Highlights
-
•
A tailgut cyst, also called retrorectal cystic hamartoma, is a rare congenital lesion that forms most commonly in the retrorectal space.
-
•
They are often misdiagnosed and can rarely transform into neuroendocrine tumours.
-
•
A complete surgical resection is required upon diagnosis, preferably by a posterior approach when feasible.
-
•
Final diagnosis is made with the histopathological examination of the resected mass and appropriate follow-up must be ensured.
Keywords: Tailgut cyst, Neuroendocrine carcinoma, Malignant transformation
Abstract
Introduction
A tailgut cyst, also called retrorectal cystic hamartoma, is a rare congenital lesion that forms most commonly in the retrorectal space. It is presumed to arise from remnants of early embryogenesis.
Presentation of Case
The following report describes a unique case of a retrorectal cystic hamartoma in a 53 year-old French Canadian man with a history of low back pain. The tumour underwent malignant transformation into a well-differentiated neuroendocrine carcinoma three years after the beginning of symptoms.
Discussion
This condition can be found at any age, but occurs especially among middle-aged women. Not only is it frequently misdiagnosed, but also several complications associated to the cyst have been reported such as infection and malignant transformation. This is why complete surgical excision of the tailgut cyst is currently recommended.
1. Introduction
Tailgut cysts, or retrorectal cystic hamartomas, are uncommon congenital lesions occurring most of the time in the retrorectal space and among middle-aged women [1,2]. They have also rarely been found in the perirenal [[3], [4], [5]] and the prerectal spaces [1,5,6].
This lesion is thought to origin from an incomplete regression of a true tail located caudally to the future anus during early embryogenesis [9]. Although the cysts appear with non-specific signs and symptoms, an early diagnosis is the best way to avoid malignant transformation of the lesion, which is a rare but a possible complication [7]. Indeed, the following case report describes a 53 year-old French Canadian man with malignant neuroendocrine transformation of a tailgut cyst in accordance with the SCARE criteria [8].
The following work
2. Presentation of case
A 53-year-old man with a history of kidney surgery for a congenital malformation and a family history of prostate cancer, complained of low back pain for almost three years. Initially, the pain was located in the lumbar area only in the seated position. However, six months prior to consultation, the pain progressed and was also felt while walking. The physical examination did not reveal any apparent mass but palpation of the sacrococcygeal articulation provoked some pain to the patient. Initial investigation with magnetic resonance imaging (MRI) revealed a mass of 1.9 × 1.8 × 2.7 cm anterior to the coccyx. The mass was heterogeneous with a cystic part. On the T1-weighted images, a hyperintense cyst was visualized. The results then suggested either a possible chordoma or an exophytic giant cell tumour. The computed tomography (CT) scan, done two months later, confirmed the results of the MRI, also showing a cleavage plane between the rectum and the mass. Moreover, no calcification was seen in it. The patient underwent a coccygectomy with en bloc posterior surgical resection of the mass with negative resection margins. The rectum was left intact since the tumor was not invading it.
The gross examination showed a resected specimen measuring 7.6 × 3.9 × 2.5 cm. The resected coccyx was 3.7 cm of height, 1.0 cm of antero-posterior diameter and approximately 2.7 cm of width. A multiloculated cystic lesion with many different epithelial elements was observed: mucinous, ciliated columnar, transitional and malpighian (Fig. 1.). Within the wall of the cyst, a tumoral proliferation of polygonal, cuboidal and columnar cells was identified with irregular borders. The cells had an abundant acidophil cytoplasm with a round-shaped nucleus containing one to two nucleoli. The mitotic rate was 4–5 mitoses per 10 fields. The immunophenotype was positive for chromogranin (Fig. 2A), and synaptophysin (Fig. 2B), and keratin Cam 5.2 (Fig. 2C) in all cells, and a focal staining was positive for keratin AE1-AE3 and CD56. The proliferation index (Ki-67) was 5–10%. The entire study of the specimen suggested a well-differentiated, grade 2/3, neuroendocrine tumour (NET) arising in a retrorectal cystic hamartoma.
