Abstract
Objective:
To describe surgical site infection (SSI) after transurethral resection of prostate (TURP) from the French national database.
Methods:
A national SSI surveillance system was implemented in 1999. Each year, the network included urology departments that included at least two months plus one month follow-up, or at least 100 consecutive targeted surgical procedures. A dataset of patients who underwent urology procedures during the six-year period 2008–2013 was made available. SSI diagnosis was made according to standardised CDC criteria. Descriptive analyses were performed using SAS software version 9.4.
Results:
A total of 12,897 TURPs were performed by 89 urology departments. The crude incidence SSI rate was 2.43 (95% confidence interval = 2.16–2.79). The mean delay for diagnosis was 11.9 ± 8.9 days. The treatment of the SSI required a new surgical intervention in 1.35%. In the multilevel multivariate analysis, ASA score and duration of follow-up were the only parameters correlated with the SSI rate.
Conclusions:
On more than 12,000 TURPs surveyed, the SSI rate was 2.43. ASA score and duration of follow-up were the only parameters correlated with the SSI rate.
Keywords: Surgical wound infection, incidence, benign prostate hyperplasia, prostate, TURP, population surveillance
Introduction
The implementation of a surveillance system (with, but even without an associated infection control program) was shown to reduce surgical site infections (SSIs) rates (Astagneau et al., 2009). In this context, many countries have developed a national system for the surveillance of nosocomial infections. According to European Association of Urology recommendations, infectious complications should be registered, and infection control is a valuable method for monitoring a department’s quality output and for feedback to surgeons (Grabe et al., 2012). In France, a national surgical site infection surveillance system (the RAISIN surveillance system) based on a pyramidal organisation (local, regional and national) was implemented in 1999. The RAISIN surveillance system relies on volunteer surgical wards from public or private hospitals that routinely collect nosocomial infections. At the early stages of the network, any type of procedure was included for the main surgical specialties and the annual number of total procedures in the database rose from 79,803 in 1999 to 106,220 in 2013 (Desenclos and RAISIN Working Group, 2009; Surveillance des infections du site opératoire France, 2011 / 2012 / Maladies infectieuses / Rapports et synthèses / Publications et outils / Accueil, n.d.). Since 2010, the RAISIN network has encouraged targeted surveillance and, therefore, prostatectomy for cancer and transurethral resection of prostate (TURP) are the surgical procedures included in urology.
TURP is known to be followed by febrile or symptomatic urinary tract infections in 5–10% of patients, and sometimes a more severe sepsis (Grabe et al., 2012). Most of the data come from studies designed to assess the efficacy of antibiotic prophylaxis in the early 2000. As no urological procedure is included in the European Centres for Disease Control network (ECDC, 2013), we assume that the French national database could provide useful information to urologists. An appropriate statistical method for analysing grouped or clustered data by multilevel modelling would estimate the coefficient variability at the department level, and estimate contextual effects after adjusting for individual variables, while accounting for the non-independence of within-group observations (Goldstein, 2010).
The aim of this study was to describe SSI after prostate surgery from the French national database ISO-RAISIN 2008-2013, targeted on TURP, to determine individual- and ward-level factors associated with SSI occurrence in such surgeries, and to discuss how such national surveillance can be used for setting research setting.
Materials and methods
Data collection
Each year, the network included voluntary participation by urology departments; to be included surveillance had to be done over at least two months plus one month of follow-up, or include at least 100 consecutive targeted surgical procedures. Details on data collection and management methods of the RAISIN system were published elsewhere (Rioux et al., 2007). SSI diagnosis was made by the surgical team according to standardised CDC criteria. Concerning TURP, these infections (UTI) and prostatitis. UTI was defined as leucocytes ≥ 104/mL, bacteriuria ≥ 103/mL, with no more than two different bacteria, and clinical symptoms. A standardised questionnaire was completed for each patient who underwent surgery. According to the French Medical Acts Classification, any other procedures but TURP were withdrawn from our database. The following characteristics were detailed: the Altemeier wound classification, American Society of Anesthesiologists physical status classification system (ASA score), duration of surgery (≤ 75th percentiles vs. > 75th percentiles, the 75th percentile was 1 h for TURP), duration of post-surgery follow-up, time of SSI occurrence, mean time between surgery and SSI diagnosis, reoperation for SSI, patient outcome (alive vs. deceased), NNIS of the surgery (NNIS is a risk classification system based on assumed and independent risk factors for infection, ASA score > 2, duration of surgery > 75th percentile and wound contamination > 2 (Culver et al., 1991)).
