Abstract
Introduction: Postoperative delirium and pain are common complications in adults, and are difficult both to prevent and treat. Obstructive sleep apnea (OSA) is prevalent in surgical patients, and has been suggested to be a risk factor for postoperative delirium and pain. OSA also might impact pain perception, and alter pain medication requirements. This protocol describes an observational study, with the primary aim of testing whether OSA is an independent predictor of postoperative complications, focusing on (i) postoperative incident delirium and (ii) acute postoperative pain severity. We secondarily hypothesize that compliance with prescribed treatment for OSA (typically continuous positive airway pressure or CPAP) might decrease the risk of delirium and the severity of pain.
Methods and analysis: We will include data from patients who have been enrolled into three prospective studies: ENGAGES, PODCAST, and SATISFY-SOS. All participants underwent general anesthesia for a non-neurosurgical inpatient operation, and had a postoperative hospital stay of at least one day at Barnes Jewish Hospital in St. Louis, Missouri, from February 2013 to December 2017. Patients included in this study have been assessed for postoperative delirium and pain severity as part of the parent studies. In the current study, determination of delirium diagnosis will be based on the 3-minute Diagnostic Confusion Assessment Method, and the Visual Analogue Pain Scale will be used for pain severity. Data on OSA diagnosis, OSA risk and compliance with treatment will be obtained from the preoperative assessment record. Other variables that are candidate risk factors for delirium and pain will also be extracted from this record. We will use logistic regression to test whether OSA independently predicts postoperative delirium and linear regression to assess OSAs relationship to acute pain severity. We will conduct secondary analyses with subgroups to explore whether these relationships are modified by compliance with OSA treatment.
Keywords: Obstructive Sleep Apnea, Postoperative Delirium, Postoperative Pain
Introduction
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing. OSA is characterized by repetitive, functional collapse of the airway leading to cyclical decrements or cessations of airflow during sleep 1. It is estimated that 20% of the general population suffers from OSA 2, 3, and among adults with OSA, up to 75% are unaware of the diagnosis 4, 5. Of relevance to perioperative medicine, there is also a high OSA prevalence in surgical patients 3. In common with the general population, many of these patients are unaware they have OSA 6, 7. Also of note, prevalence of sleep apnea often varies by type of surgery; for example, prevalence in the bariatric surgery population is estimated to be 70% 8, 9. OSA prevalence combined with ignorance of diagnosis is cause for concern given the wide range of health consequences. OSA has been causally implicated in an assortment of both acute and chronic disorders. Acutely, OSA has been associated with disrupted sleep, tiredness, and episodic hypoxia and hypercapnia during sleep 10, 11. Chronically, OSA has been linked to a multitude of co-morbidities, including ischemic heart disease and stroke 12, hypertension 13, 14, arrhythmias 15, 16, aortic dissection 17, 18, chronic fatigue 19, pulmonary hypertension 20, 21, diabetes 22, and respiratory acidosis with compensatory metabolic alkalosis 23, 24.
OSA is becoming a growing concern in the perioperative period, as there is increasing evidence linking OSA to adverse postoperative outcomes 25, 26. For example, following various surgical procedures, patients with OSA probably have more respiratory, cardiac, and neurologic complications 27– 30, as well as increased postoperative infections 31. Unsurprisingly surgical patients with OSA therefore have a higher transfer rate to the ICU 28, increased stay in the ICU 31, and increased overall length of hospital stay 27, 28.
Of particular relevance to the research focus of this protocol, certain aspects of OSA such as recurrent hypoxemia, systemic inflammation, and sleep disruption have been associated with altered pain processing and incident delirium 32– 34. A causal link between OSA and delirium would be clinically important given the negative outcomes associated with postoperative delirium. In the DSM-5, delirium is defined as a disturbance in attention, awareness, and cognition that develops over a short period of time and over the course of a day, fluctuates in severity 35. In older adults, the incidence of postoperative delirium ranges from 10–70%, depending on the type of surgery 36. Patients who experience postoperative delirium often require an extended stay in the intensive care unit 37, subsequently report decreased quality of life 38, and might be at increased risk for accidental falls, long-term cognitive decline and death after hospital discharge 39. Thus, postoperative delirium is associated with a considerable burden on patients and their families, and an increase to society in the overall cost of healthcare 40, 41.
