Abstract
Background
Electroencephalogram (EEG)-based brain network analysis is a useful biological correlate reflecting brain function. Sensor-level network analysis might be contaminated by volume conduction and does not explain regional brain characteristics. Source-level network analysis could be a useful alternative. We analyzed EEG-based source-level network in major depressive disorder (MDD).
Method
Resting-state EEG was recorded in 87 MDD and 58 healthy controls, and cortical source signals were estimated. Network measures were calculated: global indices (strength, clustering coefficient (CC), path length (PL), and efficiency) and nodal indices (eigenvector centrality and nodal CC) in six frequency. Correlation analyses were performed between network indices and symptom scales.
Results
At the global level, MDD showed decreased strength, CC in theta and alpha bands, and efficiency in alpha band, while enhanced PL in alpha band. At nodal level, eigenvector centrality of alpha band showed region dependent changes in MDD. Nodal CCs of alpha band were reduced in MDD and were negatively correlated with depression and anxiety scales.
Conclusion
Disturbances in EEG-based brain network indices might reflect altered emotional processing in MDD. These source-level network indices might provide useful biomarkers to understand regional brain pathology in MDD.
Keywords: Major depressive disorder, Electroencephalogram, Brain electrical activity mapping, Source-level brain network
Highlights
-
•
We investigated the altered function of electroencephalogram-based cortical network for major depressive disorder (MDD).
-
•
MDD showed altered cortical brain network at both global and nodal level network in alpha frequency band.
-
•
Abnormal network indices in MDD were significantly correlated with depression and anxiety symptom scale scores.
-
•
Disrupted cortical networks band might reflect altered neural pathway during emotional processing in patients with MDD.
1. Introduction
Major depressive disorder (MDD) is a serious mental disease characterized by depressed mood, and MDD patients showed loss of interest and pleasure, insomnia, concentration difficulties, fatigue, and feelings of worthlessness (Friedman et al., 2011). Previous research has reported that individuals MDD show reduced behavioral reactivity and altered brain activation in the anterior cingulate cortex (ACC), amygdala, and insula while performing goal-directed task (Bourke et al., 2010; Bylsma et al., 2008).
Recently, however, researchers have become more interested in resting-state brain functions rather than brain functions during goal-directed tasks (Dutta et al., 2014; Fingelkurts and Fingelkurts, 2015; Kaiser et al., 2015; Zeng et al., 2012). In particular, resting-state functional connectivity (FC) between brain regions could provide a more informative insight about the pathophysiology of MDD (Fingelkurts et al., 2007). The altered resting-state FC of MDD has been reported by various functional magnetic resonance imaging (fMRI) studies (Mulders et al., 2015). (Greicius et al., 2003) observed an enhanced FC between the posterior cingulate cortex (PCC) and the ventral anterior cingulate cortex (vACC) in resting-state compared with performing tasks. An additional study showed increased resting-state FC between subgenual anterior cingulate cortex (sgACC) and amygdala and sgACC and insula of MDD compared to healthy controls (HCs) (Connolly et al., 2013). Conversely, diminished FC patterns between the para-hippocampal gyrus and frontal cortex have been reported (Zhou et al., 2012); moreover, reduced FC among the ACC, PCC, medial prefrontal cortex (mPFC), and orbitofrontal cortex have also been observed (Anand et al., 2009; Bluhm et al., 2009; Cullen et al., 2009). However, it is still controversial whether FC between specific brain regions, such as the cerebellum and temporal and fusiform gyri, is enhanced or not in the context of MDD (Dutta et al., 2014; Zhou et al., 2012).
Furthermore, several studies investigated brain network based graph theoretical approaches, which would quantify dynamical complex brain network at both global and nodal levels (see the supplementary for the more detailed explanation of each index). Dynamic network measurements would help to understand brain mechanism of the psychiatric disorder including MDD. For instance, MDD showed short path length and enhanced global efficiency (Guo et al., 2014), and high local efficiency and modularity of MDD were also investigated (Ye et al., 2015). Moreover, MDD showed decreased nodal efficiency and nodal degree in the frontal area including the ACC and dorsolateral and superior frontal areas (Hou et al., 2016; Ye et al., 2016); in contrast, increased nodal efficiency was observed in limbic regions (Ye et al., 2016).
Until now, most of the previous network studies used fMRI to investigate brain network. fMRI is suitable imaging tool to investigate regional information of brain network due to its excellent spatial resolution; nevertheless, fMRI has low temporal resolution, and is limited in its ability to elucidate neural processes that occur over the course of milliseconds (Kim et al., 1997). Electroencephalogram (EEG) is a suitable tool to address the limitations of fMRI, as it can be used to observe fast neural processes because of its high temporal sensitivity (Burle et al., 2015; Wierda et al., 2012). Also, the dynamic neural oscillations could be quantified by spectrum analysis of EEG signal (Fingelkurts and Fingelkurts, 2015; Nuwer, 1997; 1988).
In particular, abnormal alpha rhythm during resting-state is known to be related to the pathologic characteristics of MDD (Fingelkurts et al., 2007; Jaworska et al., 2012; Knott et al., 2001; Olbrich and Arns, 2013). Previous studies have shown that relatively higher alpha power at left than right frontal region and altered alpha activity at the occipital area are two remarkable characteristics of MDD (Fingelkurts et al., 2007; Jaworska et al., 2012; Knott et al., 2001). Moreover, disrupted FC in theta and alpha bands were revealed by various synchrony measures such as partial directed coherence and operational synchrony index (Fingelkurts et al., 2007). Furthermore, increase in the operational synchrony within three subnets of the default mode network was found in the MDD patients when compared with healthy controls (Fingelkurts and Fingelkurts, 2017). The enhanced connectivity within the three subnets was related to first-person perspective, reflective agency and narration, and bodily representational-emotional agency (Fingelkurts and Fingelkurts, 2017).
