Abstract
17β-Estradiol (E2) attenuates hypoxia-induced pulmonary hypertension (HPH) through estrogen receptor (ER)-dependent effects, including inhibition of hypoxia-induced endothelial cell proliferation; however, the mechanisms responsible for this remain unknown. We hypothesized that the protective effects of E2 in HPH are mediated through hypoxia-inducible factor 1α (HIF-1α)-dependent increases in ERβ expression. Sprague-Dawley rats and ERα or ERβ knockout mice were exposed to hypobaric hypoxia for 2–3 weeks. The effects of hypoxia were also studied in primary rat or human pulmonary artery endothelial cells (PAECs). Hypoxia increased expression of ERβ, but not ERα, in lungs from HPH rats as well as in rat and human PAECs. ERβ mRNA time dependently increased in PAECs exposed to hypoxia. Normoxic HIF-1α/HIF-2α stabilization increased PAEC ERβ, whereas HIF-1α knockdown decreased ERβ abundance in hypoxic PAECs. In turn, ERβ knockdown in hypoxic PAECs increased HIF-2α expression, suggesting a hypoxia-sensitive feedback mechanism. ERβ knockdown in hypoxic PAECs also decreased expression of the HIF inhibitor prolyl hydroxylase 2 (PHD2), whereas ERβ activation increased PHD2 and decreased both HIF-1α and HIF-2α, suggesting that ERβ regulates the PHD2/HIF-1α/HIF-2α axis during hypoxia. Whereas hypoxic wild-type or ERα knockout mice treated with E2 demonstrated less pulmonary vascular remodeling and decreased HIF-1α after hypoxia compared with untreated hypoxic mice, ERβ knockout mice exhibited increased HIF-2α and an attenuated response to E2 during hypoxia. Taken together, our results demonstrate a novel and potentially therapeutically targetable mechanism whereby hypoxia, via HIF-1α, increases ERβ expression and the E2-ERβ axis targets PHD2, HIF-1α, and HIF-2α to attenuate HPH development.
Keywords: hypoxia-inducible factor 1α, hypoxia-inducible factor 2α, pulmonary hypertension, prolyl hydroxylase 2, 17β-estradiol
Clinical Relevance
Our study has three important clinical implications. First, we identify a novel and lung-specific estrogen receptor β response to hypoxia that may explain the protective effects driven by estrogen in hypoxia-induced pulmonary hypertension. This finding has potential therapeutic implications because although hypoxia-induced pulmonary hypertension is one of the most common forms of pulmonary hypertension worldwide, no therapeutic options exist. Second, our results are the first to link estrogen receptor to the regulation of hypoxia-inducible factor 2. Third, our results may help solve the “estrogen paradox” in pulmonary arterial hypertension.
Pulmonary hypertension (PH) encompasses a heterogeneous group of pulmonary vascular disorders characterized by increased vasoconstriction and aberrant muscularization of the pulmonary arteries (PAs) that lead to increased right ventricular afterload and, if left untreated, right ventricular failure and death (1–3). Several forms of PH are characterized by sexual dimorphism. For example, hypoxia-induced PH (HPH), one of the most common forms of PH worldwide (4–6), is less common and less severe in females (reviewed in Reference 7). On the other hand, pulmonary arterial hypertension (PAH), a progressive vasculopathy characterized by uncontrolled endothelial proliferation, obliterative PA lesions, and markedly elevated PA pressures, exhibits a female/male ratio that ranges from 1.4 to 4.1:1 (reviewed in Reference 7). These disparate observations have been attributed to dichotomous and tissue-dependent effects of 17β-estradiol (E2) and its receptors (estrogen receptor α [ERα] and ERβ). For example, although E2 may have detrimental effects on the pulmonary vasculature in the context of a BMPR2 mutation (8, 9), it has favorable ER-dependent effects on pulmonary vascular tone and PA endothelial cell (PAEC) homeostasis in hypoxic conditions (10–13). Interestingly, E2’s vasculoprotective effects on PAECs are not observed during normoxic conditions, indicating that E2 exerts specific actions during hypoxia that may not be active during nonhypoxic conditions. Further studies of the specific effects of hypoxia on E2 signaling in the pulmonary vasculature are therefore of crucial importance, as such investigations may 1) identify potential novel therapeutic targets for HPH and PH induced by chronic lung diseases, and 2) elucidate whether alterations in the E2-ER axis—similarly to recently identified alterations in estrogen metabolism (14, 15)—contribute to the predisposition of females for PAH. Given our recently reported observations of hypoxia-specific and ER-mediated protective E2 signaling in HPH (10, 16), we aimed to identify whether and how hypoxia affects ER expression and function in the pulmonary vasculature.
ERα and ERβ (encoded by the genes Esr1 and Esr2, respectively) are proteins that belong to the nuclear-receptor superfamily of transcription factors and are expressed in both sexes (17, 18). Two subtypes exist, both of which are expressed in the histologically normal lung, with ERβ purportedly being more abundant than ERα (19–22). In addition to differences in tissue expression patterns, the two ERs also differ in their biological functions (21–24). For instance, in the setting of breast and prostate cancer, ERα mediates proproliferative effects and ERβ mediates antiproliferative signaling (25–28). Interestingly, ERβ exhibits relevant effects in PAECs. Specifically, ERβ mediates E2-induced increases in endothelial nitric oxide synthase activity and prostacyclin synthesis in fetal bovine PAECs (29, 30), and attenuates ERK1/2 (p42/44 MAPK) activation in hypoxic rat PAECs (10). Administration of a selective ERβ agonist replicates E2’s inhibitory effects on hypoxic pulmonary vasoconstriction in isolated rat PA rings, and this effect is mediated in a nitric oxide– and endothelium-dependent manner (13). Lastly, in a rat model of HPH, E2-mediated decreases in pulmonary vascular remodeling were attenuated after cotreatment with a selective ERβ antagonist (10).
