Abstract
Bronchial epithelial cells (BECs) from healthy children inhibit human lung fibroblast (HLF) expression of collagen and fibroblast-to-myofibroblast transition (FMT), whereas asthmatic BECs do so less effectively, suggesting that diminished epithelial-derived regulatory factors contribute to airway remodeling. Preliminary data demonstrated that secretion of the activin A inhibitor follistatin-like 3 (FSTL3) by healthy BECs was greater than that by asthmatic BECs. We sought to determine the relative secretion of FSTL3 and activin A by asthmatic and healthy BECs, and whether FSTL3 inhibits FMT. To quantify the abundance of the total proteome FSTL3 and activin A in supernatants of differentiated BEC cultures from healthy children and children with asthma, we performed mass spectrometry and ELISA. HLFs were cocultured with primary BECs and then HLF expression of collagen I and α-smooth muscle actin (α-SMA) was quantified by qPCR, and FMT was quantified by flow cytometry. Loss-of-function studies were conducted using lentivirus-delivered shRNA. Using mass spectrometry and ELISA results from larger cohorts, we found that FSTL3 concentrations were greater in media conditioned by healthy BECs compared with asthmatic BECs (4,012 vs. 2,553 pg/ml; P = 0.002), and in media conditioned by asthmatic BECs from children with normal lung function relative to those with airflow obstruction (FEV1/FVC ratio < 0.8; n = 9; 3,026 vs. 1,922 pg/ml; P = 0.04). shRNA depletion of FSTL3 in BECs (n = 8) increased HLF collagen I expression by 92% (P = 0.001) and α-SMA expression by 88% (P = 0.02), and increased FMT by flow cytometry in cocultured HLFs, whereas shRNA depletion of activin A (n = 6) resulted in decreased α-SMA (22%; P = 0.01) expression and decreased FMT. Together, these results indicate that deficient FSTL3 expression by asthmatic BECs impairs epithelial regulation of HLFs and FMT.
Keywords: activin A, airway remodeling, asthma, epithelial cells, FSTL3
Clinical Relevance
Using a human airway epithelial cell/stromal cell ex vivo model system including primary bronchial epithelial cells from carefully characterized children with and without asthma, this study demonstrates a deficiency in follistatin-like 3 (FSTL3) production by asthmatic as compared with healthy bronchial epithelial cells, especially by epithelial cells from subjects with asthma and airway obstruction. When cocultured with epithelial cells wherein FSTL3 is knocked down, fibroblasts increase expression of collagen I and α-smooth muscle actin, suggesting deficient FSTL3 expression by asthmatic epithelium as a mechanism that may impair epithelial regulation of fibroblasts and fibroblast-to-myofibroblast transition, leading to subepithelial fibrosis.
Epidemiologic studies have revealed that compared with children without asthma, children with asthma have lower lung function during childhood that persists into adulthood (1–3). Bronchial biopsies from children with asthma demonstrate that features of airway remodeling, including excessive subepithelial matrix protein/proteoglycan deposition, are already present in early childhood (4–7). Together, these data suggest that early structural changes in the asthmatic airway contribute to lung function declines that manifest early in the natural history of asthma. Although inhaled corticosteroids reduce morbidity, they do not alter the natural history of asthma or prevent declines in lung function (8–10). An improved understanding of the mechanisms that drive airway remodeling is fundamental to the development of future clinical interventions to alter the natural course of asthma and prevent lung function decline.
Airway epithelial cells are the first point of contact between the environment and the lung. Evidence from in vivo animal models (11–14), human bronchial biopsies (14–18), and our previous studies of differentiated bronchial epithelial cells (BECs) from children with asthma (19) demonstrate that the bronchial epithelium secretes various factors, including transforming growth factor β (TGF-β) and activin A, that may regulate airway inflammation and stromal cells, including fibroblasts. In vivo and in vitro data suggest that activin-A, a TGF-β superfamily member, may play an important role in the regulation of airway inflammation in asthma (18, 20–22). However, there are conflicting published data regarding whether this cytokine has predominantly pro- or antiinflammatory effects in asthma, or whether its actions have different directionality among different cell and tissue types or milieus (23, 24). In animal models of airway remodeling and pulmonary fibrosis, TGF-β and activin A have been shown to promote fibroblast proliferation (25) and fibrosis (26–28), and drive fibroblast-to-myofibroblast transition (FMT) (29), thereby enhancing airway extracellular matrix (ECM) deposition (20, 30–33). Our group has observed that in coculture, BECs from healthy children markedly downregulate lung fibroblast expression of ECM components, including type I collagen, as well as α-smooth muscle actin (α-SMA) indicative of a myofibroblast phenotype (34), suggesting that epithelial cells directly inhibit fibroblasts, FMT, and ECM production. In contrast, asthmatic BECs are significantly less able to downregulate myofibroblasts (34, 35). A potential mechanism that might explain differential regulation of fibroblasts by asthmatic BECs is a deficiency in secreted proteins that inhibit ECM production by fibroblasts and FMT. However, to date, little is known about the potential negative inhibition of lung fibroblasts by airway epithelial cells. To investigate this mechanism, we compared proteins secreted by asthmatic BECs and healthy BECs (36).
In the present study, we expand upon observations from preliminary mass spectrometry–based experiments to characterize defects that we observed in the secretion of an activin A inhibitor, follistatin-like 3 (FSTL3), by asthmatic BECs relative to healthy BECs. Subsequent to that work, we hypothesized that BECs from children with asthma secrete less FSTL3 and/or more activin A than BECs from healthy children. Furthermore, we hypothesized that a deficiency of FSTL3 and/or increase in activin A secretion in asthmatic BECs might explain our previous observation that in coculture, asthmatic BECs are less able to downregulate human lung fibroblasts (HLFs) and FMT than healthy BECs.
