Abstract
Chronic obstructive pulmonary disease (COPD) is a syndrome that comprises several lung pathologies, but subphenotyping the various disease subtypes has been difficult. One reason may be that current efforts focused on studying COPD once it has occurred do not allow tracing back to the different origins of disease. This perspective proposes that emphysema originates when susceptible airway, endothelial, and/or hematopoietic cells are exposed to environmental toxins such as cigarette smoke, biomass fuel, or traffic emissions. These susceptible cell types may initiate distinct pathobiological mechanisms (“COPD endotypes”) that ultimately manifest the emphysematous destruction of the lung. On the basis of evidence from the “airway” endotype, we suggest that grading these endotypes by severity may allow better diagnosis of disease at early stages when intervention can be designed on the basis of the mechanisms involved. Therefore, genomic, proteomic, and metabolomic studies on at-risk patients will be important in the identification of biomarkers that help designate each endotype. Together with understanding of the involved molecular pathways that lead to disease manifestation, these efforts may lead to development of intervention strategies.
Keywords: lung cell types, susceptibility, airway, endothelia, hematopoietic
Clinical Relevance
Chronic obstructive pulmonary disease (COPD) is a complex disease that has been extremely difficult to subphenotype. COPD may originate when susceptible lung cells of different types (i.e., airway, endothelial, hematopoietic cells) are exposed to environmental toxins such as cigarette smoke, biomass fuel, or traffic emissions. These susceptible cell types may generate “COPD endotypes” that are characterized by distinct pathobiological mechanisms and comorbidities that ultimately manifest the emphysematous destruction of the lung. Studying COPD in younger cohorts may provide better distinction of the endotypes and help in the development of reliable biomarkers.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and disability, affecting more than 9% of the world population (1), and it is the third leading cause of death in the United States (2). Approximately 6.5% of adults (13.7 million) in the United States report having been diagnosed with COPD, and many more have undiagnosed COPD (3). Although all-cause death rates are decreasing in the United States, the COPD mortality rate has increased, particularly among women (4). COPD cases result in over 130,000 deaths (63.1 deaths per 100,000 people) per year. In comparison, the number of deaths per year resulting from lung cancer is estimated at 180,000 (5). However, the economic burden of COPD is substantially higher than that of lung cancer, for several reasons. COPD can affect patients over 30–40 years after initial diagnosis and can disable patients up to the time of death; therefore, the chronicity of disability can drastically reduce productivity for up to half of a lifespan. A recent estimate of total costs of hospitalization and absenteeism placed the annual cost of COPD at $36 billion, although the actual economic cost may even be double that estimate (6).
Cigarette smoking is the most widely studied risk factor for COPD, whereas the dominant risk factor for COPD worldwide is household air pollution arising from combustion of biomass and coal. Other important causes of COPD are occupational exposures from vapors, traffic emissions, dust, and fumes. The prevention and treatment of COPD remain a big challenge because of the heterogeneity of COPD and the lack of reliable biomarkers able to predict early decline in lung function. The lack of reproducibility of biomarkers may be due to the unknown early pathobiology associated with the different clinical features.
It is generally agreed that COPD should be considered a clinical syndrome (i.e., a heterogeneous collection of subphenotypes). However, thus far, attempts to achieve reproducible subphenotypes for COPD have proven elusive. An unsupervised clustering of 17,146 individuals with COPD from 10 cohorts showed that disease traits coexist in the same individual in varying degrees, making mutually exclusive COPD subphenotypes difficult to define (7). Yet, in relation to 3-year all-cause mortality, a recent work showed that it is feasible to identify reproducible subtypes of patients with COPD using unbiased algorithms (8). In another study, gene expression analyses of cells in induced sputum from 140 subjects suggested a possible clustering driven mainly by the severity of airflow limitation and the extent of emphysema (9). A systemic inflammatory phenotype of COPD has also been proposed (10). Also, comorbidities that impact the main disease characteristics that can be a target for a “treatable traits” approach have been considered. The traits are based on pulmonary, extrapulmonary, environmental, and behavioral factors that distinguish a given patient from other patients with similar clinical presentations (11–13). However, disease heterogeneity appears to be a result not only of clinical, functional, structural, and biological factors but also of dynamic changes that occur over time in individuals with COPD.
