Abstract
Natural products are vital in drug discovery and the search for anticancer agents has been significant importance to the researchers for a long time. In the present study, aqueous leaf extract of Pouteria sapota (P.sapota) was evaluated for its cytotoxic activity. The leaf extract was preliminarily screened for antioxidant activity using DPPH method for Radical Scavenging Activity, Hydrogen Peroxide Scavenging Activity and Reducing Power Activity. Further, the aqueous leaf extract was screened for cytotoxic activity against breast cancer cell lines (MCF-7) in vitro. The results of the study showed that aqueous extract of the P.sapota leaf was rich in phytochemicals, antioxidant activity and showed a significant anti-cancer activity against tested MCF-7 cell lines. The present study was designed to evaluate the anticancer potential of P.sapota leaf. The antioxidants present in P.sapota have strong cytotoxic activity suggests that it can be considered for anti-cancer treatment.
Keywords: Pouteria sapota, Anti-cancer activity, Anti-oxidants, In vitro, MCF-7
Graphical abstract
Highlights
-
•
The P. sapota showed a significant anti-cancer activity against cell lines.
-
•
The GC analysis showed many active constituents.
-
•
The active constituents exhibit biological activity.
1. Introduction
Medicinal plants are effective anticancer agents since centuries [1]. Different parts of medicinal plants were investigated in order to find out its anti-cancer agents [2]. The anti-oxidants present in the medicinal plants was possibly responsible for the anticancer activity [3]. Likewise, P.sapota is a well-known fruit crop not majorly investigated for its medicinal and biological properties. This plant is also known as “mamay” in native Central America, Mexico and in many parts of the world; the plant was majorly grown for its fruits, which are enriched with abundant of nutrients [4]. The leaf extract was found to be effective biologically against blowfly [5]. However, the parts of the plants were not deeply studied for its biological activity. Hence, the present study was designed to find the in-vitro antioxidant and cytotoxic activity of P.sapota leaf aqueous extract.
2. Materials and methods
2.1. Chemicals
All the chemicals used for this study are of analytical grade and were purchased from Sigma Aldrich, USA; Roche, Germany; and SD Fine Chemicals, India.
2.2. Sample collection
The fresh leaves of P.sapota (500 g) were collected from Botanical Garden of VIT University, Vellore, Tamil Nadu, India. Leaf samples were identified by taxonomist at Department of Biological Sciences, VIT University. A voucher specimen was deposited at VIT plant repository for further reference. Immediately after collection, leaves were cleaned with distilled water extensively, wiped with sterile cotton and shade dried under room temperature. Air dried plant leaves were then pulverized into fine powder mechanically and stored at − 20 °C until use.
2.3. Preparation of extract
Aqueous extract of P.sapota was carried out by adopting the previous methodology with slight modification [6]. In brief 500 g of pulverized P.sapota leaf powder was soaked in 250 ml of distilled water at room temperature (26 ± 1 °C) for 48 h under continuous orbital shaking (125 rpm). The resultant mixture was then filtered and concentrated by lyophilizer. The lyophilized aqueous extract weighing 26.3 g was used for further biological assay experiment.
2.4. Antioxidant Assays
2.4.1. DPPH method for radical scavenging activity
The radical scavenging activity of P.sopata leaf extract was estimated using DPPH method [7], [8], [9]. In this assay, 0.1 mM solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of the aqueous extracts of the sample at different concentrations (25, 50, 75 and 100 µg/ml) with the standard ascorbic acid. These mixtures were shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance was measured at 517 nm using UV–VIS spectrophotometer and the results obtained were inversely proportional to radical scavenging activity.
The percentage (%) of radical scavenging activity is measured by the formula
where, A1 is OD of test sample and A0 is OD of control
2.4.2. Reducing power activity
The reducing power activity of P.sopata leaf extract was estimated using standard method [10], [11]. In this method, 0.1 ml of the leaf extract of different concentrations (25, 50, 75 and 100 µg/ml) was mixed with 2.5 ml of phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferric cyanide respectively. All tubes were incubated at 50 °C for 20 min and after incubation 2.5 ml of 10% trichloroacetic acid was added to each test tube. Then the tubes were centrifuged at 10,000 rpm for 10 min, to the 5 ml supernatant (upper layer) of the centrifuged samples 5 ml distilled water was added and mixed well. To the prepared 10 ml of samples 1 ml of 0.1% ferric chloride was added correspondingly in each tube. Finally, the absorbance of each sample was measured at 700 nm against a blank (distilled water).