These findings implicate a unique pathway of ER-dependent signaling during hypoxia that inhibits pulmonary vascular remodeling. We sought to investigate whether and how hypoxia affects ER expression and function in vitro and in vivo. We demonstrate for the first time that hypoxia upregulates ERβ expression in isolated PAECs as well as in the pulmonary vasculature, and that this increase is dependent on hypoxia-inducible factor 1α (HIF-1α). Furthermore, we provide the first evidence demonstrating that ERβ decreases both HIF-1α and HIF-2α in vitro and in vivo, and is necessary for E2’s vasculoprotective effects in a murine model of HPH. This novel role of ERβ in regulating HIF-2α is of particular relevance because dysregulated HIF-2α signaling has recently been reported to be a potent driver of the formation of obliterative lesions in PH (31, 32). Finally, we provide evidence that ERβ stimulates expression of the HIF-α inhibitor prolyl hydroxylase 2 (PHD2) in PAECs, thus identifying attenuation of HIF-1α and HIF-2α signaling as a novel mechanism of E2 action in the pulmonary vasculature. Parts of this study have been previously reported in abstract form (33, 34).
Methods
Please refer to the data supplement for details regarding the materials and methods used in this work.
Animal Care
The animal studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee, and adhered to the National Institutes of Health guidelines for the care and use of laboratory animals under the Animal Welfare Act.
In Vivo Studies
The HPH model has been described previously (10). Male Sprague-Dawley rats (Charles River) or wild-type (WT), Esr1−/−, and Esr2−/− C57BL/6 mice (Jackson Laboratories) were exposed to 2–3 weeks of hypobaric hypoxia, respectively. A subgroup of mice were treated with E2 (75 μg/kg/d via subcutaneous pellets [10]; Innovative Research of America) for the duration of hypoxia exposure.
In Vitro Studies
Primary rat PAECs (from Drs. Troy Stevens and Diego Alvarez, University of South Alabama) or human PAECs (Lonza) were exposed to hypoxia (1% O2) using an InvivO2 hypoxia workstation (Ruskinn, Inc.) as described previously (10). Control cells of identical passage/confluence were grown concomitantly under room-air conditions. To verify that no detectable or only minimal amounts of E2 were present in the culture media, E2 concentrations were quantified via ELISA (Calbiotech) and by comparing levels with those from E2-treated media (see Fig. E1 in the data supplement). Cells were treated with deferoxamine (DFO; 10–100 μM; Sigma-Aldrich) for 24 hours in room air or with the selective ERβ agonist diarylpropionitrile (DPN; 1–100 nM; Tocris) for 48 hours at 1–5% O2. Cells were transfected using Lipofectamine 2000 with siHIF-1α or siERβ (ThermoFisher) for 24 hours and exposed to hypoxia for 24–48 hours.
Immunocytochemistry
Rat or human PAECs were seeded on gelatin-coated coverslips and exposed to room air or hypoxia. The EC phenotype was confirmed (Figure E2). Primary antibodies against ERα or ERβ (1:200; Santa Cruz) were used. Alexa Fluor 488 conjugated anti-rabbit secondary antibody (1:200; ThermoFisher) and DAPI (ThermoFisher) mounting media were used. Ten fields per slide were taken using a Nikon microscope at 40 × . ER expression was quantified as the average megapixel intensity per number of nuclei by ImageJ (National Institutes of Health).
Immunohistochemistry
Lung sections were stained with ERα (1:500, Santa Cruz) or ERβ (1:10; Dako) and quantified using Metamorph software. WT, Esr1−/−, and Esr2−/− mouse lungs were stained for α-smooth muscle actin (1:500; Abcam). Quantification was performed as previously described (10).
Western Blotting
Tissue/cell lysis was performed as previously described (10). The following antibodies were used: ERα (1:1,000; Santa Cruz), ERβ (1:1,000; Santa Cruz), HIF-1α and HIF2-α (1:1,000; Novus Biological), PHD2 (1:1,000; Abcam), and vinculin (1:5000; Calbiochem). Densitometry was performed via ImageJ.
Real-Time qRT-PCR
Total RNA was isolated from rat and human PAECs using the RNeasy Plus Mini Kit (Qiagen), and 1 μg total RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). TaqMan gene expression assays for Esr1, Esr2, and 18S (ThermoFisher; for assay IDs, see the data supplement) were used. Changes in mRNA expression were determined by the comparative CT (2−∆∆CT) method and expressed as the fold change compared with normoxic controls.
Statistical Analyses
Results are expressed as means ± SEM. At least three independent experiments (run in duplicates) were performed for all in vitro studies. Statistical analyses were performed with GraphPad Prism 6 (La Jolla, CA). Student’s t test or one-way ANOVA with Tukey’s post hoc or Dunnett’s correction were used when appropriate. Statistical significance was accepted at P < 0.05.
Results
Hypoxia Increases ERβ, but Not ERα, in Rat Lungs
Our previous studies in HPH rats suggested that hypoxia may upregulate ERβ protein in the pulmonary vasculature (10). To further investigate this finding, we evaluated lung ERα and ERβ protein expression in chronically hypoxic Sprague-Dawley rats by immunohistochemistry and Western blot. Although 2 weeks of hypoxia tended to decrease lung ERα abundance (Figures 1A–1C), hypoxia robustly increased the lung expression of ERβ (Figures 1A and 1D–1E). ERβ staining localized to PAECs, suggesting that ERβ is predominantly expressed in these cells. The hypoxia-induced increase in ERβ was lung specific, as no increase was noted in the right ventricle (RV), left ventricle, or liver (Figure E3). In fact, ERβ expression was significantly decreased in the RV and liver, suggesting differential effects of hypoxia on ERβ expression in the lung versus other organ systems.
Figure 1.
Hypoxia increases expression of estrogen receptor β (ERβ), but not ERα, in the lung. (A) Representative immunohistochemistry images of lung sections from normoxic and hypoxic animals stained for ERα and ERβ. Note the increase in ERβ-positive cells (arrow) in pulmonary arteries from hypoxic rats. Positive staining for ERβ was mainly present in endothelial cells, and there was no significant staining of smooth muscle cells. (B–E) Protein expression of (B) ERα and (D) ERβ in whole-lung homogenates from rats exposed to 2 weeks of normoxia (fraction of inspired oxygen [FiO2] 21%) or chronic hypobaric hypoxia (Patm = 362 mm Hg; equivalent to 10% FiO2). Quantification of (C) lung ERα and (E) ERβ by densitometry is shown. Images were obtained at × 40 magnification. Scale bars = 50 μm; n = 4/group. Error bars in scatterplots are means ± SEM. *P < 0.05 versus normoxia control by t test.