The reported impact of OSA on postoperative pain and pain perception poses further challenges to clinicians and patients. Adequate postoperative analgesia is an important component of recovery, and pain negatively impacts quality of life. Mechanistic evidence in various populations suggests that sleep deprivation promotes up-regulation of cytokines 42– 47, including interleukin-1β, interleukin-6, and tumor necrosis factor, all of which might induce excessive sensitivity to pain 45, 48. Consistent with these studies clinical evidence, including compelling data from burn victims, suggests that interrupted and inadequate sleep promotes hyperalgesia 32– 34, 49. Furthermore, Khalid et al. showed that treatment with continuous positive airway pressure (CPAP) in adults with OSA dampened their sensitivity to painful stimuli 50. Thus, whether or not people with OSA have increased pain sensitivity might to some extent depend on how effectively they are treated. To complicate matters further, people with OSA, especially if they experience episodic hypoxemia during sleep, reportedly have increased susceptibility to the respiratory depressant effects of opioid medications 51, 52. Thus, since opioids are the mainstay of therapy for severe postoperative pain, it can be especially difficult to provide safe and adequate analgesia to surgical patients with OSA.
The objectives of this study are to investigate further the relationships between OSA on the one hand, and common postoperative complications such as pain and delirium on the other hand. We hypothesize that patients with OSA experience more severe postoperative pain and have a higher incidence of postoperative delirium. We further hypothesize these negative outcomes might be mitigated by compliance with OSA treatment.
Protocol
Study design
This protocol describes an observational study, investigating the relationship between OSA as a risk factor, and postoperative delirium and acute postsurgical pain severity as adverse outcomes. The three parent studies from which the data are being obtained for the current study have all been approved by the Human Research Protection Office (HRPO) at Washington University, and patients enrolled in all three studies provided written informed consent. The HRPO has also provided approval for this current study. Data will be aggregated from the Systematic Assessment and Targeted Improvement of Services Following Yearlong Surgical Outcomes Surveys Study (SATISFY-SOS, NCT02032030); the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes study (ENGAGES, NCT02241655); and the Prevention of Delirium and Complications Associated with Surgical Treatments study (PODCAST, NCT01690988). For greater detail regarding the three parent studies, please review previously published protocols and literature 53– 57.
Patients ≥ 18 years who underwent general anesthesia for a non-neurosurgical inpatient operation at Barnes Jewish Hospital in St. Louis, Missouri, from February 2013 to December 2017, will be included in our analysis. Patients had a postoperative hospital stay of at least one day. The main outcomes of interest will include postoperative delirium and pain, assessed daily until postoperative day 3. The primary aim of this study is to investigate whether OSA is an independent predictor of postoperative delirium and acute postsurgical pain severity. We will conduct secondary analyses with subgroups to explore whether these associations are modified by compliance with OSA treatment.
This protocol is compliant with published guidelines for observational study protocols, and the conduct and reporting of this study will adhere to the RECORD and STROBE guidelines for observational studies 58– 60.
Eligibility criteria
Inclusion criteria:
(i) Enrollment in the SATISFY-SOS, ENGAGES, or PODCAST study;
(ii) Postoperative stay of at least 1 day following surgery at Barnes Jewish Hospital
(iii) General anesthesia for elective surgical procedures
Exclusion criteria:
(i) Neurosurgery
(ii) Age <18
Data Collection
i. Baseline Data. Patients undergoing elective surgery are routinely screened at the Center for Preoperative Assessment and Planning at Barnes Jewish Hospital in St. Louis, Missouri, where detailed medical history is collected and screening tests are administered, including the STOP-BANG ( Snoring, Tiredness, Observed Apnea, High Blood Pressure, Body Mass Index>35kg/m 2, Age >50, Neck circumference, male Gender) test for OSA risk. Baseline characteristics will be extracted via electronic chart review and will include but are not limited to: age, sex, race, ethnicity, smoking history, alcohol use (average per week), STOP-BANG criteria, OSA status, and pre-existing medical conditions.
ii. Delirium assessment method. Delirium is one of the primary outcomes of this study, and will be determined using the 3D-Confusion Assessment Method (3D-CAM), a validated, abbreviated assessment derived from the Confusion Assessment Method (CAM) 61. The CAM, a delirium assessment instrument used primarily by non-psychiatrists, typically takes between 15 and 30 minutes to complete 62. The 3D-CAM was developed as a method to more efficiently screen patients for delirium. It consists of a subset of the questions used in the CAM, as well as CAM scoring items that are based on patient behavior (10 cognitive testing items, 10 interviewer observations). With this approach, the 3D-CAM is intended to only take 3 minutes.
