Abstract
Animals and humans are exposed each day to a multitude of chemicals in the air, water and food. They have developed a battery of enzymes and transporters that facilitate the biotransformation and elimination of these compounds. Moreover, a majority of these enzymes and transporters are inducible due to the activation of xenobiotic receptors which act as transcription factors for the regulation of their target genes (such as xenobiotic metabolizing enzymes, see below §4 for the AhR). These receptors include several members of the nuclear/steroid receptor family (CAR for Constitutive Androstane Receptor, PXR for Pregnane X Receptor) but also the Aryl hydrocarbon Receptor or AhR, a member of the bHLH-PAS family (basic Helix-Loop-Helix - Period/ARNT/Single minded). In addition to the regulation of xenobiotic metabolism, numerous alternative functions have been characterized for the AhR since its discovery. These alternative functions will be described in this review along with its endogenous functions as revealed by experiments performed on knock-out animals.
Keywords: AhR, ARNT, AhRR, Dioxins, Src, Cytochromes P450
Highlights
-
•
The firstly discovered AhR function is its role in xenobiotic metabolism via the genomic pathway.
-
•
Endogenous functions are described, involving alternative pathways such as the non-genomic pathway.
-
•
A strong involvement in cell physiology (proliferation, adhesion and migration) was related.
-
•
AhR also have physiological roles identified by KO-models, the most common of which is vascular.
-
•
But these results in KO-models are species-specific and have to be considered with precaution.
1. Introduction
Animals and humans are exposed each day to a multitude of chemicals in the air, water and food. They have developed a battery of enzymes and transporters that facilitate the biotransformation and elimination of these compounds [1], [2]. Moreover, a majority of these enzymes and transporters are inducible due to the activation of xenobiotic receptors which act as transcription factors for the regulation of their target genes (such as xenobiotic metabolizing enzymes, see below §4 for the AhR) [3]. These receptors include several members of the nuclear/steroid receptor family (CAR for Constitutive Androstane Receptor, PXR for Pregnane X Receptor) [4] but also the Aryl hydrocarbon Receptor or AhR, a member of the bHLH-PAS family (basic Helix-Loop-Helix – Period/ARNT/Single minded) (Fig. 1). In addition to the regulation of xenobiotic metabolism, numerous alternative functions have been characterized for the AhR since its discovery. These alternative functions will be described in this review along with its endogenous functions as revealed by experiments performed on knock-out animals [5].
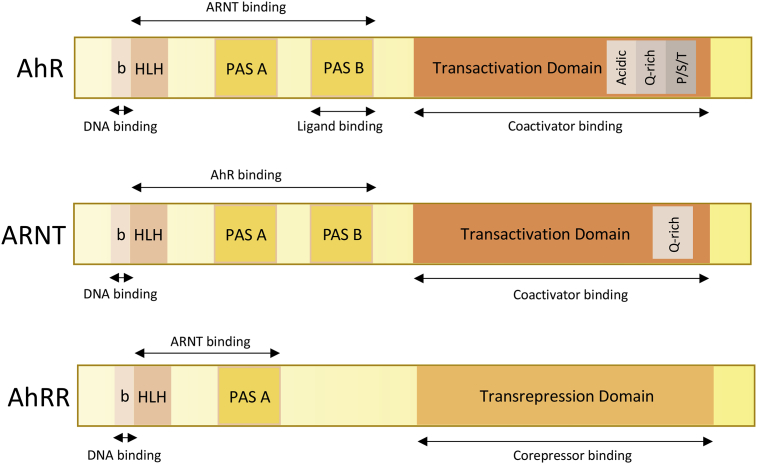
Fig. 1.
The functional domains of the AhR, ARNT and AhRR proteins. The AhR contains 1) a bHLH domain that allows the dimerization with its partner ARNT, the binding of DNA and the interactions with chaperones such as Hsp90 (Heat Shock Protein 90); it also contains sequences important for both nuclear import and export [76]; 2) a PAS domain which comprises two structural repeats A and B which are also involved in the dimerization with ARNT (PAS A) but which also allows the ligand binding (PAS B); 3) a C-terminal domain which contains three subdomains: one subdomain which is enriched with acidic residues (glutamate/aspartate), another one which is enriched with glutamine (Q-rich) and a third one which is enriched with serine, threonine & proline (S/T/P). Coactivators and co-repressors interact with the AhR via this domain [77], [78]. ARNT has a structure similar to AhR: The bHLH and PAS A domains are involved in the dimerization with AhR or AhRR and in DNA-binding. But in spite of the presence of a PAS B domain, ARNT is not able to bind ligands. AhRR also contains a DNA-binding domain (bHLH) and a dimerization domain (PAS A). The absence of the PAS B domain leads to its inability to bind ligands [79].