Regarding the department level, the following characteristics were considered: healthcare facility status (private or public); global SSI rate in urology; and SSI rate in TURPs.
Population
A dataset of patients who underwent urology procedures during the six-year period 2008–2013 was made available (17,958 TURPs, 386 urology departments, 300 healthcare facilities). To generate unbiased and accurate estimates from multilevel models, we limited our analysis to healthcare facilities that included at least 100 surgical procedures over the five years. Those that had < 10 surgical procedures for one year were removed from the analysis. To study SSI trends, a cohort of services involved during three consecutive years, 2011–2013, with a sufficient number of procedures per year, was established. The local ethical committee validated the protocol.
Statistical analysis
To consider the hierarchical structure of data, analyses were performed using a two-level (patient, ward) logistic regression model. The binary outcome was the occurrence of an SSI during the first 30 days post surgery. An empty model was built with a random intercept at patient and ward levels containing no independent variables at any level to determine the initial distribution of the variance of the dependent variable between the two levels. Patient characteristics with a P value < 0.20 in a prior univariate multilevel analysis were included in a multivariate multilevel model, allowing for the probability of SSI to vary across wards but assuming that the effects of individual explanatory variables were the same for each ward. Parameters were estimated using the simulation-based Markov Chain Monte Carlo (MCMC) procedure for discrete response multilevel models. To quantify heterogeneity between wards, the median odds ratio (MOR) was calculated. Ninety-five percent confidence intervals (CI) for the MOR were calculated using the 2.5th and 97.5th percentiles of the posterior distribution of the ward variance.
Descriptive analyses were performed using the SAS software version 9.4 and multilevel modelling was carried out using the software MLwiN version 2.23.
Results
Population
A total of 12,893 patients undergoing TURPs were included (13.3% for cancer, 74.4% for benign prostate hyperplasia [BPH] and for 12.3% of patients the information was unknown) coming from 89 urology departments belonging to 65 public or private healthcare structures.
Patient descriptions and surgical procedure characteristics are shown in Table 1.
Table 1.
Patient descriptions and surgical procedure characteristics.
| TURP | |
|---|---|
| n | 12,893 |
| Mean age ± SD (years) | 72 ± 10 |
| NNIS (%) | |
| 0 | 42.9 |
| 1 | 40.9 |
| 2, 3 | 11.4 |
| One-day surgery (%) | 0.80 |
| Mean length of stay ± SD (days) | 6.0 ± 6.9 |
| Outcome (% deaths) | 0.21 |
| Mean operative time ± SD (min) | 50 ± 33 |
| Mean follow-up ± SD (days) | 41.3 ± 37.5 |
| SSI rate (%) (95% CI) | 2.43 (2.16–2.79) |
Regarding Altemeier classification, 95.5% of TURPs were classified as clean contaminated procedures.
The mean operative time of TURPs significantly rose from 2008 to 2013 from a mean time of 47.5 ± 26.9 min to 53.4 ± 32.9 min (P < 10–4).
SSI description
A total of 313 infections were reported. The crude incidence SSI rate was 2.43% (95% CI = 2.16–2.79). The mean time to diagnosis was 11.9 ± 8.9 days (median = 9 days, range 0–30 days). The type of infection was unknown for 9.2% of the reported infections; more than half of the infections (55.0%) were UTI. The treatment of the SSI needed a new surgical intervention in 1.35% of SSIs.
The SSI rate was stable over six years.
Risk factors for SSI are described in Table 2.
Table 2.