Delirium in two of the parent studies, ENGAGES and PODCAST, was assessed using either the long form of the CAM or an abbreviated CAM designed and validated 63 for critically ill patients, often found in the intensive care unit (CAM-ICU). For this sub-study, pertinent 3D-CAM data from the long CAM assessments will be extracted for our analysis. In the third parent study (SATISFY-SOS), delirium was assessed with the 3D-CAM. The presence of delirium will be defined as a positive 3D-CAM or CAM-ICU during any postoperative assessment through postoperative day 3. In order to qualify for a diagnosis of delirium with the 3D-CAM, the following three criteria must be met: 1) either acute onset OR a fluctuating course; 2) inattention; and 3) either disorganized thinking OR altered level of consciousness. A patient will be considered positive for delirium if the patient is recorded to have had a single instance of delirium during their postoperative stay.
iii. Pain Assessment Method. Pain during hospital stay will be assessed using the Visual Analogue Scale (VAS), a validated pain assessment instrument that has been widely used in adult populations 64, 65. Patients are asked to indicate on a line 100mm in length the severity of their pain in three different situations: 1) at rest, 2) taking a deep breath or coughing, and 3) moving (sitting up, walking, or moving extremities). The patient’s mark is then measured with a ruler and recorded in mm. For our analysis, we will incorporate the highest pain score recorded on any postoperative assessment as our value of interest. As postsurgical pain is often dependent on the type of surgery, we will adjust for type of surgery in our statistical model, as well as other confounding variables described in the methods below.
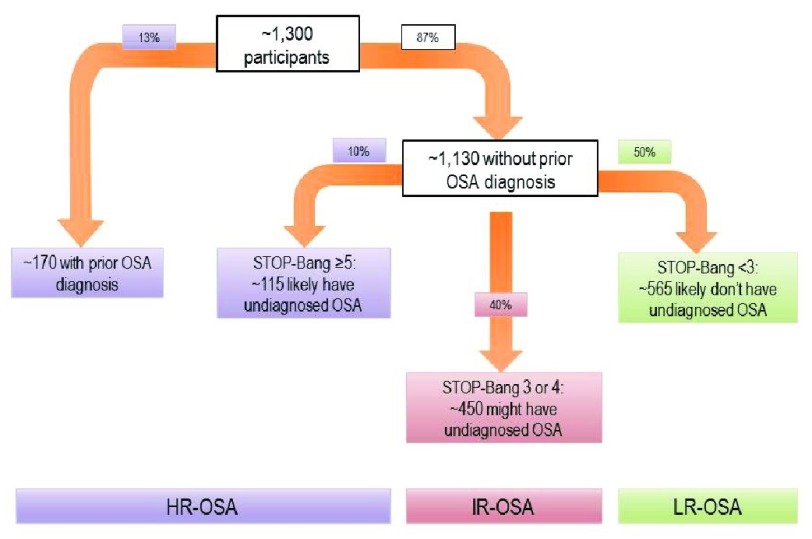
iv. OSA Classification For the primary analysis ( Figure 1), patients will be grouped into one of three categories: high risk of OSA (HR-OSA), intermediate risk of OSA (IR-OSA), and low risk of OSA (LR-OSA). Patients with a history of a positive polysomnography test will be classified as HR-OSA, whereas patients with a history of negative polysomnography will be classified as LR- OSA. Patients with no history of polysomnography testing will be classified into one of the three categories based on STOP-BANG screening status. The STOP-Bang questionnaire classifies patients into three commonly accepted categories based on scoring: 0–2 indicates low risk of OSA; 3–4 indicates intermediate risk; 5–8 indicates high risk 66. We will follow these guidelines for classifying patients as HR-OSA, IR-OSA, or LR-OSA for our primary analysis, and thus likely demonstrate important trends between and among groups.
Figure 1. Predicted groupings for OSA-risk classification in the primary analysis, based on previous data from our preoperative assessment clinic 7.
Of note, current literature classifies, often for simplicity, a STOP-Bang score of ≥3 as high risk for OSA. However, this can obscure analysis, potentially resulting in a falsely weaker association between OSA risk and risk of postoperative adverse outcomes. Therefore, we will not group intermediate risk of OSA with high risk of OSA. Also, some literature incorporates bicarbonate levels to help determine OSA risk. As baseline laboratory values are not available for each participant, we will not include this component for classifying OSA risk.