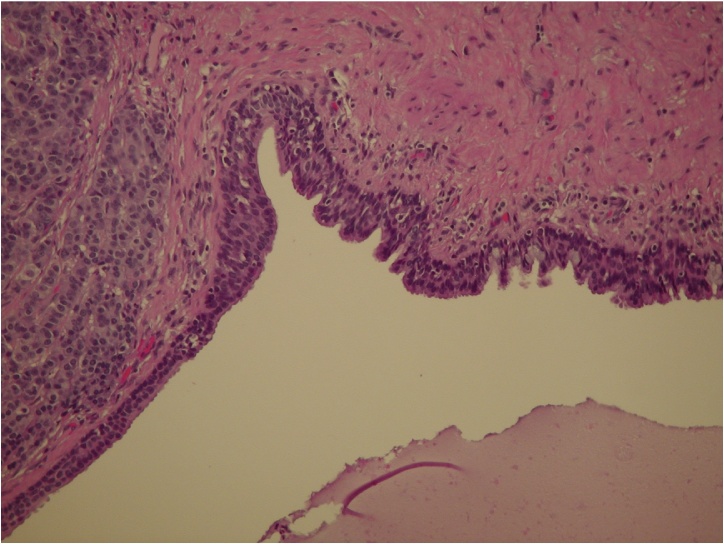
Fig. 1.
The tailgut cyst is lined here by glandular epithelial cells including goblet cells, transitional cells and ciliated columnar cells. Tumor cells adopting a trabecular pattern of growth are seen on the left.
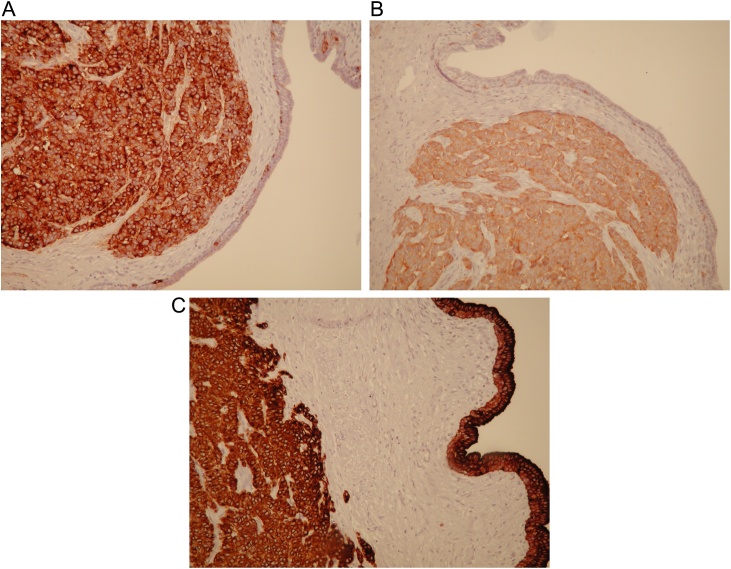
Fig. 2.
A. Tumour cells expressing chromogranin. B. Immunohistochemically, the tumour cells were positive for synaptophysin. C. The tumour cells and epithelial lining of the cyst were strongly positive for keratin Cam 5.2.
The patient was doing well and had no recurrence of pain two months following surgery. No mass was palpated upon digital rectal examination and the seric chromogranin A (CgA) level was normal. In addition to clinical history and physical exam, the patient was followed with CT scans and seric CgA.
Two years after surgical resection, the patient presented a left inguinal mass. An ultrasound revealed a potentially malignant lymph node. The patient underwent surgical resection of the lymph node that confirmed a distant recurrence of the neuroendocrine tumor. A complete metastatic workup revealed multiple, bilateral pulmonary nodules on the thoracic CT scan as well as mediastinal lymph nodes suspicious for malignancy. An octreotide scan confirmed the previously mentioned findings in addition to malignant foci in the liver.