Hypoxia Increases Expression of ERβ, but Not ERα, in Primary Rat and Human PAECs
Because ERβ appeared to be primarily expressed in PAECs and upregulated under hypoxic conditions, we next evaluated the effect of hypoxia on ER expression in primary rat PAECs. Rat PAECs were cultured under hypoxic conditions (1% O2) for 4, 24, or 72 hours, and ER expression was evaluated by immunocytochemistry. As was observed in vivo, ERα was not significantly affected by hypoxia exposure compared with normoxic controls (Figures 2A and 2B). In contrast, hypoxia time dependently increased ERβ protein compared with normoxic controls (Figures 2A and 2B). Interestingly, this hypoxia-induced increase in ERβ expression was accompanied by a localization pattern suggestive of nuclear translocation (Figures 2A and 2C). To investigate whether the hypoxia-induced upregulation of ERβ is transcriptionally regulated, we used real-time RT-PCR to quantify ERβ mRNA. As expected, ERβ mRNA was significantly increased in rat PAECs at 24 hours (but not at the earlier time point of 16 h; Figure 2D), suggesting that the hypoxia-induced increase in ERβ is mediated by a transcriptional mechanism. This increase was specific to ERβ, as similar increases in ERα mRNA expression were not observed (Figure 2D).
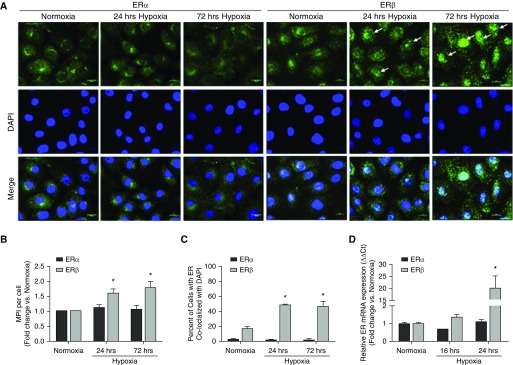
Figure 2.
Hypoxia increases expression of ERβ, but not ERα, in cultured rat pulmonary artery endothelial cells (PAECs). (A) Representative immunocytochemistry images of PAECs at 24 and 72 hours of hypoxia (1% O2) versus normoxia. ERα (left three columns) and ERβ (right three columns) are in green. Note the significant increase in ERβ staining intensity in hypoxic cells, with a pattern of ERβ staining suggestive of increased nuclear translocation (arrows). (B) Time course of hypoxia-induced expression of ERα (black bars) and ERβ (gray bars) by immunocytochemistry. ERβ expression was quantified by normalizing the megapixel intensity (MPI) by the number of cells (nuclei stained with DAPI) with ImageJ, and is expressed as the fold change of MPI at 4, 24, and 72 hours versus MPI at corresponding normoxia time points. (C) ER nuclear translocation was quantified as percent ER stain colocalized with DAPI. (D) ERα and ERβ gene expression in rat PAECs measured by real-time qRT-PCR. Scale bars = 10 μm. Data are from three independent experiments and are presented as means ± SEM. *P < 0.05 versus normoxia control by one-way ANOVA with post hoc Tukey’s test.
To corroborate these findings, we cultured human primary PAECs under hypoxic conditions for 24 or 72 hours, and evaluated ERα and ERβ expression by immunocytochemistry and Western blot. As in the rat PAECs, hypoxia selectively and time dependently increased ERβ (but not ERα) expression, with a trend toward an increase at 24 hours and a significant 25% increase at 72 hours (Figures 3A and 3B). As in the rat PAECs, immunocytochemistry studies suggested that the hypoxia-induced increase in ERβ abundance was paralleled by a change in ERβ’s cellular localization pattern consistent with translocation to the nucleus (Figures 3A and 3C). In fact, 80% of the ERβ-positive cells colocalized with DAPI at 24 hours, and this was maintained even at 72 hours. Finally, we quantified the changes in ERβ expression using real-time RT-PCR. Similar to what was observed with rat PAECs, ERβ, but not ERα, mRNA was significantly increased at 24 hours (but not at the earlier time point of 4 h; Figure 3D), suggesting that the hypoxia-induced increase in ERβ is transcriptionally regulated. Interestingly, we also observed hypoxic induction of ERβ, but not ERα, protein in human pulmonary microvascular endothelial cells (PMVECs; Figure E4) and pulmonary smooth muscle cells (data not shown) at 72 hours, indicating that the specific hypoxic regulation of ERβ is relevant to multiple cell types/phenotypes in the pulmonary vasculature. Taken together, these data indicate that hypoxia 1) selectively increases ERβ mRNA and protein in cultured primary PAECs, and 2) results in nuclear translocation of ERβ, suggesting that this receptor has biologically relevant effects in the context of hypoxia.
Figure 3.
Hypoxia increases expression of ERβ, but not ERα, in primary human PAECs. (A) Representative immunocytochemistry images of human PAECs at 24 and 72 hours of hypoxia (1% O2) versus normoxia. ERα (left three columns) or ERβ (right three columns) are in green. Note a pattern of ERβ staining suggestive of increased nuclear translocation (arrows). (B) Time course of hypoxia-induced expression of ERα (black bars) and ERβ (gray bars). ER expression was quantified by normalizing the MPI by the number of cells (nuclei stained with DAPI) with ImageJ, and is expressed as the fold change of MPI at 4, 24, and 72 hours versus MPI at corresponding normoxia time points. (C) ER nuclear translocation was quantified as percent ER stain colocalized with DAPI. (D) ERα and ERβ gene expression in rat PAECs measured by real-time qRT-PCR. Scale bars = 10 μm. Data are from three independent experiments and are presented as means ± SEM. *P < 0.05 versus normoxia control by one-way ANOVA with post hoc Tukey’s test.