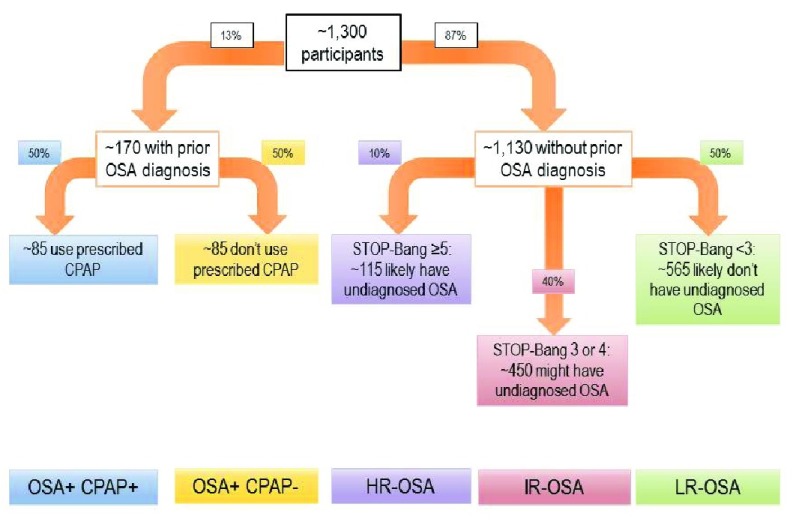
For secondary analysis ( Figure 2), we will analyze delirium incidence and pain severity among five patient groupings: confirmed OSA + report using prescribed CPAP, confirmed OSA + report not using prescribed CPAP, high risk for OSA (STOP-Bang 5–8), intermediate risk for OSA (STOP-Bang 3–4), low risk for OSA (STOP-Bang <3). Thus, secondary analysis will likely demonstrate if reported CPAP adherence mitigates these adverse outcomes.
Figure 2. Predicted groupings for OSA-risk classification in the secondary analysis, based on previous data from our preoperative assessment clinic 7.
v. Sample Size. We estimate that we will have data with complete outcomes (pain severity and incident delirium) and information on OSA status for approximately 1,300 patients. We estimate that 260 ( ~20%) of these patients will have incident postoperative delirium. We will have patient reported pain outcomes for all participants. We will use logistic and linear regression, including potential confounder variables, to test for an independent association between OSA as a risk factor and postoperative delirium and pain severity as outcomes of interest. We estimate that it will be appropriate to include up to 25 variables in each of the regression models.
Data Management
All electronic data collected during this study, as well as the SATISFY-SOS, ENGAGES, and PODCAST databases, are hosted on a firewall-secured network server. This server is managed and maintained by the IT team of the Department of Anesthesiology, and is securely housed behind two locked doors in the departmental offices. The Project Informaticist, Data Manager, and Director(s) are the only individuals with full access to these password-protected and encrypted databases. Delirium and pain assessments are first completed using paper surveys, which are then securely stored in locked cabinets within the department. Results are entered into a Research Electronic Data Capture (REDCap) tool hosted at Washington University School of Medicine in St. Louis 67. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
i. Statistical considerations. Continuous variables will be graphically evaluated with histograms, boxplots, and q-q plots, and numerically with measures of skewness, kurtosis, and Kolmogorov-Smirnov tests. Outliers will be excluded, and approximate normality will be ensured before parametric statistics are applied. Perioperative variables will be described with mean ± SD, median [IQR], and numbers/proportions, as appropriate. Differences in patient and other perioperative factors between groups will be evaluated with chi-squared, t-tests, ANOVA, Kruskal-Wallis, and/or Wilcoxon-Mann-Whitney tests, as appropriate. Participants missing outcome data will be excluded from analysis.
ii. Delirium. Logistic Regression will be used to assess the relationship between OSA as a risk factor and incident postoperative delirium as an outcome. For our analyses, we will include no more than 1 variable for every 8 outcomes. With an estimated incident postoperative delirium rate of 20%, we plan to include up to 25 pre-specified candidate predictor variables in the primary regression models, including the most clinically relevant interaction terms. Variables for our primary analysis have been selected based on existing evidence, and will likely include: OSA status; Age; Sex; Type of Surgery; Charlson Comorbidity Index; Procedural Cardiac Risk; ASA physical status; Alcohol use. We will also include a history of any of the following comorbidities: Previous Surgeries; Stroke; Dementia or Mild Cognitive Impairment; Visual Impairment; Depression; Anxiety; Chronic Pain; and Diabetes Mellitus. We hope to include BMI and age independently of the OSA risk classification since they are continuous variables, and their inclusion in the regressions might improve the models. We also hope to include the variable ‘tiredness’ in the models since this particular symptom could plausibly independently predict both delirium and pain.