Despite the promise of using EEG to better understand brain networks, most of the previous EEG studies have some limitations (sensor/electrode-level analysis, a small number of electrodes, and/or a small number of participants) that may affect interpretations of the obtained results. Also, poor spatial sensitivity by volume conduction effect caused spurious connection and may provide inaccurate regional information (Cho et al., 2015; van den Broek et al., 1998). Source localization would become a solution to overcome volume conduction (Cho et al., 2015; Haufe et al., 2013; Shim et al., 2014). Thus, the source-level, multi-channels, and large-scale studies are necessary for EEG-based brain network analysis.
The present study explored the altered cortical brain networks in MDD during resting-state using a source-level network analysis of EEG data. The source-level brain network could evaluate disrupted global network patterns of information on specific cortical regions. Furthermore, the relationships between psychiatric symptom scales and the altered cortical network could be revealed by correlation analysis.
2. Material and methods
2.1. Participants
Eighty-seven patients with MDD (33 male and 54 female subjects) and 58 healthy control (HC) (30 male and 28 female subjects) were recruited for this study. The patients were diagnosed based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) Axis I Psychiatric Disorders (First et al., 1997) by a psychiatrist. Patients were excluded if they accorded with the following criteria: 1) disease of the central nervous system, 2) medical history of alcohol or drug abuse, 3) mental retardation, 4) a history of head injuries with loss of consciousness and experience with electrical therapy, 5) other significant psychiatric illness such as schizophrenia, bipolar disorder, and anxiety disorders. All the subjects were recruited and their depressive symptoms were examined during their screening period in a drug naive state. The electrophysiological assessment was performed from zero to fourteen (mean ± S.D. = 5.6 ± 3.5) days after the commencement of the antidepressant treatment. Since the present study was naturalistic in design, most of the patients needed to take their medication before the scheduled electrophysiological assessment. Data on the antidepressant treatment at the time of EEG measurement was as follows: no medication (n = 11, 12.7%), escitalopram (n = 31, 36.0%), paroxetine (n = 21, 24.4%), fluoxetine (n = 8, 9.3%), and vortioxetine (n = 15, 17.4%). Benzodiazepine use were restricted as lorazepam and alprazolam as needed. HC were recruited from the local community through local newspapers and posters. All subjects provided written informed consent, and the study protocol was approved by the Institutional Review Board of Inje University Ilsan Paik Hospital (2015-07-048-002). Participant demographics reported in Table 1.
Table 1.
Demographic data of patients with major depressive disorder and healthy controls.
| MDD | HC | p | |
|---|---|---|---|
| Cases (N) | 87 | 58 | |
| Gender (male/female) | 33/54 | 30/28 | 0.124 |
| Age (years) | 42.14 ± 10.48 | 39.98 ± 11.63 | 0.248 |
| Education (years) | 13.23 ± 3.32 | 14.45 ± 3.37 | 0.069 |
| Symptom score | |||
| HAM-A | 22.09 ± 6.95 | ||
| HAM-D | 25.82 ± 8.74 | ||
| BDI | 25.63 ± 10.16 | ||
| BAI | 24.06 ± 9.41 | ||
MDD, Major depressive disorder; HCs, Healthy controls; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory.
2.2. Psychological evaluation
Hamilton Anxiety Rating Scale (HAM-A; Cronbach α = 0.84) (Hamilton, 1959; Park et al., 2016) and Beck Anxiety Inventory (BAI; Cronbach α = 0.92) (Beck and Steer, 1990; Lee et al., 2016) were used to evaluate anxiety symptoms. Hamilton Depression Rating Scale (HAM-D; Cronbach α = 0.79) (Hamilton, 1986; Yi et al., 2005) and Beck Depression Inventory (BDI; Cronbach α = 0.89) (Beck et al., 1996; Lee et al., 2017) were used to investigate depressive symptoms.
2.3. EEG recordings and pre-processing
EEG signals were recorded using a NeuroScan SynAmps2 amplifier (Compumedics USA, El Paso, TX, USA) from 62 Ag/AgCl scalp electrodes that were evenly mounted on a QuikCap according to the extended international 10−20 system. Electrode impedances were <5 kΩ. The ground electrode was placed on the forehead, and the reference electrodes were attached at the Cz electrode. The vertical electrooculogram channels were located above and below the right eye, and the horizontal electrooculogram channels were placed on the outer canthus of each eye. The EEG data were recorded with a 0.1−100-Hz bandpass filter at a sampling rate of 1000 Hz, with 60 Hz noise removed using a notch filter.
Resting-state EEGs were recorded for 5 min with the eyes closed. Eye-related artifacts were corrected using the standard correction algorithms implemented in the preprocessing software (Roh et al., 2016). Gross artifacts, such as movement artifacts, were rejected by visual inspection by a skilled expert. After rejecting artifacts, the data were bandpass filtered at 1−55 Hz and segmented into epochs with a duration of 4.096 s. The epochs were rejected if they contained significant physiological artifacts (amplitude exceeding ±75 μV) at any site over all electrodes, and ten artifact-free epochs were used for each subject for source-level network analysis (Shim et al., 2017). Epoch length was determined considering both efficiency and reliability based on previous research findings (Gudmundsson et al., 2007). The analysis pipeline for cortical network used in the present study is the same as in our previous paper (Shim et al., 2017). We used Matlab (Mathworks Inc.) while performing network analyses procedures.