Methods
Subjects
We recruited and enrolled children with atopic asthma and healthy, nonatopic children between 6 and 18 years of age who were scheduled for an elective surgery that required endotracheal intubation. A complete medical history was reviewed to ensure that each subject met the study inclusion and exclusion criteria. Children with asthma had ≥ 1 year history of physician-diagnosed asthma, physician-documented asthma symptoms in the year before study enrollment, short-acting β-agonist use ≥ twice monthly or daily use of an inhaled corticosteroid or leukotriene receptor antagonist, and birth at ≥ 36 weeks gestation. Healthy children were born at full term; lacked a history of reactive airway disease, asthma, chronic daily cough, or physician-diagnosed chronic lung disease; birth at ≥ 36 weeks gestation; and had no history of treatment with oxygen, bronchodilators, or systemic or inhaled corticosteroids. Subjects with asthma had a history of at least one of the following features of atopy: clinician-diagnosed eczema, clinician-diagnosed allergic rhinitis, history of confirmed aeroallergen sensitivity by either skin-prick test or radioallergosorbent testing (RAST), or elevated serum IgE (>100 IU/ml). Healthy children did not have a history of any of the atopic features described above.
Blood samples were drawn and RAST allergen-specific IgE levels in response to cat epithelium, timothy grass, Alternaria tenuis, Aspergillus fumigatus, Dermatophagoides farinae, and D. pteronyssinus, as well as total serum IgE, were measured. Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and forced expiratory flow, midexpiratory phase (FEF25–75%) were measured with the VMAX series 2130 spirometer (VIASYS Healthcare, Hong Kong) and the fraction of exhaled nitric oxide (FENO) was measured with a NIOX MINO nitric oxide analyzer (Aerocrine, Sweden) in accordance with American Thoracic Society guidelines (37). Spirometric measurements were repeated in subjects with asthma 15 minutes after administration of albuterol (two puffs).
The parents of the subjects provided written consent and children over 7 years of age provided assent. The study was approved by the Seattle Children’s Hospital Institutional Review Board.
Establishment of BEC Cultures and BEC-Fibroblast Cocultures
The methods used for BEC/HLF cocultures have been described previously (see data supplement) (34, 35). Briefly, BECs were obtained from donors after intubation for elective surgery. Cells were expanded in submerged culture and then passaged into Transwells and differentiated at the air–liquid interface (ALI) for 3 weeks. Cocultures were established using commercially available pediatric HLFs (Lonza) and were maintained for 96 hours, at which point samples were isolated as described.
Mass Spectrometry Analysis of the BEC Secretome
Differentiated BEC cultures were switched to PneumaCult ALI maintenance media lacking growth factor supplementation. After 72 hours of culture, the supernatants were removed and denatured via the addition of 2.5 M urea, and a mass spectrometry approach was used to compare the secretomes of asthmatic and healthy BEC cultures (see data supplement for detailed methods). To verify a subset of these results, we used label-free mass spectrometry to quantify FSTL3 in BEC media (see data supplement).
RNA Extraction and Real-Time PCR
Total RNA was isolated from HLF cells cocultured with BECs grown at the ALI as previously described (34). RT-PCR was performed using validated TaqMan probes (Life Technologies) for collagen I (COL1A1), α-smooth muscle actin (α-SMA), and GAPDH using standard methodologies (see data supplement).
Flow Cytometry
After coculture experiments, HLFs from experimental groups were mechanically detached from the culture wells and suspended in PBS. Samples were snap-frozen in liquid nitrogen so that samples from each group were run in parallel. Flow cytometry was used to quantify the percentage of myofibroblasts among HLFs as determined by cells staining positive for cytoskeletal α-SMA, as we have previously described (see data supplement) (35).
shRNA Knockdown of FSTL3 and Activin A
To block BEC production of FSTL3 and activin A, we selectively knocked down activin A and FSTL3 in differentiated BECs using shRNA with a doxycycline (Dox)-inducible lentiviral shRNA expression system (Tet-On 3G Inducible System, Thermo Scientific) that also expresses a red fluorescent protein reporter and the puromycin resistance gene. BECs in submerged culture conditions were transduced with the lentivirus vector at a multiplicity of infection of 1.0 when cells were 40% confluent. Forty-eight hours after transduction, puromycin was added to the cultures (0.1 μg/ml) to kill nontransduced cells. To initiate knockdown, we exposed BEC cultures to Dox (0.5 μg/ml). In preliminary experiments (data not shown), three independent shRNAs were tested for both FSTL3 and activin A knockdown in BECs, and the shRNA with highest efficiency in knocking down each gene was used in BEC-HLF coculture experiments. For BEC-HLF coculture experiments, 72 hours before initiation of cocultures, the BEC cultures were exposed to Dox (0.5 μg/ml). Dox exposure was maintained throughout the coculture experiments.
ELISA Analyses
Basolateral medium from each condition was sampled from triplicate Transwells and pooled. FSTL3 and activin A protein levels in basolateral conditioned media were measured using Duoset ELISA kits (R&D Systems) according to the manufacturer’s recommendations. Measurements were completed in duplicate. When sample concentrations were below the detection level of the assay, they were assigned a value of one-half the lower limit of detection for analysis.
Statistical Analysis
Protein levels are presented as means ± standard deviation (SD) when all groups (e.g., healthy, asthmatic, and HLF alone) were normally distributed, and as medians with the interquartile range if one or more groups were not normally distributed. To determine whether the data were normally distributed, the Kolmogorov-Smirnov test was used. To compare the distributions of protein levels in coculture media across the three subject groups, one-way ANOVA or the Kruskal-Wallis test was used if the data were nonnormally distributed in one or more groups. Post hoc comparisons between pairs of subject groups (e.g., between asthmatic and healthy cocultures) were made using Dunn’s multiple-comparisons test (significance level set at P < 0.05). For age, sex, lung function parameters, FENO, and IgE levels, a paired t test or the Wilcoxon signed-rank test for nonnormally distributed data was used for comparisons between subjects with asthma and healthy subjects. Prism 6.0 software (GraphPad Software Inc.) was used to analyze clinical data and protein levels in ALI cultures. COL1A1 and α-SMA relative expression was standardized using GAPDH as a nonregulated housekeeping gene. GenEx version 5.0.1 was used to analyze real-time qPCR results (MultiD Analyses AB) based on methods described by Pfaffl (38). Statistical significance was set at P < 0.05.