iii. Pain. Linear Regression will be used to examine OSA’s potential relationship to postoperative pain. For this analysis, the outcome is continuous rather than binary, and will apply to all 1,300 patients. It will be reasonable to include up to 25 pre-specified candidate predictor variables in the linear regression models, including interaction terms. As risk factors for delirium and pain are overlapping, the same candidate predictor variables will be used in this regression. Sensitivity analyses will be conducted to address limitations regarding pain. Since it is important to consider delirious patients might be unable to report pain accurately, we plan to conduct a sensitivity analysis with pain as the outcome, excluding all the patients who were diagnosed with postoperative delirium. Additionally, since our primary analysis will not consider duration of severe pain or distinguish between rest and provoked pain, we plan to conduct a sensitivity analysis with median provoked pain during hospital stay (up to postoperative day 3) as the outcome. The responses to two VAS questions (pain when (i) taking a deep breath or coughing, and (ii) moving (sitting up, walking, or moving extremities)) will be compiled to represent provoked pain during hospital stay.
Anticipated results
We expect that patients with OSA will experience greater postoperative pain severity, and have a higher risk for postoperative delirium following surgical procedures. For our secondary analyses, we propose that these adverse outcomes might be modified by compliance with CPAP treatment. We predict patients with diagnosed OSA who do not use prescribed CPAP will experience a higher incidence of delirium and increased pain. We also expect a step-wise increase in these adverse outcomes (delirium incidence and pain severity) when analyzing patients based on their STOP-Bang assessment groups (high risk vs. intermediate risk vs. low risk).
Discussion
OSA is a common and frequently undiagnosed perioperative problem. This observational study will help to clarify whether or not OSA is an independent predictor of postoperative incident delirium and acute postoperative pain. Secondary analyses may show if these adverse outcomes might be modified by compliance with OSA treatment.
In this study, we will attempt to replicate the reported finding showing that OSA is an independent predictor of postoperative delirium and acute postsurgical pain severity 32– 34. This study will have important strengths compared to the existing literature; most notably the database including routine structured preoperative screening for OSA, and postoperative delirium and pain assessments on a broad surgical population. The researchers who collected data for this study were all expertly trained in administering delirium and pain assessments. In an effort to improve methodological rigor, we have pre-specified independent variables for regression models, and have described our statistical analyses.
This study will also have important limitations. Although we will have thorough medical histories routinely collected from preoperative clinic assessments, we will not know severity of OSA or other comorbidities. In common with any observational study, this study will be unable to distinguish association from causation. In particular, if we do find in this study that OSA is associated with either increased delirium incidence or pain severity, we will not be able to determine (i) whether OSA is causally implicated or (ii) whether there is another explanatory factor associated with both OSA and these outcomes. Regarding the outcome of delirium, this study will address on the crude association with incident delirium as a binary outcome. It might be more important to focus on either the duration or severity of delirium. Regarding pain, it is important to consider that delirious patients might be unable to report pain accurately. This limitation is common to all studies evaluating postoperative pain. To mitigate this to an extent, we plan to conduct a sensitivity analysis with highest VAS pain score as the outcome, excluding all the patients who were diagnosed with postoperative delirium. Also in relation to pain, our primary outcome will be most severe pain reported in postoperative days 1–3. This approach will not consider duration of severe pain or distinguish between rest and provoked pain. To mitigate this to an extent, we plan to conduct a sensitivity analysis exploring median provoked pain through postoperative day 3 as the outcome. Additionally, it will be important to include analgesic medication as potential confounders in the regression analyses, and accurate data on these might not be available.
In conclusion, while likely providing stronger evidence regarding the impact of OSA on postoperative delirium and pain, this study might also discern interventional strategies for treatment and prevention. For example, in relation to delirium, we could test perioperative delirium prevention bundles in patients with OSA or we could investigate whether preoperative initiation of CPAP treatment decreases this complication. The role of CPAP therapy in relation to improved analgesia should also be clarified. Regarding pain, we could further develop analgesic plans especially for surgical patients with OSA, such as emphasizing regional analgesia or non-opioid analgesics. We could also implement procedures intended to improve the safety of patients with OSA receiving respiratory depressant medications in the perioperative period. With emerging knowledge about biased signaling with opioids 68, it is possible that certain opioids (e.g. morphine) are safer than others (e.g., Fentanyl) for patients with OSA in terms of their propensity to provide analgesia rather than to cause respiratory depression. We hope to use the foundational work proposed in this observational study to guide the design of such trials and clinical plans, with the goals of reducing postoperative delirium and acute postoperative pain severity for the large number of patients at risk due to OSA.