2.4. Source localization
To estimate a time-series of source activities, the minimum-norm estimation was used, which was implemented in the eConnectome toolbox (Biomedical Functional Imaging and Neuroengineering Laboratory, University of Minnesota, Minneapolis, MN, USA) (He et al., 2011). A three-layer boundary element method (BEM) model, constructed from the Montreal Neurological Institute (MNI) 152 standard template, was used to compute the lead field matrix. Cortical current density values at 7850 cortical vertices were evaluated for every time-point of each epoch. After estimating the cortical current density at every time-point, 66 nodes were selected from among the original cortical vertices. In our previous studies, we chose 314 nodes as evenly as possible (Shim et al., 2014); however, we found that these were too numerous to allow efficient estimation of the brain regions. Thus, here, we evenly selected 66 nodes (33 per hemisphere) that were sampled based on the Brodmann areas (BA), excluding areas located deep in the brain (see supplement method). The representative value of each node was evaluated by averaging the cortical sources located in each node. A time-series of the cortical sources at each of the 66 nodes were bandpass filtered and divided into six frequency bands (delta [1−4 Hz], theta [4−8 Hz], alpha [8−12 Hz], low-beta [12−22 Hz], high-beta [22−30 Hz], and gamma [30−55 Hz]).
2.5. Connectivity and network analysis
The FC between each pair of nodes was evaluated using phase-locking values (PLVs). PLVs were used as the measure of synchronization because PLVs range from 0 to 1; thus, they can be directly used to represent the connection strength in a weighted network analysis, without any further modification (Lachaux et al., 1999; Shim et al., 2017; 2014).
The weighted network was quantitatively analyzed based on the graph theory (Bullmore and Sporns, 2009b; Sporns et al., 2004). In the present study, we selected representative network measurements at both the global and nodal levels. Strength, clustering coefficient, path length, and efficiency were calculated for the global level network, and nodal clustering coefficient and eigenvector centrality were evaluated for the nodal level network. The network measurements are defined as follows: 1) Strength represents the degree of connection strength in the network. A higher strength value means that the whole brain is strongly connected. 2) Clustering coefficients (CC) represent the degree in which a node is clustered with its neighbor's nodes. The enhanced CC indicated the well-segregated network between the relevant brain regions. 3) Path length (PL) is the summation of lengths between two nodes within the network. The PL of a well-integrated network is shorter than a randomly organized network, which is related to the speed of information processing. 4) Efficiency is the effectiveness of information processing in the brain; low efficiency means the network performs at a lesser work rate. 5) Eigenvector Centrality (EC) represents the influencing power of hub of the network. EC is calculated by considering both degree and strength of connection of brain network. High EC refers to the vital node in the network. CC and EC are computed at each node and then averaged over all values for quantifying global level network. For more details (e.g. equations) on these mentioned network measures please see the supplementary information and references.
2.6. Statistical analysis
The differences in cortical network characteristics at the global level between patients with MDD and HCs were investigated for each frequency band using independent t-tests. Since four network values were repeatedly tested at each frequency, 4 independent t-test were performed with adjusted p-values using the false discovery rate (FDR) method to limit type I error (Benjamini and Hochberg, 1995). At the nodal level characteristics, since the number of nodes are sixty six, 66 independent t-tests were performed for each frequency band with an adjusted p-values using the FDR method. When significant differences were found in the network indices between the two groups, the effect size (eta squared, η2) was calculated, and significantly different nodes were defined with a 0.06-threshold (medium effect) (Green and Salkind, 2010). Correlation analyses were performed to investigate the relationships between the network indices and the symptom severity scores in patients with MDD with adjusted p-values using FDR.
3. Results
3.1. Demographic data
The patients with MDD and HC did not differ in gender (p = 0.124), age (p = 0.248), and education (p = 0.069). The average and standard deviation of psychiatric scores in MDD are as follows: HAM-A, 22.09 ± 6.95; HAM-D, 25.82 ± 8.74; BDI, 25.63 ± 10.16; and BAI, 24.06 ± 9.41. Demographic and psychological characteristics of participants are shown in Table 1. All patients were receiving medications during the study: selective serotonin reuptake inhibitors (N = 69), venlafaxine (N = 15), mirtazapine (N = 5), lorazepam (N = 32), clonazepam (N = 22), diazepam (N = 10), and alprazolam (N = 20).
3.2. Global level network
The global level network indices of strength, CC, PL, and global efficiency showed significant differences between patients with MDD and HCs (Table 2). Strength, CC, and efficiency were significantly decreased in MDD compared to HCs in the theta band (strength: 29.52 ± 5.33 vs. 31.50 ± 5.40, p = 0.031; CC: 0.42 ± 0.09 vs. 0.45 ± 0.09, p = 0.037, FDR corrected) and alpha band (strength: 36.08 ± 7.55 vs. 39.45 ± 7.81, p = 0.010; CC: 0.51 ± 0.12 vs. 0.58 ± 0.13, p = 0.010; efficiency: 0.57 ± 0.10 vs. 0.61 ± 0.11, p = 0.013, FDR corrected). On the other hand, PL was significantly longer in MDD compared to HCs only in the alpha band (2.17 ± 0.47 vs. 1.99 ± 0.45, p = 0.019, FDR corrected).
Table 2.
Mean and standard deviation values of the global network indices of strength, clustering coefficient, path length, and efficiency in theta and alpha band frequencies.
| MDD | HC | Corrected p value | |
|---|---|---|---|
| Theta band | |||
| Strength | 29.52 ± 5.33 | 31.50 ± 5.40 | 0.031⁎ |
| Clustering coefficient | 0.42 ± 0.09 | 0.45 ± 0.09 | 0.037⁎ |
| Path length | 2.63 ± 0.38 | 2.51 ± 0.41 | 0.068 |
| Efficiency | 0.47 ± 0.08 | 0.50 ± 0.07 | 0.052 |
| Alpha band | |||
| Strength | 36.08 ± 7.55 | 39.45 ± 7.81 | 0.010⁎ |
| Clustering coefficient | 0.51 ± 0.12 | 0.58 ± 0.13 | 0.010⁎ |
| Path length | 2.17 ± 0.47 | 1.99 ± 0.45 | 0.019⁎ |
| Efficiency | 0.57 ± 0.10 | 0.61 ± 0.11 | 0.013⁎ |
Major depressive disorder, MDD; Healthy control, HC.
p < 0.05.