Normoxic HIF-α Stabilization Is Sufficient to Increase ERβ in Rat PAECs
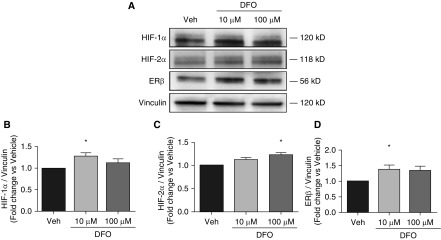
We next sought to determine whether the hypoxia-induced increase in rat PAEC ERβ expression is driven by HIF-1α, a master regulator of hypoxia-driven responses (35). We treated rat PAECs with the iron chelator DFO, which leads to normoxic HIF-α stabilization (Figures 4A and 4B). Indeed, DFO treatment (10 μM for 24 h) resulted in a robust 25% increase in ERβ (Figures 4A and 4D). DFO leads to stabilization of HIF-2α and, similar to the case with HIF-1α, we detected the stabilization of HIF-2α in rat PAECs after DFO treatment (Figures 4A and 4C), indicating that normoxic HIF-α stabilization is sufficient to increase ERβ expression.
Figure 4.
Hypoxia-inducible factor 1α (HIF-1α) stabilization under normoxic conditions increases ERβ expression in cultured rat PAECs. Effects of treatment with deferoxamine (DFO; 10 or 100 μM for 24 h) or vehicle (veh; H2O) on HIF-1α (A and B), HIF-2α (A and C), and ERβ (A and D) expression are shown in representative Western blots (A) and densitometry (B–D) from three independent experiments. Data are means ± SEM. *P < 0.05 versus vehicle control using one-way ANOVA with post hoc Tukey’s test.
HIF-1α Is Necessary for the Hypoxia-induced Increase in ERβ Expression in Rat PAECs
To examine whether HIF-1α is necessary for the hypoxia-induced increase in ERβ, we used siRNA directed against HIF-1α in rat PAECs grown under hypoxic conditions (Figures 5A and 5B). As expected, knockdown of HIF-1α attenuated the hypoxia-induced increase in ERβ at 24 and 48 hours of hypoxia (Figures 5A and 5C), suggesting that HIF-1α indeed is necessary for hypoxia to increase ERβ.
Figure 5.
HIF-1α and ERβ regulate HIF-2α and prolyl hydroxylase 2 (PHD2) expression in hypoxic rat PAECs. (A) Western blot and (B–E) densitometry of HIF-1α (B), ERβ (C), HIF-2α (D), and PHD2 (E) protein expression after knockdown of HIF-1α or ERβ by siRNA. Scrambled control oligos were used as negative control. Cells were grown for 24 or 48 hours under hypoxic conditions (1% O2). Data represent three independent experiments and are expressed as means ± SEM. *P < 0.05 versus scrambled control; ^P < 0.05 versus normoxic control using one-way ANOVA with post hoc Tukey’s test. Scr = scrambled.
ERβ Decreases HIF-2α and Increases PHD2 in Rat PAECs
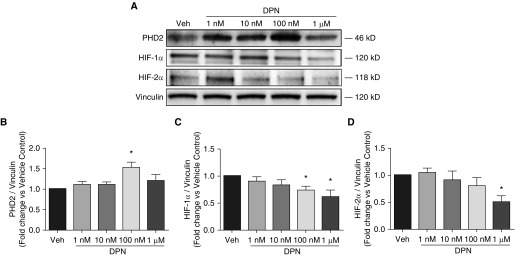
To determine whether ERβ in turn affects HIF-1α expression during hypoxia, we used siRNA directed against ERβ. Interestingly, knockdown of ERβ during hypoxia resulted in decreased expression of HIF-1α at 24 (but not 48) hours of hypoxia exposure (Figures 5A and 5B), suggesting that HIF-1α is time dependently under the control of ERβ. We next assessed whether the HIF-α family member HIF-2α is regulated by ERβ. Although HIF-2α was decreased after 24 hours of ERβ knockdown, we noted a significant 30% increase in HIF-2α protein 48 hours after ERβ knockdown (Figures 5A and 5D), suggesting that ERβ regulates and time dependently decreases HIF-2α. Because PHD2 is a major inhibitor of HIF-α (by mediating HIF-α degradation) (36), we next sought to identify whether ERβ also affects PHD2 expression. Indeed, knockdown of ERβ during hypoxia resulted in 50% decreased expression of PHD2 at both 24 and 48 hours (Figures 5A and 5E), indicating that ERβ regulates PHD2 expression. We corroborated these findings by treating hypoxic rat PAECs for 48 hours with the ERβ-selective agonist DPN (Figure 6). We noted a dose-dependent increase in PHD2 expression in cells treated with 100 nM of DPN (Figures 6A and 6B), indicating that, indeed, ERβ increases PHD2 expression in rat PAECs. We also observed dose-dependent decreases in HIF-1α (Figures 6A and 6C) and HIF-2α (Figures 6A and 6D) expression in cells treated with DPN. Given the potential inactivation of PHD2 in severe hypoxic conditions (37), we also tested the effect of DPN on PHD2, HIF-1α, and HIF-2α expression under less severe hypoxic conditions (5% O2; Figure E5). Similar to our observations at 1% O2, we observed a dose-dependent increase in PHD2 expression (Figures E5A and E5B), a decrease in HIF-2α expression (Figures E5A and E5D), and a trend toward decreased HIF-1α expression (Figures E5A and E5C). In summary, these data suggest that in hypoxic rat PAECs, HIF-1α increases ERβ, and that ERβ in turn decreases HIF-2α and increases its inhibitor, PHD2. The data for DPN, but not those for siERβ, suggest that ERβ may also decrease HIF-1α. This discrepancy may be due to a compensatory upregulation of ERα or other factors after ERβ knockdown, or it may indicate a time-dependent differential effect of ERβ on HIF-1α expression.
Figure 6.
Selective ERβ agonism with diarylpropionitrile (DPN) dose dependently increases PHD2 and decreases HIF-1α and HIF-2α in cultured rat PAECs. (A) Representative Western blots showing the effects of DPN (1, 10, or 100 nM, or 1 μM) or vehicle (DMSO) on PHD2 protein expression during hypoxia (1% O2; 48 h). (B–D) The protein expression of PHD2 (B), HIF-1α (C), and HIF-2α (D) was quantified with densitometry. Data represent the mean ± SEM from three independent experiments. *P < 0.05 versus vehicle control using one-way ANOVA with post hoc Dunnett’s test.