Results
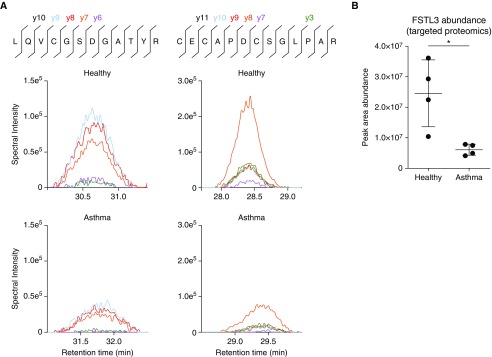
In a preliminary analysis of the secretomes from asthmatic and healthy epithelium by mass spectrometry, we observed that the relative abundance of the activin A inhibitor FSTL3 was smaller in media conditioned by asthmatic as compared with healthy BECs (data not shown). To verify these results, we used a label-free targeted proteomics method to quantify FSTL3. Using this method, we found that FSTL3 peptides were more abundant (Figures 1A and 1B) in healthy (n = 4) versus asthmatic (n = 4) BECs.
Figure 1.
Quantification of follistatin-like 3 (FSTL3) via targeted proteomics in primary bronchial epithelial cells (BECs) derived from healthy donors and donors with asthma. (A) The peptide primary sequence, locations and numbers of cleavage ions, and representative images showing histograms of the indicated product ions quantified from two independent FSTL3 peptides in isolates from media samples obtained from healthy BECs compared with asthmatic BECs. (B) FSTL3 abundance was assessed by summing the peak area quantification of the product ions from two technical replicates from two independent experiments using healthy donors (n = 4) and donors with asthma (n = 4). FSTL3 abundance was decreased in media samples obtained from asthmatic BECs compared with healthy BECs. Significance was assessed using an unpaired Student’s t test (*P < 0.05).
Next, we conducted confirmatory experiments in a larger sample of differentiated BEC lines obtained from 21 healthy subjects and 21 subjects with asthma. The clinical characteristics of the subjects are summarized in Table 1. Briefly, the healthy subjects and subjects with asthma were of similar age (healthy: 11.99 yr; asthmatic: 12.03 yr) and sex (healthy: 48% female; asthmatic: 55% female; P = 0.8). The majority of subjects with asthma were using daily inhaled corticosteroids (60%) at the time of enrollment. Eighty-five percent of the subjects with asthma had positive RAST responses to a specific aeroallergen, and subjects with asthma had significantly greater serum IgE levels than healthy subjects (median 138.5 IU/ml in subjects with asthma vs. 22 IU/ml in healthy subjects; P = 0.003). Finally, the FEV1/FVC ratio and FEF25–75% were significantly lower in subjects with asthma than in healthy subjects (FEV1/FVC 0.82 vs. 0.87, P = 0.02; FEF25–75% 77.3% vs. 98.7%, P = 0.01), and FENO levels were significantly higher in subjects with asthma (24.7 ppb ± 5.3 vs. 10.7 ppb ± 0.9; P = 0.008).
Table 1.
Subject Characteristics
| Healthy Control Subjects N = 21 | Subjects with Asthma N = 21 | P value | |
|---|---|---|---|
| Age, yr (mean ± SD) | 11.99 (0.7) | 12.03 (0.8) | 1 |
| Female sex (%) | 10 (48%) | 11 (55%) | 0.8 |
| Current use of inhaled steroids (yes; %) | 12 (60%) | ||
| History of eczema (yes; %) | 6 (30%) | ||
| History of allergic rhinitis (yes; %) | 15 (75%) | ||
| Positive RAST (yes; %) | 17 (85%) | ||
| IgE IU/ml (median ± IQR) | 22 (10 – 35) | 138.5 (3–528) | 0.003 |
| FVC% predicted (mean ± SD) | 111.8 (15.2) | 103.5 (17.7) | 0.2 |
| FEV1/FVC ratio (mean ± SD) | 0.87 (0.04) | 0.82 (0.05) | 0.02 |
| FEV1% predicted (mean ± SD) | 104.7 (12.2) | 100.4 (21.4) | 0.5 |
| FEF25–75% predicted (mean ± SD) | 98.7 (21.7) | 77.3 (33.1) | 0.01 |
| FENO ppb (mean ± SD) | 10.7 (0.9) | 24.7 (5.3) | 0.008 |
Definition of abbreviations: FEF25–75% = forced expiratory flow, midexpiratory phase; FENO = fraction of exhaled nitric oxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; ppb = parts per billion; RAST = radioallergosorbent testing.
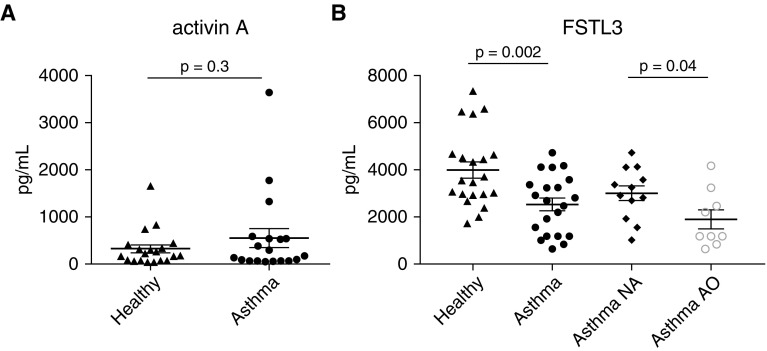
We found that secreted FSTL3 protein levels quantified by ELISA in BEC ALI culture media were significantly greater in BEC cultures from healthy subjects than in those from subjects with asthma (4,012 vs. 2,553 pg/ml, P = 0.002; Figure 2B). When the group with asthma was dichotomized by the presence or absence of airway obstruction, FSTL3 levels were higher in BEC media from children with asthma and normal lung function (n = 12) compared with subjects with asthma and airflow obstruction (FEV1/FVC ratio <0.8; n = 9; 3,026 vs. 1922 pg/ml, P = 0.04; Figure 2B). We observed no significant difference in activin A concentration in BEC media from healthy cell lines compared with asthmatic cell lines (323 vs. 549 pg/ml, P = 0.3; Figure 2A). Together, these data suggest that secretion of FSTL3, but not activin A, is diminished in asthmatic BECs, and that this effect is more pronounced in BECs isolated from patients exhibiting evidence of airway obstruction.
Figure 2.