Moreover, we checked the differences of global level network indices by severity in MDD. MDD was divided into three groups using BDI scale as follows: mild (BDI ≤15), moderate (BDI: 16–23), and severe (24 ≤ BDI). One-way analysis of variances (ANOVA) with Bonferroni correction was performed to estimate the differences among three groups. As a result, in alpha frequency band, severe group showed significantly decreased strength, CC, and efficiency and increased PL compared to mild group (strength: 34.18 ± 7.55 vs. 40.58 ± 8.17, p = 0.023; CC: 0.50 ± 0.12 vs. 0.60 ± 0.13, p = 0.022; efficiency: 0.54 ± 0.11 vs. 0.64 ± 0.12, p = 0.023; PL: 2.28 ± 0.47 vs. 1.91 ± 0.45, p = 0.04, FDR corrected).
3.3. Nodal level network
Nodal level network indices were investigated in the theta and alpha bands because the significant differences in the global level network were found in these two frequency bands.
3.3.1. Eigenvector centrality
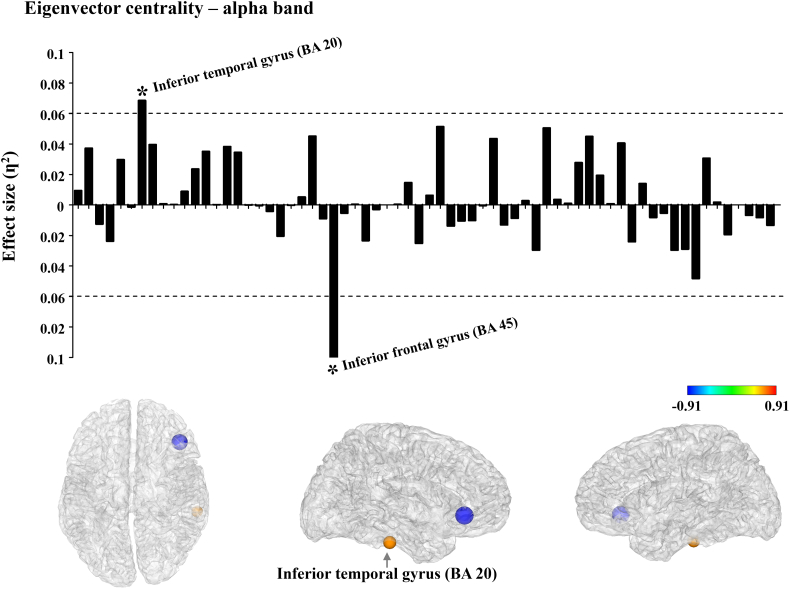
Eigenvector centrality of MDD was significantly different only in the alpha band (p < 0.05, FDR corrected). Among the regions showing significant differences, only two brain areas exceeded the effect size of 0.06. Eigenvector centrality of the inferior frontal gyrus (BA45) was significantly decreased in MDD compared to HCs while, eigenvector centrality of MDD in the inferior temporal gyrus (BA20) was significantly enhanced compared to HCs (Fig. 1).
Fig. 1.
Effect-size of differences of eigenvector centrality between patients with MDD and HCs in alpha frequency bands. The meaning of each bar is the effect-size at each node. The threshold value was set as 0.06 (medium effect). In the brain model, the density of colors and size of circles represent the difference direction and effect size, respectively (* ƞ2 > 0.06).
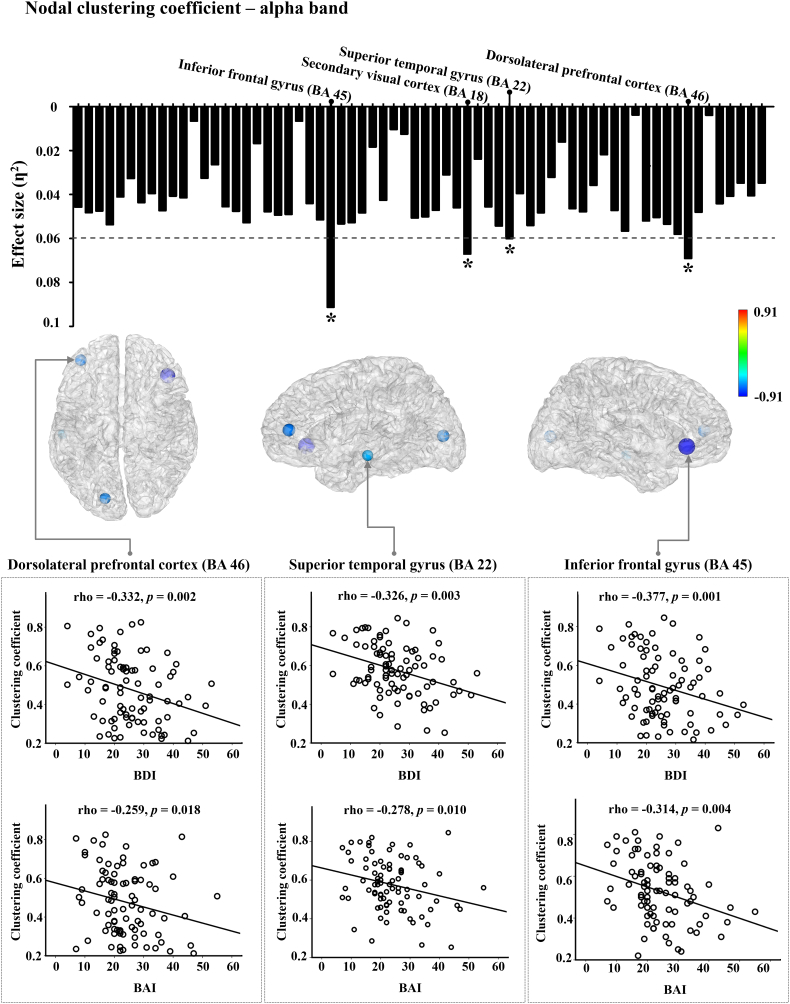
3.3.2. Nodal clustering coefficients
The patients with MDD showed significantly decreased nodal CC at various regions only in the alpha band compared to HCs (p < 0.05, FDR corrected). The brain regions exceeding the effect size of 0.06 were as follows: the inferior frontal gyrus (BA45), secondary visual cortex (BA18), superior temporal gyrus (BA22), and dorsolateral prefrontal cortex (BA46) (Fig. 2). In addition, nodal CC showed significant negative correlations with symptom severity scores: the dorsolateral prefrontal cortex with BDI (rho = −0.332, p = 0.002) and BAI (rho = −0.259, p = 0.018) scores respectively, the superior temporal gyrus with BDI (rho = −0.326, p = 0.003) and BAI (rho = −0.278, p = 0.010) scores respectively, and the inferior frontal gyrus with BDI (rho = −0.377, p = 0.001) and BAI (rho = −0.314, p = 0.004) scores in MDD (Fig. 2).