ERβ Is Necessary for E2 to Attenuate Hypoxia-induced Pulmonary Vascular Remodeling and Decrease Lung HIF-1α and HIF-2α Expression
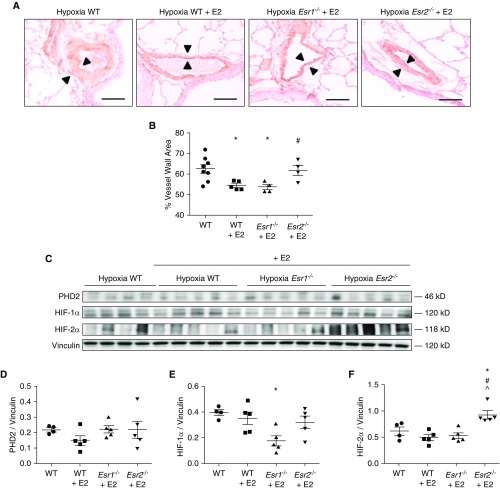
To corroborate our findings in vivo, we treated Esr1−/− or Esr2−/− mice (mice containing germline null mutations for ERα or ERβ, respectively) and WT mice with E2 (75 μg/kg/day [10]), and exposed them to 3 weeks of hypoxia (Patm = 362 mm Hg). An additional group of untreated WT mice were placed in hypoxia to serve as controls. As previously noted in chronically hypoxic rats, compared with hypoxic WT controls, E2 treatment in WT mice significantly decreased hypoxia-induced pulmonary vascular remodeling by 40% (Figures 7A and 7B). Importantly, this reduction in pulmonary vascular remodeling was observed in E2-treated Esr1−/−, but not Esr2−/−, mice (Figures 7A and 7B), suggesting that ERβ is required for E2 to attenuate hypoxia-induced pulmonary vascular remodeling. Contrary to our hypothesis, PHD2 was not affected by E2 or loss of ERα or ERβ (Figures 7C and 7D), suggesting that PHD2 is not solely regulated by E2-ER signaling. Alternatively, it is possible that E2-ER–mediated changes in PHD2 expression in PAECs in vivo were not captured at the time point investigated, or were masked by surrounding tissue in whole-lung homogenates. On the other hand, E2 treatment in hypoxic WT mice tended to decrease lung HIF-1α and HIF-2α expression (Figures 7C, 7E, and 7F), and in the case of HIF-1α, resulted in a significant 50% decrease in E2-treated Esr1−/− mice (where E2 signaling occurs through ERβ; Figures 7C and 7E). However, E2 did not lower HIF-1α abundance in Esr2−/− mice (where E2 signaling occurs through ERα; Figures 7C and 7E). In parallel, E2 treatment led to increased HIF-2α expression in Esr2−/− mice (Figures 7C and 7F). This suggests that ERβ is required for E2 to decrease HIF-2α and HIF-1α expression. However, in the latter case, this effect can only occur in the absence of ERα, indicating that 1) ERα has effects opposite to those of ERβ and actually increases HIF-1α, and 2) ERα and ERβ compete with each other for E2 binding. Taken together, these studies demonstrate that ERβ is required for E2 to attenuate hypoxia-induced pulmonary vascular remodeling, is necessary for E2 to decrease lung HIF-α expression, and prevents increases in lung HIF-2α expression.
Figure 7.
ERβ is necessary for E2-mediated attenuation of hypoxic pulmonary vascular remodeling and HIF-1α and HIF-2α expression in mice. (A) Representative immunohistochemical images of lung sections stained for smooth muscle actin from untreated wild-type (WT) (solid circles), E2-treated WT (solid squares), E2-treated Esr1−/− (solid triangles), and E2-treated Esr2−/− (solid inverted triangles) mice exposed to chronic hypobaric hypoxia (Patm = 362 mm Hg; equivalent to 10% FiO2; 3 wk). Note the decrease in vascular wall thickness (arrowheads) in E2-treated WT and Esr1−/− mice, but not in E2-treated Esr2−/− mice compared to WT controls. Scale bars = 50 μm. (B) Quantification of the wall thickness of small- and medium-sized pulmonary arteries (less than 200 μM). (C) PHD2, HIF-2α, and HIF-1α protein expression in mouse lung homogenates from WT, E2-treated WT, E2-treated Esr1−/−, and E2-treated Esr2−/− mice exposed to chronic hypobaric hypoxia. (D–F) Expression levels of lung PHD2, HIF-2α, and HIF-1α quantified by densitometry; n = 4–5 per group. Scatterplots include means ± SEM. *P < 0.05 versus WT; #P < 0.05 versus WT + E2; ^P < 0.05 versus Esr1−/− (one-way ANOVA with post hoc Tukey’s test). E2 = 17β-estradiol.
Discussion
Our studies reveal that ERβ, but not ERα, is upregulated in hypoxic rat lung, cultured hypoxic rat and human conduit PAECs, and PMVECs. We show for the first time that HIF-α chemical stabilization with DFO is sufficient to induce ERβ expression and that knockdown of HIF-1α by siRNA reduces ERβ expression in hypoxic PAECs, identifying that the hypoxia-induced increase in ERβ expression occurs via a HIF-α–dependent mechanism. Additionally, we provide novel evidence suggesting that HIF-2α is downregulated and PHD2 is upregulated by ERβ in vitro. siRNA directed against ERβ leads to the robust upregulation of HIF-2α and downregulation of PHD2 in hypoxic PAECs, and treatment with the ERβ agonist DPN upregulates PHD2 expression and decreases HIF-1α and HIF-2α in a dose-dependent manner. Lastly, we demonstrate that ERβ is necessary for E2 to attenuate hypoxia-induced pulmonary vascular remodeling, decrease lung HIF-1α, and prevent increases in HIF-2α expression. However, the decrease in HIF-1α in vivo only occurred in the absence of ERα. Taken together, these novel data implicate ERβ as a mediator of protective E2 signaling in the hypoxic pulmonary vasculature. A schematic of the putative interaction among hypoxia, ERβ, HIF-1α, HIF-2α, and PHD2 in the pulmonary vasculature is provided in Figure E6.