Secreted levels of activin A and FSTL3 in media obtained from primary BECs derived from healthy donors and donors with asthma. (A) No significant difference was observed in activin A secretion between the healthy (n = 21) and asthmatic (n = 21) BECs (323 vs. 549 pg/ml; P = 0.3). (B) FSTL3 secretion from asthmatic BECs was significantly lower than that from healthy BECs (4,012 vs. 2,553 pg/ml; P = 0.002). When the asthmatic BECs were dichotomized into BECs from subjects with normal airflow (NA, n = 12) and BECs from subjects with airflow obstruction (AO, n = 9), asthmatic BECs from donors with AO (FEV1/FVC < 0.8) demonstrated significantly less secretion of FSTL3 compared with asthmatic BECs from donors with normal airflow (3,026 vs. 1,922 pg/ml; P = 0.04).
To determine whether there was a relationship between BEC production of FSTL3 or activin A and airway eosinophilic inflammation among BEC donors, we assessed associations between the concentrations of FSTL3 and activin A in supernatant from differentiated BECs from both donors with asthma and healthy donors and donor FENO levels. We did not observe a correlation between BEC-secreted FSTL3 concentrations and donor FENO levels among all subjects (r = −0.2, P = 0.2) or among subjects with asthma (r = −0.06, P = 0.8). Similarly, there was no correlation between BEC activin A concentrations and donor FENO levels among all subjects (r = −0.1, P = 0.6) or among subjects with asthma (r = 0.07, P = 0.8).
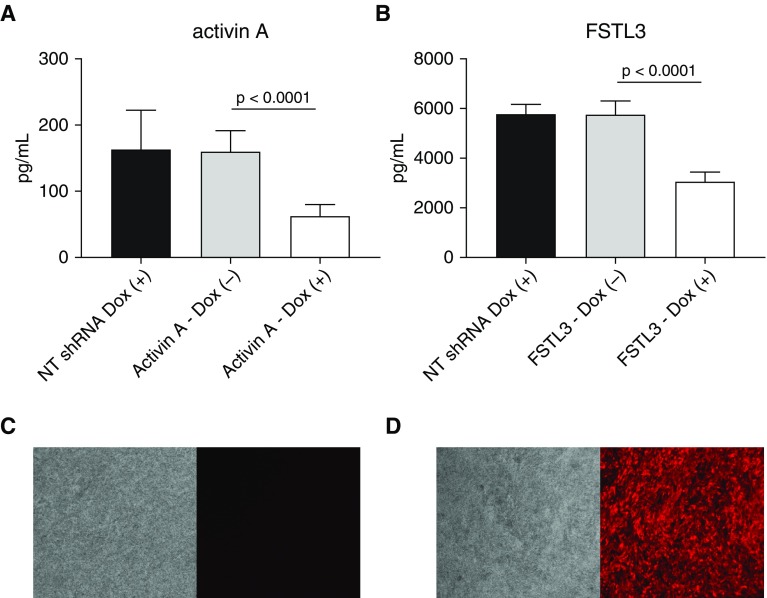
To artificially manipulate FSTL3 and activin A secretion by BECs, we next determined the knockdown efficiency using shRNAs. We observed that Dox-induced shRNA knockdown of activin A in BECs (n = 11 asthmatic BEC lines, n = 5 healthy BEC lines) reduced secreted activin A protein concentrations in BEC culture media by 61%, from a mean of 159.8 pg/ml to 62.6 pg/ml (P < 0.0001; Figure 3A) Similarly, we evaluated the shRNA knockdown efficiency of FSTL3 in BECs (n = 6 asthmatic BEC lines, n = 11 healthy BEC lines) and found that secreted FSTL3 protein concentrations in BEC culture media were reduced by 47%, from a mean of 5,750 pg/ml to 3,048 pg/ml (P < 0.0001; Figure 3B).
Figure 3.
Knockdown studies using a red fluorescent protein (RFP)-tagged, doxycycline (Dox)-inducible lentiviral shRNA targeted against activin A and FSTL3 in primary BECs. (A) Secretion of activin A by primary BECs transduced with Dox-inducible shRNA targeting activin A and nontargeting (NT) control shRNA (n = 10). Addition of Dox to the media led to a 68% reduction of activin A secretion (n = 11 asthmatic BEC lines, n = 5 healthy BEC lines; 62.6 pg/ml vs. 159.8 pg/ml; P < 0.0001). (B) Secretion of FSTL3 by primary BECs transduced with Dox-inducible shRNA targeting FSTL3 and NT control shRNA. Addition of Dox to the media led to a 46% reduction of FSTL3 secretion (n = 6 asthmatic BEC lines, n = 11 healthy BEC lines; 3,048 pg/ml vs. 5,750 pg/ml; P < 0.0001). (C and D) RFP expression in FSTL3 shRNA lentiviral-transfected BECs without and with the addition of Dox, respectively.