Data availability
No data is associated with this article.
Acknowledgements
The authors would like to acknowledge and thank other experts and advisors involved in the study including: Angela Mickle, M.S.; Hannah Maybrier, B.S.; Thaddeus Budelier, M.D.; Jamila Burton, B.S.; Jordan Oberhaus, B.S.; Daniel Park, B.S.; Amrita Aranake-Chrisinger, M.D., MSCI; Bradley Fritz, M.D., MSCI; and Mark Willingham, M.D., MSCI; of Washington University School of Medicine, Department of Anesthesiology (660 S Euclid Ave Campus Box 8054, St. Louis, MO, 63110).
Funding Statement
Research reported in this publication was supported by the National Center For Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number TL1TR002344. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by grants from the National Heart, Lung, and Blood Institute of the NIH under Award Numbers 5R21HL123666 and 5T35HL007815, as well as the 2017-Washington University School of Medicine Meharry Summer Research Program, Stipend Name: Lilly.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 1 approved
Compliance and Ethical considerations
As this study is an observational data analysis of patients enrolled in Satisfy-SOS, ENGAGES, and PODCAST there is no direct burden placed upon the patients in the study and procedures for monitoring exposure compliance are not necessary. There is low risk of breach of confidential health information. However, all data are hosted on a firewall-secured network server, managed by the Department of Anesthesiology, which is securely stored behind two locked doors within the departmental office suite. Only Health Insurance Portability Accountability Act (HIPPA)-trained employees of the Department of Anesthesia or Barnes Jewish Healthcare have access to resources on the private network server. The three parent studies from which the data are being obtained for the current study have all been approved by the Human Research Protection Office (HRPO) at Washington University, and patients enrolled in all three studies provided written informed consent. The HRPO has also provided approval for this current study.
Registration, Reporting, and dissemination
The three parent studies from which data for this study are being used have all been registered at clinicaltrials.gov. SATISFY-SOS is registered as NCT02032030. ENGAGES is registered as NCT02241655. PODCAST is registered as NCT01690988j. Results of this study will be presented at national meetings and published in a scientific journal. Participants will not be individually notified regarding the results of this study.
References
- 1. Dempsey JA, Veasey SC, Morgan BJ, et al. : Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ: Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 3. Peppard PE, Young T, Barnet JH, et al. : Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simpson L, Hillman DR, Cooper MN, et al. : High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013;17(3):967–973. 10.1007/s11325-012-0785-0 [DOI] [PubMed] [Google Scholar]
- 5. Lee W, Nagubadi S, Kryger MH, et al. : Epidemiology of obstructive sleep apnea: A population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. 10.1586/17476348.2.3.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finkel KJ, Searleman AC, Tymkew H, et al. : Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10(7):753–758. 10.1016/j.sleep.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 7. Lockhart EM, Willingham MD, Abdallah AB, et al. : Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med. 2013;14(5):407–415. 10.1016/j.sleep.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frey WC, Pilcher J: Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13(5):676–683. 10.1381/096089203322509228 [DOI] [PubMed] [Google Scholar]
- 9. O'Keeffe T, Patterson EJ: Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14(1):23–26. 10.1381/096089204772787248 [DOI] [PubMed] [Google Scholar]
- 10. Brown KA: Intermittent hypoxia and the practice of anesthesia. Anesthesiology. 2009;110(4):922–927. 10.1097/ALN.0b013e31819c480a [DOI] [PubMed] [Google Scholar]
- 11. Fassbender P, Herbstreit F, Eikermann M, et al. : Obstructive Sleep Apnea-a Perioperative Risk Factor. Dtsch Arztebl Int. 2016;113(27–28):463–469. 10.3238/arztebl.2016.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaggi HK, Concato J, Kernan WN, et al. : Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 13. Peppard PE, Young T, Palta M, et al. : Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 14. Pedrosa RP, Barros IML, Drager LF, et al. : OSA is common and independently associated with hypertension and increased arterial stiffness in consecutive perimenopausal women. Chest. 2014;146(1):66–72. 10.1378/chest.14-0097 [DOI] [PubMed] [Google Scholar]
- 15. Tung P, Levitzky YS, Wang R, et al. : Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6(7): pii: e004500. 10.1161/JAHA.116.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cadby G, McArdle N, Briffa T, et al. : Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148(4):945–952. 10.1378/chest.15-0229 [DOI] [PubMed] [Google Scholar]
