.
Recent studies identified genetic variants that appear to alter risk and mortality in ARDS by influencing platelet numbers and their decline (89, 90). Wei and colleagues discovered a SNP in the gene for a cytoskeletal scaffold protein that influences actin assembly and disassembly, LRRC16A, and found that it is associated with platelet number (platelet count) and with altered risk for ARDS. Using mediation analysis, a method to examine variables that are potentially linked, they concluded that LRRC16A variants are associated with ARDS risk and that a portion of the association is via influence of LRRC16A on platelet number (90, 92). In a subsequent analysis, these investigators also found that the LRRC16A genotype influences the rate of decline in platelet number in patients with ARDS in the 28 days after ICU admission, as well as that an attenuated platelet count decline is associated with greater survival (89). Mediation analysis was again used to evaluate the relationship of decline in platelet number and the association of an apparent functional LRRC16A SNP with mortality. Together, these observations (88–90) provide new insights regarding genetic influences on platelet count in critically ill patients and new evidence that platelet number is a key variable in ARDS outcomes. The mechanisms involved in decline in platelet number, as well as influences of platelet functional alterations on outcomes in ARDS, were not evaluated and are important issues for future investigation (93).
Changes in Function and Phenotype of Platelets in ARDS: Largely Uncharted Territory
In addition to changes in platelet number, qualitative characteristics of platelets and changes in their functional repertoire may influence ALI and the natural history of ARDS. “Classic” markers of platelet activation, as well as display of receptors on circulating platelets, were found to be altered in samples from subjects with ARDS in limited earlier studies (reviewed in [14]). More recently, activated platelets were identified in blood samples from patients with ARDS caused by influenza A (94). These limited observations suggest that activities of platelets are altered in subjects with ALI, but correlation with key variables that influence the course of ARDS has not been done. In addition to traditional functions, “new,” nontraditional, biologic activities of platelets (12) may also be altered and may influence outcomes in ARDS and its predisposing conditions. For example, it is now known that human and murine platelets have extensive and diverse transcriptomes (95). Furthermore, the platelet transcriptome and proteome are dynamic and can change in clinical disorders (96–98). Alterations in platelet RNAs (see Platelets in Sepsis and Septic ALI section below), biologically significant proteins, and protein synthetic functions occur in sepsis (E. A. Middleton and colleagues, unpublished results), and they may influence events in septic ARDS. Similar, but perhaps distinct, changes in platelet transcriptome and proteome signatures likely occur in other underlying conditions that trigger ARDS, and possibly at different stages in the natural history of the syndrome (62, 99). Nevertheless, these issues are just beginning to be explored.
Platelets in Specific Conditions That Trigger ARDS and Experimental ALI
Epidemiologic observations that span global boundaries and the 50 years since the classic description of ARDS (100) indicate that four underlying conditions—bacterial or viral pneumonia, nonpulmonary sepsis, aspiration, and trauma and its sequelae—are the common predispositions that establish risk for the clinical syndrome, followed by a group of less frequent insults (62, 99, 101). There is evidence that platelets contribute to each of the four major etiologies of ARDS, much of it derived from surrogate experimental models (Table 2).
Table 2.
Recent Studies of Platelets in Murine Models of Acute Lung Injury
| Model | Major Platelet Activities Examined and/or Variables Influenced by Platelets | Reference |
|---|---|---|
| LPS-induced ALI (aerosolized) | Lung neutrophil accumulation, increased alveolar–capillary permeability | 66 |
| LPS-induced ALI (intranasal) | Alveolar–capillary barrier function, inflammation-induced alveolar hemorrhage | 77 |
| LPS-induced ALI (intratracheal) | Intraalveolar platelet sequestration, platelet–neutrophil aggregate formation, increased alveolar–capillary permeability | 43 |
| LPS-induced ALI (intranasal) | Leukocyte recruitment to the lungs | 36-38 |
| LPS-induced ALI (aerosolized) | Neutrophil recruitment to lungs, increased alveolar–capillary permeability | 170 |
| LPS-induced ALI (intranasal) | Inflammation-induced alveolar hemorrhage | 81 |
| Klebsiella pneumoniae pneumonia, “pneumosepsis” | Bacterial burden in lungs, blood, systemic organs; inflammation-induced alveolar hemorrhage; leukocyte recruitment to lungs; NET formation; endothelial activation; cytokine concentrations; coagulation | 76, 115 |
| Streptococcus pneumoniae pneumonia | Bacterial burden in lungs, blood, spleen; cytokine concentrations; coagulation | 102 |
| Escherichia coli pneumonia | Platelet–leukocyte interaction, neutrophil recruitment to lungs, transcellular metabolism, platelet-endothelial signaling | 39 |
| H7N7 influenza A | Platelet accumulation in alveolar compartments, platelet–leukocyte interaction, platelet activation markers in BAL fluid, natural history of infection | 104 |
| H1N1 influenza A | Activation of platelets by influenza virus | 106 |
| CLP | Lung neutrophil accumulation, increased alveolar–capillary permeability | 66 |
| CLP | Lung neutrophil accumulation, lung edema, alveolar inflammation by histologic score | 117 |
| CLP | Lung neutrophil accumulation, lung edema, alveolar inflammation by histologic score | 118 |
| Acid-induced ALI | Increased alveolar–capillary permeability | 32 |
| Acid-induced ALI | Lung neutrophil accumulation, increased alveolar–capillary permeability | 66 |
| Acid-induced ALI | Lung endothelial cell activation, development of procoagulant lung endothelium | 133 |
| Acid-induced ALI | Platelet–neutrophil aggregation, alterations in lung barrier integrity induced by intravascular platelet–neutrophil interactions | 132 |
| Acid-induced ALI | Alveolar neutrophil accumulation, increased alveolar–capillary permeability, alveolar inflammation by histologic score | 65 |
| TRALI | Platelet sequestration in lungs, platelet–neutrophil interaction, increased alveolar–capillary permeability | 32 |
| TRALI | Platelet sequestration in lungs, NET formation, increased alveolar–capillary permeability | 58 |
| TRALI | Platelet–neutrophil interaction, increased alveolar–capillary permeability | 43 |
| TRALI | Neutrophil–platelet interaction, NET formation, pulmonary edema | 33 |
| TRALI | Platelets not required for increased alveolar–capillary permeability and decreased oxygenation but influenced alveolar hemorrhage in this study | 142 |
| VILI | Oxygenation, lung neutrophil accumulation, increased alveolar–capillary permeability | 60 |
Definition of abbreviations: ALI = acute lung injury; CLP = cecal ligation and puncture; NET = neutrophil extracellular trap; TRALI = transfusion-related ALI; VILI = ventilator-induced lung injury.
The list of studies of platelets in experimental models of ALI in this table is not comprehensive, and other relevant investigations using animal models are mentioned in the text. Major variables and platelet activities examined in the studies are indicated, but many of the individual reports contain additional useful findings (e.g., effect on survival). In the listed studies, approaches to indicate contributions by platelets included induced or genetic thrombocytopenia, genetic alterations in platelet adhesion or signaling molecules, inhibition of platelet mediators, and/or administration of antiplatelet drugs or aspirin-triggered lipoxins. Earlier studies of platelets in experimental animal models are mentioned in the text and reviewed in References 12 and 14.
Platelets in ALI and ARDS caused by bacterial and viral pneumonia
Earlier observations suggested that platelets have complex influences in ARDS and experimental ALI induced by “direct” bacterial or viral infection (reviewed in [14]). In more recent investigations, mice with severe thrombocytopenia hemorrhaged into the lungs, but not distant organs, in a model of Klebsiella pneumoniae pneumonia with systemic involvement (76), providing evidence for critical activities of platelets in the maintenance of pulmonary vascular barrier integrity in infectious lung inflammation (see Platelets Have Complex Effects on Pulmonary and Systemic Endothelial Permeability and Vascular Barrier Integrity section above). Mice with severe thrombocytopenia also had increased bacterial burdens in the lungs, blood, spleen, and liver; greater plasma proinflammatory cytokine concentrations; and increased mortality. Animals with less severe, but substantial, alterations in platelet counts had less pronounced patterns of lung and systemic involvement (76). Thrombocytopenia did not reduce intrapulmonary neutrophil accumulation or formation of NETs in this model, a finding that was unexpected on the basis of other experimental observations (12, 39, 66). In a parallel report from the same group, thrombocytopenia in mice with Streptococcus pneumoniae pneumonia resulted in reduced survival; higher bacterial loads in the lungs, blood, and spleen; and altered plasma cytokine concentrations (102). Interestingly, the P2Y12 inhibitor clopidogrel did not alter lung or systemic organ bacterial counts, although it reduced tail bleeding times. As noted in the Platelets Are Inflammatory and Immune Effector Cells in the Lungs and Other Organs section above, platelets and neutrophils were reported to have complex molecular interactions that also involve the lung endothelium (Figure 2) in experimental E. coli pneumonia (39). Although it is unknown if the observations in these studies can be extended to clinical pneumonia causing ARDS or to other etiologies of experimental pneumonia, these models in aggregate nonetheless demonstrate intricate activities of platelets in parenchymal lung infection in laboratory animals.