2. The AhR ligands
Numerous ligands (Fig. 2) for the AhR have been described. Xenobiotics, which are mostly aromatic hydrocarbons (including dioxins or PCBs “polychlorinated biphenyls”) were the first ligands discovered. The main source of human exposure (>90%) to aromatic hydrocarbons is through contaminated food. Acute exposure to high doses of dioxins in the workplace or due to industrial accidents can cause skin lesions such as chloracne. Long-term environmental exposure results in more extensive toxic effects among which are immunotoxicity, neurodevelopmental abnormalities, thyroid dysfunction, disruption of steroid hormones and reproductive functions. Experiments in animals have demonstrated carcinogenicity, with multiple cancer sites, in a large number of species (recent epidemiological studies on occupationally exposed persons are in agreement with these findings). The International Agency for Research on Cancer (IARC) has classified TCDD in group 1 (carcinogenic to humans) whereas PCBs are classified in an intermediate group, 2A (probably carcinogenic to humans). Recently, natural compounds which are found in food have been characterized as AhR ligands. Flavonoids such as quercetin and resveratrol, the most abundant class of polyphenols, are found in fruits and vegetables. Indoles such as indole-3-carbinol (I3C) are derived from cruciferous vegetables such as broccoli or Brussels sprouts. Finally, molecules in the body which are formed by endogenous metabolism, such as FICZ (formylindolo [3,2-b] carbazole), indirubin, indigo, metabolites of arachidonic acid or kynurenine pathway metabolites, also have been described as AhR ligands. In the central nervous system, the catabolism of tryptophan leads to the production of NAD+, neuroactive metabolites such as kynurenic acid, glutamatergic agonists (NMDA) or neurotoxins (quinolinic acid). In mammals, three enzymes catalyze the first limiting step of catabolism of tryptophan to N-formyl-kynurenine: TDO2 (“tryptophan-2,3-dioxygenase”) and IDO1 and 2 (“indoleamine-2,3-dioxygenases”) [6].
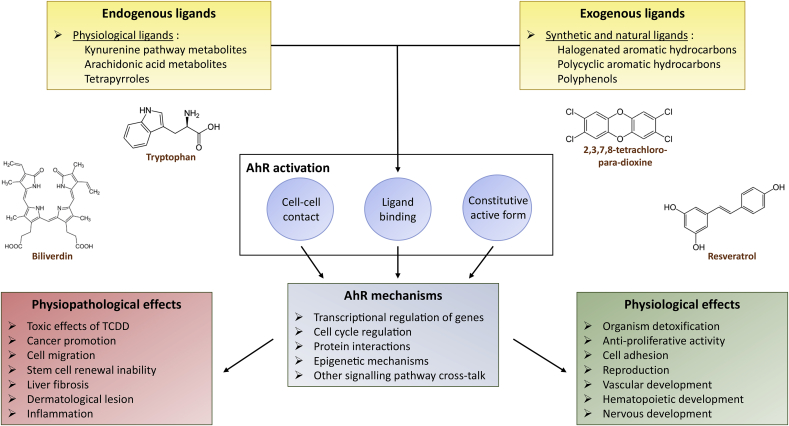
Fig. 2.
The functional relationship between the AhR ligands and the regulatory roles of this receptor in physiology and pathophysiology. Synthetic ligands such as Halogenated Aromatic Hydrocarbons (HAHs) such as PCBs (“Polychlorobiphenyls”) and PCDD (polychlorinateddibenzo-para-dioxins) or PAHs (Polycyclic Aromatic Hydrocarbons) which include benzo (a) pyrene (B(a)P) or 3-methylcholanthrene (3-MC) were among the first molecules to be identified as AhR ligands. These molecules are present in the air or in foods as complex mixtures, they are very stable; some may accumulate in the body (TCDD has a half-life of about seven years in humans) and they are powerful inducers of AhR [80]. Dioxins or PCBs are highly soluble in fats and can, therefore, reach high concentrations in fatty foods such as dairy products, fishes, meats and seafood. More recently, ligands of natural origins (food and endogenous ligands) such as flavonoids or indole derivatives also have been characterized as AhR ligands. Flavonoids are found in fruits and vegetables and represent the most abundant class of polyphenols. Among them, quercetin and resveratrol activate the AhR [8], [81] and exert both agonist and antagonistic effects. Indoles such as indole-3-carbinol (I3C), which are derived from cruciferous plants such as broccoli or Brussels sprouts, are reported to have anti-cancer properties. Part of the effects of I3C occurs via activation of the AhR [82]. In addition, physiological endogenous ligands of the AhR such as indole amino acid metabolites (tryptophan, tryptamine, indole acetic acid) recently have been characterized. A photoproduct of tryptophan also has been identified through structural and chromatographic studies [83]: FICZ (6-formylindolo [3,2-b] carbazole) [84]. Indirubin and indigo represent another group of indoles [85] which are detected in human urine under normal physiological conditions and, therefore, are present in our organisms, and are strong inducers of the AhR [85], [86]. Physiological ligands also include metabolites of arachidonic acid (lipoxin A4, some prostaglandins (PGG2)) [87], [88], tetrapyroles (bilirubin, a degradation product), heme and biliverdin [89]).
3. The AhR complex
The non-activated form of the AhR is cytoplasmic and it forms a complex with several chaperones [7] among which are two HSP90 (Heat Shock Protein 90), a co-chaperone p23, a XAP-molecule 2 (hepatitis B Virus X-associated protein 2). Some studies suggest that the Src tyrosine kinase also is a member of the complex. These proteins maintain the correct folding of the AhR, allow a proper recognition of the ligand by the receptor and, subsequently, ensure indirectly an efficient transcriptional effect [8].