Fig. 2.
Effect-size of differences of eigenvector centrality between patients with MDD and HCs in alpha frequency bands. The meaning of each bar is the effect-size at each node. The threshold value was set as 0.06 (medium effect). In the brain model, the density of colors and size of circles represent the difference direction and effect size, respectively (* ƞ2 > 0.06). The relationships between nodal clustering coefficient and psychiatric symptoms (MDD, Major depressive disorder; HCs, Healthy controls; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory).
4. Discussion
The cortical brain networks during resting-state were investigated by EEG source-level network analysis based on graph theory in patients with MDD (Bondy and Murty, 1976; Bullmore and Sporns, 2009a). The following were observed in patients with MDD: 1) in the global indices, the strength and CC were significantly decreased in both theta and alpha bands and enhanced PL and reduced efficiency were found in the alpha band; 2) the nodal eigenvector centrality of the alpha band was significantly decreased at the inferior frontal gyrus while increased in the inferior temporal gyrus; 3) the nodal CCs were significantly reduced in the alpha band, and they showed significant negative correlations with depression and anxiety scores.
4.1. Global level network
The patients with MDD showed significantly decreased strength and CC in both theta and alpha bands, while, prolonged PL and reduced efficiency were revealed only in the alpha band.
Dynamic neuronal oscillations could be quantified as specific brain patterns by spectral analysis of EEG signals (Fingelkurts and Fingelkurts, 2015). Unique EEG patterns in psychiatric patients were revealed during resting-state, and these patterns were related to the pathological characteristics (Hughes and John, 1999; Sponheim et al., 2000). In particular, abnormal theta and alpha frequency patterns have been continuously reported to be involved in MDD (Fingelkurts et al., 2006; Klimesch, 1999; Li et al., 2015; Metzger et al., 2010; Mölle et al., 2002; Quirk and Beer, 2006). These two type of frequency oscillations are also known to relate to emotion processing (Aftanas and Golocheikine, 2001; Aftanas et al., 2002). Thus, altered emotion processing found in MDD would be closely associated with abnormal two brain rhythms, a hypothesis that has been supported by a number of quantitative EEG studies. For instance, altered alpha power in both absolute and relative values of MDD has been observed (Pollock and Schneider, 1990; Rosenfeld et al., 1995), while another study documented the relationships between abnormal alpha oscillation and diminished emotion arousal (Olbrich and Arns, 2013). Additionally, increased frontal and parietal alpha power have been reported, which was closely related to emotion processing (Jaworska et al., 2012), while on the other hand, decreased theta power and its negative correlation with severity of depression have also been reported (Spronk et al., 2011). Moreover, there were several connectivity studies, but the results of these studies were inconsistent (Fingelkurts et al., 2007; Leuchter et al., 2012). Some research reported the decreased functional connectivity between the frontal and temporal area in the alpha frequency band (Park et al., 2007); otherwise, increased functional connectivity in the theta and alpha frequency bands were also found (Fingelkurts et al., 2007), and altered functional connectivity was significantly correlated with depression symptom scores and three components (first-person agency, self-reflection and narration, and bodily representational-emotional agency) of selfhood (Fingelkurts and Fingelkurts, 2017).
Meanwhile, disrupted global network indices in patients with MDD were revealed by fMRI. The patients with MDD showed enhanced global efficiency and reduced PL compared to HCs (Zhang et al., 2011). On the contrary, decreased global efficiency in MDD was found (Park et al., 2013). Also, reduced PL and CC in patients with MDD were found (Guo et al., 2014), and altered global network indices are significantly correlated with depression severity scale scores. Even though previous studies showed inconsistent results regarding whether or not the global indices were increased, it is obvious that patients with MDD show altered brain network as compared to HCs and the altered networks would lead to inefficient information propagation.
In the present study, altered brain networks were observed (decrease – strength, CC, and efficiency; increase – PL) at the theta and alpha frequency bands in patients with MDD. Reduced CC and enhanced PL could imply disrupted segregation and integration of brain networks, and diminished strength could represent a weak connection between brain areas. That is, the brain network of MDD is composed of weak and low-level connectivity and it directly connected to inefficient processing. In particular, MDD showed noticeable disrupt network characteristics at theta and alpha rhythms, which are closely related to abnormal emotion processing of MDD. That is, the altered network of MDD at the theta and alpha frequency bands might cause the altered emotional response or emotional arousal.
4.2. Nodal level network
The results of the nodal level could provide more specific regional information of brain network, rather than global level information. In the present study, patients with MDD showed two different directions of eigenvector centralities; increased activity in the inferior temporal gyrus and decreased activity in the inferior frontal gyrus. Moreover, significantly reduced nodal CCs were revealed in the dorsolateral prefrontal cortex, inferior frontal gyrus, and superior temporal gyrus areas in patients with MDD. These nodal CCs showed negative correlations with depression and anxiety scores.