Influenza is a major cause of clinical viral pneumonia and ARDS (62, 103). Experimental observations indicate that platelets have complex activities in influenza infection and influenza-induced ALI. Histopathologic studies demonstrated “massive” accumulation of activated platelets and platelet–leukocyte aggregates in vascular and extravascular compartments of the lungs of influenza A–infected mice (104). Viral proteins were detected in platelets in BAL fluid. In addition, serotonin and IL-1β were present in BAL samples from infected animals and were taken as biomarkers of platelet activation. Serotonin and IL-1β are pleiotropic immune modulators with effects on endothelial permeability, endothelial cell activation, and responses of multiple target inflammatory cells (12). Whereas serotonin is stored in platelets and released on activation, IL-1β is produced by an intricate post-transcriptional synthetic and inflammasome-mediated mechanism in activated platelets (12, 105), although its biosynthesis was not examined in influenza-infected mice. Genetic deficiency of integrin αIIbβ3, an αIIbβ3 antagonist, and pharmacologic inhibitors of platelet activation each reduced histologic markers of ALI and mortality in infected animals. In contrast, activation of protease-activated receptor 4, a key platelet receptor for thrombin in mice, worsened ALI and increased mortality, indicating that platelets contributed to lung damage in this model and suggesting that antiplatelet drugs may interrupt inflammatory injury in influenza pneumonia (104). Observations in a second study indicated that H1N1 influenza activates platelets via a complex mechanism that includes the FcγRIIA immunoreceptor and thrombin generation, thus involving both innate and adaptive immune pathways (106). This is one of many observations indicating that FcγRIIA is important in platelet biology, although it is not expressed on murine platelets (reviewed in [7, 107]). It was also suggested that interactions between influenza virus and platelets take place in the blood during viremia (106).
Studies of human cells, blood samples, and patients with clinical influenza complement observations in murine models. Thrombocytopenia is a risk factor for death in clinical ALI caused by H1N1 influenza A (108). Human platelets respond directly to influenza virus in vitro, contributing to evidence that platelets are sensors of viral infection in vivo (7, 106). Platelet–monocyte aggregates form in the blood of human subjects after influenza A vaccination, potentially expanding a population of proinflammatory monocytes (109). In addition, activated platelets and platelet–monocyte aggregates were present in venous blood samples from patients with ARDS caused by H1N1 influenza A (94), indicating that, as in experimental influenza in mice, platelets may be effector cells in lung injury caused by clinical influenza infection. Lung hemorrhage, microvascular thrombosis, and DAD are observed in severe clinical influenza, generating the proposal that activated platelets mediate aberrant hemostasis and maladaptive, “hyper” inflammation by interacting with leukocytes and endothelial cells in severe influenza infection (110). Infection of lung endothelial cells by virus, resulting in adhesion of platelets, is suggested to be a mechanism in influenza-induced ALI on the basis of parallel in vitro studies of human lung microvascular endothelial cells and mice infected with H3N2 influenza A (111). ASA improved survival in the in vivo model described in this report, suggesting that antiplatelet therapy may be a useful adjunctive strategy in severe influenza (111).
Platelets in sepsis and septic ALI
The contributions of platelets to the dysregulated systemic inflammation and disordered hemostasis that characterize sepsis have been reviewed previously (26–29, 85, 112, 113). Molecular sensing and signal transduction systems that allow platelets to detect and respond to pathogens and their toxins may be central to the pathogenesis of sepsis (reviewed in [12, 26, 114]). Engagement of platelet TLRs and other receptors that recognize microbial signals results in activation of “traditional” and more recently discovered effector responses of platelets (Table 1) (12). Thrombocytopenia, which is multifactorial in nature but due in part to deposition of activated platelets in microvascular thrombi, is a key facet of the pathophysiology of sepsis (85). On the basis of recent experimental observations, altered platelet number and function are central in “pneumosepsis” resulting from primary bacterial lung infection (76, 115). Recent experimental findings also demonstrate that platelets influence lung neutrophil recruitment and injury in polymicrobial sepsis modeled by cecal ligation and puncture (66, 116–118). Parallel clinical observations indicate that platelets contribute to dysregulated host responses in sepsis resulting from infection in the lungs, abdominal organs, and other tissues (91).
Histologic analysis and surrogate plasma assays indicate that NETs (Figure 3) form in lung microvessels and in vessels in other organs in experimental sepsis and that they contribute to physiologic dysfunction and outcomes (119–124). Concentrations of plasma cell–free DNA, taken as a marker of NET formation, correlated with incidence and severity of ARDS in one study (120). It is not clear, however, that plasma DNA was specific for NETs under these conditions, because DNA is released from injured cells of a variety of types in addition to PMNs undergoing NETosis.
In a recent report, platelets and NETs were central in microvascular thrombin generation, fibrin deposition, and impaired microvascular perfusion in mice challenged with intraperitoneal E. coli LPS (122). Additional observations suggested that NETs, together with platelets, thrombin, and tissue factor (TF), were critical in the disseminated thrombosis and microvascular dysfunction (122) that are fundamental features of sepsis. This study focused on the microvasculature of the liver, but in vivo imaging also indicated that generation of intravascular thrombin activity was associated with NET formation in lung microvessels. Intravascular fibrin deposition and formation of platelet-fibrin thrombi are key features of septic ALI in humans (reviewed in [14]), providing clinical relevance. NETs did not directly induce coagulation of human plasma in recent in vitro studies, however, suggesting that there may be species differences or other relevant variables (125). Agents with the ability to inhibit NETosis or dismantle NETs after their formation have been identified (55, 59, 123, 124, 126), suggesting potential adjuvant therapeutic approaches in sepsis and septic ARDS.
Evolving evidence indicates that the platelet transcriptome and proteome (95) (Table 1) are altered in sepsis and other infectious and inflammatory diseases. Platelets from patients with sepsis expressed spliced mRNA encoding TF and accelerated plasma clotting in a TF-dependent manner, whereas spliced TF mRNA was absent and the unspliced, precursor pre-mRNA was present in platelets from healthy age-matched control subjects (127). Live bacteria, LPS, and staphylococcal α-toxin triggered splicing of the TF pre-mRNA, generation of the mature translatable mRNA, and generation of TF-dependent procoagulation activity in platelets from control subjects studied in vitro, suggesting that signals in the septic “milieu” induced splicing and translation of TF mRNA in circulating platelets in patients with sepsis. The findings extended earlier observations demonstrating the presence of pre-mRNAs, in addition to mRNAs, and a functional spliceosome in human platelets (7, 128, 129). Sepsis may also alter the transcriptional profiles of megakaryocytes (97), thereby inducing changes in the transcriptome and proteome of circulating platelets in functionally significant ways (127, 130, and M. T. Rondina and colleagues, unpublished results). These issues are only beginning to be examined in platelets from patients with septic ARDS.
Aspiration-induced ALI
Instillation of low-pH acid into the tracheobronchial tree is considered to be a relevant model of aspiration-induced ALI, although, as with all surrogate animal models, it does not mimic the clinical condition completely (131). In an often-cited study (69), intratracheal insufflation of 1 M HCl in mice induced P-selectin–dependent platelet–neutrophil aggregation; accumulation of platelet–leukocyte aggregates in lung capillaries; and ALI that was reduced by platelet depletion, anti–P-selectin antibodies, ASA, or a TXA2 receptor antagonist. The findings indicated that intercellular signaling between platelets, neutrophils, and endothelial cells involving TXA2 mediated acid-induced ALI. Cell–cell interactions involving platelets, neutrophils, endothelial cells, and TXA2 were also detected in an E. coli pneumonia model (39), as mentioned in the Platelets Are Inflammatory and Immune Effector Cells in the Lungs and Other Organs section above, demonstrating that this multifactorial mechanism is not unique to sterile lung inflammation triggered by acid injury.