4. Activation and modulation of the AhR
Several signaling pathways can be activated by the AhR. The first pathway to be described was the genomic pathway (Fig. 3) and it is now well-characterized. After a ligand is bound, the AhR translocates into the nucleus and it binds to ARNT to form an active heterodimer. This heterodimer modulates the expression of targets by binding to xenobiotic responsive elements (XRE) and coregulators. The amount of protein expressed from targeted genes is reduced by 80–95% in many cell culture models within 4 h of treatment by a ligand [9], [10], [11]. After being exported out of the nucleus, the AhR is rapidly degraded in the cytoplasmic compartment by the proteasome [12]. Proteasomal degradation of the AhR involves its binding of ubiquitin covalently. Other post-translational modifications of the AhR have been observed. SUMOylation enhances AhR stability through inhibition of its ubiquitinylation. However, this may suppresses its transactivating activity [13], [14]. The different ligands of the AhR may activate the receptor differentially. We have shown that resveratrol does not strongly activate the expression of CYP1A1 in a human hepatocellular cell line. However, resveratrol does activate the expression of paraoxonase 1 (PON1) which might allow the detoxication of oxidized lipids and which could explain, partly, the beneficial effects of exposure to resveratrol on the incidence of cardiovascular diseases. This may be due to the binding of the AhR to alternative XREs [15]. Since TCDD does not activate PON1 expression, this suggests that different ligands might activate different gene clusters.
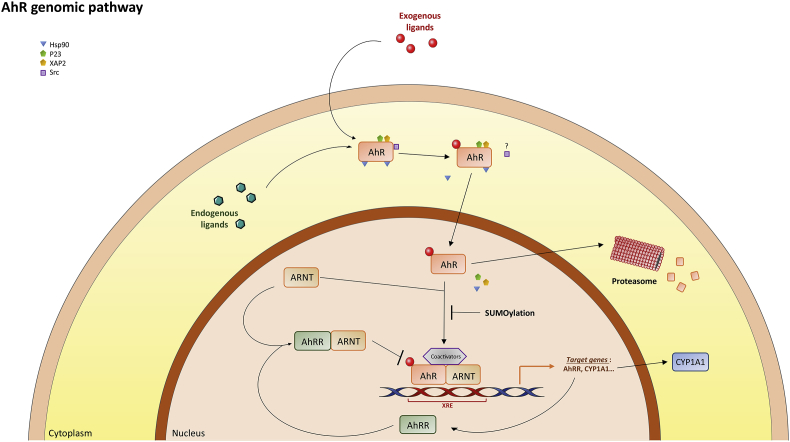
Fig. 3.
The AhR genomic pathway. When a ligand crosses the plasma membrane (passive diffusion), it binds to the AhR (the PAS B domain). This allows the translocation of the ligand-receptor complex into the nucleus and the dissociation of the receptor complex. In the nucleus, the complex heterodimerizes with its partner ARNT (AhR Nuclear Translocator also called HIF-1β) [90]. The heterodimer binds specific DNA sequences located in the promoter regions of target genes named xenobiotic response elements (XRE, 5′-TA/TGCGTG-3′) [80]. AhR-ARNT induces the transcription of target genes by the recruitment of various components of the transcriptional machinery [91] such as CBP/p300 (cAMP response element-binding protein binding protein) [80], [81], [82], SRC-1 (steroid receptor coactivator 1), p160/bHLH-PAS, NCoA2/GRIP1/TIF2 (Nuclear receptor Coactivator 2/Glucocorticoid Receptor Interacting Protein 1/Transcriptional Intermediate Factor 2) and p/CIP (p300/CBP/CoIntegrator-associated Protein) [95] as well as other transcriptional coactivators such as RIP140 (Receptor Interacting Protein 140) [96], [97] or ATP-dependent chromatin remodeling components such as BRG-1 (Brahma Related Gene). After being exported out of the nucleus, the AhR is rapidly degraded in the cytoplasmic compartment by the proteasome [12]. Other post-translational modifications such as SUMOylation of the human AhR have been observed [13].
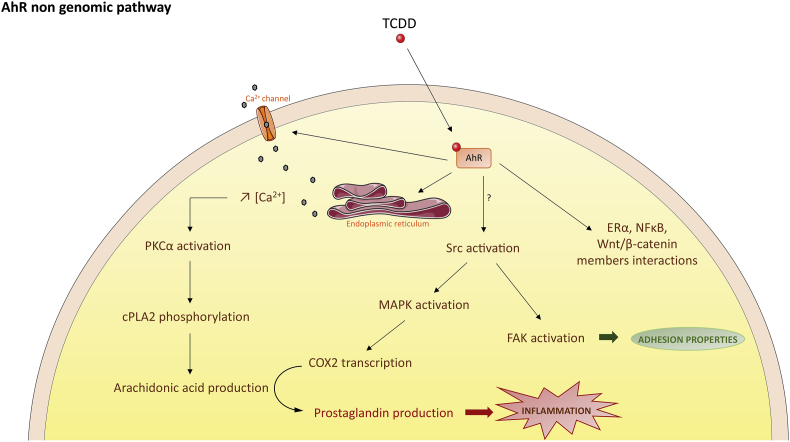
One of the key target genes activated in the AhR genomic pathway is the AhR repressor, AhRR [16], [17], [18] (Fig. 1). The AhRR protein is similar to the AhR but it cannot bind ligands due to the absence of the PAS B domain in the N-terminal region [18], [19]. Further, the AhRR differs from AhR and ARNT in that the C-terminal domain, which is a transactivation domain in AhR and ARNT, is a transrepression domain in AhRR. It allows the binding of corepressors which are involved in a negative regulatory loop for AhR. Following induction, AhRR suppresses AhR activity by binding to ARNT and XRE (AhRR-ARNT complex) [20], [21]. AhRR, thus, is able to modulate the transcription of AhR-dependent genes. This negative regulatory loop and the proteosomal degradation of the receptor protect biological systems from the consequences of overstimulation by agonists and provide a temporal control of the signaling (Fig. 3). In addition to the genomic pathway, several non-genomic pathways have been identified recently (Fig. 4). For instance, following exposure to TCDD, there is a rapid increase in intracellular calcium concentration (from both extracellular and endoplasmic reticulum sources). TCDD also leads to the functional activation of the tyrosine kinase Src by releasing it from the AhR complex [22]. This could be accompanied by the activation of the Focal Adhesion Kinase and by the modification of the adhesion properties of the cell through disruption of focal adhesion points [22], [23]. Src activation could be accompanied also very rapidly by the activation of MAP kinases, ERK1 and ERK2. All these processes may converge to regulate pathophysiological processes such as inflammation. Indeed, the calcium influx causes the activation of protein kinase C (PKCα) which phosphorylates a serine residue of a cytosolic enzyme, phospholipase A2 (cPLA2) with the subsequent production of arachidonic acid. The parallel activation of MAP kinases by Src leads to the transcription of cyclooxygenase 2 (COX2) which uses arachidonic acid to produce prostaglandins that can cause inflammation. Thus, these two signaling pathways, which were initially activated by TCDD, converge towards the stimulation of inflammation [24]. Moreover, the AhR interacts with Wnt/β-catenin, ER-alpha or NF-kB and strongly modulates their actions [25], [26], [27], [28]. On the other hand, these transcription factors also impact AhR signaling. For example, β-catenin is now described as a co-activator of this receptor [29].