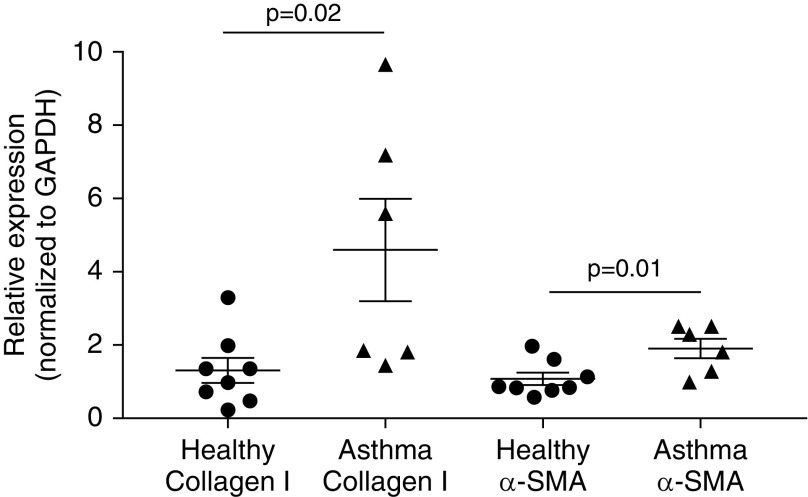
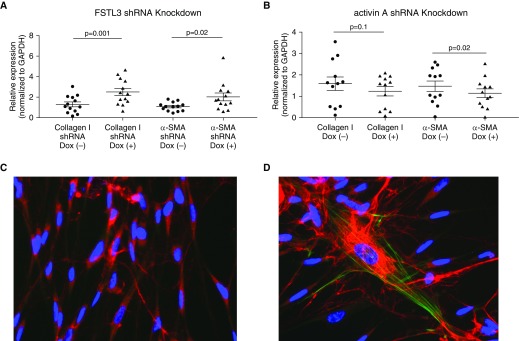
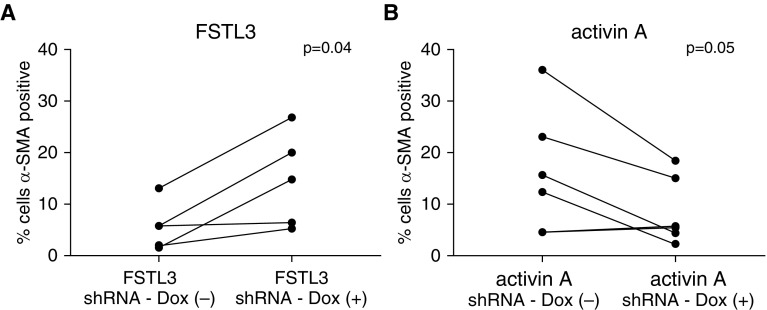
Next, we conducted experiments to assess the effect of shRNA knockdown of FSTL3 or activin A in differentiated BECs on FMT and the expression of collagen I and α-SMA by HLFs that were cocultured with BECs. In baseline BEC-HLF cocultures that were not transduced with lentivirus carrying shRNA, collagen I expression was fourfold greater by HLFs cocultured with asthmatic as compared with healthy BECs (P = 0.02; Figure 4), and α-SMA expression was twofold greater by HLFs cocultured with asthmatic as compared with healthy BECs (P = 0.01). After knockdown of FSTL3 in healthy BECs (n = 13), we found that cocultured HLFs exhibited a 92% increase in expression of collagen I (P = 0.001; Figure 5A) and an 88% increase in expression of α-SMA (P = 0.02) compared with HLFs cocultured with BECs transduced with shRNA against FSTL3 that that were not activated with Dox. Conversely, after knockdown of activin A in asthmatic BECs (n = 12), we found that cocultured HLFs exhibited a nonsignificant 23% decrease in expression of collagen I (P = 0.1; Figure 5B) and a 22% decrease in expression of α-SMA (P = 0.02) compared with HLFs cocultured with shRNA-transduced BECs that were not activated with Dox. To further assess the effect of shRNA knockdown of FSTL3 or activin A in differentiated BECs on FMT in HLFs cocultured with BECs, we used flow cytometry to quantify the percentage of myofibroblasts among HLFs as determined by cells staining positive for cytoskeletal α-SMA. In healthy BEC-HLF cocultures (n = 5), shRNA knockdown of FSTL3 in BECs increased the percentage of HLFs staining for cytoskeletal α-SMA by flow cytometry as a myofibroblast marker from 5.7% (± SD 4.6) to 14.7% (± SD 9.1) (P = 0.04; Figure 6A). In asthmatic BEC-HLF cocultures (n = 6), shRNA knockdown of activin A in BECs decreased the percentage of HLFs that stained for cytoskeletal α-SMA by flow cytometry from 15.9% (± SD 12) to 8.5% (± SD 6.5) (P = 0.05). Based on these combined results, we conclude that alterations in the ratio of secreted FSTL3 to secreted activin A can enhance or inhibit HLF production of ECM components and FMT.
Figure 4.
Relative expression of collagen I and α-smooth muscle actin (α-SMA) by human lung fibroblasts (HLFs) conditioned with primary BECs derived from healthy donors and donors with asthma. Collagen I expression was fourfold greater in HLFs cocultured with asthmatic BECs (n = 6) compared with HLFs cocultured with healthy BECs (n = 8; P = 0.02), and α-SMA expression was twofold greater in HLFs cocultured with asthmatic BECs compared with HLFs cocultured with healthy BECs (P = 0.01).
Figure 5.
(A) Relative expression of collagen I and α-SMA by HLFs conditioned with primary BECs derived from healthy donors (n = 13) after inhibition of BEC secretion of FSTL3 via Dox-inducible lentiviral shRNA knockdown. After knockdown of FSTL3 production by healthy BECs, collagen I expression was 92% greater in HLFs cocultured with BECs exposed to Dox compared with BECs not exposed to Dox (P = 0.001). α-SMA expression was 88% greater in HLFs cocultured with BECs exposed to Dox compared with BECs not exposed to Dox (P = 0.02). (B) Expression of collagen I and α-SMA by HLFs conditioned with primary BECs derived from donors with asthma (n = 12) after inhibition of BEC secretion of activin A via Dox-inducible lentiviral shRNA knockdown. After knockdown of activin A secretion by asthmatic BECs, collagen I expression was not significantly lower in HLFs cocultured with BECs exposed to Dox compared with BECs not exposed to Dox (P = 0.1). α-SMA expression was 22% lower in HLFs cocultured with BECs exposed to Dox compared with BECs not exposed to Dox (P = 0.02). (C and D) Representative immunostaining for collagen I (red), α-SMA (green), and DAPI (blue) in a representative healthy BEC-HLF coculture FSTL3 shRNA knockdown experiment (C, Dox-negative; D, Dox-positive).
Figure 6.
(A) In healthy BEC-HLF cocultures (n = 5), shRNA knockdown of FSTL3 in BECs increased the percentage of HLFs that stained for cytoskeletal α-SMA by flow cytometry as a myofibroblast marker from 5.7% (± SD 4.6) to 14.7% (± SD 9.1) (P = 0.04). (B) In asthmatic BEC-HLF cocultures (n = 6), shRNA knockdown of activin A in BECs decreased the percentage of HLFs staining for cytoskeletal α-SMA by flow cytometry from 15.9% (± SD 12) to 8.5% (± SD 6.5) (P = 0.05).