The patient had neither carcinoid syndrome nor any other symptom of any functional NET. Considering the absence of respiratory symptoms and the extent of metastases, no surgical treatment nor local radiotherapy were offered. A long-acting somatostatin analog was prescribed in an attempt to limit tumour progression. Six months later, the patient received systemic Streptozocin/5-Fluorouracil chemotherapy treatment because of tumor progression. The tumor was controlled for 12 months but subsequent scans revealed progression of the pulmonary and liver metastases. The patient then received everolimus for 4 months but the tumor continued to progress on the scan.
The patient is currently receiving Peptide Receptor Radionuclide Therapy (PPRT) with close follow-up.
3. Discussion
Including this report, only 29 cases have been reported in the literature (Table 1). Tailgut cysts, also known as retrorectal cystic hamartomas, are uncommon congenital multicystic lesions occurring typically in the retrorectal space. They are bordered posteriorly by the sacrum, anteriorly by the rectum, superiorly by the peritoneal reflection, and inferiorly by the anal and coccygeus muscles. Many different lesions in addition to cystic hamartoma may be found in the retrorectal space. The most frequent in this area are teratomas for children and chordomas for adults [9].
Table 1.
Reported cases of neuroendocrine tumours arising in tailgut cysts.
| Case | Main author | Year (yr) | Age (yr) | Gender (M/F) |
|---|---|---|---|---|
| 1 | Hood [30] | 1988 | NAa | NA |
| 2 | Hood [30] | 1988 | 50 | F |
| 3 | Lin [31] | 1992 | 18 | F |
| 4 | Schnee [32] | 1994 | 62 | M |
| 5 | Hornstein [33] | 1998 | 19 | F |
| 6 | Prasad [2] | 1999 | 69 | F |
| 7 | Oyama [34] | 2000 | 52 | M |
| 8 | Mourra [35] | 2003 | 68 | M |
| 9 | Jacob [36] | 2004 | NA | NA |
| 10 | Song [37] | 2004 | 41 | F |
| 11 | Mathieu [38] | 2005 | 49 | F |
| 12 | Lee [39] | 2007 | 40 | F |
| 13 | Liang [14] | 2008 | 51 | F |
| 14 | La Rosa [40] | 2010 | 73 | F |
| 15 | Mathis [11] | 2010 | NA | NA |
| 16 | Wöhlke [41] | 2011 | 55 | F |
| 17 | Spada [42] | 2011 | 63 | F |
| 18 | Spada [42] | 2011 | 41 | F |
| 19 | Misawa [43] | 2013 | 53 | F |
| 20 | Damato [44] | 2013 | NA | F |
| 21 | Kim [45] | 2014 | 49 | M |
| 22 | Charalampakis [7] | 2014 | 35 | M |
| 23 | Abukar [46] | 2014 | 61 | M |
| 24 | Sharma [47] | 2014 | 30 | F |
| 25 | Mitsuyama [15] | 2015 | 53 | M |
| 26 | Jehangir [48] | 2016 | 74 | M |
| 27 | Mora-Guzmán [49] | 2017 | 56 | F |
| 28 | Singh [50] | 2018 | 63 | M |
| 29 (Present case) |
Al Khaldi | 2018 | 53 | M |
NA: not available.
Although the origin of the retrorectal cystic hamartoma is unknown, its lining cells and its main location in the retrorectal space gave rise to the hypothesis that it originates from the remnant of the normally regressing hindgut tail present in the embryo in early embryogenesis. Between days 28 and 35 of the embryogenesis, the embryo possesses a true tail which further differentiates into various organs. The hindgut extends into this tail and the name tailgut is given to this entity. The tailgut is then distal to the future anus and normally undergoes involution later in the embryogenesis. It is thought that the remnant of this structure could be the origin of the retrorectal cystic hamartoma [1,2,10].