Fig. 4.
The AhR non-genomic pathways. In recent years, numerous observations indicate that additional AhR pathways can be activated by the AhR. For instance, TCDD rapidly increases intracellular calcium concentrations or the activity of the Src tyrosine kinase and Focal Adhesion Kinase with functional consequences for other pathways (MAPK) or cellular functional properties (adhesion and migration). One outcome of such activations could be inflammation through the subsequent production of arachidonic acid and then prostaglandins. Finally, the AhR is able to interact with several other transcriptional regulators such as β-catenin, NF-kB or the estrogen receptor alpha.
Finally, after exposure to a ligand, the level of the AhR protein has been found, both in vitro and in vivo, to decrease rapidly without the level of the messenger RNA being altered [9].
5. Regulation of cellular functions by the AhR
The best-characterized AhR function to date is the establishment of a protective adaptive response to xenobiotics through induction of the synthesis of xenobiotic metabolism enzymes. Aromatic hydrocarbons activate the AhR which induces family 1-P450 cytochromes (1A1, 1A2, 1B1) the functions of which deal mostly with hydrocarbon detoxication. This elegant regulatory loop protects xenobiotic-exposed animals by detecting and then metabolizing these substances. However, the high degree of conservation of this receptor among species [21], its pattern of expression during development and in adult tissues [30] and the phenotypic alterations observed in AhR-deficient mice [31], [32], [33] suggest a strong involvement of the AhR in cell physiology which is independent of the metabolism of xenobiotics. The detoxification function of the AhR may have been acquired late in evolution.
5.1. Cell proliferation
One of the most intriguing and exciting aspects of AhR biology is its ability to promote or inhibit cell proliferation. For example, AhR KO mouse embryonic fibroblasts exhibit slow growth and accumulation in the G2/M phase of the cell cycle [34]. In human hepatoma cells (HepG2), AhR-siRNAs block the G1/S transition of the cell cycle and decrease cyclins D1 and E as well as CDK2/4-dependent cyclin kinases. This supports a pro-proliferative role for the receptor [35]. TCDD also can affect the expression of genes involved in cell proliferation (TGF-β, IL-1β and PAI-2), regulation of the cell cycle (JunB and JunD [36]) and inflammation [37], [38], [39]. In human breast cancer cells (MCF-7), NF-kB, via its RelA subunit, physically interacts with AhR [25] which results in transactivation of the c-myc proto-oncogene. With respect to the cell cycle, the expression of JunD and subsequently cyclin A, which blocks cell contact inhibition and favors proliferation [36], is triggered by TCDD-activated AhR via a novel ARNT-independent pathway. The role of AhR as a cancer promoter has been demonstrated in murine models which overexpress a constitutively active form of the AhR [40], [41]. All these results suggest that the AhR favors cell proliferation. However, other studies have revealed an anti-proliferative activity of the AhR. AhR stimulates the transcription of the tumor suppressor gene, p27Kip1, in non-proliferative hepatoma cells or in the fetal thymus [42], [43]. The AhR also regulates the function of the pro-proliferative factor E2F (E2F factors can be inhibited by direct interaction with retinoblastoma protein, pRb, and their function also depends on the presence of co-activators p300) in 3 different ways: 1) TCDD activates the physical interaction between the AhR and pRb which promotes its binding to E2F and stops the cell cycle [44], 2) in addition, TCDD stimulates the interaction between AhR and p300, which leads to displacement of p300 from E2F sites [45], 3) finally, a direct inhibitory interaction was detected between the AhR and E2F with potential implications for stem cell renewal [46], [47].
Overall, the activity of the AhR on cell proliferation is probably dependent on the cell type, the timing of the cell cycle (and the expression of interacting partners such as RelA or pRb), the developmental period (if considering an animal model). Therefore, any specific action which would include the use of an AhR ligand to control the cell cycle, needs to be carefully evaluated in regard to these parameters.