Several recent reports add evidence that platelets and platelet–neutrophil interactions contribute to acid-induced ALI. Intratracheal challenge of mice with 0.1 M HCl caused lung neutrophil accumulation, pulmonary edema, and intraalveolar release of neutrophil elastase that were reduced by pre- or post-treatment with a small-molecule inhibitor of the platelet chemokines CCL5 and CXCL4 (66). Extensive parallel experiments focused on platelet chemokines in ALI in other models (LPS challenge, cecal ligation and puncture) were also included in this report. In a different investigation, platelet-specific CXCL4 and CXCL7 were reported to mediate acid-induced ALI in a complementary fashion that induced neutrophil accumulation and disrupted alveolar–capillary barrier integrity. Genetic approaches resulting in altered chemokine expression revealed differential effects of CXCL4 and CXCL7 on neutrophil sequestration and alveolar–capillary barrier disruption (65).
In a study that examined effects of ASA-induced mediators in lung inflammation, ASA-triggered resolvin D1 (AT-RvD1) and its receptor were detected in injured lung tissue in a murine model of self-limited ALI involving selective intrabronchial instillation of HCl (132). Animals treated with AT-RvD1 had decreased numbers of platelet–neutrophil aggregates in right ventricular blood samples, partial alveolar–capillary barrier stabilization, and improved lung mechanics. The experiments were interpreted to indicate that AT-RvD1 reduced P-selectin–dependent platelet–neutrophil interactions and thereby ameliorated PMN-mediated alveolar damage in acid-induced ALI (132).
On the basis of results with platelet depletion protocols and other findings, platelets were reported to trigger endothelial TF expression in the lungs of mice challenged by intranasal HCL instillation (133). The in vivo observations were supported by experiments with isolated, blood-perfused lung preparations. Additional experiments using genetically altered mice indicated that platelets are activated by reactive oxygen species in acid-induced ALI, resulting in TF, von Willebrand factor, and P-selectin surface expression on both platelets and endothelial cells. The findings suggest that platelet-dependent mechanisms mediate secondary intravascular changes in aspiration-triggered ALI, an important observation because the injury is initiated on the epithelial side of the alveolar–capillary membrane (131). Formation of platelet–neutrophil aggregates in mice with acid-induced ALI (69, 132) also indicates that the blood compartment is a site of intercellular signaling after acid-induced alveolar epithelial damage.
Trauma-induced ALI and TRALI
Platelets are critical in hemostatic responses to penetrating and blunt trauma (134), and they also have biologic activities that may influence subsequent clinical phenotypes and complications, including the development of trauma-induced ARDS (135). In a recent report, early transfusion of platelets was associated with increased risk for ARDS in patients with severe isolated traumatic brain injury (136). The mechanisms accounting for this intriguing observation are yet to be determined, and it is not clear if the transfused platelets, underlying factor(s) contributing to the indications for platelet transfusion, or both were the pathophysiologic culprits. Alterations in vascular barrier stabilizing functions of platelets (63, 137), in addition to traditional hemostatic activities, may be central to their contributions to complex pathophysiologic responses to trauma (see Platelets Have Complex Effects on Pulmonary and Systemic Endothelial Permeability and Vascular Barrier Integrity section above).
Experimental observations suggest that activities of platelets contribute to trauma-induced ALI. In studies using a protocol of hemorrhagic shock and resuscitation in mice, neutrophil accumulation and tissue damage were decreased in the lungs and livers of platelet-depleted mice, suggesting that platelets mediated injury to the involved organs by facilitating neutrophil infiltration (68). Although this report indicates a key role for platelets, their contributions to tissue injury in hemorrhagic shock secondary to focal or multiple trauma are largely uncharacterized. Mechanisms involving other inflammatory effector cells may provide clues. For example, traumatic tissue injury can release circulating endogenous DAMPs that are recognized by receptors on target inflammatory cells, including TLRs (138, 139). Mitochondrial DAMPs released in this fashion were reported to bind to PMNs in part via TLR9, inducing activation and a sepsis-like state (139). Platelets also sense and respond to DAMPs via their repertoire of surface TLRs, including TLR9 (reviewed in [12]), but it is unknown if platelet TLR9 is activated by DAMPs in trauma or trauma-induced ARDS.
TRALI is a potential complication of transfusion of plasma-containing blood products in trauma and surgery, although it also occurs in nontraumatic medical conditions (140). A variety of observations indicate that platelets and platelet–neutrophil interactions contribute substantially to the pathogenesis of TRALI. A “two-hit” mouse model involving inflammatory priming of the animals followed by infusion of antileukocyte antibodies (32) has been widely employed in the field, and it has revealed important insights into platelet–neutrophil interactions, as mentioned in previous sections of this review, as well as insights into mechanisms of ALI (33, 41, 43, 58, 59, 141). Platelet-induced NET formation (Figure 3) is a central event in lung damage under these experimental conditions, and platelet depletion or targeting of platelets with pharmacologic agents, including ASA, inhibitors of integrin αIIbβ3, or ASA-triggered lipoxin A4, reduces ALI (43, 59). In vitro experiments in which NET formation and increased endothelial permeability were triggered by activated platelets and reduced by a TXA2 inhibitor also indicated a mechanism involving platelets (59). Nevertheless, not all investigators agree on a requirement for platelets in experimental TRALI (58, 142), suggesting that additional effector mechanisms may be involved. Platelets may have differential influences on alveolar permeability to plasma components and on alveolar extravasation of RBCs (Figure 4) in murine TRALI (142).
Platelets in uncommon causes of ARDS: global pathogen–associated syndromes as examples
Although primary bacterial or viral pneumonia, aspiration, sepsis, and trauma cause the majority of cases of ARDS in ICUs worldwide on a day-to-day basis, less common etiologies are also important from both biologic and management perspectives. Infectious diseases with global impact, particularly systemic pathogen–induced syndromes, are useful examples. Of these, severe malaria is of major significance. Malaria is transmitted in over 100 countries, and imported malaria in travelers and emigrants occurs with substantial frequency in Europe and North America. Malaria-associated acute respiratory distress syndrome (MA-ARDS) is most commonly caused by Plasmodium falciparum but can be caused by each of the five malarial parasites that infect humans, and it is one of several life-threatening malarial syndromes that can occur alone or in combination (143). The pathophysiology of MA-ARDS includes increased-permeability pulmonary edema (Figure 5) and other features that are characteristic of more common causes of ARDS (144–146). Animal models of malaria-associated acute lung injury (MA-ALI), particularly mice infected with rodent malarial strains, provide useful insights regarding lung involvement (146) and other aspects of the immunopathogenesis of malaria and its severe complications (147). Experimental and clinical observations indicate that monocytes and macrophages are critical leukocyte subtypes in inflamed alveoli in MA-ARDS and MA-ALI (146, 148) (Figure 5), an important difference from the neutrophilic inflammation that dominates more common etiologies of ARDS (31) (Figure 2). Inflammation in malaria is largely manifested as an inflammatory vasculopathy involving accumulation of monocytes, macrophages, lymphocyte subsets, platelets, and infected RBCs in vessels (Figure 5) (reviewed in [114]), although leukocytes are sometimes found in the interstitium and alveolar space in MA-ALI and MA-ARDS (146). It is possible that MA-ARDS is a unique subphenotype that differs from inflammatory subphenotypes induced by other underlying causes of ARDS (149), although this has not been examined.
Figure 5.