- 17. Saruhara H, Takata Y, Usui Y, et al. : Obstructive sleep apnea as a potential risk factor for aortic disease. Heart Vessels. 2012;27(2):166–173. 10.1007/s00380-011-0135-3 [DOI] [PubMed] [Google Scholar]
- 18. Naito R, Sakakura K, Kasai T, et al. : Aortic dissection is associated with intermittent hypoxia and re-oxygenation. Heart Vessels. 2012;27(3):265–270. 10.1007/s00380-011-0149-x [DOI] [PubMed] [Google Scholar]
- 19. Mariman A, Delesie L, Tobback E, et al. : Undiagnosed and comorbid disorders in patients with presumed chronic fatigue syndrome. J Psychosom Res. 2013;75(5):491–496. 10.1016/j.jpsychores.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 20. Ismail K, Roberts K, Manning P, et al. : OSA and pulmonary hypertension: Time for a new look. Chest. 2015;147(3):847–861. 10.1378/chest.14-0614 [DOI] [PubMed] [Google Scholar]
- 21. Javaheri S, Javaheri S, Javaheri A: Sleep apnea, heart failure, and pulmonary hypertension. Curr Heart Fail Rep. 2013;10(4):315–320. 10.1007/s11897-013-0167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Punjabi NM, Beamer BA: Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179(3):235–240. 10.1164/rccm.200809-1392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang T, Eskandari D, Zou D, et al. : Increased carbonic anhydrase activity is associated with sleep apnea severity and related hypoxemia. Sleep. 2015;38(7):1067–1073. 10.5665/sleep.4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan H, Pinto SJ, Huang J, et al. : Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep. 2012;35(9):1257–1267. 10.5665/sleep.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam KK, Kunder S, Wong J, et al. : Obstructive sleep apnea, pain, and opioids: Is the riddle solved? Curr Opin Anaesthesiol. 2016;29(1):134–140. 10.1097/ACO.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasu TS, Grewal R, Doghramji K: Obstructive sleep apnea syndrome and perioperative complications: A systematic review of the literature. J Clin Sleep Med. 2012;8(2):199–207. 10.5664/jcsm.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta RM, Parvizi J, Hanssen AD, et al. : Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897–905. 10.4065/76.9.897 [DOI] [PubMed] [Google Scholar]
- 28. Kaw R, Pasupuleti V, Walker E, et al. : Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441. 10.1378/chest.11-0283 [DOI] [PubMed] [Google Scholar]
- 29. Hwang D, Shakir N, Limann B, et al. : Association of sleep-disordered breathing with postoperative complications. Chest. 2008;133(5):1128–1134. 10.1378/chest.07-1488 [DOI] [PubMed] [Google Scholar]
- 30. Kaw R, Golish J, Ghamande S, et al. : Incremental risk of obstructive sleep apnea on cardiac surgical outcomes. J Cardiovasc Surg (Torino). 2006;47(6):683–689. [PubMed] [Google Scholar]
- 31. Liao P, Yegneswaran B, Vairavanathan S, et al. : Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56(11):819–828. 10.1007/s12630-009-9190-y [DOI] [PubMed] [Google Scholar]
- 32. Haack M, Lee E, Cohen DA, et al. : Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145(1–2):136–141. 10.1016/j.pain.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith MT, Klick B, Kozachik S, et al. : Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497–506. 10.1016/j.pain.2008.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roehrs T, Hyde M, Blaisdell B, et al. : Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. 10.1093/sleep/29.2.145 [DOI] [PubMed] [Google Scholar]
- 35. Meagher DJ, Morandi A, Inouye SK, et al. : Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12:164. 10.1186/s12916-014-0164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whitlock EL, Vannucci A, Avidan MS: Postoperative delirium. Minerva Anestesiol. 2011;77(4):448–456. [PMC free article] [PubMed] [Google Scholar]
- 37. Inouye SK: Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. 10.1056/NEJMra052321 [DOI] [PubMed] [Google Scholar]
- 38. Koster S, Hensens AG, Schuurmans MJ, et al. : Consequences of delirium after cardiac operations. Ann Thorac Surg. 2012;93(3):705–711. 10.1016/j.athoracsur.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 39. Scholz AF, Oldroyd C, McCarthy K, et al. : Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21–8. 10.1002/bjs.10062 [DOI] [PubMed] [Google Scholar]