5.2. Adhesion and cell migration
The contribution of the AhR in adhesion processes, which involve cell-cell and cell-extracellular matrix interactions, has recently emerged. Cell density also influences the compartmentalization of the AhR and low densities lead to a nuclear localization of the AhR [48]. These interactions are also very important for metastatic processes.
Knock-out models seem to confirm this involvement of the AhR in cell migration. Immortalized mammary fibroblasts derived from AhR KO mice, display decreased migration which is associated with an increased formation of cytoskeleton stress fibers and a reduction in the formation of lamellipods [49]. Signaling pathways which regulate cell migration are also inhibited in AhR-deficient cells which exhibit weaker activation of focal adhesion kinase (FAK), PKB/Akt (protein kinase B), ERK1 (extracellular signal-regulated kinase 1) and Rac-1 (Ras-related C3 botulinum toxin substrate 1). In addition, these fibroblasts induce fewer tumors in vivo in immune-deficient NOD-SCID mice (non-obese diabetic/severe combined immunodeficiency) than in wild-type mice [49].
The involvement of AhR in mobility and cellular plasticity also has been demonstrated by studies based on xenobiotic treatments. Exposure of human MCF-7 or HepG2 cells to TCDD causes morphological changes such as the appearance of lamellipodia, which cause greater cell adhesion and motility. This is associated with a reorganization of the cytoskeleton mainly due to a redistribution of actin and vinculin and to the activation of the FAK and Src kinases (Fig. 4) [23]. These cellular effects are accompanied by changes in the expression of certain genes such as E-cadherin and by the activation of JNKs [23]. E-Cadherin downregulation is a hallmark of epithelial-mesenchymal transition which is triggered by transcription factors such as Slug, a direct AhR target gene [50], [51].
6. Physiological roles of the Ah receptor
AhR KO mice display developmental abnormalities which highlight the roles of the receptor in female fertility [52], perinatal growth [18], [31], regulation of blood pressure, production of peripheral lymphocyte counts [31], [32], [53] and increased susceptibility to colitis [54]. Recently, it has been shown in a mouse model of induced-colitis, that FICZ (a high-affinity endogenous AhR ligand) prevents intestinal barrier function via AhR activation by suppressing IL-6 and claudin-2 expression [55].
Depending upon the model, AhR KO mice develop cardiac hypertrophy [56], dermatological lesions, portal vascular hypertrophy [32] and pyloric hyperplasia of the gastrointestinal tract [56]. One of the most common phenotypes to all AhR KO models is vascular. The mice exhibit a systematic persistence of ductus venosus [57], a porto-fetal shunt of the developing liver, which normally closes immediately after birth [58], [59]. These abnormalities result in reduced liver size associated with portal fibrosis and early lipid accumulation [60], [61]. A candidate gene that could explain this liver phenotype is Transforming Growth Factor ß (TGF-β). The AhR KO mouse livers have increased levels of TGF-β in the portal space [62] which could contribute to the development of fibrosis and to a low proliferative capacity as a result of the pro-fibrogenic and anti-proliferative activities of this cytokine. Additional studies have shown that this elevation of TGF-β is related to the accumulation of retinoic acid and to a reduction in retinoic acid metabolism, which lead to a decrease in CYP2C39 [63]. Other vascular abnormalities have been observed in these mice, such as the persistence of the hyaloid artery and an impairment of limbic vascularization in the developing eye [57]. The AhR KO mice also develop an ocular pathology which consists of a horizontal pendular nystagmus which is associated with myelin defects of the optic nerve and a local inflammation [64]. Similar myelin defects also have been identified recently in the peripheral nervous system [65]. Moreover, the AhR is involved in neuroendocrine pathways such as those that control the brain-pituitary-interrenal and gonadal axes. Treatment of rainbow trout with β-naphtoflavone and resveratrol, agonist and antagonist of AhR, respectively, has elucidated the role of the receptor in the disruption of steroid production after PCB exposure [66].
Studies in KO models suggest that AhR ligands activate AhR-independent pathways but these results require thoughtful interpretation as to the mechanisms involved. For example, in AhR KO rats, treatment with alpha-naphtoflavone (an AhR antagonist) causes an increase in the rate of ovulation and in follicular growth [67]. Abnormalities in the immune system also have been explored. After administration of TCDD, AhR KO rats displayed changes in immune phenotypes such as a decrease in CD8+ T cells and CD11+ but an increase in NKT cells [68].
Although similar consequences frequently are found in different species (such as the insensitivity to the effects of TCDD in AhR-deficient animals as compared to the wild-type), some AhR KO phenotypes are species-specific, such as the differences that occur between mice and rats [69], [70]. For example, vascular phenotypes that are found in AhR KO mice, such as the persistent ductus venosus of the liver or hyaloid artery in the eye, are not observed in AhR KO rats. Or, alterations of the urinary tract (renal dilatation or degenerative changes and ureter dilatation) have been identified in AhR KO rats, but not in AhR KO mice.
7. Conclusion
In recent years, new functions of the AhR have been identified both in vertebrate and invertebrate models. In vertebrates, the AhR regulates the functions of transposable elements (including retrotransposons) which are suspected to regulate a large number of gene expression patterns [71], [72], chromatin functions (insulators) and also epigenetic mechanisms through the regulation of SIRT1 activity or miR expression [5]. This could have potential impacts in terms of evolution. The AhR is also a protein whose functions have been modified throughout evolution. In invertebrates, no ligand for the AhR has been identified to present [73], [74], [75]. This suggests that the protein has acquired detoxication functions over time.