The percentage inhibition was calculated by the equation.
where, A1 is OD of test sample and A0 is OD of control
2.4.3. Hydrogen peroxide Scavenging Activity
The Hydrogen peroxide Scavenging Activity was determined according to the standard method [12], [13]. In this method, 1 ml of the sample in different concentrations of (25, 50, 75 and 100 µg/ml) was mixed with 2 ml hydrogen peroxide solution respectively. These tubes were incubated at room temperature for 10 min. After incubation absorbance of the samples were checked at 230 nm in a spectrophotometer.
The percentage inhibition was calculated by the equation
where, A1 is OD of test sample and A0 is OD of control
2.5. MTT assay
The human breast cancer cell lines (MCF-7) obtained from National Centre for Cell Sciences (NCCS), Pune, India was used for MTT ((3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrzolim bromide) assay [14], [15]. The MCF-7 cell lines were grown and maintained in MEM medium in a 5% CO2 incubator at 37 °C respectively. Further, the cell suspension was harvested by centrifugation and the adherent cells were released from their substrate by trypsinization or scraping. Later the cells were resuspended in the medium at a quantity of 1 × 106 per ml. Further, a serial dilution was done to dilute the cells from 1 × 106 to 1 × 103 cells per ml respectively. Thereby, 0.1 ml of the above dilutions was plated out into the wells of a microtiter plate and control was maintained with medium alone. The cells were incubated for 12 h at 37 °C and 0.01 ml of MTT reagent (prior to the experiment MTT was dissolved in phosphate buffered saline (pH 7.4) and stored at 4 °C) was added to each well including controls and incubated for another 4 h correspondingly. Then the cells were periodically viewed under a microscope for the presence of intracellular punctuates purple precipitate. When the purple precipitate was clearly visible 0.1 ml of solubilization solution [It is a combination of 40% dimethylformamide in 2% glacial acetic acid mixed with 16% SDS (pH 4.7) and stored at room temperature] was added to all the wells, including controls and mixed well without shaking. The absorbance was recorded at 570 nm and the percentage of cell viability is calculated by using the following equation.