Past research has shown that MDD show impaired emotional information processing (Bylsma et al., 2008; Fingelkurts et al., 2007). Several steps, such as recognition and regulation, are involved in emotional information processing. Emotion recognition is an ability to identify emotional stimuli (Phillips et al., 2003), and the temporal lobes such as the amygdala, insula, inferior temporal gyrus, and superior temporal gyrus are involved in emotion recognition (Bourke et al., 2010; Keightley et al., 2003; Langenecker et al., 2005; Stuhrmann et al., 2011b). Altered responses to emotional stimuli and disrupted temporal, amygdala, and insular activities during emotion recognition are distinct characteristics of patients with MDD (Langenecker et al., 2005; Stuhrmann et al., 2011a).
Alternations in neural processes characteristic of MDD appear to greatly disrupt individuals' ability to effectively regulate their emotions. Emotion regulation is defined as processes of monitoring, evaluating, and modifying for emotional features (Gross, 1998; Thompson, 1994). This emotion regulatory process is largely controlled by the frontal lobe, in regions such as the ACC, dorsolateral prefrontal cortex, and inferior frontal gyrus (Grecucci et al., 2013; Hallam et al., 2015; Ochsner et al., 2004; Rive et al., 2013a). Several studies have reported that abnormal frontal cortex (including prefrontal and inferior frontal gyrus) and anterior cingulate gyrus activities are closely related to disrupted emotional regulation of MDD (Domes et al., 2010; Joormann et al., 2012; Rive et al., 2013b). According to resting-state fMRI studies, increased functional connectedness of limbic regions including the amygdala and hippocampus as well as subcortical thalamic nuclei were observed in patients with MDD; on the other hand, the decreased nodal efficiency of cognitive control regions such as the dorsolateral prefrontal cortex and ACC have also been reported in patients with MDD compared to HCs (Hou et al., 2016; Ye et al., 2016).
The Eigenvector centrality indicates a measure of the influence of a node in a network. If one node has a higher value of eigenvector centrality, it indicates this node is hyper-influencing compared to other nodes in a network. That is, in the present study, the reduced eigenvector centrality of the inferior frontal gyrus implies diminished hub function (diminished influencing power) in this area, which might related to impaired emotion regulation. On the other hand, the enhanced eigenvector centrality of the inferior temporal gyrus represents the hyperactive hub function (increased influencing power), which might associate with the increased influx of emotional information.
Moreover, altered nodal CCs could support our hypothesis. The patients with MDD showed decreased nodal CC of the dorsolateral prefrontal cortex, inferior frontal gyrus, and superior temporal gyrus, and these regions are concerned in emotional processing. Namely, reduced nodal CC may indicate weak (inefficient) networks strength around these brain regions, and it might be affect information processing. Also, in the present study, as the severity scales of depression and anxiety worsened, the indices of nodal CC diminished. It supported that these three regions might be important to understand the altered emotional function in patients with MDD.
In sum, the results from eigenvector centrality imply reduced influencing power for emotion regulation of the frontal lobe and enhanced influencing power for emotion recognition of the temporal lobe. Moreover, reduced CC reflects weak network strength in the frontotemporal regions in patients with MDD, and it might help to understand about ridiculous information flow of MDD during emotion processing.
4.3. Limitations
The use of medication was not controlled for in the present study, and because of this, the results should be interpreted with caution. Our result showed opposite side compared to previous studies controlled medication of patients, such as decreased strength. We thought that this finding might be influenced by medications of patients. Second, we did not use individual head models for EEG source imaging, as the individual MRI data were not available.
5. Conclusion
We found disturbances in EEG-based brain network index, which might reflect altered emotional processing of MDD. Weak and inefficient brain networks were revealed in the theta and alpha bands. Especially, the increased influencing power of the inferior temporal gyrus might imply enhanced emotion recognition at this region of the patients with MDD. These source level network analyses might provide useful biomarkers to understand the regional brain pathology of MDD. More study still need to explore brain networks of the MDD patients. In present study, we focused on only static brain network of MDD; however, some recent studies augured that the brain network of MDD is change over time (Cheng et al., 2016; Zhang et al., 2016; Zhang et al., 2014). EEG is suitable imaging tools for exploring change of brain network due to its high temporal resolution. Thus, we'll keep going explore the dynamic brain network of MDD changed over time as a future direction.
Acknowledgments
Acknowledgement
This work was supported by a grant from the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2018R1A2A2A05018505), and by the Ministry of Science, ICT & Future Planning (NRF-2015M3C7A1028252).
Declaration of interest
The authors have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.06.012.