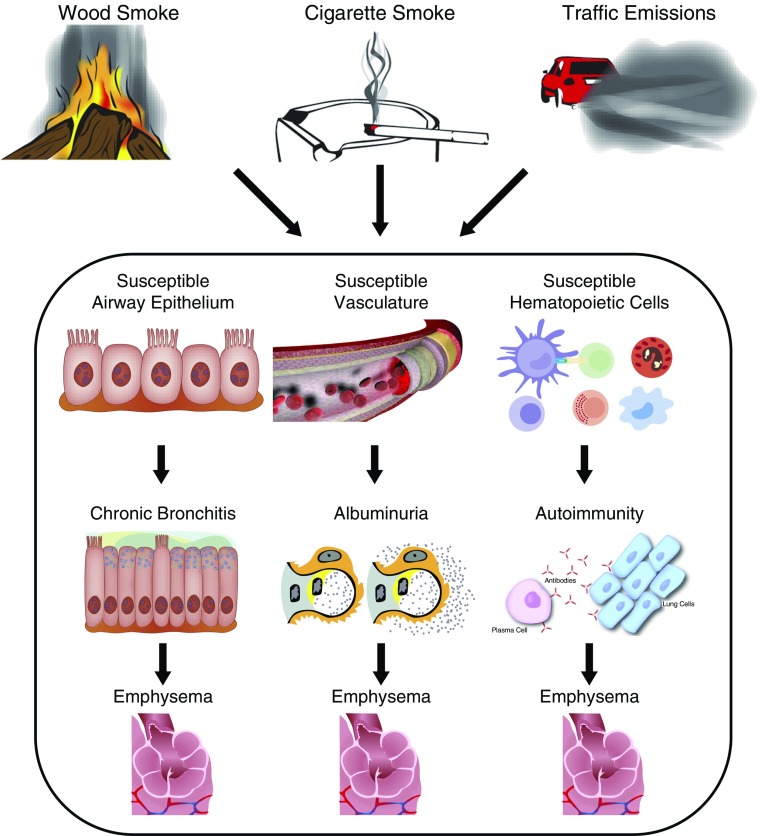
To reliably subphenotype COPD, we propose that it is critical to study younger cohorts of subjects at risk for developing COPD so that the pathobiological mechanisms (endotypes) can be identified. Exposure to causative factors such as cigarette smoke, biomass smoke, or traffic emissions may affect different cell types in the lung, depending on susceptibility (Figure 1). Individuals with susceptible airway epithelial cells may be prone to increased mucin gene expression that leads to phlegm production and emphysema owing to certain genetic polymorphisms (14). For other exposed patients, susceptible endothelial cells of the lung and/or systemic vasculature may lead to pulmonary and renal endothelial cell injury, resulting in albuminuria or pulmonary vascular disease (15) and emphysema. Others may have susceptible hematopoietic cells of the lung (macrophages or dendritic or T cells) that initiate abnormal responses of B cells, ultimately leading to the destruction of the alveolar structures and emphysema (16) (Figure 1). Although the pathology and clinical presentation of the established disease may be identical, current diagnostic tools do not allow tracing back of the vastly different origins. Therefore, early endotyping is necessary to have the tools to stratify patients with COPD by the pathobiological origins (i.e., COPD due to alteration of the airway epithelium, of the vascular endothelium, or of the hematopoietic compartment).
Figure 1.
Early detection of disease helps distinguish the chronic obstructive pulmonary disease endotypes. Although ultimately all endotypes lead to emphysema, the cells and the pathways involved in causing this pathology may be different.
Currently, severity of chronic airflow obstruction (CAO) is classified on the basis of spirometry, typically performed after the administration of an adequate dose of a short-acting inhaled bronchodilator to exclude airflow limitation caused by airway constriction, as well as to minimize overall variability (2). Specific spirometric cutpoints have been defined by consensus for purposes of simplicity. When the ratio of FEV1 to FVC falls below 0.7, it is taken to be an indicator of obstructed pulmonary function (17). Other experts advocate the use of a statistically defined lower limit of normal value for the ratio, such as the fifth percentile, rather than an arbitrary cutpoint of 0.7 (18). Severity of established CAO is then staged depending on the degree of reduction below the predicted FEV1 value on the basis of sex, height, and race/ethnicity. The Global Initiative for Chronic Obstructive Lung Disease criteria classify COPD into the following stages: stage 0 (FEV1/ FVC, ≥0.7), stage 1 (FEV1 ≥80% of predicted value and obstructed pulmonary function), stage 2 (FEV1, 50% to <80% of predicted value), stage 3 (FEV1, 30% to <50% of predicted value), and stage 4 (FEV1, <30% of predicted value) (17).