Risk factors for SSI.
| Variable | Coding | n | SSI | SSI rate (%) | OR | 95% CI OR | P value |
|---|---|---|---|---|---|---|---|
| Age (years) | ≤ 72 | 6547 | 144 | 2.20 | Ref | ||
| > 72 | 6346 | 169 | 2.66 | 1.22 | 0.97–1.52 | 0.09 | |
| Preoperative stay (days) | ≤ 2 | 6810 | 167 | 2.45 | Ref | ||
| > 2 | 817 | 23 | 2.82 | 1.15 | 0.74–1.79 | 0.53 | |
| Wound classification | 2 | 12,312 | 298 | 2.42 | Ref | ||
| 3 | 440 | 14 | 3.18 | 1.32 | 0.77–2.28 | 0.31 | |
| ASA score | 1 and 2 | 8357 | 178 | 2.13 | Ref | ||
| 3 and 4 | 4132 | 122 | 2.96 | 1.40 | 1.11–1.77 | 0.005 | |
| Operative time (min) | ≤ P75 | 9075 | 223 | 2.46 | Ref | ||
| > P75 | 3818 | 90 | 2.36 | 0.96 | 0.75–1.23 | 0.74 | |
| NNIS | 0 | 5528 | 121 | 2.19 | Ref | ||
| 1 | 5268 | 127 | 2.47 | 1.10 | 0.86–1.42 | 0.31 | |
| 2 and 3 | 1474 | 50 | 3.39 | 1.57 | 1.12–2.19 | 0.01 | |
| Length of stay (days) | ≤ 5 | 9941 | 193 | 1.94 | Ref | ||
| > 5 | 2952 | 120 | 4.07 | 2.14 | 1.70–2.70 | < 10–4 | |
| Follow-up (days) | ≤ 36 | 2289 | 124 | 4.69 | Ref | ||
| > 36 | 5339 | 66 | 0.03 | 0.01 | 0.00–0.03 | < 10–4 |
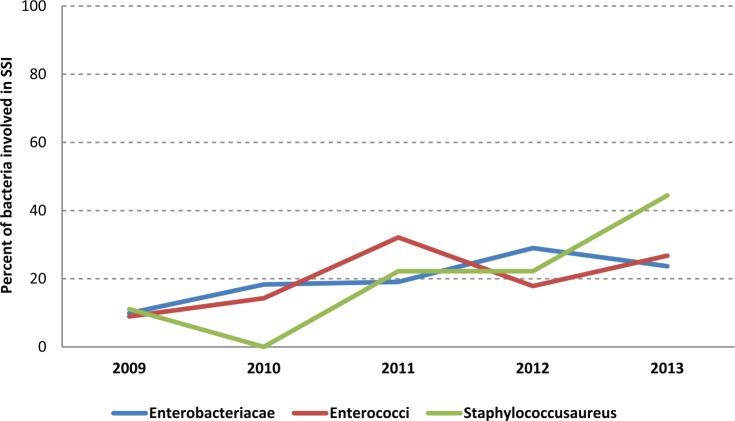
Since 2009, microbiological documentation of SSI has been included in the national database. Over the last five years, 255 SSIs had bacterial documentation (Table 3). Gram-negative bacilli were found in more than half of the documented SSI (Table 3, Figure 1).
Table 3.
Microbiological documentation of SSI.
| Bacteria | n | % |
|---|---|---|
| Enterobacteriacae | 109 | 42.7 |
| Escherichia coli | 78 | 30.6 |
| Enterobacter cloacae | 12 | 4.7 |
| Proteus mirabilis | 7 | 2.6 |
| Klebsiella oxytoca | 6 | 2.4 |
| Klebsiella pneumoniae | 6 | 2.4 |
| Pseudomonas aeruginosa | 19 | 7.5 |
| Enterococci | 61 | 23.9 |
| Enterococcus faecalis | 42 | 16.5 |
| Other enterococcus | 19 | 7.5 |
| Staphylococci | 22 | 8.6 |
| Staphylococcusaureus | 9 | 3.5 |
| Other staphylococcus | 13 | 5.1 |
| Other bacterias | 44 | 17.3 |
Figure 1.
Evolution of bacteria involved in post-TURP SSIs.
SSI due to S. aureus has risen over the past four years. Two multi-resistant S. aureus (MRSA) were found among SSIs, in 2011 and 2013.