Recurrent infection, perianal fistulas or malignant transformation may complicate tailgut cysts. In a recent study focused on the surgical outcome of tailgut cysts [11], malignant transformation occured in 13% of the 31 patients followed, which is much greater than the previously established risk of 2% [1]. Malignant transformation results most commonly in an adenocarcinoma or a neuroendocrine carcinoma [12].
Interestingly, tailgut cysts have been reported to be seen three times more often in middle-aged women than in men [13]. Liang et al. proposed that female predominance for carcinoid tumours arising in the cyst could probably be explained by a hormone-related phenomenon because of the numerous estrogen receptors that could be found on the tumour cells. However, other studies are needed to validate this hypothesis [14].
The clinical diagnosis of this entity is difficult because it is mostly asymptomatic or it manifests with non-specific mass effect symptoms, such as low back pressure or pain with defecation, rectal bleeding or neurological symptoms associated with spinal cord tethering [1,2,9,15].
Tailgut cysts are usually misdiagnosed and mistaken for an abscess or a perianal fistula. They can be palpated as fluctuant masses on digital rectal examination. On CT scan or MRI, tailgut cysts are visualized as retrorectal cystic masses, most frequently multilocular with a large central cyst and smaller cysts in the periphery. Signs of underlying infection or malignancy include indistinct cyst margins, nodular wall thickening and enhancement, intracystic vegetations, surrounding lymphadenopathy and superior extension above the level of S3 [16].
A preoperative biopsy of the mass is not recommended because of the risk of infection, spilling malignant cells into the abdominal cavity or seeding of the biopsy tract [17].
Complete surgical resection of tailgut cysts is recommended to prevent malignant transformation [11]. Three different surgical approaches for resection of retrorectal tumours have been described: the posterior approach, the anterior approach and the combined anteroposterior approach [18]. The first approach, also known as the transperineal or transacrococcygeal approach, is recommended for tumours confined to the S3 level or below and when there is a doubt about bone involvement. It permits direct exposure of the lesion and adds the possibility of performing a coccygectomy. The second approach has the advantage of offering a good view of all pelvic structures with the abdominal exposition and can be considered for relatively high-lying lesions (above S3) without sacral involvement. The third approach is the anteroposterior one, which is a combination of both techniques, consisting of opening the sacrum posteriorly and joining the abdominal dissection of the front. This approach provides a better visualization of all the involved structures and a wider range of resection in a restrained area such as the retrorectal space [14,19,20]. In the study conducted by Kye et al., all patients underwent complete excision of the mass. The posterior approach for benign cysts has been shown to cause fewer complications in comparison to the two other approaches. Furthermore, a coccygectomy is not necessary in most cases, provided the mass is not adherent to the coccyx.
A laparoscopic tailgut cyst resection has also been attempted in a few cases and was both feasible and safe [21], as well as a transanal endoscopic microsurgery technique [22]. However, more studies are needed to evaluate these minimally-invasive approaches.
Follow-up of tailgut cysts without signs of malignancy is mainly clinical. Imaging is used if recurrence is suspected upon clinical history and physical examination [16]. However, in malignant cases, considering the ability of neuroendocrine tumors to metastasize, post-surgical follow-up requires regular imaging as well as plasma CgA level measurement [23].
Currently, there is no standard systemic therapy for metastatic neuroendocrine tumors arising from tailgut cysts. Indeed, systemic treatment is mostly limited to neuroendocrine tumors of gastroenteropancreatic origin [24]. In addition to treating symptoms caused by NETs, somatostatin analogs exhibit an antitumor effect, lengthening the time to tumor progression [15,25].
Another treatment modality, Peptide Receptor Radionuclide Therapy (PRRT), a molecular radioisotope therapy for patients with metastatic carcinoid tumors refractory to octreotide treatment, is associated with better clinical outcome [26].
Several systemic chemotherapy regimens have also been studied in the litterature. In this case, the patient received a combination of 5-Fluorouracil with Streptozotocin, a regimen that most oncologists prescribe in the case of advanced, progressive and non-resectable carcinoid tumours [27]. A combination of capecitabine and temozolomide may prolong survival in patients with metastatic NET to the liver who have progressed on previous therapies [28].