Chronic bronchitis (CB) is recognized to be important when present concurrently with CAO, because these patients with COPD are especially prone to developing exacerbations (19, 20). CB is defined by a patient’s report of a cough producing phlegm that has persisted for at least 3 months per year for a minimum of two consecutive years (21). Both persistent and newly developed CB among patients with COPD are associated with greater respiratory symptoms, worse health-related quality of life, worse lung function, and greater exacerbation frequency (22). The main reason for CB causing exacerbations in patients with CAO is believed to be the higher risk for infections when mucus is present in airways (23). Patients with frequent respiratory exacerbations are prone to a more rapid disease progression (24), and at the time of diagnosis, often the line between the two classic major COPD phenotypes—symptomatic phlegm/cough and CAO—can become blurred. Therefore, the definition of COPD based on airflow measurements has been qualified with markers of symptoms based on a standardized questionnaire, the COPD Assessment Test (25).
Unfortunately, most histopathological and genetic studies on human COPD are conducted using tissues from patients who have established COPD and in whom tissue remodeling has already occurred (26). However, we propose that each of the endotypes should be considered as a disease entity with severity grading according to symptom scores. For example, the “airway” COPD endotype may be graded as mild when airway epithelial cells are exposed to cigarette smoke, biomass smoke, or other environmental pollutants that in susceptible individuals lead to chronic airway inflammation and sustained increase in numbers of goblet cells and enlarged submucosal glands (27). These chronic changes to the airway epithelium cause unabated mucous hypersecretion, occluding the conducting airways and causing chronic cough and phlegm production. This productive cough stage, characterized by increased production of mucus, can progress to chronic phlegm production and cough when the irritation triggered by neuroendocrine epithelial cells causes the cough reflex. Over time, these stages could be accompanied by airway remodeling (i.e., thickening of the basement membrane and smooth muscle). This airway remodeling may reduce the elasticity of the airways, and increased mucus production with reduced ciliary clearance could reduce the airflow and cause the symptom of wheeze, although the airway lumen may not constrict as is standard for patients with asthma. Over time, persistent inflammation of the small airways could lead to the destruction of the alveolar walls, pathologically characterized by the emphysema. Also, whole-lung computed tomographic scans suggest that small airway disease precedes emphysema with increasing COPD severity (26, 28). The narrowing and disappearance of small conducting airways before the onset of emphysematous destruction was observed by multidetector computed tomography, suggesting that airway inflammation and destruction affect alveolar structures (26). These findings suggest that over the course of an adult life, airway inflammation may affect the underlying course of airway disease activity and lead to rapid decline in lung function. Despite these reports, the mechanisms underlying airway inflammation leading to the destruction of alveolar walls are still not understood.
Although this perspective describes the “airway” endotype and the grading by severity in more detail, we propose that in the future, the “endothelial” and “hematopoietic” endotypes should also be graded on the basis of disease severity starting at early stages to better define their phenotypes and symptoms. At the early stages of the disease, these putative endotypes may be easier to differentiate. However, as the disease progresses, other cell types and structures would be affected that make the distinction of each endotype more difficult.