3. Results
3.1. Antioxidant assays
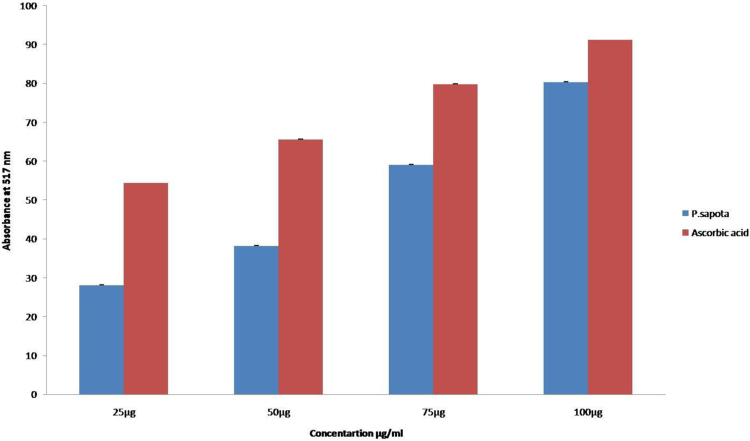
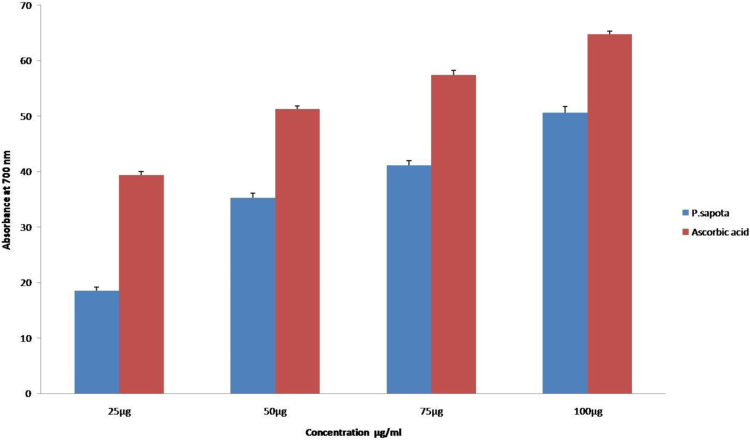
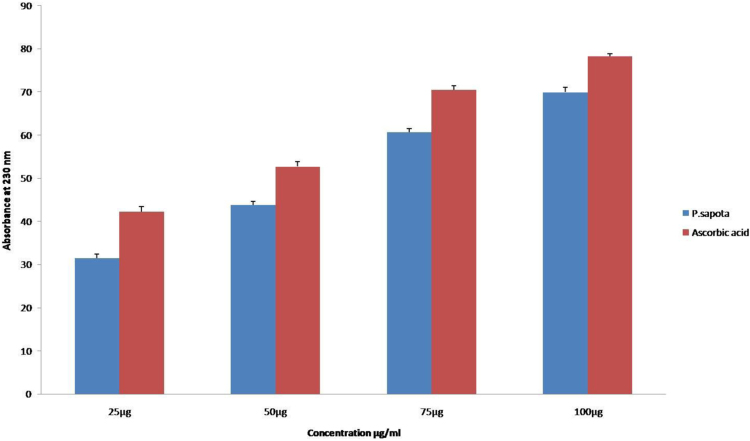
The DPPH assay shows the presence of antioxidant capacity among the concentrations of the sample simultaneously. The antioxidant activity showed an equivalent strength to that of the standard, ascorbic acid comparatively (Fig. 1). The antioxidants present in the P.sopata leaf extract reduce ferric cyanide to ferrous compound with a strong reducing power capacity at all the concentrations (25 µg, 50 µg, 75 µg and 100 µg) respectively (Fig. 2). The leaf extract of P.sopata showed a strong ability to scavenge hydrogen peroxide with the standard ascorbic acid (Fig. 3). The antioxidants present in the leaf extract were responsible for the scavenging activity, thus all the concentrations of the sample (25 µg, 50 µg, 75 µg, and 100 µg) showed inhibitory action for the production of free hydroxyl radicals.
Fig. 1.
The graphical representation of the DPPH antioxidant activity of P.sapota leaf extract with the standard ascorbic acid.
Fig. 2.
The graphical representation of the Ferric acid reducing assay of P.sapota leaf extract with the standard ascorbic acid.
Fig. 3.
The graphical representation of the hydrogen peroxide radical scavenging assay of P.sapota leaf extract with the standard ascorbic acid.
3.2. MTT Assay
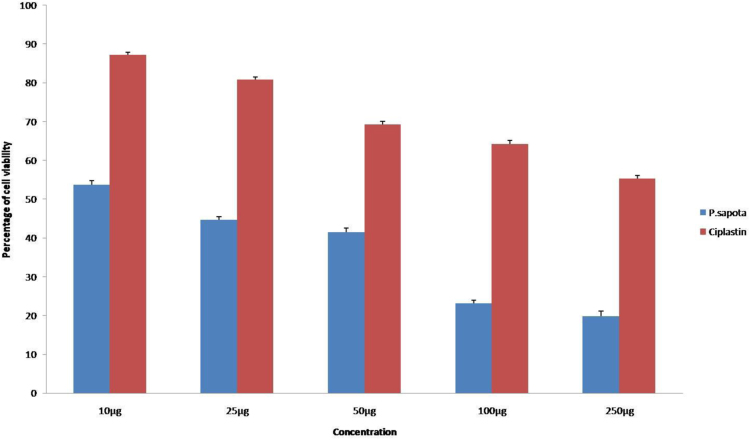
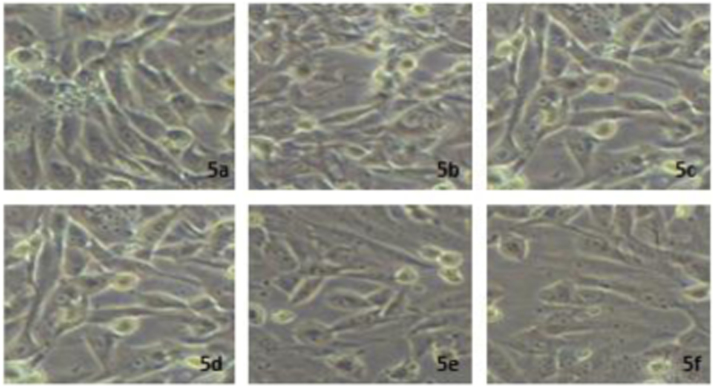
Using MTT assay, the effect of P.sapota leaf extract on breast cancer cell lines (MCF-7) cell proliferation was evaluated [16]. The assay has exposed the cytotoxic effect of leaf extract on cancer cells with cisplatin as the control (Fig. 4) apparently inducing its cell proliferation. The results showed changes in the cell morphology in all concentrations (25 µg–125 µg) (Fig. 5).