Platelets may be central in experimental malaria-associated ALI (MA-ALI) and clinical malaria-associated ARDS (MA-ARDS). One of the sequelae of severe malaria is a syndrome of increased permeability pulmonary edema and other pathophysiologic features that are characteristic of ARDS, termed MA-ARDS. MA-ARDS can be modeled by MA-ALI in mice. MA-ARDS and experimental MA-ALI are vasculopathies with major accumulation of inflammatory effector cells in lung vessels. Monocytes are major leukocyte subtypes found in histologic samples in malarial vasculopathies, in contrast to ALI and ARDS induced by bacterial pathogens and other common underlying conditions in which polymorphonuclear leukocytes are the dominant leukocyte type (see Figure 2). Intravascular monocyte-to-macrophage transition may occur in MA-ALI and MA-ARDS. In addition, lymphocytes may be key effectors of alveolar injury on the basis of observations in models of another major complication of severe malaria, malarial cerebral edema. Platelets are believed to be central in malarial cerebral edema and may be pivotal in MA-ALI and ARDS, but their activities in malarial lung injury have not been defined and should be studied more precisely. In other infectious and inflammatory conditions, platelets interact with and signal endothelial cells and each of the key leukocyte types believed to be involved in MA-ALI, MA-ARDS, and cerebral malaria (Table 1; reviewed in [7 and 12]). Plasmodium-infected red blood cells (IRBC) can directly activate platelets and induce relevant functional responses, including the release of platelet factor 4 (15). Human platelets are activated and form platelet–monocyte aggregates in response to the malarial toxin hemozoin (unpublished results). See text and References 12, 15, 114, and 146 for additional details.
Thrombocytopenia is common in severe malarial syndromes (143). Platelets respond in complex and controversial ways to malarial infection (150, 151), and they are suggested to contribute to the pathophysiology of MA-ARDS (146) (Figure 5). Nevertheless, knowledge in this area is superficial and needs to be expanded. Platelets sequester in the lungs in murine MA-ALI caused by Plasmodium berghei ANKA (152). CD40 and CD40L, which are important immune modulators in platelet biology (12, 29, 35), may be central in this experimental model (146, 153). In parallel, there is evidence that platelets are important in the inflammatory vasculopathy of cerebral malaria (15, 114), another major condition in the spectrum of severe malaria (143). The activities of platelets and their interactions with other immune effector cells (Figure 5) have not, however, been clearly defined in cerebral malaria, experimental MA-ALI, or clinical MA-ARDS and need further study. Organ-specific intravascular infiltrates and inflammation are suggested to occur in severe malaria (154), and it is possible that contributions of platelets are distinct in malarial inflammation in the lung and brain (146). A confounding factor in ultimately determining the contributions of platelets to clinical MA-ARDS is that concomitant bacterial pneumonia and sepsis, syndromes in which platelets may have activities both unique and overlapping with those in severe malaria (see the previous sections in this review), occur with significant frequency in patients with malaria-induced alveolar injury (144). Thus, mechanistic investigations employing the animal models may be particularly useful in addressing this issue.
Additional pathogen-associated systemic inflammatory syndromes that are uncommon in North American and European ICUs but are major problems in other parts of the world also cause ARDS, including dengue and leptospirosis. Thrombocytopenia is a central feature of each (155, 156). Circulating platelets are activated in dengue (157), although this has not been investigated in leptospirosis. Symptomatic pulmonary complications are relatively uncommon in dengue, but alveolar involvement and pleural effusion occur (158). Platelets from patients with dengue, or exposed to dengue virus in vitro, release IL-1β in microparticles that increase permeability of cultured endothelium (157). Thus, circulating platelets could contribute to alveolar and pleural vascular leak in this infection. DAD and clinical features consistent with ARDS have been reported in fatal cases (158–160). Coinfection with dengue and influenza A leading to severe, fatal ALI has also been reported (161). Similarly, characteristic clinical and histologic features of ARDS occur in severe leptospirosis (156, 160). Platelets and platelet aggregates accumulate in alveolar capillaries (156), and activated platelets may contribute to the lung injury, but there are no definitive studies. Even less is known about platelets in severe acute respiratory syndrome, Middle East respiratory syndrome, and other uncommon and emerging infectious syndromes that cause ARDS.
Platelets in supportive measures used in ARDS
Platelets contribute to comorbid conditions that frequently affect patients with ARDS, regardless of the frequency of the underlying cause (reviewed in [16, 162]). In addition, inappropriate measures of systemic or respiratory support, including ventilation with excessive Vt and high concentrations of inspired oxygen, are potential causes of morbidity and mortality in patients with ARDS (62, 163). Platelets may also contribute to these iatrogenic complications (14). Previously, on the basis of assays of endothelial cells isolated from blood-perfused rat lungs and conditions of platelet depletion compared with normal platelet numbers, it was reported that platelets transfer proadhesive factors to endothelium in high-Vt ventilation (164). More recently, VILI was reported to involve formation of platelet–neutrophil aggregates in circulating blood, generation of NETs in lung microvessels, and sequestration of platelets in alveolar regions in which NETs were present (60). Circulating NET components were also detected in blood samples from mice ventilated with high Vt. Depletion of platelets reduced each of these variables and, in parallel, resulted in decreased neutrophil accumulation in lung blood, interstitial, and alveolar compartments; blunted deterioration of gas exchange; and decreased protein content in BAL fluid samples (60). On the basis of results with blocking protocols, platelet chemokines and neutrophil MAC1 (integrin αMβ2) mediated platelet–neutrophil interaction, NET formation, and ALI in this model. These observations indicate that platelet–neutrophil interactions and NET formation drive sterile inflammatory lung damage in experimental VILI, raising the possibility that similar events occur in clinical respiratory support involving excessive Vt. In contrast, NETosis was induced, but NETs did not seem to make a major contribution to VILI, in a two-hit model (LPS, high Vt) based on the results of DNase treatment (165). Platelets were not examined in this study.
Hyperoxia induces pulmonary platelet sequestration and platelet-fibrin thrombi formation, is a classic cause of DAD, and may accelerate the progression of ARDS (72, 73, 163). Nevertheless, platelet depletion did not markedly alter lung injury resulting from exposure of hybrid (CBAxC57BL/10) mice to 100% oxygen for various time periods, yielding the conclusion that platelets are not requisite for oxygen toxicity (166). A more recent study identified a survival disadvantage when c-mpl−/− mice with genetic thrombocytopenia were challenged with hyperoxia (32).
In addition to high-Vt ventilation and excessive inspired oxygen, excessive fluid administration resulting in increased lung microvascular pressure can worsen extravasation of fluid and protein across the leaky alveolar–capillary membrane and may drive progression of ALI to ARDS (61, 163). In earlier studies of alveolar responses to hydrostatic stresses, increased pressure in venular capillaries in isolated, blood-perfused rat lungs induced surface P-selectin expression on endothelial cell– (167) and P-selectin–dependent leukocyte accumulation in lung postcapillary venules (168). Because adherent, activated neutrophils can capture platelets under some circumstances (see Platelets Are Inflammatory and Immune Effector Cells in the Lungs and Other Organs section above), these findings suggest a potential mechanism for platelet accumulation and alveolar thromboinflammation resulting from excessive fluid administration. Platelets were not deposited in lung microvessels under these experimental conditions (167), however, suggesting that counterregulatory events may have been at play.