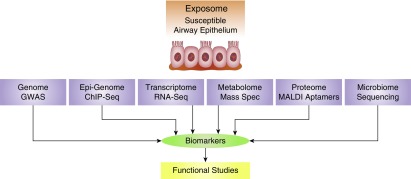
Currently, cessation of causative exposure is the main effective intervention strategy for individuals at risk for COPD in occupational, community-based, or clinical cohorts. Oxygen supplementation remains one of the few treatments that can mitigate morbidity and mortality (29). Furthermore, currently available therapies are only able to partially mitigate symptoms; some ameliorate lung function decline (30) but do little to alleviate morbidity and mortality for patients with COPD. New approaches should be devised by intervening at the stage of productive cough for the airway endotype or at the stage of albuminuria for the endothelial endotype. Also, the combination of poor lung growth and rapid decline trajectory of lung function (31) may place patients with CB at particularly high risk for developing COPD. To identify these early stages of disease in individuals with otherwise normal lung function, either improved lung imaging techniques with higher resolution or biomarkers able to predict disease with more accuracy preceding lung function decline are needed. This approach will establish the relationship between early disease and destruction of parenchymal alveoli. Samples from patients with these endotypes can be interrogated using various technologies to identify biomarkers of early disease (Figure 2). The genome can be analyzed using genome-wide association studies, the epigenome using chromosome immunoprecipitation and sequencing analyses, RNA expression using RNA-sequencing approaches, the proteome using matrix-assisted laser desorption/ionization and aptamer technology, the metabolome using mass spectrometry, and the microbiome using DNA sequencing of the 16S subunit of ribosomal RNA. In addition, it is important that functional studies be conducted to clarify how representative these biomarkers are of the endotype and to determine their relevance to disease pathogenesis, because detailed understanding of these mechanisms will provide novel targets useful to reverse disease before irreversible damage is established. For example, genome-wide association studies found significant association of SNPs within the FAM13A and EFCAB4A, CHID1 and AP2A2, RPL31P11, and ATF6 genes with CB (32). Low levels of circulating CC16 are associated with increased risk for CB (33), and a SNP in the TP53 gene modifies Pro72 to Arg by affecting expression of SPDEF (SAM pointed domain containing Ets transcription factor) and Bcl-2 and thereby mucin gene expression (14). More recently, detailed quantitation of MUC5AC and MUC5B protein in sputum predicted CB with 70–80% accuracy (34). Increased levels of endothelial cell advanced glycation end products and receptor for advanced glycation end products and albuminuria could identify patients with COPD in whom angiotensin-converting enzyme inhibitor therapy improves renal and lung function by reducing endothelial injury (15). Another study found enrichment in B cell–related genes in patients with COPD with emphysema (16).
Figure 2.
Discovery of predictive biomarkers for early-stage chronic bronchitis. aptamer = single-stranded DNA or RNA that binds protein targets with high selectivity and specificity; ChIP-Seq = chromosome immunoprecipitation followed by sequencing of antibody-associated DNA molecules; GWAS = genome-wide association studies; MALDI = matrix-assisted laser desorption/ionization; Mass Spec = mass spectromentry; RNA-Seq = sequencing of all RNA species.
Future analyses of a combination of these and other biomarkers will better identify individuals with each endotype. These early changes will need to show a strong correlation with the development of CAO, because certain patients may yet maintain their lung function owing to some of these early inflammatory changes being able to be compensated by higher peak lung function attained in adulthood. The ultimate goal of precision medicine is to provide the right treatment given at the right time and tailored to a patient’s individual needs. Early detection–based biomarkers derived from “omic” findings are needed for individual patients with late-stage COPD, who may present either with a single endotype or with a combination of endotypes. Unfortunately, although omic studies have in part identified reproducible associations, with minimal effect sizes (35, 36) the causative role of these association is far from certain. Therefore, functional studies to clarify the molecular mechanisms that lead to disease are crucially needed and should be supported to better achieve effective intervention strategies.
Acknowledgments
Acknowledgment
The authors thank Elise Calvillo for preparing the figures.
Footnotes
Supported by National Institutes of Health grants HL068111 and ES015482.