Fig. 4.
The quantitative comparison of cytotoxicity effect of P.sopata leaf extract with the standard Ciplastin.
Fig. 5.
MTT images show the effect of P.sapota leaf extract on breast cancer cell line MCF-7 at different concentarions with ciplastin as the control (5a) Control; 5b) 10 µg; 5c) 25 µg; 5d) 50 µg; 5e) 100 µg; 5 F) 250 µg).
4. Discussion
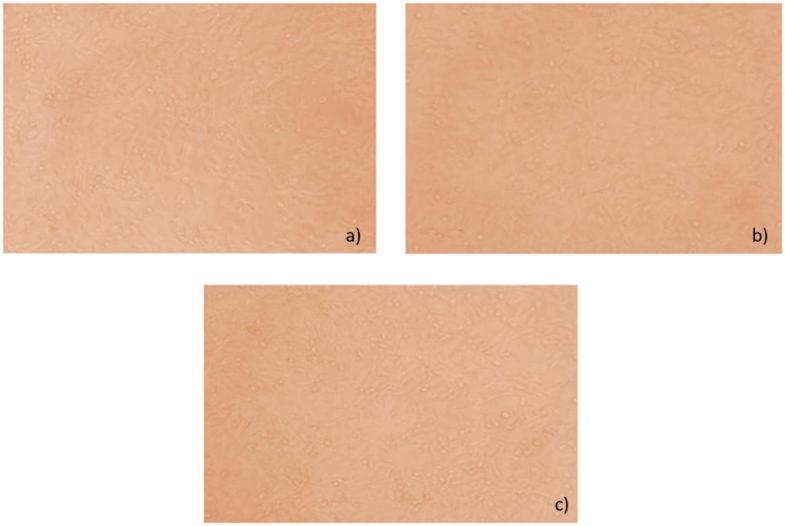
Antioxidants are capable of either suppressing or inhibiting the oxidation processes that are occurring in the presence of atmospheric oxygen or any reactive oxygen species [17]. DPPH assay was the most reliable antioxidant assay to determine the antioxidants present in medicinal plants [18], [19], [20]. So far, the antioxidant capacity of P.sapota leaf has been not reported in the literature. This study found the strong antioxidant activity using DPPH assay in four different concentrations (25 µg, 50 µg, 75 µg, and 100 µg). The results were similar to the antioxidant activity results that were reported in earlier studies [21], [22], [23], [24]. In addition to this, the reducing power activity directly reflects the antioxidant capacity of the sample [25], [26]. The reducing power activity of P.sopata leaf extract was due to the presence of antioxidants that are capable to break the free radicals by donating its hydrogen atom and rapid decomposition of hydrogen peroxide into oxygen resulting in the neutralization of water [10]. Further, the hydrogen peroxide scavenging activity confirms the ability of antioxidants present in the P.sapota leaf extract, where decreasing the levels of prooxidants was noticed [27], [28]. The resultant inhibitory activities are shown by P. sapota leaf extract in hydrogen peroxide scavenging assay increases along with the concentration of the extract. Which means the antioxidant activity depends on the concentration of the extract. Fig. 6
Fig. 6.
MTT images show the effect of P.sapota leaf extract on breast cancer cell line MDAMB-231 at different concentarions with ciplastin as the control (6a) Control; 6b) 10 µg; 6c) 320 µg).
Once the presence of rich antioxidants was confirmed using the antioxidant assays, anticancer activity was evaluated using MTT assay. MTT assay is a commonly used calorimetric assay to evaluate the metabolic activity of the cells [29]. This assay measures the cytotoxic activity caused by leaf extract under optimum conditions, at the end of the assay, the purple color product was formed due to the enzymatic reduction of tetrazolium dye to an insoluble form, formazan [30], [31]. So far, quercetin, a bioactive compound was isolated from P.sapota fruit and its anticancer activity was proved in cancer cell lines, but not from the leaves [32]. Similarly, many medicinal plants were evaluated for anticancer activity against breast cancer cell lines (MCF-7) [33], [34], [35], [36], [37], [38]. The present study reveals the potency of antioxidant activity increases with its concentration. The antioxidant activity of the leaf extract was believed to be the primarily responsible for cytotoxicity activity against the cancer lines. This procasts a new perception for the leaf extract and it can be used as an efficient adjuvant in the treatment of cancer.