Antiplatelet Agents in Prevention and Therapy of ARDS
ASA, other pharmacologic platelet inhibitors, and endogenous ASA-induced lipoxins ameliorate ALI in a variety of experimental models, as demonstrated in reports profiled in this review and others we have not discussed (32, 43, 57, 59, 69, 104, 111, 132, 169, 170). In addition, some but not all retrospective and observational studies suggest that antiplatelet agents are beneficial in clinical ARDS and in predisposing conditions (171–176). These observations generated a rationale for prospective evaluation of ASA in ARDS (177–179). A randomized clinical study of low-dose ASA as a preventative agent, the LIPS-A (Lung Injury Prevention with Aspirin) trial, was subsequently completed and had negative results (180). There are a number of potential reasons for a lack of effect of ASA in this trial, including timing of administration and dosing of the drug, which are ubiquitous issues in clinical and experimental studies of pharmacologic intervention in ALI and ARDS (163, 181, 182). The dosing regimen in the LIPS-A trial was rationally chosen from the literature, predicting that it would elevate plasma antiinflammatory lipoxin activity and reduce plasma thromboxane concentrations on the basis of analysis of patients with other disorders (180), but these variables as direct indices of ASA efficacy were not reported in the LIPS-A study. Of note, an unexpectedly low number of control patients developed ARDS in this trial, reducing power to detect a beneficial effect of ASA (180, 183). Another possible factor in the outcome is that, although ASA inhibits “traditional” hemostatic responses such as platelet aggregation and TXA2 synthesis and has antithrombotic activities (2), it is not a complete antiplatelet agent and does not block some inflammatory effector activities of platelets. For example, ASA did not attenuate surface translocation of P-selectin by activated platelets or formation of platelet–monocyte and platelet–neutrophil aggregates in human whole blood treated with a variety of prothrombotic and proinflammatory agonists (184, 185). In addition, ASA alone did not block synthesis of MCP (monocyte chemoattractant protein)-1 or IL-8, release of active matrix metalloproteinase 9, or synthesis of cyclooxygenase 2 when thrombin-stimulated human platelets adhered to and signaled human monocytes in vitro (186). Because P-selectin translocation and P-selectin binding to monocyte PSGL-1 are critical for platelet–leukocyte aggregate formation and intercellular signaling under these conditions (reviewed in [7]; also see the Platelets Are Inflammatory and Immune Effector Cells in the Lungs and Other Organs section above), this result is consistent with lack of inhibition of P-selectin translocation by ASA noted in other experiments (184, 185). These studies suggest that there may be major defects in the therapeutic profile of ASA administered as a single agent to interrupt inflammatory events that are critical to the pathogenesis of ARDS on the basis of multiple experimental observations cited in this review. Nevertheless, 7 days of low- or high-dose ASA pretreatment reduced indices of alveolar inflammation in volunteers subjected to low-intensity LPS inhalation challenge, and short-term ASA reduced alveolar neutrophilic inflammation in ex vivo perfused and ventilated human lungs (187). This important result demonstrates that ASA alone inhibits some components of acute lung inflammation in humans and human tissues under some conditions, but it may not be sufficient to alter the natural history of ARDS.
An additional possibility regarding the negative LIPS-A outcome is that platelets have barrier-protective activities (Figure 4) and alveolar reparative potential in the injured lung (14, 188–190) that might be blunted by antiplatelet agents such as ASA (12). As a corollary, evidence that platelets have defensive activities against pathogens continues to evolve (7, 15, 26, 28, 191); these functions could also be reduced by inhibitory drugs. “Amicus or adversary” issues of this nature will need to be taken into account in future considerations of ASA or other antiplatelet agents, alone or in combination with additional pharmacologic interventions, in ARDS (169).
Conclusions
As outlined in the first section of this review, platelets have traditional and more recently discovered biologic activities that confer broad functions in host defense but also establish them as effector cells in thrombosis, vascular barrier dysregulation, and complex inflammatory responses, which are key processes that underlie ALI and ARDS. Consistent with this “amicus or adversary” paradox, the second section of the review outlines evidence from experimental models and limited clinical information that supports contributions of platelets to common and less frequent causes of ARDS and to complications of its supportive management. New genetic observations indicate that platelet numbers influence the natural history and outcomes in ARDS, suggesting the possibility that platelet function and phenotype may have similar influences. Yet, the number, function, and characteristics, including “new” biologic features such as the transcriptome, protein synthetic potential, and malleable proteome, of platelets in common and uncommon subphenotypes of ARDS remain undefined. Contributions of lung megakaryocytes to inflammation and thrombosis in experimental ALI and clinical ARDS are also unexplored, and new discoveries of the lungs as sites of regulated thrombopoiesis have not yet been plumbed in conditions of acute lung injury and inflammation. Differences in the results of studies of antiplatelet agents in experimental ALI, as well as retrospective analysis of platelet-inhibitory drugs in clinical lung injury populations considered in the context of the results of the recent prospective LIPS-A trial of ASA as a preventative agent, raise issues regarding platelets as therapeutic targets in ARDS. Pursuit of these and related questions (Table 3) in the next decade will almost certainly reveal additional unexpected features of platelets and megakaryocytes and their relationship to lung biology, as well as their activities in lung inflammation and injury.
Table 3.
Some Outstanding Questions Regarding Platelets, Megakaryocytes, Acute Lung Injury, and Acute Respiratory Distress Syndrome
| • What are the biologic signals and factors that regulate platelet number, production, survival, and distribution in ALI and ARDS? |
| • What is the role of the lung in regulating thrombopoiesis in ALI, ARDS, and other critical illnesses? |
| • Do lung and marrow megakaryocytes have inflammatory and hemostatic activities in ALI, ARDS, and critical illness that complement their activities as thrombopoietic precursors? |
| • What are the functional characteristics of circulating platelets in common (pneumonia, sepsis, aspiration, trauma) and uncommon (MA-ARDS, others) subphenotypes of ARDS and animal models of these subphenotypes? |
| • Do functional characteristics (transcriptome, proteome, synthetic capacity, surface phenotype) of platelets change during the natural history of ALI and ARDS? |
| • What are the differences in function of circulating platelets and platelets sequestered in microvessels in the lungs in ALI and ARDS? |
| • What activities do platelets have in lung interstitial and intraalveolar compartments in ALI and ARDS? |
| • What are the precise mechanisms by which platelets regulate alveolar–capillary barrier integrity in ALI and ARDS? Do platelets regulate lymphatic barrier activity in these syndromes? |
| • What are the pivotal molecular consequences of platelet–leukocyte interactions in common and uncommon subphenotypes of ARDS? |
| • Do platelets have critical interactions with endogenous or therapeutically delivered stem cells or other reparative cell populations in lung injury? |
| • Can “adversary” functions of platelets be therapeutically targeted while preserving key “amicus” functions that are critical for lung defense and repair in ARDS? |
| • What tools can be developed (e.g., imaging methodologies, “in vivo cell biology assays”) to aid in posing and answering these questions in clinically meaningful ways? |
Definition of abbreviations: ALI = acute lung injury; ARDS = acute respiratory distress syndrome; MA-ARDS = malaria-associated acute respiratory distress syndrome.
Acknowledgments
Acknowledgment
The authors thank Kendra Richardson for invaluable efforts in preparation of the manuscript; Diana Lim for creative contributions and preparation of the figures; Estelle Harris and Rob Paine for critical reading of the manuscript; and many faculty colleagues, fellows, and students for helpful discussions.
Footnotes
This work and studies cited in this article were supported by National Heart, Lung, and Blood Institute (NHLBI) grants R37HL044525, HL112311, HL066277, HL077671, HL091754, HL090870, and HL130541; by the Ben B. and Iris M. Margolis Foundation; and by NHLBI grant T32 HL105321 during fellowship (E.A.M.).