Discussion
In this study, we first conducted an exploratory secretome analysis using mass spectrometry of proteins secreted by differentiated BECs that were obtained from carefully phenotyped healthy children and children with asthma, and observed a high abundance of FSTL3 with lower concentrations in supernatant from subjects with asthma as compared with healthy subjects. We then confirmed this novel observation using ELISA of supernatants from BECs obtained from a larger cohort of children with asthma and healthy children. Furthermore, we observed lower levels of FSTL3 secreted by BECs from subjects with asthma and airway obstruction as compared with BECs from subjects with asthma and normal lung function. Previously, we found that that in coculture, BECs from healthy children downregulated HLF expression of type I collagen, as well as α-SMA indicative of a myofibroblast phenotype (34). In contrast, we observed significantly less downregulation when HLFs were cocultured with asthmatic BECs (34, 35). To determine whether a deficiency in asthmatic BEC production of FSTL3 might partially explain this observation of aberrant regulation of HLFs by asthmatic BECs, we conducted a series of experiments wherein we used shRNA to knock down expression of FSTL3 or activin A in differentiated BECs that were then cocultured with normal HLFs. We were able to significantly reduce the concentrations of secreted FSTL3 and activin A in primary BECs from children that were differentiated at an ALI using a shRNA knockdown approach. Finally, when BECs were cocultured with HLFs, knockdown of FSTL3 in BECs from healthy children led to a significant increase in HLF expression of both collagen I and α-SMA, as well as increased FMT, as quantified by flow cytometry. Knockdown of activin A in asthmatic BECs led to a modest but significant decrease in expression of α-SMA by cocultured HLFs, as well as a decrease in FMT as quantified by flow cytometry. Together, these data suggest deficient FSTL3 expression by asthmatic BECs as a mechanism that may contribute to impaired epithelial regulation of HLF ECM production and FMT leading to subepithelial fibrosis and ECM deposition. Reduced production of FSTL3 removes an inherent regulatory inhibition of activin A activity, thereby allowing activin A profibrotic effects to occur with less opposition. Together, these data suggest that in the healthy state, FSTL3 serves a protective role and prevents airway remodeling, whereas in some patients with asthma, deficient epithelial FSTL3 production results in increased activin A activity and therefore increased airway fibroblast activation and FMT that may contribute to airway remodeling.
The bioactivity of activin A is complex, and conflicting regulatory effects on inflammation have been observed depending on the anatomic site studied (26, 39). Activin A has been proposed to be a regulator of inflammation in multiple cell types and tissues, and diseases as varied as skin and brain injuries, inflammatory arthropathies, arteriosclerosis, and inflammatory bowel disease (40, 41). In murine in vivo asthma models, there is conflicting evidence as to whether activin A acts in a proinflammatory or antiinflammatory fashion (23). In a house dust mite (HDM) asthma model, inhibition of TGF-β and activin A via antibody blockade reduced activin A–dependent T-helper cell type 9 (Th9) cell differentiation, eosinophil and mast cell recruitment to the lung, and IL-13 secretion by pulmonary lymph node cells (21). Furthermore, in a mouse model of allergic asthma exacerbation, inhibition of activin A signaling by administration of intranasal follistatin (FST) before acute allergen exposure reduced pulmonary mucus secretion in the lung and production of Th2 cytokines (20). Finally, clinical studies have demonstrated higher serum levels of activin A in subjects with severe asthma as compared with healthy subjects or subjects with mild asthma (18), as well as greater expression by subjects with allergic asthma of activin A, the activin receptor, and CD4+ T cells (22). In contrast, other groups have reported experiments wherein activin A blockade increased Th2 cytokine secretion by pulmonary lymph nodes and led to increased concentrations of airway eosinophils. Taken together, the data in the literature seem to support both proinflammatory and immunosuppressive roles for activin A in allergic asthma (24).
Evidence obtained in humans consistently shows that activin A is profibrotic across various tissues and diseases, including in wound repair (23), interstitial pulmonary fibrosis (42), and kidney disease and liver cirrhosis (40). Independently of TGF-β, activin A induces lung fibroblast proliferation (18) and drives the differentiation of pulmonary fibroblasts into myofibroblasts (29), potent producers of collagen I and III in fibrosis (43–45). Consistent with these findings, murine modeling also supports a proremodeling role for activin A in asthma. For example, enhanced activin A expression and airway remodeling was observed after intranasal HDM exposure in mice overexpressing Smad2 in the airways, and this effect was inhibited by prior injection of an activin A–neutralizing antibody (33). In an HDM murine asthma model, monoclonal antibody blockade of activin A led to decreased mucus secretion and subepithelial collagen deposition (21). Furthermore, in a murine ovalbumin asthma model, treatment with FST (46) inhibited activin A bioactivity and reduced subepithelial collagen deposition (20). Our observations in a human airway epithelial cell/stromal cell ex vivo model system are consistent with evidence from murine models and suggest that the TGF-β superfamily member activin A promotes lung fibrosis (26–28).
FST is a protein whose primary recognized function is the neutralization of TGF-β superfamily members, including activins (47). FST is a 31–39 kD single-chain protein. FSTL3 is a more recently identified distinct glycoprotein that has homology with FST and also binds and inhibits TGF-β ligands, including activin A (46, 48). Activin A can stimulate expression of FSTL3, resulting in an inhibitory feedback loop to ameliorate activin activity (46, 48). FSTL3 is similar to FST in both structure and function; however, unlike FST, which is expressed at the cell surface and secreted, FSTL3 is both actively secreted and nuclear (49). Furthermore, expression of FSTL3 is more tissue specific than that of FST (50). Although FSTL3 immunoreactivity was first noted to be strong in lung epithelial cells more than a decade ago (51), data regarding the role of FSTL3 in the lung are limited. Here, we show that FSTL3 is strongly expressed by human airway epithelial cells at both RNA and protein levels. Further, our observations suggest that an imbalance of activin A–FSTL3 signaling by asthmatic epithelial cells is likely to contribute to subepithelial fibrosis and ECM deposition.
In apparent contrast to our findings, Samitas and colleagues found that compared with healthy patients, patients with mild-to-moderate and severe asthma had elevated activin-A levels in BAL and serum, and increased activin-A immunostaining in airway epithelial cells from biopsies, whereas bronchial mucosal immunostaining for activin-A receptor proteins was reduced in asthma (52). Samitas and colleagues also observed a strong correlation between angiogenesis in biopsies and activin-A expression in epithelial and subepithelial cells. However, using in vitro experiments with human umbilical vein and pulmonary microvascular endothelial cell lines, they found that exogenous activin-A reduced spontaneous as well as vascular endothelial growth factor–enhanced endothelial proliferation. There are several potential explanations for these seemingly discordant findings from human in vivo samples and mechanistic in vitro experiments. First, it is important to recognize the limitations of using cell lines as opposed to primary human cells when conducting mechanistic experiments. Second, a cytokine such as activin A may have different effects in different cell types and tissues. Third, activin A may have varying regulatory and counterregulatory effects in vivo and in vitro depending on whether studies are conducted in a “baseline” state or in a setting of allergen exposure or viral infection. In contrast to the study by Sumitas and colleagues, in our study we included only BECs from pediatric subjects with mild-to-moderate asthma, we assessed the production of activin A and FSTL3 by primary airway epithelial cells two passages removed from donors and therefore isolated from the influence of immune cell influences, and we studied the effects of activin A and FSTL3 signaling on fibroblasts as opposed to endothelial cells. Finally, in our model system, we studied the distinct activin A antagonist FSTL3, whereas Sumitas and colleagues did not investigate the role of FSTL3 or FST. A limitation of our study is that we did not study the effects of viral infection or allergen exposure on airway epithelial production of activin A or FSTL3, and epithelial regulation of fibroblasts—areas of future investigation by our group and others.