HPH is prevalent globally; it affects millions of people living at high altitude and millions of patients afflicted with a wide spectrum of chronic lung disease and sleep-disordered breathing (5). HPH is characterized by significant pulmonary vascular remodeling that may culminate in right ventricular failure and death (4, 38). Although it occurs much more frequently than PAH (39), no specific treatment for HPH exists. In fact, therapeutics used for PAH frequently worsen oxygenation in HPH (40–42), indicating a need for novel therapeutic approaches to HPH. Several clinical and preclinical studies have identified that female sex confers a protective phenotype during acute or chronic hypoxia exposure (reviewed in Reference 7). For example, in multiple species, females exhibit less hypoxic pulmonary vasoconstriction and are protected from HPH. These effects have been linked to vasodilator and antiproliferative effects of E2 (7). For example, pregnancy attenuates hypoxic pulmonary vasoconstriction, ovariectomy worsens HPH, and E2 repletion attenuates HPH (reviewed in References 7 and 43). A better understanding of the effects of E2 and its receptors in the hypoxic pulmonary vasculature could provide the rationale and basis for future studies focused on developing novel therapeutic strategies for HPH.
We previously demonstrated that E2 attenuates HPH via an ER-dependent mechanism (10), and recently expanded upon that finding by discovering an ER-dependent genome in lungs from HPH rats (16). Of note, our previous data showed that E2 exerts specific antiproliferative effects during hypoxia that are not evident during normoxia (10). For example, E2-ER dependently decreased bromodeoxyuridine uptake and vascular endothelial growth factor secretion in hypoxic rat PAECs, but not in PAECs grown in room air. Our current data expand upon these findings and suggest that hypoxia-enabled increases in ERβ expression may allow for E2 to exert its hypoxia-specific effects. Such a finding may explain why E2 has consistently been shown to be protective in hypoxia models of PH, whereas its effects in other types of PH have been less consistent (reviewed in Reference 7).
If ERβ signaling can be protective in the pulmonary vasculature, this raises the possibility that alterations in ERβ signaling may contribute to the female predominance noted in PAH. Of note, SNPs in ESR2−/− have been associated with LV structural differences in women with hypertension (44) and with increased arterial stiffness in Framingham Offspring Study participants (45). This suggests that genetic alterations in ESR2−/− could also contribute to abnormalities in the pulmonary vasculature. Alternatively, in the absence of hypoxia-enabled increases in ERβ expression, E2’s protective signaling pathways may not be engaged, allowing for the development of pulmonary vascular disease, especially in the context of otherwise predisposing conditions for PAH development (e.g., genetic abnormalities and environmental exposures).
To the best of our knowledge, our studies are the first to implicate HIF-1α as a regulator of ERβ in PAECs. Given that HIF-1α is a key regulator of hypoxia-mediated effects, this finding is not entirely surprising. Our findings are consistent with results from studies in HEK293 cells, which demonstrated that HIF-1α transcriptionally activates ERβ expression in the absence of E2, and the addition of E2 did not further enhance the upregulation of ERβ (46). Subsequent studies in other cell types have linked HIF-1α to the induction of ERβ independently of E2 (47–49). For instance, in human ER-positive breast cancer cells, hypoxia stimulated ERβ expression and reduced ERα expression (48), whereas in human stromal cells isolated from patients with endometriosis, hypoxia strongly induced ERβ (47), both in the absence of E2. Additionally, steroid receptor coactivator-1 and CREB-binding protein comprise part of the HIF-1α transcriptional coactivator complex and can promote ERβ transcription in the absence of E2 (49). Building on these observations, we performed PROMO and TRANSFAC in silico promoter analyses, which identified two putative HIF-1α binding sites in the ESR2 promoter (Ensembl ENSG00000140009) to be validated in future studies. Interestingly, we did not observe a significant increase in ERβ mRNA expression until 16 hours (human PAECs) or 24 hours (rat PAECs) after hypoxia. One explanation for this slow upregulation is that because ERβ expression is regulated by multiple coactivators and corepressors, there may be a transcriptional repressor complex at the ERβ promoter upon the first, acute induction of HIF-1α due to hypoxia that is not present at a later, chronic hypoxia time point. Finally, we cannot completely rule out that the trace amounts of E2 detected in rat PAEC media (Fig. E1; likely arising as a result of endogenous PAEC E2 synthesis via aromatase [50]) activated ERβ. However, this seems unlikely, as no E2 was detected in human PAEC media, yet hypoxia increased ERβ expression and induced its translocation in this cell type as well. Together, these data suggest that ERβ, independently of the presence of E2, is encoded by a hypoxia-responsive gene.
The finding that ERβ in turn regulates HIF-1α and HIF-2α in vitro and in vivo reveals a novel and potentially therapeutically targetable mechanism of action in the hypoxic pulmonary vasculature that may explain in part why E2 attenuates HPH and why females are protected against the development of HPH. This putative negative-feedback loop of ERβ on HIF-α likely occurs via direct binding of HIF-1α to ERβ, followed by the nuclear translocation of ERβ and binding to estrogen response elements of target genes. Interestingly, at least one study has shown that the ERβ protein is able to bind directly to HIF-1α (46). Alternatively, hypoxia could indirectly facilitate ERβ nuclear translocation via direct activation of ERβ by a HIF-1α–induced growth factor, e.g., epidermal growth factor (51). The negative-feedback mechanism we identified is consistent with data from the oncology literature, which demonstrates that ERβ inhibits HIF-1α transcriptional activity and promotes its proteasome degradation in prostate cancer cells (52). On the other hand, to the best of our knowledge, we are not aware of any prior data demonstrating that ERβ decreases HIF-2α. This is of particular importance because dysregulated HIF-2α signaling has been linked to the formation of obliterative pulmonary vascular lesions in PH (32). However, the effects of ERβ on HIF-1α and HIF-2α were not clear-cut. At 24 hours of ERβ knockdown, both HIF-1α and HIF-2α were decreased rather than increased, suggesting a time dependence of ERβ effects on these transcription factors. In addition, ERβ knockdown did not lead to increased HIF-1α at the 48-hour time point. This phenomenon may be explained by competing effects of ERα and ERβ on HIF expression: ERα (or another, as yet unidentified factor) may compensate for the loss of ERβ and repress HIF-α. On the other hand, the finding that selective ERβ activation decreased both HIF-1α and HIF-2α clearly indicates that ERβ exerts inhibitory effects on these mediators.