Author Contributions: E.A.M.: writing, editing, and internal review of the manuscript; M.T.R.: editing and internal review of the manuscript; H.S.: editing and internal review of the manuscript; and G.A.Z.: writing, revising, editing, and senior author review of the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0420TR on March 19, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Italiano JE, Jr, Hartwig JH. Megakaryocyte development and platelet formation. In: Michelson AD, editor. Platelets. 3rd ed. Amsterdam: Elsevier; 2013. pp. 27–49. [Google Scholar]
- 2.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 3.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 4.Greinacher A, Selleng S. How I evaluate and treat thrombocytopenia in the intensive care unit patient. Blood. 2016;128:3032–3042. doi: 10.1182/blood-2016-09-693655. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, Wheeler AP. Coagulopathy in critically ill patients: part 1: platelet disorders. Chest. 2009;136:1622–1630. doi: 10.1378/chest.08-2534. [DOI] [PubMed] [Google Scholar]
- 6.McFadyen JD, Kaplan ZS. Platelets are not just for clots. Transfus Med Rev. 2015;29:110–119. doi: 10.1016/j.tmrv.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34:5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 9.Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17:666–676. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 11.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96:1211–1259. doi: 10.1152/physrev.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira-de-Abreu A, Rondina MT, Weyrich AS, Zimmerman GA. Inflammation. In: Michelson AD, editor. Platelets. 3rd ed. Amsterdam: Elsevier; 2013. pp. 733–766. [Google Scholar]
- 17.Fuentes R, Wang Y, Hirsch J, Wang C, Rauova L, Worthen GS, et al. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120:3917–3922. doi: 10.1172/JCI43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looney MR, Bhattacharya J. Live imaging of the lung. Annu Rev Physiol. 2014;76:431–445. doi: 10.1146/annurev-physiol-021113-170331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwertz H, Köster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, et al. Anucleate platelets generate progeny. Blood. 2010;115:3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–874. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin JF, Slater DN, Trowbridge EA. Abnormal intrapulmonary platelet production: a possible cause of vascular and lung disease. Lancet. 1983;1:793–796. doi: 10.1016/s0140-6736(83)91851-2. [DOI] [PubMed] [Google Scholar]
- 23.Jiang S, Levine JD, Fu Y, Deng B, London R, Groopman JE, et al. Cytokine production by primary bone marrow megakaryocytes. Blood. 1994;84:4151–4156. [PubMed] [Google Scholar]
- 24.Rocca B, Secchiero P, Ciabattoni G, Ranelletti FO, Catani L, Guidotti L, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc Natl Acad Sci USA. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunin P, Penke LR, Thon JN, Monach PA, Jones T, Chang MH, et al. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J Clin Invest. 2017;127:1714–1724. doi: 10.1172/JCI84598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- 27.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12:1764–1775. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Yang F, Dunn S, Gross AK, Smyth SS. Platelets as immune mediators: their role in host defense responses and sepsis. Thromb Res. 2011;127:184–188. doi: 10.1016/j.thromres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapur R, Zufferey A, Boilard E, Semple JW. Nouvelle cuisine: platelets served with inflammation. J Immunol. 2015;194:5579–5587. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman GA, Weyrich AS. Immunology: arsonists in rheumatoid arthritis. Science. 2010;327:528–529. doi: 10.1126/science.1185869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan ZS, Zarpellon A, Alwis I, Yuan Y, McFadyen J, Ghasemzadeh M, et al. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors GPIb and PAR4. Nat Commun. 2015;6:7835. doi: 10.1038/ncomms8835. [DOI] [PubMed] [Google Scholar]
- 35.Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pörnbacher M, Lauber K, Krombach F, et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 2016;14:e1002459. doi: 10.1371/journal.pbio.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amison RT, Arnold S, O’Shaughnessy BG, Cleary SJ, Ofoedu J, Idzko M, et al. Lipopolysaccharide (LPS) induced pulmonary neutrophil recruitment and platelet activation is mediated via the P2Y1 and P2Y14 receptors in mice. Pulm Pharmacol Ther. 2017;45:62–68. doi: 10.1016/j.pupt.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Pan D, Amison RT, Riffo-Vasquez Y, Spina D, Cleary SJ, Wakelam MJ, et al. P-Rex and Vav Rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood. 2015;125:1146–1158. doi: 10.1182/blood-2014-07-591040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riffo-Vasquez Y, Somani A, Man F, Amison R, Pitchford S, Page CP. A non-anticoagulant fraction of heparin inhibits leukocyte diapedesis into the lung by an effect on platelets. Am J Respir Cell Mol Biol. 2016;55:554–563. doi: 10.1165/rcmb.2015-0172OC. [DOI] [PubMed] [Google Scholar]
- 39.Rossaint J, Kühne K, Skupski J, Van Aken H, Looney MR, Hidalgo A, et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun. 2016;7:13464. doi: 10.1038/ncomms13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dengler V, Downey GP, Tuder RM, Eltzschig HK, Schmidt EP. Neutrophil intercellular communication in acute lung injury: emerging roles of microparticles and gap junctions. Am J Respir Cell Mol Biol. 2013;49:1–5. doi: 10.1165/rcmb.2012-0472TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA. Cell-cell interactions: leukocyte-endothelial interactions. Curr Opin Hematol. 2003;10:150–158. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124:2625–2634. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sørensen OE, Borregaard N. Neutrophil extracellular traps—the dark side of neutrophils. J Clin Invest. 2016;126:1612–1620. doi: 10.1172/JCI84538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nauseef WM. Editorial: Nyet to NETs? A pause for healthy skepticism. J Leukoc Biol. 2012;91:353–355. doi: 10.1189/jlb.1011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 48.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saffarzadeh M, Preissner KT. Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr Opin Hematol. 2013;20:3–9. doi: 10.1097/MOH.0b013e32835a0025. [DOI] [PubMed] [Google Scholar]
- 51.Andrews RK, Arthur JF, Gardiner EE. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb Haemost. 2014;112:659–665. doi: 10.1160/TH14-05-0455. [DOI] [PubMed] [Google Scholar]
- 52.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122:1205–1208. doi: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 54.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123:3818–3827. doi: 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yost CC, Schwertz H, Cody MJ, Wallace JA, Campbell RA, Vieira-de-Abreu A, et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J Clin Invest. 2016;126:3783–3798. doi: 10.1172/JCI83873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, et al. Lung Transplant Outcomes Group Investigators. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191:455–463. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossaint J, Herter JM, Van Aken H, Napirei M, Döring Y, Weber C, et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 61.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 62.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baimukanova G, Miyazawa B, Potter DR, Muench MO, Bruhn R, Gibb SL, et al. Platelets regulate vascular endothelial stability: assessing the storage lesion and donor variability of apheresis platelets. Transfusion. 2016;56(Suppl 1):S65–S75. doi: 10.1111/trf.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bdeir K, Gollomp K, Stasiak M, Mei J, Papiewska-Pajak I, Zhao G, et al. Platelet-specific chemokines contribute to the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2017;56:261–270. doi: 10.1165/rcmb.2015-0245OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grommes J, Alard JE, Drechsler M, Wantha S, Mörgelin M, Kuebler WM, et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185:628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tvedten HW, Till GO, Ward PA. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol. 1985;119:92–100. [PMC free article] [PubMed] [Google Scholar]
- 68.Tweardy DJ, Khoshnevis MR, Yu B, Mastrangelo MA, Hardison EG, López JA. Essential role for platelets in organ injury and inflammation in resuscitated hemorrhagic shock. Shock. 2006;26:386–390. doi: 10.1097/01.shk.0000227907.56060.2b. [DOI] [PubMed] [Google Scholar]
- 69.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cloutier N, Paré A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, et al. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 71.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care. 2005;11:29–36. doi: 10.1097/00075198-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 74.Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O’Donnell E, Zhao BQ, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9(Suppl 1):56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Stoppelaar SF, van ’t Veer C, Claushuis TA, Albersen BJ, Roelofs JJ, van der Poll T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood. 2014;124:3781–3790. doi: 10.1182/blood-2014-05-573915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123:908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boulaftali Y, Hess PR, Kahn ML, Bergmeier W. Platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling and vascular integrity. Circ Res. 2014;114:1174–1184. doi: 10.1161/CIRCRESAHA.114.301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 2015;126:1017–1026. doi: 10.1182/blood-2014-12-617159. [DOI] [PubMed] [Google Scholar]