Multivariate analysis
Results from the empty model revealed a significant heterogeneity of SSI occurrence between individual wards (P < 0.0001). Patient and ward levels were therefore kept in the subsequent analyses. In the resulting two-level empty model, ward-level variance could be characterised by a MOR of 2.50 (95% CI = 1.99–3.20).
Multivariate results are presented in Table 4. In the final model, SSI occurrence was significantly higher among patients with an ASA score ≥ 3 (P < 0.01) and in contrast, SSI occurrence was lower among patients with a follow-up > 36 days after surgery (P < 0.0001). When including these patient-level variables, ward-level variance remained significantly different from zero with a MOR of 2.94 (95% CI = 2.33–3.84).
Table 4.
Results of the multilevel logistic regression models.
| Variable | Empty model OR (95% CI) |
Multivariate model OR (95% CI) | P value |
|---|---|---|---|
| ASA score (1, 2 vs. 3, 4) |
1.40 (1.11–1.78) | 0.005 | |
| Duration of follow-up (≤ 36 vs. > 36 days) |
0.01 (0.01–0.03) | < 0.0001 | |
| MOR (95% CI) | 2.50 (1.99–3.20) | 2.94 (2.33–3.84) |
OR, odds ratio; CI, confidence interval; MOR, median odds ratio.
Discussion
We report the largest series analysis of infectious complications for a specific urological surgery. In the RAISIN database, over 1 million procedures were followed, nearly 20,000 urological interventions with an average postoperative infection rate of 2.5–4.5%. Several risk factors have been identified. ASA score and the Altemeier classification had already been identified as risk factors. Regarding ASA score, data collection should be improved with automated anaesthesia records. Twenty-five percent of TURPs were classified as clean procedures which tends to demonstrate that French urologists are not familiar with Altemeir’s classification. In contrast, the ambulatory character that had been suggested as a protective factor towards SSIs in orthopaedic surgery (Owens et al., 2014) is not found in our study as decreasing the SSI risk in urology. The literature on ambulatory surgery may lack precision regarding strictly urological procedures. A Danish study reported the experience of 16,048 patients treated in ambulatory care, of which only 3252 were urology (901 cystoscopies, 527 resection of bladder tumours, 729 scrotal surgeries, 963 vasectomies and 132 others) (Engbaek et al., 2006). The complication rate reported was 0.11% after cystoscopy, 1.37% after scrotal surgery and 0.57% after transurethral resection of bladder; the overall rate of complications (after orthopaedic, gynaecological or digestive surgery) was 0.73%. There is no mention in this publication of general urological complications. In particular, only one UTI is reported without knowing the prior surgery.
The second criticism lies in the incompleteness of the data mainly for the ASA score. Institutions kept their choice of methodology, which makes the dataset somewhat heterogeneous. Over the years, the methodology has changed, which makes it difficult to analyse the evolution of SSI rates. However, in the multivariate analysis, this type of intervention does not seem to influence the rates. National guidelines for antibiotic prophylaxis, a major issue, recommend the use of cefuroxim but no data are available in our study about adherence or deviation to guidelines. Data collection did not include any information about antibiotic prophylaxis, its dose or its duration. As previously demonstrated, antibiotic prophylaxis significantly decreases the incidence of bacteriuria and clinical septicaemia in men with preoperative sterile urine undergoing TURP resection (Berry and Barratt, 2002).
With many participating centres and nearly 20,000 urological interventions included, this study is one of the most important regarding SSIs in urology. Regarding operative time conventionally used in the NNIS score, this one brings additional bias but is used in all articles on SSI. Indeed, the operating time that increased over years in our study depends on many factors: learning curve; surgeon; patient characteristics; technique used. Recently, surgical management of BPH had seen the arrival of new technologies. Photovaporisation or enucleation are validated alternatives to TURP; until this year, French Medical Acts Classification was unable to differentiate between the procedures. We can guess that such technologies that vary the operative time may influence the SSI rate. Nevertheless, this bias exists in all studies using this criterion. Despite several biases, our series is one that includes the largest number of patients and enables us to have a reference SSI rate. Furthermore, the conventional way to classify postoperative infections as deep, superficial or incisional is not applicable to endoscopic procedures. Post-TURP infection can simply be classified as a urinary infection without prostatitis that makes the classification obsolete.