Papaporfyriou and colleagues found that activin A levels were higher in sputum from subjects with asthma as compared with healthy subjects, and that activin A levels were associated with reticular basement membrane thickness, suggestive of an association with airway remodeling (53). They also found no difference in FST levels between sputum from patients with asthma and healthy subjects. It is difficult to compare the results from Papaporfyriou and colleagues with our observations, for several reasons. First, the sputum and BAL activin A and FST levels measured in their study reflect a different anatomic compartment of the airways (apical/luminal as opposed to basally secreted in our ex vivo coculture model). Second, although they are related, FST and FSTL3 are not the same protein, and FSTL3 was not measured by Papaporfyriou and colleagues. Of note, although we observed significantly lower secretion of FSTL3 by asthmatic BECs, we did not observe differences in FST in our secretome comparison of supernatant between asthmatic and healthy BECs (data not shown). Finally, Papaporfyriou and colleagues studied adults, whereas we conducted our study using primary cells from children with and without asthma.
There are several limitations inherent to our research design. The children with asthma in our study generally had only mild obstruction of airflow by pulmonary function testing, consistent with milder asthma phenotypes independent of treatment. An alternative explanation for their mild airway obstruction may be that a relatively high proportion of our subjects with asthma reported use of inhaled corticosteroids on a daily basis. However, the children with asthma in our study did in fact have lower lung function than healthy children, as demonstrated by lower FEV1/FVC ratios and FEF25–75% values. Given that the primary BECs used in our BEC-HLF model system were multiple passages beyond the initial airway brushings, it is unlikely that asthma controller medications used by the subjects at study enrollment would have affected our results; however, we cannot completely exclude this possibility. Another limitation of this study is that the efficiency of shRNA knockdown of FSTL3 in BECs, as assessed by the effect on levels of secreted FSTL3 in supernatant, was only ∼50%. However, this would bias toward the null hypothesis of finding no effect of BEC FSTL3 knockdown on FMT by HLFs or expression of collagen by HLFs cocultured with BECs. Yet, despite the relatively modest knockdown of FSTL3 in BECs from healthy children, we observed a significant increase in the expression of collagen I and α-SMA by HLFs cocultured with healthy BECs wherein FSTL3 was knocked down.
In summary, in an exploratory secretome analysis of proteins secreted by differentiated BECs obtained from children, we observed an abundance of FSTL3 yet significantly lower concentrations secreted by BECs from subjects with asthma as compared with healthy subjects. We confirmed this observation in BEC supernatants from a larger cohort of children with asthma and healthy children, including BECs from children with asthma and airway obstruction. Subsequently, using a human airway epithelial cell/stromal cell ex vivo model system including primary BECs from carefully characterized children with and without asthma, we demonstrated that when cocultured with BECs wherein FSTL3 is knocked down, fibroblasts increase expression of collagen I and α-SMA, suggesting deficient FSTL3 expression by asthmatic epithelium as a mechanism that may impair epithelial regulation of fibroblasts and FMT leading to subepithelial fibrosis. There is a paucity of data in the literature regarding regulation of FSTL3 expression, production, and secretion by airway epithelial cells. Although it was beyond the scope of this study, future investigations should seek to further elucidate epithelial regulation of FSTL3. A better understanding of the role of activin A–FSTL3 signaling in airway remodeling and fibrosis may lead to novel approaches to the treatment and prevention of airway remodeling in asthma and other airway diseases.
Footnotes
Supported by National Institutes of Health grants R01HL12836 (principal investigator [PI]: J.S.D.) and 5R00HL103768 (PI: R.G.J.).
Author Contributions: R.G.J., S.R.R., and J.S.D. designed experiments and drafted/edited the manuscript. K.A.B., M.P.W., V.A.G., C.H., and D.S. conducted experiments and maintained the model system.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0025OC on February 2, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964-1999. J Allergy Clin Immunol. 2002;109:189–194. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 3.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014;133:1572–1578. doi: 10.1016/j.jaci.2013.12.1033. e3. [DOI] [PubMed] [Google Scholar]
- 4.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 5.Malmström K, Pelkonen AS, Malmberg LP, Sarna S, Lindahl H, Kajosaari M, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66:157–162. doi: 10.1136/thx.2010.139246. [DOI] [PubMed] [Google Scholar]
- 6.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 7.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 8.Szefler S, Weiss S, Tonascia J, Adkinson NF, Bender B, Cherniack R, et al. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 9.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 10.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A IFWIN study team. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006;368:754–762. doi: 10.1016/S0140-6736(06)69285-4. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, et al. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 12.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004;34:567–575. doi: 10.1111/j.1365-2222.2004.1917.x. [DOI] [PubMed] [Google Scholar]
- 13.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, et al. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kariyawasam HH, Semitekolou M, Robinson DS, Xanthou G. Activin-A: a novel critical regulator of allergic asthma. Clin Exp Allergy. 2011;41:1505–1514. doi: 10.1111/j.1365-2222.2011.03784.x. [DOI] [PubMed] [Google Scholar]