- 80.Hillgruber C, Pöppelmann B, Weishaupt C, Steingräber AK, Wessel F, Berdel WE, et al. Blocking neutrophil diapedesis prevents hemorrhage during thrombocytopenia. J Exp Med. 2015;212:1255–1266. doi: 10.1084/jem.20142076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deppermann C, Kraft P, Volz J, Schuhmann MK, Beck S, Wolf K, et al. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood. 2017;129:1702–1706. doi: 10.1182/blood-2016-12-750711. [DOI] [PubMed] [Google Scholar]
- 82.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124:273–284. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navarro-Núñez L, Langan SA, Nash GB, Watson SP. The physiological and pathophysiological roles of platelet CLEC-2. Thromb Haemost. 2013;109:991–998. doi: 10.1160/TH13-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Stoppelaar SF, van ’t Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112:666–677. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 86.Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139:271–278. doi: 10.1378/chest.10-2243. [DOI] [PubMed] [Google Scholar]
- 87.Moreau D, Timsit JF, Vesin A, Garrouste-Orgeas M, de Lassence A, Zahar JR, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 88.Wang T, Liu Z, Wang Z, Duan M, Li G, Wang S, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One. 2014;9:e94124. doi: 10.1371/journal.pone.0094124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei Y, Tejera P, Wang Z, Zhang R, Chen F, Su L, et al. A missense genetic variant in LRRC16A/CARMIL1 improves acute respiratory distress syndrome survival by attenuating platelet count decline. Am J Respir Crit Care Med. 2017;195:1353–1361. doi: 10.1164/rccm.201605-0946OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei Y, Wang Z, Su L, Chen F, Tejera P, Bajwa EK, et al. Platelet count mediates the contribution of a genetic variant in LRRC16A to ARDS risk. Chest. 2015;147:607–617. doi: 10.1378/chest.14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Claushuis TA, van Vught LA, Scicluna BP, Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, et al. Molecular Diagnosis and Risk Stratification of Sepsis Consortium. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062–3072. doi: 10.1182/blood-2015-11-680744. [DOI] [PubMed] [Google Scholar]
- 92.Reilly JP, Christie JD. Linking genetics to ARDS pathogenesis: the role of the platelet. Chest. 2015;147:585–586. doi: 10.1378/chest.14-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones TK, Meyer NJ. What’s in a number? Platelet count dynamics as a novel mediator of acute respiratory distress syndrome survival. Am J Respir Crit Care Med. 2017;195:1285–1287. doi: 10.1164/rccm.201611-2264ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rondina MT, Brewster B, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES, et al. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1) Chest. 2012;141:1490–1495. doi: 10.1378/chest.11-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19:385–391. doi: 10.1097/MOH.0b013e328357010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montenont E, Echagarruga C, Allen N, Araldi E, Suarez Y, Berger JS. Platelet WDR1 suppresses platelet activity and is associated with cardiovascular disease. Blood. 2016;128:2033–2042. doi: 10.1182/blood-2016-03-703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rondina MT, Weyrich AS. Regulation of the genetic code in megakaryocytes and platelets. J Thromb Haemost. 2015;13(Suppl 1):S26–S32. doi: 10.1111/jth.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trugilho MRO, Hottz ED, Brunoro GVF, Teixeira-Ferreira A, Carvalho PC, Salazar GA, et al. Platelet proteome reveals novel pathways of platelet activation and platelet-mediated immunoregulation in dengue. PLoS Pathog. 2017;13:e1006385. doi: 10.1371/journal.ppat.1006385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pham T, Rubenfeld GD. Fifty Years of Research in ARDS. The epidemiology of acute respiratory distress syndrome: a 50th birthday review. Am J Respir Crit Care Med. 2017;195:860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 100.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 101.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 102.van den Boogaard FE, Schouten M, de Stoppelaar SF, Roelofs JJ, Brands X, Schultz MJ, et al. Thrombocytopenia impairs host defense during murine Streptococcus pneumoniae pneumonia. Crit Care Med. 2015;43:e75–e83. doi: 10.1097/CCM.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 103.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lê VB, Schneider JG, Boergeling Y, Berri F, Ducatez M, Guerin JL, et al. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am J Respir Crit Care Med. 2015;191:804–819. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 105.Hottz ED, Monteiro AP, Bozza FA, Bozza PT. Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases? Mediators Inflamm. 2015;2015:435783. doi: 10.1155/2015/435783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boilard E, Paré G, Rousseau M, Cloutier N, Dubuc I, Lévesque T, et al. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood. 2014;123:2854–2863. doi: 10.1182/blood-2013-07-515536. [DOI] [PubMed] [Google Scholar]
- 107.Arman M, Krauel K. Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis. J Thromb Haemost. 2015;13:893–908. doi: 10.1111/jth.12905. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Delgado JC, Rovira A, Esteve F, Rico N, Mañez Mendiluce R, Ballús Noguera J, et al. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly. 2013;143:w13788. doi: 10.4414/smw.2013.13788. [DOI] [PubMed] [Google Scholar]
- 109.Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 2011;6:e25595. doi: 10.1371/journal.pone.0025595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol. 2016;13:432–442. doi: 10.1038/cmi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sugiyama MG, Gamage A, Zyla R, Armstrong SM, Advani S, Advani A, et al. Influenza virus infection induces platelet-endothelial adhesion which contributes to lung injury. J Virol. 2015;90:1812–1823. doi: 10.1128/JVI.02599-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katz JN, Kolappa KP, Becker RC. Beyond thrombosis: the versatile platelet in critical illness. Chest. 2011;139:658–668. doi: 10.1378/chest.10-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Middleton E, Rondina MT. Platelets in infectious disease. Hematology Am Soc Hematol Educ Program. 2016;2016:256–261. doi: 10.1182/asheducation-2016.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Stoppelaar SF, van’t Veer C, Roelofs JJ, Claushuis TA, de Boer OJ, Tanck MW, et al. Platelet and endothelial cell P-selectin are required for host defense against Klebsiella pneumoniae-induced pneumosepsis. J Thromb Haemost. 2015;13:1128–1138. doi: 10.1111/jth.12893. [DOI] [PubMed] [Google Scholar]
- 116.Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OO, Jeppsson B, et al. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med. 2009;37:1389–1396. doi: 10.1097/CCM.0b013e31819ceb71. [DOI] [PubMed] [Google Scholar]
- 117.Hwaiz R, Rahman M, Syk I, Zhang E, Thorlacius H. Rac1-dependent secretion of platelet-derived CCL5 regulates neutrophil recruitment via activation of alveolar macrophages in septic lung injury. J Leukoc Biol. 2015;97:975–984. doi: 10.1189/jlb.4A1214-603R. [DOI] [PubMed] [Google Scholar]
- 118.Rahman M, Gustafsson D, Wang Y, Thorlacius H, Braun OO. Ticagrelor reduces neutrophil recruitment and lung damage in abdominal sepsis. Platelets. 2014;25:257–263. doi: 10.3109/09537104.2013.809520. [DOI] [PubMed] [Google Scholar]
- 119.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 120.Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 124.Tanaka K, Koike Y, Shimura T, Okigami M, Ide S, Toiyama Y, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One. 2014;9:e111888. doi: 10.1371/journal.pone.0111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Healy LD, Puy C, Fernández JA, Mitrugno A, Keshari RS, Taku NA, et al. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem. 2017;292:8616–8629. doi: 10.1074/jbc.M116.768309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rondina MT, Schwertz H, Harris ES, Kraemer BF, Campbell RA, Mackman N, et al. The septic milieu triggers expression of spliced tissue factor mRNA in human platelets. J Thromb Haemost. 2011;9:748–758. doi: 10.1111/j.1538-7836.2011.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Freishtat RJ, Natale J, Benton AS, Cohen J, Sharron M, Wiles AA, et al. Sepsis alters the megakaryocyte-platelet transcriptional axis resulting in granzyme B-mediated lymphotoxicity. Am J Respir Crit Care Med. 2009;179:467–473. doi: 10.1164/rccm.200807-1085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, et al. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Emin MT, Sun L, Huertas A, Das S, Bhattacharya J, Bhattacharya S. Platelets induce endothelial tissue factor expression in a mouse model of acid-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1209–L1220. doi: 10.1152/ajplung.00189.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76:255–256; discussion 262–263. doi: 10.1097/TA.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, et al. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc. 2014;11:728–736. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hendrickson CM, Howard BM, Kornblith LZ, Conroy AS, Nelson MF, Zhuo H, et al. The acute respiratory distress syndrome following isolated severe traumatic brain injury. J Trauma Acute Care Surg. 2016;80:989–997. doi: 10.1097/TA.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baimukanova G, Miyazawa B, Potter DR, Gibb SL, Keating S, Danesh A, et al. The effects of 22°C and 4°C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56(Suppl 1):S52–S64. doi: 10.1111/trf.13455. [DOI] [PubMed] [Google Scholar]