Conclusion
The harmonisation of SSI monitoring has helped to build a large database in France since 1999. This surveillance estimates the incidence rate of SSI through monitoring the most representative interventions by specialty. The network continued to grow and has collected data for 330,281 interventions over 20,000 in urology alone this year. In more than 12,000 TURPs surveyed, the SSI rate was 2.43. ASA score and duration of follow-up were the only parameters correlated with the SSI rate. The evolution of the incidence assesses the impact of preventive measures implemented in departments and at a national level as well. The downward trend of SSI was confirmed in urology for TURP. In comparison, France is in the lowest limit of SSI rate in European countries for most interventions. To improve the performance of the ISO-RAISIN network, further work is being finalised with the ultimate aim of lowering SSI rates, and ISO-RAISIN data collection should address TURP-specific characteristics and risk factors, in order to better motivate urologists and obtain more reliability and usefulness.
Acknowledgments
We thank the urological and infection control teams of all healthcare facilities for providing data to the French RAISIN surveillance network.
Footnotes
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Astagneau P, L’Hériteau F, Daniel F, Parneix P, Venier A-G, Malavaud S, Jarno P, Lejeune B, Savey A, Metzger M-H, Bernet C, Fabry J, Rabaud C, Tronel H, Thiolet J-M, Coignard B. ISO-RAISIN Steering Group. (2009) Reducing surgical site infection incidence through a network: results from the French ISO-RAISIN surveillance system. Journal of Hospital Infection 72: 127–134. [DOI] [PubMed] [Google Scholar]
- Berry A, Barratt A. (2002) Prophylactic antibiotic use in transurethral prostatic resection: a meta-analysis. Journal of Urology 167: 571–577. [DOI] [PubMed] [Google Scholar]
- Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, Banerjee SN, Edwards JR, Tolson JS, Henderson TS. (1991) Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. American Journal of Medicine 91: 152S–157S. [DOI] [PubMed] [Google Scholar]
- Desenclos J-C. RAISIN Working Group. (2009) RAISIN - a national programme for early warning, investigation and surveillance of healthcare-associated infection in France. Euro Surveillance 14: 19408. [PubMed] [Google Scholar]
- ECDC. (2013) Surveillance of surgical site infections in Europe 2010–2011. Stockholm: ECDC. [Google Scholar]
- Engbaek J, Bartholdy J, Hjortsø N-C. (2006) Return hospital visits and morbidity within 60 days after day surgery: a retrospective study of 18,736 day surgical procedures. Acta Anaesthesiologica Scandinavica 50: 911–919. [DOI] [PubMed] [Google Scholar]
- Goldstein H. (2010) Multilevel statistical models. 4th ed. Chichester: John Wiley & Sons, Ltd. [Google Scholar]
- Grabe M, Botto H, Cek M, Tenke P, Wagenlehner FME, Naber KG, Bjerklund Johansen TE. (2012) Preoperative assessment of the patient and risk factors for infectious complications and tentative classification of surgical field contamination of urological procedures. World Journal of Urology 30: 39–50. [DOI] [PubMed] [Google Scholar]
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, Hooton TM. (1985) The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. American Journal of Epidemiology 121: 182–205. [DOI] [PubMed] [Google Scholar]
- Owens PL, Barrett ML, Raetzman S, Maggard-Gibbons M, Steiner CA. (2014) Surgical site infections following ambulatory surgery procedures. JAMA 311: 709–716. [DOI] [PubMed] [Google Scholar]
- Rioux C, Grandbastien B, Astagneau P. (2007) Impact of a six-year control programme on surgical site infections in France: results of the INCISO surveillance. Journal of Hospital Infection 66: 217–223. [DOI] [PubMed] [Google Scholar]
- Surveillance des infections du site opératoire France, 2011 / 2012 / Maladies infectieuses / Rapports et synthèses / Publications et outils / Accueil. n.d. Available at: http://www.invs.sante.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-infectieuses/2012/Surveillance-des-infections-du-site-operatoire-France-2011 (accessed 6 June 2014).