Finally, Everolimus, an inhibitor of mammalian target of rapamycin (mTOR), has been demonstrated as an effective systemic therapy for patients with advanced NETs of the lung and gastrointestinal tract [29].
4. Conclusion
We herein presented the case of a 53-year-old French Canadian man with a well-differentiated neuroendocrine carcinoma arising in a tailgut cyst. Including this report, only 29 cases have been reported in the literature to this day and most of them concerned middle-aged women. Tailgut cysts are rare congenital lesions that manifest non-specific signs on clinical history. They are often misdiagnosed and can sometimes exhibit malignant transformation. A complete surgical resection is required upon diagnosis, preferably by a posterior approach when feasible. Final diagnosis is made with the histopathological examination of the resected mass and appropriate follow-up must be ensured.
Conflicts of interest
There are no conflicts of interest to disclose.
Funding
None.
Ethical approval
Ethical approval has been exempted by our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
- Maher Al Khaldi (primary author): conception and design of the article, acquisition of data, analysis and interpretation of data, drafting of the article, critical revision for important intellectual content.
- Amanda Mesbah (secondary author): conception and design of the article, acquisition of data, analysis and interpretation of data, drafting of the article, critical revision for important intellectual content.
- Josée Doyon: acquisition of data, analysis and interpretation of data, drafting of the article, critical revision for important intellectual content.
- Marc Isler: acquisition of data, analysis and interpretation of data.
- Andrew Mitchell: acquisition of data, analysis and interpretation of data.
- Pierre Dubé: critical revision for important intellectual content, approval of the final version to be published.
- Lucas Sideris (senior author): conception and design of the article, acquisition of data, analysis and interpretation of data, drafting of the article, critical revision for important intellectual content, approval of the final version to be published.
All cited authors have agreed to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Registration of Research Studies
None.
Guarantor
Lucas Sideris.
Acknowledgements
We thank all physicians for their commendable efforts for caring for the patient.
References
- 1.Hjermstad B.M., Helwig E.B. Tailgut cysts: report of 53 cases. Am. J. Clin. Pathol. 1988;89(February (2)):139–147. doi: 10.1093/ajcp/89.2.139. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A.R., Amin M.B., Randolph T.L., Lee C.S., Ma C.K. Retrorectal cystic hamartoma: report of 5 cases with malignancy arising in 2. Arch. Pathol. Lab. Med. 2000;124(May (5)):725–729. doi: 10.5858/2000-124-0725-RCH. [DOI] [PubMed] [Google Scholar]
- 3.Sung M.T., Ko S.F., Niu C.K., Hsieh C.S., Huang H.Y. Perirenal tailgut cyst (cystic hamartoma) J. Pediatr. Surg. 2003;38(September (9)):1404–1406. doi: 10.1016/s0022-3468(03)00408-1. [DOI] [PubMed] [Google Scholar]
- 4.Kang J.W., Kim S.H., Kim K.W., Moon S.K., Kim C.J., Chi J.G. Unusual perirenal location of a tailgut cyst. Korean J. Radiol. 2002;3(December (4)):267–270. doi: 10.3348/kjr.2002.3.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills S.E., Walker A.N., Stallings R.G., Allen J.M. Retrorectal cystic hamartoma. Report of three cases, including one with a perirenal component. Arch. Pathol. Lab. Med. 1984;108(September (9)):737–740. [PubMed] [Google Scholar]