Conflict of interest
There is no conflict of interest for all authors.
Acknowledgements
This work was supported by INSERM (Institut National de la Santé et de la Recherche Médicale); CNRS (Centre National de la Recherche Scientifique); Université Paris Descartes (Bourse de l’école doctorale MTCI); The authors declare they have no actual or potential competing financial interests. We use the Servier Medical Art Service for drawing and would like to thank this service (https://smart.servier.com/). We thank Dr. Lawrence Aggerbeck for the critical reading of this manuscript.
References
- 1.Gonzalez F.J., Nebert D.W. Evolution of the P450 gene superfamily: animal-plant “warfare”, molecular drive and human genetic differences in drug oxidation. Trends Genet. TIG. 1990;6:182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- 2.Nebert D.W., Gonzalez F.J. P450 genes: structure, evolution, and regulation. Annu. Rev. Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 3.Xu C., Li C.Y.-T., Kong A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 4.Waxman D.J. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 5.Mulero-Navarro S., Fernandez-Salguero P.M. New trends in aryl hydrocarbon receptor biology. Front. Cell. Dev. Biol. 2016;4:45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Lara L., Pérez-Severiano F., González-Esquivel D., Elizondo G., Segovia J. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J. Neurosci. Res. 2015 doi: 10.1002/jnr.23595. [DOI] [PubMed] [Google Scholar]
- 7.Petrulis J.R., Perdew G.H. The role of chaperone proteins in the aryl hydrocarbon receptor core complex, Chem. Biol. Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 8.Guyot E., Chevallier A., Barouki R., Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov. Today. 2013;18:479–486. doi: 10.1016/j.drudis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Giannone J.V., Li W., Probst M., Okey A.B. Prolonged depletion of AH receptor without alteration of receptor mRNA levels after treatment of cells in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem. Pharmacol. 1998;55:489–497. doi: 10.1016/s0006-2952(97)00493-0. [DOI] [PubMed] [Google Scholar]
- 10.Giannone J.V., Okey A.B., Harper P.A. Characterization of polyclonal antibodies to the aromatic hydrocarbon receptor. Can. J. Physiol. Pharmacol. 1995;73:7–17. doi: 10.1139/y95-002. [DOI] [PubMed] [Google Scholar]
- 11.Pollenz R.S. The aryl-hydrocarbon receptor, but not the aryl-hydrocarbon receptor nuclear translocator protein, is rapidly depleted in hepatic and nonhepatic culture cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1996;49:391–398. [PubMed] [Google Scholar]
- 12.Davarinos N.A., Pollenz R.S. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J. Biol. Chem. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 13.Xing X., Bi H., Chang A.K., Zang M.-X., Wang M., Ao X., Li S., Pan H., Guo Q., Wu H. SUMOylation of AhR modulates its activity and stability through inhibiting its ubiquitination. J. Cell. Physiol. 2012;227:3812–3819. doi: 10.1002/jcp.24092. [DOI] [PubMed] [Google Scholar]
- 14.Müller S., Matunis M.J., Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouédard C., Barouki R., Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell Biol. 2004;24:5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba T., Mimura J., Gradin K., Kuroiwa A., Watanabe T., Matsuda Y., Inazawa J., Sogawa K., Fujii-Kuriyama Y. Structure and expression of the Ah receptor repressor gene. J. Biol. Chem. 2001;276:33101–33110. doi: 10.1074/jbc.M011497200. [DOI] [PubMed] [Google Scholar]
- 17.Korkalainen M., Lindén J., Tuomisto J., Pohjanvirta R. Effect of TCDD on mRNA expression of genes encoding bHLH/PAS proteins in rat hypothalamus. Toxicology. 2005;208:1–11. doi: 10.1016/j.tox.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Mimura J., Ema M., Sogawa K., Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korkalainen M., Tuomisto J., Pohjanvirta R. Primary structure and inducibility by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) of aryl hydrocarbon receptor repressor in a TCDD-sensitive and a TCDD-resistant rat strain. Biochem. Biophys. Res. Commun. 2004;315:123–131. doi: 10.1016/j.bbrc.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Hahn M.E., Allan L.L., Sherr D.H. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem. Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn M.E. Aryl hydrocarbon receptors: diversity and evolution. Chem. Biol. Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 22.Tomkiewicz C., Herry L., Bui L.-C., Métayer C., Bourdeloux M., Barouki R., Coumoul X. The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene. 2013;32:1811–1820. doi: 10.1038/onc.2012.197. [DOI] [PubMed] [Google Scholar]
- 23.Diry M., Tomkiewicz C., Koehle C., Coumoul X., Bock K.W., Barouki R., Transy C. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–5574. doi: 10.1038/sj.onc.1209553. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem. Pharmacol. 2009;77:608–626. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Tian Y., Ke S., Denison M.S., Rabson A.B., Gallo M.A. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 26.Faust D., Vondráček J., Krčmář P., Smerdová L., Procházková J., Hrubá E., Hulinková P., Kaina B., Dietrich C., Machala M. AhR-mediated changes in global gene expression in rat liver progenitor cells. Arch. Toxicol. 2013;87:681–698. doi: 10.1007/s00204-012-0979-z. [DOI] [PubMed] [Google Scholar]