- 40. Flink BJ, Rivelli SK, Cox EA, et al. : Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116(4):788–796. 10.1097/ALN.0b013e31824b94fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirrakhimov AE, Yen T, Kwatra MM: Delirium after cardiac surgery: Have we overlooked obstructive sleep apnea?. Med Hypotheses. 2013;81(1):15–20. 10.1016/j.mehy.2013.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krueger JM, Clinton JM, Winters BD, et al. : Involvement of cytokines in slow wave sleep. Prog Brain Res. 2011;193:39–47. 10.1016/B978-0-444-53839-0.00003-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chennaoui M, Sauvet F, Drogou C, et al. : Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56(2):318–324. 10.1016/j.cyto.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 44. Krueger JM: The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14(32):3408–3416. 10.2174/138161208786549281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawasaki Y, Zhang L, Cheng JK, et al. : Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. 10.1523/JNEUROSCI.3338-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irwin MR, Wang M, Campomayor CO, et al. : Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. 10.1001/archinte.166.16.1756 [DOI] [PubMed] [Google Scholar]
- 47. Vgontzas AN, Zoumakis E, Bixler EO, et al. : Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. 10.1210/jc.2003-031562 [DOI] [PubMed] [Google Scholar]
- 48. Ren K, Dubner R: Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. 10.1038/nm.2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith MT, Edwards RR, McCann UD, et al. : The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. 10.1093/sleep/30.4.494 [DOI] [PubMed] [Google Scholar]
- 50. Khalid I, Roehrs TA, Hudgel DW, et al. : Continuous positive airway pressure in severe obstructive sleep apnea reduces pain sensitivity. Sleep. 2011;34(12):1687–1691. 10.5665/sleep.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doufas AG, Tian L, Padrez KA, et al. : Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8(1):e54807. 10.1371/journal.pone.0054807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown KA, Laferrière A, Lakheeram I, et al. : Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105(4):665–669. 10.1097/00000542-200610000-00009 [DOI] [PubMed] [Google Scholar]
- 53. Avidan MS, Fritz BA, Maybrier HR, et al. : The prevention of delirium and complications associated with surgical treatments (PODCAST) study: Protocol for an international multicentre randomised controlled trial. BMJ Open. 2014;4(9):e005651. 10.1136/bmjopen-2014-005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wildes TS, Winter AC, Maybrier HR, et al. : Protocol for the electroencephalography guidance of anesthesia to alleviate geriatric syndromes (ENGAGES) study: A pragmatic, randomised clinical trial. BMJ Open. 2016;6(6):e011505. 10.1136/bmjopen-2016-011505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Helsten DL, Ben Abdallah A, Avidan MS, et al. : Methodologic considerations for collecting patient-reported outcomes from unselected surgical patients. Anesthesiology. 2016;125(3):495–504. 10.1097/ALN.0000000000001217 [DOI] [PubMed] [Google Scholar]
- 56. Kronzer VL, Ben Abdallah A, McKinnon SL, et al. : Ability of preoperative falls to predict postsurgical outcomes in non-selected patients undergoing elective surgery at an academic medical centre: Protocol for a prospective cohort study. BMJ Open. 2016;6(9):e011570. 10.1136/bmjopen-2016-011570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kronzer VL, Jerry MR, Avidan MS: Assessing change in patient-reported quality of life after elective surgery: Protocol for an observational comparison study. F1000Res. 2016;5:976. 10.12688/f1000research.8758.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benchimol EI, Smeeth L, Guttmann A, et al. : The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. Z Evid Fortbild Qual Gesundhwes. 2016;115–116:33–48. 10.1016/j.zefq.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Elm E, Altman DG, Egger M, et al. : The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 60. PLOS Medicine Editors: Observational studies: Getting clear about transparency. PLoS Med. 2014;11(8):e1001711. 10.1371/journal.pmed.1001711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marcantonio ER, Ngo LH, O'Connor M, et al. : 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554–561. 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marcantonio ER, Goldman L, Mangione CM, et al. : A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. 10.1001/jama.1994.03510260066030 [DOI] [PubMed] [Google Scholar]
- 63. Ely EW, Margolin R, Francis J, et al. : Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379. 10.1097/00003246-200107000-00012 [DOI] [PubMed] [Google Scholar]
- 64. Hawker GA, Mian S, Kendzerska T, et al. : Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP)). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 65. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP: Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. 10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 66. Chung SA, Yuan H, Chung F: A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107(5):1543–1563. 10.1213/ane.0b013e318187c83a [DOI] [PubMed] [Google Scholar]
- 67. Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmid CL, Kennedy NM, Ross NC, et al. : Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell. 2017;171(5):1165–1175.e13. 10.1016/j.cell.2017.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]