- 6.Jang S.H., Jang K.S., Song Y.S., Min K.W., Han H.X., Lee K.G., Paik S.S. Unusual prerectal location of a tailgut cyst: a case report. World J. Gastroenterol. 2006;12(August (31)):5081. doi: 10.3748/wjg.v12.i31.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charalampakis V., Stamatiou D., Christodoulakis M., Kafousi M., Chryssou E., de Bree E., Melissas J. A large presacral tailgut cyst with a carcinoid tumor in a male: report of a case. Surg. Today. 2014;44(May (5)):961–966. doi: 10.1007/s00595-012-0482-4. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Dahan H., Arrivé L., Wendum D., le Pointe H.D., Djouhri H., Tubiana J.M. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics. 2001;21(May (3)):575–584. doi: 10.1148/radiographics.21.3.g01ma13575. [DOI] [PubMed] [Google Scholar]
- 10.Tampi C., Lotwala V., Lakdawala M., Coelho K. Retrorectal cyst hamartoma (tailgut cyst) with malignant transformation. Gynecol. Oncol. 2007;105(April (1)):266–268. doi: 10.1016/j.ygyno.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Mathis K.L., Dozois E.J., Grewal M.S., Metzger P., Larson D.W., Devine R.M. Malignant risk and surgical outcomes of presacral tailgut cysts. Br. J. Surg. 2010;97(April (4)):575–579. doi: 10.1002/bjs.6915. [DOI] [PubMed] [Google Scholar]
- 12.Lu N.H., Tseng M.J. Laparoscopic management of tailgut cyst: case report and review of literature. J. Minim. Invasive Gynecol. 2010;17(November (6)):802–804. doi: 10.1016/j.jmig.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Au E., Anderson O., Morgan B., Alarcon L., George M.L. Tailgut cysts: report of two cases. Int. J. Colorectal Dis. 2009;24(March (3)):345–350. doi: 10.1007/s00384-008-0598-6. [DOI] [PubMed] [Google Scholar]
- 14.Liang J.J., Alrawi S., Fuller G.N., Tan D. Carcinoid tumors arising in tailgut cysts may be associated with estrogen receptor status: case report and review of the literature. Int. J. Clin. Exp. Pathol. 2008;1(6):539. [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsuyama T., Kubota M., Nakamura Y., Yuzurihara M., Hoshi K., Okada Y. Neuroendocrine tumor arising from tailgut cyst with spinal cord tethering: case report and literature review. Spine J. 2015;15(February (2)):e1–e8. doi: 10.1016/j.spinee.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Shetty A.S., Loch R., Yoo N., Mellnick V., Fowler K., Narra V. Imaging of tailgut cysts. Abdom. Imaging. 2015;40(October (7)):2783–2795. doi: 10.1007/s00261-015-0463-3. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz R.E., Lyda M., Lew M., Paz I.B. A carcinoembryonic antigen-secreting adenocarcinoma arising within a retrorectal tailgut cyst: clinicopathological considerations. Am. J. Gastroenterol. 2000;95(May (5)):1344. doi: 10.1111/j.1572-0241.2000.02023.x. [DOI] [PubMed] [Google Scholar]
- 18.Kye B.H., Kim H.J., Cho H.M., Chin H.M., Kim J.G. Clinicopathological features of retrorectal tumors in adults: 9 years of experience in a single institution. J. Korean Surg. Soc. 2011;81(August (2)):122–127. doi: 10.4174/jkss.2011.81.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchs N., Taylor S., Roche B. The posterior approach for low retrorectal tumors in adults. Int. J. Colorectal Dis. 2007;22(April (4)):381–385. doi: 10.1007/s00384-006-0183-9. [DOI] [PubMed] [Google Scholar]