- 27.Procházková J., Kabátková M., Bryja V., Umannová L., Bernatík O., Kozubík A., Machala M., Vondrácek J. The interplay of the aryl hydrocarbon receptor and β-catenin alters both AhR-dependent transcription and Wnt/β-catenin signaling in liver progenitors. Toxicol. Sci. Offic. J. Soc. Toxicol. 2011;122:349–360. doi: 10.1093/toxsci/kfr129. [DOI] [PubMed] [Google Scholar]
- 28.Vondráček J., Machala M. Environmental ligands of the aryl hydrocarbon receptor and their effects in models of adult liver progenitor cells. Stem Cell. Int. 2016;2016:4326194. doi: 10.1155/2016/4326194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaas S., Kreft L., Schwarz M., Braeuning A. Cooperation of structurally different aryl hydrocarbon receptor agonists and β-catenin in the regulation of CYP1A expression. Toxicology. 2014;325:31–41. doi: 10.1016/j.tox.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Abbott B.D., Birnbaum L.S., Perdew G.H. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev. Dyn. Offic. Publ. Am. Assoc. Anat. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Salguero P., Pineau T., Hilbert D.M., McPhail T., Lee S.S., Kimura S., Nebert D.W., Rudikoff S., Ward J.M., Gonzalez F.J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt J.V., Su G.H., Reddy J.K., Simon M.C., Bradfield C.A. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T.N., Nakao K., Ema M., Sogawa K., Yasuda M., Katsuki M., Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells Devoted Mol. Cell Mech. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 34.Elizondo G., Fernandez-Salguero P., Sheikh M.S., Kim G.Y., Fornace A.J., Lee K.S., Gonzalez F.J. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol. Pharmacol. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 35.Abdelrahim M., Smith R., Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol. Pharmacol. 2003;63:1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 36.Weiss C., Faust D., Schreck I., Ruff A., Farwerck T., Melenberg A., Schneider S., Oesch-Bartlomowicz B., Zatloukalová J., Vondráček J., Oesch F., Dietrich C. TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene. 2008;27:2198–2207. doi: 10.1038/sj.onc.1210859. [DOI] [PubMed] [Google Scholar]
- 37.Gaido K.W., Maness S.C. Regulation of gene expression and acceleration of differentiation in human keratinocytes by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1994;127:199–208. doi: 10.1006/taap.1994.1154. [DOI] [PubMed] [Google Scholar]
- 38.Hoffer A., Chang C.Y., Puga A. Dioxin induces transcription of fos and jun genes by Ah receptor-dependent and -independent pathways. Toxicol. Appl. Pharmacol. 1996;141:238–247. doi: 10.1006/taap.1996.0280. [DOI] [PubMed] [Google Scholar]
- 39.Kim M.J., Pelloux V., Guyot E., Tordjman J., Bui L.-C., Chevallier A., Forest C., Benelli C., Clément K., Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moennikes O., Loeppen S., Buchmann A., Andersson P., Ittrich C., Poellinger L., Schwarz M. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–4710. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 41.Andersson P., McGuire J., Rubio C., Gradin K., Whitelaw M.L., Pettersson S., Hanberg A., Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolluri S.K., Weiss C., Koff A., Göttlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puga A., Maier A., Medvedovic M. The transcriptional signature of dioxin in human hepatoma HepG2 cells. Biochem. Pharmacol. 2000;60:1129–1142. doi: 10.1016/s0006-2952(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 44.Huang G., Elferink C.J. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol. Pharmacol. 2005;67:88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- 45.Marlowe J.L., Knudsen E.S., Schwemberger S., Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J. Biol. Chem. 2004;279:29013–29022. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- 46.Laiosa M.D., Tate E.R., Ahrenhoerster L.S., Chen Y., Wang D. Effects of developmental activation of the aryl hydrocarbon receptor by 2,3,7,8-Tetrachlorodibenzo-p-dioxin on long-term self-renewal of murine hematopoietic stem cells. Environ. Health Perspect. 2016;124:957–965. doi: 10.1289/ehp.1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Kurita H., Carreira V., Ko C.-I., Fan Y., Zhang X., Biesiada J., Medvedovic M., Puga A. Ah receptor activation by dioxin disrupts activin, BMP, and WNT signals during the early differentiation of mouse embryonic stem cells and inhibits cardiomyocyte functions. Toxicol. Sci. Offic. J. Soc. Toxicol. 2016;149:346–357. doi: 10.1093/toxsci/kfv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikuta T., Kobayashi Y., Kawajiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J. Biol. Chem. 2004;279:19209–19216. doi: 10.1074/jbc.M310492200. [DOI] [PubMed] [Google Scholar]