Appendix A. Supplementary data
Supplementary material
References
- Aftanas L., Golocheikine S. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci. Lett. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Aftanas L.I., Varlamov A.A., Pavlov S.V., Makhnev V.P., Reva N.V. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int. J. Psychophysiol. 2002;44:67–82. doi: 10.1016/s0167-8760(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. Neuroimaging. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A. Psychological Corporation; San Antonio, TX: 1990. Manual for the Beck Anxiety Inventory. [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. San Antonio; TX: 1996. Beck Depression Inventory-II. (78204-72498) [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995:289–300. [Google Scholar]
- Bluhm R., Williamson P., Lanius R., Théberge J., Densmore M., Bartha R., Neufeld R., Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin. Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Bondy J.A., Murty U.S.R. Citeseer; 1976. Graph Theory with Applications. [Google Scholar]
- Bourke C., Douglas K., Porter R. Processing of facial emotion expression in major depression: a review. Aust. N. Z. J. Psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burle B., Spieser L., Roger C., Casini L., Hasbroucq T., Vidal F. Spatial and temporal resolutions of EEG: is it really black and white? A scalp current density view. Int. J. Psychophysiol. 2015;97:210–220. doi: 10.1016/j.ijpsycho.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma L.M., Morris B.H., Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin. Psychol. Rev. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Qiu J., Liu W., Tang Y., Huang C.-C., Wang X., Zhang J., Lin W., Zheng L. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139:3296–3309. doi: 10.1093/brain/aww255. [DOI] [PubMed] [Google Scholar]
- Cho J.-H., Vorwerk J., Wolters C.H., Knösche T.R. Influence of the head model on EEG and MEG source connectivity analyses. NeuroImage. 2015;110:60–77. doi: 10.1016/j.neuroimage.2015.01.043. [DOI] [PubMed] [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., Hoeft F., Wolkowitz O., Eisendrath S., Frank G., Hendren R., Max J.E., Paulus M.P. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., Camchong J., Bell C.J., Houri A., Kumra S. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Schulze L., Böttger M., Grossmann A., Hauenstein K., Wirtz P.H., Heinrichs M., Herpertz S.C. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.W. Resting state networks in major depressive disorder. Psychiatry Res. Neuroimaging. 2014;224:139–151. doi: 10.1016/j.pscychresns.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol. Psychiatry. 2015;77:1050–1060. doi: 10.1016/j.biopsych.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A. Three-dimensional components of selfhood in treatment-naive patients with major depressive disorder: a resting-state qEEG imaging study. Neuropsychologia. 2017;99:30–36. doi: 10.1016/j.neuropsychologia.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A., Rytsälä H., Suominen K., Isometsä E., Kähkönen S. Composition of brain oscillations in ongoing EEG during major depression disorder. Neurosci. Res. 2006;56:133–144. doi: 10.1016/j.neures.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A., Rytsälä H., Suominen K., Isometsä E., Kähkönen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum. Brain Mapp. 2007;28:247–261. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. American Psychiatric Pub; 1997. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version. [Google Scholar]
- Friedman M.J., Resick P.A., Bryant R.A., Brewin C.R. Considering PTSD for DSM-5. Depression Anx. 2011;28:750–769. doi: 10.1002/da.20767. [DOI] [PubMed] [Google Scholar]
- Grecucci A., Giorgetta C., Bonini N., Sanfey A.G. Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front. Hum. Neurosci. 2013;7:523. doi: 10.3389/fnhum.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.B., Salkind N.J. Prentice Hall Press; 2010. Using SPSS for Windows and Macintosh: Analyzing and Understanding Data. [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. Sharpening the focus: emotion regulation, arousal, and social competence. Psychol. Inq. 1998;9:287–290. [Google Scholar]
- Gudmundsson S., Runarsson T.P., Sigurdsson S., Eiriksdottir G., Johnsen K. Reliability of quantitative EEG features. Clin. Neurophysiol. 2007;118:2162–2171. doi: 10.1016/j.clinph.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Guo H., Cheng C., Cao X., Xiang J., Chen J., Zhang K. Resting-state functional connectivity abnormalities in first-onset unmedicated depression. Neural Regen. Res. 2014;9:153. doi: 10.4103/1673-5374.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam G.P., Webb T.L., Sheeran P., Miles E., Wilkinson I.D., Hunter M.D., Barker A.T., Woodruff P.W., Totterdell P., Lindquist K.A. The neural correlates of emotion regulation by implementation intentions. PLoS ONE. 2015;10:e0119500. doi: 10.1371/journal.pone.0119500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Springer; 1986. The Hamilton Rating Scale for Depression. Assessment of Depression; pp. 143–152. [Google Scholar]
- Haufe S., Nikulin V.V., Müller K.-R., Nolte G. A critical assessment of connectivity measures for EEG data: a simulation study. NeuroImage. 2013;64:120–133. doi: 10.1016/j.neuroimage.2012.09.036. [DOI] [PubMed] [Google Scholar]
- He B., Dai Y., Astolfi L., Babiloni F., Yuan H., Yang L. eConnectome: a MATLAB toolbox for mapping and imaging of brain functional connectivity. J. Neurosci. Methods. 2011;195:261–269. doi: 10.1016/j.jneumeth.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Wang Z., Jiang W., Yin Y., Yue Y., Zhang Y., Song X., Yuan Y. Divergent topological architecture of the default mode network as a pretreatment predictor of early antidepressant response in major depressive disorder. Sci. Rep. 2016;6 doi: 10.1038/srep39243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R., John E.R. Conventional and quantitative electroencephalography in psychiatry. J. Neuropsychiatr. Clin. Neurosci. 1999;11:190–208. doi: 10.1176/jnp.11.2.190. [DOI] [PubMed] [Google Scholar]
- Jaworska N., Blier P., Fusee W., Knott V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J. Psychiatr. Res. 2012;46:1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Cooney R.E., Henry M.L., Gotlib I.H. Neural correlates of automatic mood regulation in girls at high risk for depression. J. Abnorm. Psychol. 2012;121:61. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psych. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley M.L., Winocur G., Graham S.J., Mayberg H.S., Hevenor S.J., Grady C.L. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Kim S.G., Richter W., Uǧurbil K. Limitations of temporal resolution in functional MRI. Magn. Reson. Med. 1997;37:631–636. doi: 10.1002/mrm.1910370427. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knott V., Mahoney C., Kennedy S., Evans K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. Neuroimaging. 2001;106:123–140. doi: 10.1016/s0925-4927(00)00080-9. [DOI] [PubMed] [Google Scholar]