- 138.Calfee CS, Matthay MA. Clinical immunology: culprits with evolutionary ties. Nature. 2010;464:41–42. doi: 10.1038/464041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al. TRALI Study Group. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hechler B, Maître B, Magnenat S, Heim V, El Mdawar MB, Gachet C, et al. Platelets are dispensable for antibody-mediated transfusion-related acute lung injury in the mouse. J Thromb Haemost. 2016;14:1255–1267. doi: 10.1111/jth.13335. [DOI] [PubMed] [Google Scholar]
- 143.Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: severe malaria. Crit Care. 2003;7:315–323. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mohan A, Sharma SK, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis. 2008;45:179–193. [PubMed] [Google Scholar]
- 145.Taylor WRJ, Hanson J, Turner GDH, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest. 2012;142:492–505. doi: 10.1378/chest.11-2655. [DOI] [PubMed] [Google Scholar]
- 146.Van den Steen PE, Deroost K, Deckers J, Van Herck E, Struyf S, Opdenakker G. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 2013;29:346–358. doi: 10.1016/j.pt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Lamb TJ, Brown DE, Potocnik AJ, Langhorne J. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010581. [DOI] [PubMed] [Google Scholar]
- 148.de Azevedo-Quintanilha IG, Vieira-de-Abreu A, Ferreira AC, Nascimento DO, Siqueira AM, Campbell RA, et al. Integrin αDβ2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS) Malar J. 2016;15:393. doi: 10.1186/s12936-016-1447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gramaglia I, Velez J, Combes V, Grau GE, Wree M, van der Heyde HC. Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response. Blood. 2017;129:1669–1679. doi: 10.1182/blood-2016-08-733519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morrell CN. Platelets: killers of parasites or patients? Blood. 2017;129:1571–1572. doi: 10.1182/blood-2017-01-764621. [DOI] [PubMed] [Google Scholar]
- 152.Piguet PF, Da Laperrousaz C, Vesin C, Tacchini-Cottier F, Senaldi G, Grau GE. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infect Immun. 2000;68:3822–3829. doi: 10.1128/iai.68.7.3822-3829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Piguet PF, Kan CD, Vesin C, Rochat A, Donati Y, Barazzone C. Role of CD40-CVD40L in mouse severe malaria. Am J Pathol. 2001;159:733–742. doi: 10.1016/s0002-9440(10)61744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schofield L. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol Cell Biol. 2007;85:130–137. doi: 10.1038/sj.icb.7100040. [DOI] [PubMed] [Google Scholar]
- 155.Hottz E, Tolley ND, Zimmerman GA, Weyrich AS, Bozza FA. Platelets in dengue infection. Drug Discov Today Dis Mech. 2011;8:e33–e38. [Google Scholar]
- 156.Nicodemo AC, Duarte MI, Alves VA, Takakura CF, Santos RT, Nicodemo EL. Lung lesions in human leptospirosis: microscopic, immunohistochemical, and ultrastructural features related to thrombocytopenia. Am J Trop Med Hyg. 1997;56:181–187. doi: 10.4269/ajtmh.1997.56.181. [DOI] [PubMed] [Google Scholar]
- 157.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodrigues RS, Brum AL, Paes MV, Póvoa TF, Basilio-de-Oliveira CA, Marchiori E, et al. Lung in dengue: computed tomography findings. PLoS One. 2014;9:e96313. doi: 10.1371/journal.pone.0096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Póvoa TF, Alves AM, Oliveira CA, Nuovo GJ, Chagas VL, Paes MV. The pathology of severe dengue in multiple organs of human fatal cases: histopathology, ultrastructure and virus replication. PLoS One. 2014;9:e83386. doi: 10.1371/journal.pone.0083386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.von Ranke FM, Zanetti G, Hochhegger B, Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013;191:9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perdigão AC, Ramalho IL, Guedes MI, Braga DN, Cavalcanti LP, Melo ME, et al. Coinfection with influenza A(H1N1)pdm09 and dengue virus in fatal cases. Mem Inst Oswaldo Cruz. 2016;111:588–591. doi: 10.1590/0074-02760160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. 2017;91:352–364. doi: 10.1016/j.kint.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 163.Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is acute respiratory distress syndrome a preventable disease? Am J Respir Crit Care Med. 2017;195:725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- 164.Yiming MT, Lederer DJ, Sun L, Huertas A, Issekutz AC, Bhattacharya S. Platelets enhance endothelial adhesiveness in high tidal volume ventilation. Am J Respir Cell Mol Biol. 2008;39:569–575. doi: 10.1165/rcmb.2007-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yildiz C, Palaniyar N, Otulakowski G, Khan MA, Post M, Kuebler WM, et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology. 2015;122:864–875. doi: 10.1097/ALN.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 166.Barazzone C, Tacchini-Cottier F, Vesin C, Rochat AF, Piguet PF. Hyperoxia induces platelet activation and lung sequestration: an event dependent on tumor necrosis factor-α and CD11a. Am J Respir Cell Mol Biol. 1996;15:107–114. doi: 10.1165/ajrcmb.15.1.8679214. [DOI] [PubMed] [Google Scholar]
- 167.Kuebler WM, Ying X, Singh B, Issekutz AC, Bhattacharya J. Pressure is proinflammatory in lung venular capillaries. J Clin Invest. 1999;104:495–502. doi: 10.1172/JCI6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ichimura H, Parthasarathi K, Issekutz AC, Bhattacharya J. Pressure-induced leukocyte margination in lung postcapillary venules. Am J Physiol Lung Cell Mol Physiol. 2005;289:L407–L412. doi: 10.1152/ajplung.00048.2005. [DOI] [PubMed] [Google Scholar]
- 169.Panka BA, de Grooth HJ, Spoelstra-de Man AM, Looney MR, Tuinman PR. Prevention or treatment of ARDS with aspirin: a review of preclinical models and meta-analysis of clinical studies. Shock. 2017;47:13–21. doi: 10.1097/SHK.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tilgner J, von Trotha KT, Gombert A, Jacobs MJ, Drechsler M, Döring Y, et al. Aspirin, but not tirofiban displays protective effects in endotoxin induced lung injury. PLoS One. 2016;11:e0161218. doi: 10.1371/journal.pone.0161218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boyle AJ, Di Gangi S, Hamid UI, Mottram LJ, McNamee L, White G, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139:289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harr JN, Moore EE, Johnson J, Chin TL, Wohlauer MV, Maier R, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013;41:399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kor DJ, Erlich J, Gong MN, Malinchoc M, Carter RE, Gajic O, et al. U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators. Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Crit Care Med. 2011;39:2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O’Neal HR, Jr, Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Valerio-Rojas JC, Jaffer IJ, Kor DJ, Gajic O, Cartin-Ceba R. Outcomes of severe sepsis and septic shock patients on chronic antiplatelet treatment: a historical cohort study. Crit Care Res Pract. 2013;2013:782573. doi: 10.1155/2013/782573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kor DJ, Talmor DS, Banner-Goodspeed VM, Carter RE, Hinds R, Park PK, et al. US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A) Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2:e001606. doi: 10.1136/bmjopen-2012-001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, et al. US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A) Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-A randomized clinical trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reilly JP, Christie JD. Is it possible to prevent ARDS? JAMA. 2016;315:2403–2405. doi: 10.1001/jama.2016.5988. [DOI] [PubMed] [Google Scholar]
- 182.Tuinman PR, Müller MC, Jongsma G, Hegeman MA, Juffermans NP. High-dose acetylsalicylic acid is superior to low-dose as well as to clopidogrel in preventing lipopolysaccharide-induced lung injury in mice. Shock. 2013;40:334–338. doi: 10.1097/SHK.0b013e3182a384f0. [DOI] [PubMed] [Google Scholar]
- 183.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5:524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 184.Li N, Hu H, Hjemdahl P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by whole blood flow cytometry. Thromb Res. 2003;111:165–170. doi: 10.1016/j.thromres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 185.Rinder CS, Student LA, Bonan JL, Rinder HM, Smith BR. Aspirin does not inhibit adenosine diphosphate-induced platelet α-granule release. Blood. 1993;82:505–512. [PubMed] [Google Scholar]
- 186.Weyrich AS, Denis MM, Kuhlmann-Eyre JR, Spencer ED, Dixon DA, Marathe GK, et al. Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation. 2005;111:633–642. doi: 10.1161/01.CIR.0000154607.90506.45. [DOI] [PubMed] [Google Scholar]
- 187.Hamid U, Krasnodembskaya A, Fitzgerald M, Shyamsundar M, Kissenpfennig A, Scott C, et al. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax. 2017;72:971–980. doi: 10.1136/thoraxjnl-2016-208571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mammoto T, Chen Z, Jiang A, Jiang E, Ingber DE, Mammoto A. Acceleration of lung regeneration by platelet-rich plasma extract through the low-density lipoprotein receptor-related protein 5-Tie2 pathway. Am J Respir Cell Mol Biol. 2016;54:103–113. doi: 10.1165/rcmb.2015-0045OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet-rich plasma extract prevents pulmonary edema through angiopoietin-Tie2 signaling. Am J Respir Cell Mol Biol. 2015;52:56–64. doi: 10.1165/rcmb.2014-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rafii S, Cao Z, Lis R, Siempos II, Chavez D, Shido K, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171:1368–1382.e23. doi: 10.1016/j.cell.2017.11.001. [DOI] [PubMed] [Google Scholar]