- 20.Localio S.A., Eng K., Ranson J.H. Abdominosacral approach for retrorectal tumors. Ann. Surg. 1980;191(May (5)):555. doi: 10.1097/00000658-198005000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim S.W., Huh J.W., Kim Y.J., Kim H.R. Laparoscopy-assisted resection of tailgut cysts: report of a case. Case Rep. Gastroenterol. 2011;5(1):22–27. doi: 10.1159/000322912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serra Aracil X., Gómez Díaz C., Bombardó Junca J., Mora López L., Alcántara Moral M., Ayguavives Garnica I., Navarro Soto S. Surgical excision of retrorectal tumour using transanal endoscopic microsurgery. Colorectal Dis. 2010;12(June (6)):594–595. doi: 10.1111/j.1463-1318.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong J.F., Teo C.C. An unusual case of presacral carcinoid tumor and the approach of management. Am. J. Can. Case Rep. 2013;1(March (1)):28–34. [Google Scholar]
- 24.Basu B., Sirohi B., Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr. Relat. Cancer. 2010;17(March (1)):R75–90. doi: 10.1677/ERC-09-0108. [DOI] [PubMed] [Google Scholar]
- 25.Sidéris L., Dubé P., Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist. 2012;17(June (6)):747–755. doi: 10.1634/theoncologist.2011-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., Bushnell D. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. New Eng. J. Med. 2017;376(January (2)):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rougier P., Mitry E. Chemotherapy in the treatment of neuroendocrine malignant tumors. Digestion. 2000;62(Suppl. 1):73–78. doi: 10.1159/000051859. [DOI] [PubMed] [Google Scholar]
- 28.Fine R.L., Gulati A.P., Krantz B.A., Moss R.A., Schreibman S., Tsushima D.A., Mowatt K.B., Dinnen R.D., Mao Y., Stevens P.D., Schrope B. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the pancreas Center at Columbia University experience. Cancer Chemother. Pharmacol. 2013;71(March (3)):663–670. doi: 10.1007/s00280-012-2055-z. [DOI] [PubMed] [Google Scholar]
- 29.Yao J.C., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., Tomasek J., Raderer M., Lahner H., Voi M., Pacaud L.B. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(March (10022)):968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood D.L. Retrorectal cystic hamartoma; report of five cases with carcinoid tumor arising in two. Am. J. Clin. Pathol. 1988;89:433. [Google Scholar]
- 31.Lin S.L., Yang A.H., Liu H.C. Tailgut cyst with carcinoid: a case report. Zhonghua yi xue za zhi. Chin. Med. J. Free China Ed. 1992;49(January (1)):57–60. [PubMed] [Google Scholar]
- 32.Schnee C.L., Hurst R.W., Curtis M.T., Friedman E.D. Carcinoid tumor of the sacrum: case report. Neurosurgery. 1994;35(December (6)):1163–1167. doi: 10.1227/00006123-199412000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Horenstein M.G., Erlandson R.A., Gonzalez-Cueto D.M., Rosai J. Presacral carcinoid tumors: report of three cases and review of the literature. Am. J. Surg. Pathol. 1998;22(February (2)):251–255. doi: 10.1097/00000478-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Oyama K., Embi C., Rader A.E. Aspiration cytology and core biopsy of a carcinoid tumor arising in a retrorectal cyst: a case report. Diag. Cytopathol. 2000;22(June (6)):376–378. doi: 10.1002/(sici)1097-0339(200006)22:6<376::aid-dc9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Mourra N., Caplin S., Parc R., Flejou J.F. Presacral neuroendocrine carcinoma developed in a tailgut cyst. Dis. Colon. Rectum. 2003;46(March (3)):411–413. doi: 10.1007/s10350-004-6564-7. [DOI] [PubMed] [Google Scholar]
- 36.Jacob S., Dewan Y., Joseph S. Presacral carcinoid tumour arising in a tailgut cyst--a case report. Indian J. Pathol. Microbiol. 2004;47(January (1)):32–33. [PubMed] [Google Scholar]