5. Conclusion
The work aimed at studying the in vitro antioxidant and cytotoxic activity of P.sapota aqueous extract. The antioxidant extract showed cytotoxic activity at different concentrations against the cancer cell lines (MCF-7) with the standards comparatively. This allows for a new perspective of its use in a situation that involves oxidative stress and cell proliferation. In future, the extract can be formulated and it can be used in the treatment of cancer. However, in vivo experiments should be carried out for a better understanding of the mechanism.
Acknowledgment
The author thanks the VIT University for providing VIT SEED GRANT for carrying out this research work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.06.004.
Appendix A. Transparency document
Supplementary material
References
- 1.Ahmad R., Ahmad N., Naqvi A.A., Shehzad A., Al-Ghamdi M.S. Role of traditional Islamic and Arabic plants in cancer therapy. J. Tradit. Complement. Med. 2017;7(2):195–204. doi: 10.1016/j.jtcme.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordell G.A., Beecher C.W., Pezzuto J.M. Can ethnopharmacology contribute to the development of new anticancer drugs? J. Ethnopharmacol. 1991;32(1–3):117–133. doi: 10.1016/0378-8741(91)90110-y. [DOI] [PubMed] [Google Scholar]
- 3.Gupta P., Jain V., Pareek A., Kumari P., Singh R., Agarwal P. Evaluation of effect of alcoholic extract of heartwood of Pterocarpus marsupium on in vitro antioxidant, anti-glycation, sorbitol accumulation and inhibition of aldose reductase activity. J. Tradit. Complement. Med. 2016 doi: 10.1016/j.jtcme.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma-Orozco G., Ortiz-Moreno A., Dorantes-Álvarez L., Sampedro J.G., Nájera H. Purification and partial biochemical characterization of polyphenol oxidase from mamey (Pouteria sapota) Phytochemistry. 2011;72(1):82–88. doi: 10.1016/j.phytochem.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Carriço C., Pinto Z.T., Dutok C., Caetano R.L., Pessanha R.R., Chil-Nuñez I. Biological activity of Pouteria sapota leaf extract on post-embryonic development of blowfly Chrysomya putoria (Wiedemann, 1818)(Calliphoridae) Rev. Bras. De. Farmacogn. 2014;24(3):304–308. [Google Scholar]
- 6.Rahmani A.H., Aly S.M., Ali H., Babiker A.Y., Srikar S. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 2014;7(3):483. [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma O.P., Bhat T.K. DPPH antioxidant assay revisited. Food Chem. 2009;113(4):1202–1205. [Google Scholar]
- 8.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958 [Google Scholar]
- 9.Singh M., Pandey N., Agnihotri V., Singh K., Pandey A. Antioxidant, antimicrobial activity and bioactive compounds of Bergenia ciliata Sternb.: a valuable medicinal herb of Sikkim Himalaya. J. Tradit. Complement. Med. 2017;7(2):152–157. doi: 10.1016/j.jtcme.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira I.C., Baptista P., Vilas-Boas M., Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100(4):1511–1516. [Google Scholar]
- 11..!!! INVALID CITATION!!!.
- 12.Ruch R.J., Cheng S.-j., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 13.Sroka Z., Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003;41(6):753–758. doi: 10.1016/s0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 14.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods. 1986;94(1–2):57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 15.Xu Jm, Song St, Tang Zm, Jiang Zf, Liu Xq, Zhou L. Predictive chemotherapy of advanced breast cancer directed by MTT assay in vitro. Breast Cancer Res. Treat. 1999;53(1):77–85. doi: 10.1023/a:1006122912146. [DOI] [PubMed] [Google Scholar]
- 16.T. Stump, B. Santee, L. Williams, C. Heinze, R. Kunze, S. Amos, et al. The Effects of Apigenin on Cell Proliferation Apoptosis in Glioblastoma Multiforme 2016.