- 15.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-β isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–313. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetta A, Zanini A, Foresi A, D’Ippolito R, Tipa A, Castagnaro A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005;35:1437–1442. doi: 10.1111/j.1365-2222.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 17.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–1912. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 18.Karagiannidis C, Hense G, Martin C, Epstein M, Rückert B, Mantel PY, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-β-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–997. doi: 10.1016/j.jaci.2011.11.035. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy CL, Nguyen HA, Mohamud R, Yao J, Oh DY, Plebanski M, et al. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax. 2013;68:9–18. doi: 10.1136/thoraxjnl-2011-201128. [DOI] [PubMed] [Google Scholar]
- 21.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-β promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129:1000–1010. doi: 10.1016/j.jaci.2011.12.965. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber S, Schramm C. Role of activin A in the induction of Foxp3+ and Foxp3− CD4+ regulatory T cells. Crit Rev Immunol. 2011;31:53–60. doi: 10.1615/critrevimmunol.v31.i1.50. [DOI] [PubMed] [Google Scholar]
- 23.Hardy CL, Rolland JM, O’Hehir RE. The immunoregulatory and fibrotic roles of activin A in allergic asthma. Clin Exp Allergy. 2015;45:1510–1522. doi: 10.1111/cea.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, et al. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J Exp Med. 2009;206:1769–1785. doi: 10.1084/jem.20082603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostettler KE, Roth M, Burgess JK, Gencay MM, Gambazzi F, Black JL, et al. Airway epithelium-derived transforming growth factor-β is a regulator of fibroblast proliferation in both fibrotic and normal subjects. Clin Exp Allergy. 2008;38:1309–1317. doi: 10.1111/j.1365-2222.2008.03017.x. [DOI] [PubMed] [Google Scholar]
- 26.Walton KL, Makanji Y, Harrison CA. New insights into the mechanisms of activin action and inhibition. Mol Cell Endocrinol. 2012;359:2–12. doi: 10.1016/j.mce.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Apostolou E, Stavropoulos A, Sountoulidis A, Xirakia C, Giaglis S, Protopapadakis E, et al. Activin-A overexpression in the murine lung causes pathology that simulates acute respiratory distress syndrome. Am J Respir Crit Care Med. 2012;185:382–391. doi: 10.1164/rccm.201105-0784OC. [DOI] [PubMed] [Google Scholar]
- 28.Matsuse T, Fukuchi Y, Eto Y, Matsui H, Hosoi T, Oka T, et al. Expression of immunoreactive and bioactive activin A protein in adult murine lung after bleomycin treatment. Am J Respir Cell Mol Biol. 1995;13:17–24. doi: 10.1165/ajrcmb.13.1.7541220. [DOI] [PubMed] [Google Scholar]
- 29.Ohga E, Matsuse T, Teramoto S, Katayama H, Nagase T, Fukuchi Y, et al. Effects of activin A on proliferation and differentiation of human lung fibroblasts. Biochem Biophys Res Commun. 1996;228:391–396. doi: 10.1006/bbrc.1996.1672. [DOI] [PubMed] [Google Scholar]
- 30.Michalik M, Pierzchalska M, Legutko A, Ura M, Ostaszewska A, Soja J, et al. Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture. Med Sci Monit. 2009;15:BR194–BR201. [PubMed] [Google Scholar]
- 31.Roche WR. Fibroblasts and asthma. Clin Exp Allergy. 1991;21:545–548. doi: 10.1111/j.1365-2222.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 32.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 33.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves SR, Kolstad T, Lien TY, Elliott M, Ziegler SF, Wight TN, et al. Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components. J Allergy Clin Immunol. 2014;134:663–670. doi: 10.1016/j.jaci.2014.04.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves SR, Kolstad T, Lien TY, Herrington-Shaner S, Debley JS. Fibroblast-myofibroblast transition is differentially regulated by bronchial epithelial cells from asthmatic children. Respir Res. 2015;16:21. doi: 10.1186/s12931-015-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debley JS, Reeves SR, Glukhova V, Kolstad T, Gallaher A, Haghighi C, et al. Role of follistatin-like-3 (FSTL3) secretion by asthmatic epithelial cells in regulating lung fibroblast extracellular matrix expression and fibroblast-to-myofibroblast transition. Am J Respir Crit Care Med. 2015;191:A2642. [Google Scholar]
- 37.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedger MP, de Kretser DM. The activins and their binding protein, follistatin—diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–295. doi: 10.1016/j.cytogfr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011;85:255–297. doi: 10.1016/B978-0-12-385961-7.00013-5. [DOI] [PubMed] [Google Scholar]
- 41.de Kretser DM, O’Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Matsuse T, Ikegami A, Ohga E, Hosoi T, Oka T, Kida K, et al. Expression of immunoreactive activin A protein in remodeling lesions associated with interstitial pulmonary fibrosis. Am J Pathol. 1996;148:707–713. [PMC free article] [PubMed] [Google Scholar]
- 43.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-β1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 45.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 46.Schneyer A, Schoen A, Quigg A, Sidis Y. Differential binding and neutralization of activins A and B by follistatin and follistatin like-3 (FSTL-3/FSRP/FLRG) Endocrinology. 2003;144:1671–1674. doi: 10.1210/en.2002-0203. [DOI] [PubMed] [Google Scholar]
- 47.Phillips DJ, de Kretser DM, Hedger MP. Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev. 2009;20:153–164. doi: 10.1016/j.cytogfr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Robertson RD, Mukherjee A. Synexpression group analyses identify new functions of FSTL3, a TGFβ ligand inhibitor. Biochem Biophys Res Commun. 2012;427:568–573. doi: 10.1016/j.bbrc.2012.09.098. [DOI] [PubMed] [Google Scholar]
- 49.Tortoriello DV, Sidis Y, Holtzman DA, Holmes WE, Schneyer AL. Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology. 2001;142:3426–3434. doi: 10.1210/endo.142.8.8319. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchida K, Nakatani M, Matsuzaki T, Yamakawa N, Liu Z, Bao Y, et al. Novel factors in regulation of activin signaling. Mol Cell Endocrinol. 2004;225:1–8. doi: 10.1016/j.mce.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-β family. J Biol Chem. 2000;275:40788–40796. doi: 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- 52.Samitas K, Poulos N, Semitekolou M, Morianos I, Tousa S, Economidou E, et al. Activin-A is overexpressed in severe asthma and is implicated in angiogenic processes. Eur Respir J. 2016;47:769–782. doi: 10.1183/13993003.00437-2015. [DOI] [PubMed] [Google Scholar]
- 53.Papaporfyriou A, Bakakos P, Kostikas K, Papatheodorou G, Hillas G, Trigidou R, et al. Activin A and follistatin in patients with asthma. Does severity make the difference? Respirology. 2017;22:473–479. doi: 10.1111/resp.12937. [DOI] [PubMed] [Google Scholar]