- 37.Song D.E., Park J.K., Hur B., Ro J.Y. Carcinoid tumor arising in a tailgut cyst of the anorectal junction with distant metastasis: a case report and review of the literature. Arch. Pathol. Lab. Med. 2004;128(May (5)):578–580. doi: 10.5858/2004-128-578-CTAIAT. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu A., Chamlou R., Le Moine F., Maris C., Van De Stadt J., Salmon I. Tailgut cyst associated with a carcinoid tumor: case report and review of the literature. Histol. Histopathol. 2005;20(October (4)):1065–1070. doi: 10.14670/HH-20.1065. [DOI] [PubMed] [Google Scholar]
- 39.Lee C.M., Lee S.H., Jeon C.W., Ahn B.K., Baek S.U. Carcinoid tumor arising within a tailgut cyst-a case report. J. Korean Soc. Coloproctol. 2007;23(February (1)):65–67. [Google Scholar]
- 40.La Rosa S., Boni L., Finzi G., Vigetti D., Papanikolaou N., Tenconi S.M., Dionigi G., Clerici M., Garancini S., Capella C. Ghrelin-producing well-differentiated neuroendocrine tumor (carcinoid) of tailgut cyst. Morphological, immunohistochemical, ultrastructural, and RT-PCR study of a case and review of the literature. Endocr. Pathol. 2010;21(September (3)):190–198. doi: 10.1007/s12022-010-9127-6. [DOI] [PubMed] [Google Scholar]
- 41.Wöhlke M., Sauer J., Dommisch K., Görling S., Valdix A., Hinze R. Primary metastatic well-differentiated neuroendocrine tumor arising in a tailgut cyst. Der. Pathol. 2011;32(March (2)):165–167. doi: 10.1007/s00292-010-1390-2. [DOI] [PubMed] [Google Scholar]
- 42.Spada F., Pelosi G., Squadroni M., Lorizzo K., Farris A., De Braud F., Fazio N. Neuroendocrine tumour arising inside a retro-rectal tailgut cyst: report of two cases and a review of the literature. Ecancermedicalscience. 2011;5 doi: 10.3332/ecancer.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misawa S.I., Horie H., Yamaguchi T., Kobayashi S., Kumano H., Lefor A.T., Yasuda Y. A unique retrorectal tumor with neuroendocrine differentiation: case report and review of the literature. Int. J. Surg. Pathol. 2013;21(June (3)):271–277. doi: 10.1177/1066896913476738. [DOI] [PubMed] [Google Scholar]
- 44.Damato A., Pusceddu S., Milione M., Mazzaferro V., Magli M., Seregni E., De Braud F., Buzzoni R. Well-differentiated neuroendocrine tumor of tailgut cyst. A rare entity with controversial medical opportunities. Tumori J. 2013;99(4):148–151. doi: 10.1177/030089161309900422. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.H., Jin S.Y., Hong S.S., Lee T.H. A carcinoid tumour arising within a tailgut cyst: a diagnostic challenge. Scott. Med. J. 2014;59(Frbruary (1)):e14–e17. doi: 10.1177/0036933013519029. [DOI] [PubMed] [Google Scholar]
- 46.Abukar A.A., Parcell B.J., Lim C.B., Patil P.V., Ramsanahie A., Carey F., Steele R.J., Thaha M.A. Malignancy within a tail gut cyst: a case of retrorectal carcinoid tumour. Case Rep. Surg. 2014;2014 doi: 10.1155/2014/454502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma M., Madan M., Manjari M., Garg S. Primary pre-sacral carcinoid tumor: a rare entity. Glob. J. Med. Res. 2014;(November (5)) [Google Scholar]
- 48.Jehangir A., Le B.H., Carter F.M. A rare case of carcinoid tumor in a tailgut cyst. J. Community Hosp. Intern. Med. Perspect. 2016;6(January (30):31410. doi: 10.3402/jchimp.v6.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mora-Guzmán I., Alonso-Casado A., Rodríguez Sánchez A., Bermejo Marcos E. Neuroendocrine tumour arising inside a tailgut cyst. Ann. R. Coll. Surg. Engl. 2017;99(February (2)):e91–e93. doi: 10.1308/rcsann.2016.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh A., Karnik S., Khedkar B., Deshmukh S., Deodhar K. A well-differentiated neuroendocrine tumor (grade I) arising in a tailgut cyst. J. Cancer Res. Ther. 2018 doi: 10.4103/0973-1482.189236. http://www.cancerjournal.net/preprintarticle.asp?id=189236 ahead of print. [DOI] [PubMed] [Google Scholar]