The in vivo effects of E2 and ERβ on HIF-α were complex. E2 did not statistically significantly decrease HIF-1α in hypoxic WT rats (even though there was a trend), and a significant decrease occurred only in E2-treated Esr1−/− mice. Although this could be the result of a type II error, it may also suggest that ERα increases HIF-1α. Specifically, ERα and ERβ may have opposing effects on HIF-1α in vivo, allowing ERβ to decrease HIF-1α only in the absence of ERα. Such a paradigm would be consistent with the known proproliferative effects of ERα in prostate cancer (28) and in PA smooth muscle cells (53). An alternative hypothesis is that the absence of ERα increases the abundance of E2 at ERβ, thus driving a more prominent ERβ signal. This would suggest that ERα and ERβ compete with each other for binding to E2. Such a scenario is likely, given that in the absence of ERβ there is no increase in HIF-1α (Figures 7C and 7F). Similarly, E2 did not statistically significantly decrease HIF-2α in vivo, yet, again, there was a trend. In contrast to HIF-1α, HIF-2α markedly increased in E2-treated Esr2−/− mice. This indicates that ERβ exerts inhibitory effects on HIF-2α and suggests that ERα and ERβ may have opposing effects on HIF-2α in vivo, with ERα exerting stimulatory effects and ERβ preventing this increase.
Our data suggest that the inhibitory effect of ERβ on HIF-1α and HIF-2α may be mediated by PHD2. PHDs hydroxylate HIF-1α and HIF-2α, leading to von Hippel–Lindau protein-dependent ubiquitination and rapid proteasomal degradation (35). Three main isoforms exist, with PHD2 being the most prominent isoform (36). Interestingly, we found that ERβ induces PHD2 in hypoxic PAECs. This may, in turn, downregulate HIF-1α and HIF-2α. This finding is supported by recently published data from prostate epithelial cells demonstrating that ERβ promotes HIF-1α degradation by increasing PHD2 transcription and by identifying an estrogen response element in the PHD2 promoter (52). Given the recent report that PHD2 expressed in PAECs protects against PAH development (32), our identification of PHD2 as a target of ERβ is of particular interest, as it suggests a novel mechanism whereby E2 attenuates HPH development.
ERβ may also inhibit HIF-1α and HIF-2α through PHD2-independent mechanisms. For example, ERβ was shown to inhibit HIF-1α transcriptional activity via downregulation of ARNT (HIF-1β) (54). In another study, ERβ was shown to destabilize HIF-1α protein via proteasomal degradation (55). It is possible that similar mechanisms regulate ERβ’s effects on HIF-1α in the hypoxic pulmonary vasculature. Future studies will focus on identifying the exact molecular mechanisms of ERβ-mediated attenuation of HIF-1α and HIF-2α in the hypoxic lung and in hypoxic PAECs.
Our study has several limitations. First, although our in vitro data robustly identify PHD2 as a target of ERβ, we did not detect any changes in PHD2 expression in Esr1−/− or Esr2−/− mice treated with E2. Although it is possible that PHD2 is not regulated by E2-ERβ signaling in vivo, we consider it more likely that E2-ERβ–mediated changes in PHD2 expression in PAECs in vivo were not captured at the time point investigated, or were masked by surrounding tissue in whole-lung homogenates. This is supported by the observation that the effects we noted on HIF-1α and HIF-2α were robust in vitro as well as in vivo. Second, our in vitro studies used a severe level of hypoxia (1% O2). This severe level of hypoxia can inhibit PHD2 activity at least acutely (at 4 h), but perhaps not chronically (36, 37, 56), which could be a source of variability in our data; however, we were able to corroborate our DPN findings using less severe hypoxic conditions (5% O2), during which PHD2 should be active (Figure E3). Second, in our studies we used in vitro rat and human primary cells, and conducted in vivo studies in rats and mice; however, it should be noted we did not use human cells for all studies. Future investigations will focus on expanding upon these mechanisms in human PAECs and PMVECS, and in patients with HPH under less severe hypoxic conditions. Third, although we confirmed that hypoxia upregulates ERβ in several cell types and in two species, we did not corroborate our mechanistic studies in human cells. We did, however, confirm the relevance of this signaling pathway in vivo, making the rat PAEC studies relevant. Mechanistic studies dissecting the interplay among HIFs, PHD, and ERβ in PAECs from healthy donors and patients with PH are currently ongoing in our laboratory. To eliminate potentially confounding effects of estrogens or the estrous cycle on ER expression, we performed the current study in male animals, and therefore do not provide information on ER expression in hypoxic females. Similarly, our commercially obtained human PAECs and PMVECs were derived from male donors. Studies in females are currently underway, and a sex-specific comparison of ER expression will be the focus of future investigations. Lastly, our studies focused on hypoxia-induced changes in the pulmonary vasculature and are therefore relevant for HPH and WHO group 3 PH, but cannot necessarily be extrapolated to PAH. Given that HIF-1α, HIF-2α, and female sex play major roles in PAH development, the cross-talk among HIF-1α, HIF-2α, E2, and ERβ in this disease needs to be explored further. The protective ERβ signaling noted in hypoxia may be lost or altered in PAH.
In summary, we provide the first evidence of a novel HIF-1α–dependent hypoxia pathway that induces ERβ, but not ERα, in PAECs and PMVECs. We show that the hypoxia-induced upregulation of ERβ is specific to the lung and pulmonary vasculature, as evidenced by the decrease in ERβ expression in the hypoxic RV and liver. We demonstrate that ERβ induces expression of the HIF-α inhibitor PHD2 and decreases HIF-1α and HIF-2α expression in vitro. In vivo, ERβ prevents increases in HIF-2α and is necessary for E2 to decrease lung HIF-1α and hypoxia-induced pulmonary vascular remodeling. Harnessing these pathways may facilitate the development of specific therapeutic strategies for patients with HPH and WHO group 3 PH, for whom there are no pharmacological treatment options, and help unravel the “estrogen paradox” in PAH.