Authors Contributions: Drafting of manuscript for important intellectual content: Y.T. and H.P.; and conception: H.P., R.V.G., P.M., A.S., and Y.T.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0002PS on March 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999-2011. Chest. 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannino DM, Kiriz VA. Changing the burden of COPD mortality. Int J Chron Obstruct Pulmon Dis. 2006;1:219–233. doi: 10.2147/copd.2006.1.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer. 2001;1:82–86. doi: 10.1038/35094091. [DOI] [PubMed] [Google Scholar]
- 6.Mannino DM, Higuchi K, Yu TC, Zhou H, Li Y, Tian H, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148:138–150. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaldi PJ, Benet M, Petersen H, Rafaels N, Finigan J, Paoletti M, et al. Do COPD subtypes really exist? COPD heterogeneity and clustering in 10 independent cohorts. Thorax. 2017;72:998–1006. doi: 10.1136/thoraxjnl-2016-209846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgel PR, Paillasseur JL, Janssens W, Piquet J, Ter Riet G, Garcia-Aymerich J, et al. A simple algorithm for the identification of clinical COPD phenotypes. Eur Respir J. 2017;50:1701034. doi: 10.1183/13993003.01034-2017. [DOI] [PubMed] [Google Scholar]
- 9.Menche J, Sharma A, Cho MH, Mayer RJ, Rennard SI, Celli B, et al. A diVIsive Shuffling Approach (VIStA) for gene expression analysis to identify subtypes in chronic obstructive pulmonary disease. BMC Syst Biol. 2014;8(Suppl 2):S8. doi: 10.1186/1752-0509-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingleton J, Hardy J, Beasley R. Treatable traits of chronic airways disease. Curr Opin Pulm Med. 2018;24:24–31. doi: 10.1097/MCP.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 12.Kocks JWH, Seys SF, van Duin TS, Diamant Z, Tsiligianni IG. Assessing patient-reported outcomes in asthma and COPD patients: which can be recommended in clinical practice? Curr Opin Pulm Med. 2018;24:18–23. doi: 10.1097/MCP.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 13.Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 14.Chand HS, Montano G, Huang X, Randell SH, Mebratu Y, Petersen H, et al. A genetic variant of p53 restricts the mucous secretory phenotype by regulating SPDEF and Bcl-2 expression. Nat Commun. 2014;5:5567. doi: 10.1038/ncomms6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polverino F, Laucho-Contreras ME, Petersen H, Bijol V, Sholl LM, Choi ME, et al. A pilot study linking endothelial injury in lungs and kidneys in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1464–1476. doi: 10.1164/rccm.201609-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faner R, Cruz T, Casserras T, López-Giraldo A, Noell G, Coca I, et al. Network analysis of lung transcriptomics reveals a distinct b-cell signature in emphysema. Am J Respir Crit Care Med. 2016;193:1242–1253. doi: 10.1164/rccm.201507-1311OC. [DOI] [PubMed] [Google Scholar]
- 17.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 18.Güder G, Brenner S, Angermann CE, Ertl G, Held M, Sachs AP, et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir Res. 2012;13:13. doi: 10.1186/1465-9921-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, et al. Initiatives Bronchopneumopathie Chronique Obstructive (BPCO) Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 20.de Oca MM, Halbert RJ, Lopez MV, Perez-Padilla R, Tálamo C, Moreno D, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40:28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 21.NHLBI, NIH. Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, global strategy for the diagnosis, management and prevention of chronic obstructive lung disease: NHLBI/WHO workshop report. NIH Publication 2701. Bethesda, MD: NIH. 2001 [Google Scholar]
- 22.Kim V, Zhao H, Boriek AM, Anzueto A, Soler X, Bhatt SP, et al. COPDGene Investigators. Persistent and newly developed chronic bronchitis are associated with worse outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:1016–1025. doi: 10.1513/AnnalsATS.201512-800OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha JA. Mechanisms of chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2015;12(Suppl 2):S157–S159. doi: 10.1513/AnnalsATS.201507-427AW. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 26.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim V, Oros M, Durra H, Kelsen S, Aksoy M, Cornwell WD, et al. Chronic bronchitis and current smoking are associated with more goblet cells in moderate to severe COPD and smokers without airflow obstruction. PLoS One. 2015;10:e0116108. doi: 10.1371/journal.pone.0116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestbo J TORCH Study Group. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J. 2004;24:206–210. doi: 10.1183/09031936.04.00120603. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 31.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Cho MH, Hersh CP, McDonald ML, Crapo JD, Bakke PS, et al. COPDGene and ECLIPSE Investigators. Genetic susceptibility for chronic bronchitis in chronic obstructive pulmonary disease. Respir Res. 2014;15:113. doi: 10.1186/s12931-014-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen H, Leng S, Belinsky SA, Miller BE, Tal-Singer R, Owen CA, et al. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur Respir J. 2015;46:1501–1503. doi: 10.1183/13993003.00682-2015. [DOI] [PubMed] [Google Scholar]
- 34.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berndt SI, Skibola CF, Joseph V, Camp NJ, Nieters A, Wang Z, et al. Genome-wide association study identifies multiple risk loci for chronic lymphocytic leukemia. Nat Genet. 2013;45:868–876. doi: 10.1038/ng.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, et al. ECLIPSE, ICGN, and COPDGene Investigators. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]