- Lachaux J.-P., Rodriguez E., Martinerie J., Varela F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker S.A., Bieliauskas L.A., Rapport L.J., Zubieta J.-K., Wilde E.A., Berent S. Face emotion perception and executive functioning deficits in depression. J. Clin. Exp. Neuropsychol. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Lee H.-K., Lee E.-H., Hwang S.-T., Hong S.-H., Kim J.-H. Psychometric properties of the Beck anxiety inventory in the community-dwelling sample of Korean adults. Korean J. Clin. Psych. 2016;35:822–830. [Google Scholar]
- Lee E.-H., Lee S.-J., Hwang S.-T., Hong S.-H., Kim J.-H. Reliability and validity of the Beck depression inventory-II among Korean adolescents. Psych. Investig. 2017;14:30–36. doi: 10.4306/pi.2017.14.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter A.F., Cook I.A., Hunter A.M., Cai C., Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Long C., Yang L. Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/810548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger C.D., Eckert U., Steiner J., Sartorius A., Buchmann J.E., Stadler J., Tempelmann C., Speck O., Bogerts B., Abler B. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front. Neuroanat. 2010;4:138. doi: 10.3389/fnana.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Marshall L., Fehm H.L., Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur. J. Neurosci. 2002;15:923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Nuwer M.R. Quantitative EEG: I. Techniques and problems of frequency analysis and topographic mapping. J. Clin. Neurophysiol. 1988;5:1–44. [PubMed] [Google Scholar]
- Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American clinical neurophysiology society. Neurology. 1997;49:277–292. doi: 10.1212/wnl.49.1.277. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D., Gross J.J. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olbrich S., Arns M. EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. Int. Rev. Psych. 2013;25:604–618. doi: 10.3109/09540261.2013.816269. [DOI] [PubMed] [Google Scholar]
- Park C.-A., Kwon R.-J., Kim S., Jang H.-R., Chae J.-H., Kim T., Jeong J. World Congress on Medical Physics and Biomedical Engineering 2006. Springer; 2007. Decreased phase synchronization of the EEG in patients with major depressive disorder; pp. 1095–1098. [Google Scholar]
- Park C.-H., Wang S.-M., Lee H.-K., Kweon Y.-S., Lee C.T., Kim K.-T., Kim Y.-J., Lee K.-U. 2013. Affective State-Dependent Changes in the Brain Functional Network in Major Depressive Disorder. Social Cognitive and Affective Neuroscience, nst126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Jeong H.S., Im J.J., Jeon Y., Ma J., Choi Y., Ban S., Kim S., Yu S., Lee S. Reliability and validity of the Korean version of the post-traumatic stress disorder checklist in public firefighters and rescue workers. Korean J. Biol. Psych. 2016;23:29–36. [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pollock V., Schneider L. Topographic quantitative EEG in elderly subjects with major depression. Psychophysiology. 1990;27:438–444. doi: 10.1111/j.1469-8986.1990.tb02340.x. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Roh S.-C., Park E.-J., Shim M., Lee S.-H. EEG beta and low gamma power correlates with inattention in patients with major depressive disorder. J. Affect. Disord. 2016;204:124–130. doi: 10.1016/j.jad.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J.P., Cha G., Blair T., Gotlib I.H. Operant (biofeedback) control of left-right frontal alpha power differences: potential neurotherapy for affective disorders. Biofeed. Self-Regul. 1995;20:241–258. doi: 10.1007/BF01474516. [DOI] [PubMed] [Google Scholar]
- Shim M., Kim D.-W., Lee S.-H., Im C.-H. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophr. Res. 2014;156:197–203. doi: 10.1016/j.schres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Shim M., Im C., Lee S. Disrupted cortical brain network in post-traumatic stress disorder patients: a resting-state electroencephalographic study. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim S.R., Clementz B.A., Iacono W.G., Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol. Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- Sporns O., Chialvo D.R., Kaiser M., Hilgetag C.C. Organization, development and function of complex brain networks. Trends Cogn. Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Spronk D., Arns M., Barnett K., Cooper N., Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: a pilot study. J. Affect. Disord. 2011;128:41–48. doi: 10.1016/j.jad.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A., Suslow T., Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol. Mood Anx. Dis. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A., Suslow T., Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol. Mood Anx. Dis. 2011;1:1. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.A. Emotion regulation: a theme in search of definition. Monogr. Soc. Res. Child Dev. 1994;59:25–52. [PubMed] [Google Scholar]
- van den Broek S.P., Reinders F., Donderwinkel M., Peters M. Volume conduction effects in EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 1998;106:522–534. doi: 10.1016/s0013-4694(97)00147-8. [DOI] [PubMed] [Google Scholar]
- Wierda S.M., van Rijn H., Taatgen N.A., Martens S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proc. Natl. Acad. Sci. 2012;109:8456–8460. doi: 10.1073/pnas.1201858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Yang T., Qing P., Lei X., Qiu J., Liu G. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting-state fMRI. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0133775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Qing P., Zhang K., Liu G. Altered network efficiency in major depressive disorder. BMC Psych. 2016;16:450. doi: 10.1186/s12888-016-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.S., Bae S.O., Ahn Y.M., Park D.B., Noh K.S., Shin H.K., Woo H.W., Lee H.S., Han S.I., Kim Y.S. Validity and reliability of the Korean version of the Hamilton depression rating scale (K-HDRS) J. Korean Neuropsych. Assoc. 2005;44:456–465. [Google Scholar]
- Zeng L.-L., Shen H., Liu L., Wang L., Li B., Fang P., Zhou Z., Li Y., Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kendrick K.M., Lu G., Feng J. The fault lies on the other side: altered brain functional connectivity in psychiatric disorders is mainly caused by counterpart regions in the opposite hemisphere. Cereb. Cortex. 2014;25:3475–3486. doi: 10.1093/cercor/bhu173. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cheng W., Liu Z., Zhang K., Lei X., Yao Y., Becker B., Liu Y., Kendrick K.M., Lu G. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain. 2016;139:2307–2321. doi: 10.1093/brain/aww143. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang Z., Wan J.-Q., Sun Y.-W., Su S.-S., Ding W.-N., Xu J.-R. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS ONE. 2012;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material