Acknowledgments
Acknowledgment
The authors thank Dr. Robert G. Presson, Jr., for equipment support, Ms. Crystal Sorg and Mr. John Fierst for technical assistance, and Drs. Angelia Lockett and Kelly Schweitzer for technical advice.
Footnotes
Supported by VA Merit grant 1I01BX002042-01A2 (T.L.) and National Institutes of Health grants 5T32HL091816-05 (A.L.F.), NCATS 5TL1TR001107-02 (A.L.F.), and R01HL077328 (I.P.).
Author Contributions: A.L.F., M.S., I.P. and T.L. designed experiments, analyzed and interpreted data, and wrote the manuscript. A.L.F., M.S., J.A.W., M.A., B.Y., and T.L. performed experiments.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0167OC on February 2, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med. 2013;34:639–650. doi: 10.1016/j.ccm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 5.Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25) Suppl:D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Bärtsch P, Gibbs JSR. Effect of altitude on the heart and the lungs. Circulation. 2007;116:2191–2202. doi: 10.1161/CIRCULATIONAHA.106.650796. [DOI] [PubMed] [Google Scholar]
- 7.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307:L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 8.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, et al. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res. 2011;12:159. doi: 10.1186/1465-9921-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, et al. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ. 2013;3:564–577. doi: 10.1086/674312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, et al. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, et al. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab. 2007;293:E865–E871. doi: 10.1152/ajpendo.00201.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, et al. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, et al. Selective estrogen receptor-alpha and estrogen receptor-β agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1486–R1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 16.Frump AL, Albrecht ME, McClintick JN, Lahm T. Estrogen receptor-dependent attenuation of hypoxia-induced changes in the lung genome of pulmonary hypertension rats. Pulm Circ. 2017;7:232–243. doi: 10.1177/2045893217702055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 19.Mollerup S, Jørgensen K, Berge G, Haugen A. Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 20.Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of α and β estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol. 2002;188:125–140. doi: 10.1016/s0303-7207(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 21.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson JA. Estrogen receptor β—a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- 24.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: Estrogen receptor α and estrogen receptor β: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 26.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, et al. Estrogen receptor-β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc Natl Acad Sci USA. 2010;107:3123–3128. doi: 10.1073/pnas.0905524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slusarz A, Jackson GA, Day JK, Shenouda NS, Bogener JL, Browning JD, et al. Aggressive prostate cancer is prevented in ERαKO mice and stimulated in ERβKO TRAMP mice. Endocrinology. 2012;153:4160–4170. doi: 10.1210/en.2012-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 30.Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, et al. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol. 2002;26:610–616. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- 31.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, et al. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol. 2016;36:1584–1594. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Z, Li M, Wharton J, Zhu MM, Zhao Y-Y. PHD2 deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through HIF-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selej MW, Wood J, Lockett AD, Albrecht M, Schweitzer K, Petrache I, et al. Hypoxia increases expression of estrogen receptor (ER)-β in vivo and in vitro. Am J Resp Crit Care Med. 2013;187:A2257. [Google Scholar]
- 34.Selej MLA, Albrecht M, Petrache I, Lahm T. Hypoxia increases estrogen receptor β expression in cultured rat pulmonary artery endothelial cells. Am J Respir Crit Care Med. 2012;185:A4817. [Google Scholar]
- 35.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 38.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 39.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1) Suppl:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 41.Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32:619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- 42.Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]
- 43.Austin ED, Lahm T, West J, Tofovic SP, Johansen AK, Maclean MR, et al. Gender, sex hormones and pulmonary hypertension. Pulm Circ. 2013;3:294–314. doi: 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter I, Shearman AM, Vasan RS, Zucker DR, Schmid CH, Demissie S, et al. Association of estrogen receptor beta gene polymorphisms with left ventricular mass and wall thickness in women. Am J Hypertens. 2005;18:1388–1395. doi: 10.1016/j.amjhyper.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Peter I, Kelley-Hedgepeth A, Huggins GS, Housman DE, Mendelsohn ME, Vita JA, et al. Association between arterial stiffness and variations in oestrogen-related genes. J Hum Hypertens. 2009;23:636–644. doi: 10.1038/jhh.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim W, Cho J, Kwon HY, Park Y, Rhyu M-R, Lee Y. Hypoxia-inducible factor 1 α activates and is inhibited by unoccupied estrogen receptor β. FEBS Lett. 2009;583:1314–1318. doi: 10.1016/j.febslet.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Wu M-H, Lu C-W, Chang F-M, Tsai S-J. Estrogen receptor expression affected by hypoxia inducible factor-1α in stromal cells from patients with endometriosis. Taiwan J Obstet Gynecol. 2012;51:50–54. doi: 10.1016/j.tjog.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Wolff M, Kosyna FK, Dunst J, Jelkmann W, Depping R. Impact of hypoxia inducible factors on estrogen receptor expression in breast cancer cells. Arch Biochem Biophys. 2017;613:23–30. doi: 10.1016/j.abb.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Tremblay A, Giguère V. Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor β. J Steroid Biochem Mol Biol. 2001;77:19–27. doi: 10.1016/s0960-0760(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 50.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mak P, Chang C, Pursell B, Mercurio AM. Estrogen receptor β sustains epithelial differentiation by regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci USA. 2013;110:4708–4713. doi: 10.1073/pnas.1221654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright AF, Ewart MA, Mair K, Nilsen M, Dempsie Y, Loughlin L, et al. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res. 2015;106:206–216. doi: 10.1093/cvr/cvv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim W, Park Y, Cho J, Park C, Park J, Park YK, et al. Estrogen receptor beta inhibits transcriptional activity of hypoxia inducible factor-1 through the downregulation of arylhydrocarbon receptor nuclear translocator. Breast Cancer Res. 2011;13:R32. doi: 10.1186/bcr2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ginouvès A, Ilc K, Macías N, Pouysségur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFαand protects cells from necrosis. Proc Natl Acad Sci USA. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]