- 17.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudonné S., Vitrac X., Coutiere P., Woillez M., Mérillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 19.Floegel A., Kim D.-O., Chung S.-J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24(7):1043–1048. [Google Scholar]
- 20.Masisi K., Beta T., Moghadasian M.H. Antioxidant properties of diverse cereal grains: a review on in vitro and in vivo studies. Food Chem. 2016;196:90–97. doi: 10.1016/j.foodchem.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Fuentealba C., Gálvez L., Cobos A., Olaeta J.A., Defilippi B.G., Chirinos R. Characterization of main primary and secondary metabolites and in vitro antioxidant and antihyperglycemic properties in the mesocarp of three biotypes of Pouteria lucuma. Food Chem. 2016;190:403–411. doi: 10.1016/j.foodchem.2015.05.111. [DOI] [PubMed] [Google Scholar]
- 22.França C.V., Perfeito J.P.S., Resck I.S., Gomes S.M., William C., Fagg C.F.S.C. Potential radical-scavenging activity of Pouteria caimito leaves extracts. J. Appl. Pharm. Sci. 2016;6(07):184–188. [Google Scholar]
- 23.Torres-Rodríguez A., Salinas-Moreno Y., Valle-Guadarrama S., Alia-Tejacal I. Soluble phenols and antioxidant activity in mamey sapote (Pouteria sapota) fruits in postharvest. Food Res. Int. 2011;44(7):1956–1961. [Google Scholar]
- 24.Ma J., Yang H., Basile M.J., Kennelly E.J. Analysis of polyphenolic antioxidants from the fruits of three pouteria species by selected ion monitoring liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2004;52(19):5873–5878. doi: 10.1021/jf049950k. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Shen Y., Zhu Y., Xu Z. Assessment of the correlations between reducing power, scavenging DPPH activity and anti-lipid-oxidation capability of phenolic antioxidants. LWT-Food Sci. Technol. 2015;63(1):569–574. [Google Scholar]
- 26.Canabady-Rochelle L.L., Harscoat-Schiavo C., Kessler V., Aymes A., Fournier F., Girardet J.-M. Determination of reducing power and metal chelating ability of antioxidant peptides: revisited methods. Food Chem. 2015;183:129–135. doi: 10.1016/j.foodchem.2015.02.147. [DOI] [PubMed] [Google Scholar]
- 27.Mansouri A., Makris D.P., Kefalas P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescence-based assay: structure–activity relationships. J. Pharm. Biomed. Anal. 2005;39(1):22–26. doi: 10.1016/j.jpba.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 28.Ma X., Li H., Dong J., Qian W. Determination of hydrogen peroxide scavenging activity of phenolic acids by employing gold nanoshells precursor composites as nanoprobes. Food Chem. 2011;126(2):698–704. [Google Scholar]
- 29.Rahman M.A., Akhtar J. Evaluation of anticancer activity of Cordia dichotoma leaves against a human prostate carcinoma cell line, PC3. J. Tradit. Complement. Med. 2016 doi: 10.1016/j.jtcme.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland M.W., Learmonth B.A. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic. Res. 1997;27(3):283–289. doi: 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- 31.Yahyaei H., Mohseni M., Ghanbari H., Messori M. Synthesis and characterization of polyhedral oligomeric titanized silsesquioxane: a new biocompatible cage like molecule for biomedical application. Mater. Sci. Eng.: C. 2016;61:293–300. doi: 10.1016/j.msec.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Kamalakannan K., Rayar A., Megala L. Isolation of quercetin from pouteria sapota and evaluation of its anti oxidant and cancer activities. World J. Pharm. Pharm. Sci. 2016 [Google Scholar]
- 33.Jayaprakasam B., Zhang Y., Seeram N.P., Nair M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74(1):125–132. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmi V., Bai G.V.S. In vitro anticancer activity of Clerodendrum phlomidis leaves and its silver nanoparticles on human breast cancer cell line (MCF-7) Asian J. Innov. Res. 2016;1(2):01–05. [Google Scholar]
- 35.Sarojini S., Senthilkumaar P., Ramesh V. Impact of ethanolic extract of Mikania glomerata on human breast cancer (MCF 7) cell line. Int. J. Adv. Sci. Res. 2016;2(4):94–103. [Google Scholar]
- 36.Subarnas A., Diantini A., Abdulah R., Zuhrotun A., Nugraha P.A., Hadisaputri Y.E. Apoptosis-mediated antiproliferative activity of friedolanostane triterpenoid isolated from the leaves of Garcinia celebica against MCF-7 human breast cancer cell lines. Biomed. Rep. 2016;4(1):79–82. doi: 10.3892/br.2015.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florence A., Jeeva S. In vitro anticancer activity of gmelina asiatica l. leaf against human breast cancer cell line (MCF-7) Int. J. Pharm. Sci. Res. 2016;7(5):2116. [Google Scholar]
- 38.Goldberg K.H., Yin A.C., Mupparapu A., Retzbach E.P., Goldberg G.S., Yang C.F. Components in aqueous Hibiscus rosa-sinensis flower extract inhibit in vitro melanoma cell growth. J. Tradit. Complement. Med. 2017;7(1):45–49. doi: 10.1016/j.jtcme.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material