- 49.Mulero-Navarro S., Pozo-Guisado E., Pérez-Mancera P.A., Alvarez-Barrientos A., Catalina-Fernández I., Hernández-Nieto E., Sáenz-Santamaria J., Martínez N., Rojas J.M., Sánchez-García I., Fernández-Salguero P.M. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J. Biol. Chem. 2005;280:28731–28741. doi: 10.1074/jbc.M504538200. [DOI] [PubMed] [Google Scholar]
- 50.Ikuta T., Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp. Cell Res. 2006;312:3585–3594. doi: 10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Pierre S., Chevallier A., Teixeira-Clerc F., Ambolet-Camoit A., Bui L.-C., Bats A.-S., Fournet J.-C., Fernandez-Salguero P., Aggerbeck M., Lotersztajn S., Barouki R., Coumoul X. Aryl hydrocarbon receptor-dependent induction of liver fibrosis by dioxin. Toxicol. Sci. Offic. J. Soc. Toxicol. 2014;137:114–124. doi: 10.1093/toxsci/kft236. [DOI] [PubMed] [Google Scholar]
- 52.Abbott B.D., Schmid J.E., Pitt J.A., Buckalew A.R., Wood C.R., Held G.A., Diliberto J.J. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- 53.Lahvis G.P., Bradfield C.A. Ahr null alleles: distinctive or different? Biochem. Pharmacol. 1998;56:781–787. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q., Yang K., Han B., Sheng B., Yin J., Pu A., Li L., Sun L., Yu M., Qiu Y., Xiao W., Yang H. Aryl hydrocarbon receptor inhibits inflammation in DSS-induced colitis via the MK2/p-MK2/TTP pathway. Int. J. Mol. Med. 2018;41:868–876. doi: 10.3892/ijmm.2017.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Y., Wang Q., Yu K., Fan X., Xiao W., Cai Y., Xu P., Yu M., Yang H. 6-Formylindolo(3,2- b )carbazole induced aryl hydrocarbon receptor activation prevents intestinal barrier dysfunction through regulation of claudin-2 expression. Chem. Biol. Interact. 2018;288:83–90. doi: 10.1016/j.cbi.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Salguero P.M., Ward J.M., Sundberg J.P., Gonzalez F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 57.Lahvis G.P., Pyzalski R.W., Glover E., Pitot H.C., McElwee M.K., Bradfield C.A. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol. Pharmacol. 2005;67:714–720. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- 58.Lahvis G.P., Lindell S.L., Thomas R.S., McCuskey R.S., Murphy C., Glover E., Bentz M., Southard J., Bradfield C.A. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomae T.L., Glover E., Bradfield C.A. A maternal Ahr null genotype sensitizes embryos to chemical teratogenesis, J. Biol. Chem. 2004;279:30189–30194. doi: 10.1074/jbc.M403690200. [DOI] [PubMed] [Google Scholar]
- 60.Lee J.H., Wada T., Febbraio M., He J., Matsubara T., Lee M.J., Gonzalez F.J., Xie W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis, Gastroenterology. 2010;139:653–663. doi: 10.1053/j.gastro.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonnell W.M., Chensue S.W., Askari F.K., Moseley R.H. Hepatic fibrosis in Ahr-/- mice. Science. 1996;271:223–224. [PubMed] [Google Scholar]
- 62.Zaher H., Fernandez-Salguero P.M., Letterio J., Sheikh M.S., Fornace A.J., Roberts A.B., Gonzalez F.J. The involvement of aryl hydrocarbon receptor in the activation of transforming growth factor-beta and apoptosis. Mol. Pharmacol. 1998;54:313–321. doi: 10.1124/mol.54.2.313. [DOI] [PubMed] [Google Scholar]
- 63.Andreola F., Hayhurst G.P., Luo G., Ferguson S.S., Gonzalez F.J., Goldstein J.A., De Luca L.M. Mouse liver CYP2C39 is a novel retinoic acid 4-hydroxylase. Its down-regulation offers a molecular basis for liver retinoid accumulation and fibrosis in aryl hydrocarbon receptor-null mice. J. Biol. Chem. 2004;279:3434–3438. doi: 10.1074/jbc.M305832200. [DOI] [PubMed] [Google Scholar]
- 64.Juricek L., Carcaud J., Pelhaitre A., Riday T.T., Chevallier A., Lanzini J., Auzeil N., Laprévote O., Dumont F., Jacques S., Letourneur F., Massaad C., Agulhon C., Barouki R., Beraneck M., Coumoul X. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci. Rep. 2017;7:9794. doi: 10.1038/s41598-017-09621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shackleford G., Sampathkumar N.K., Hichor M., Weill L., Meffre D., Juricek L., Laurendeau I., Chevallier A., Ortonne N., Larousserie F., Herbin M., Bièche I., Coumoul X., Beraneck M., Baulieu E.-E., Charbonnier F., Pasmant E., Massaad C. Involvement of Aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E1319–E1328. doi: 10.1073/pnas.1715999115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aluru N., Vijayan M.M. Brain transcriptomics in response to beta-naphthoflavone treatment in rainbow trout: the role of aryl hydrocarbon receptor signaling. Aquat. Toxicol. Amst. Neth. 2008;87:1–12. doi: 10.1016/j.aquatox.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Barreiro K.A., Di Yorio M.P., Artillo-Guida R.D., Paz D.A., Faletti A.G. Daily treatment with α-naphthoflavone enhances follicular growth and ovulation rate in the rat. Toxicol. Appl. Pharmacol. 2011;252:11–17. doi: 10.1016/j.taap.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Phadnis-Moghe A.S., Chen W., Li J., Crawford R.B., Bach A., D'Ingillo S., Kovalova N., Suarez-Martinez J.E., Kaplan B.L.F., Harrill J.A., Budinsky R., Rowlands J.C., Thomas R.S., Kaminski N.E. Immunological characterization of the aryl hydrocarbon receptor (AHR) knockout rat in the presence and absence of 2,3,7,8-tetrachlorodibenzo- p -dioxin (TCDD) Toxicology. 2016;368–369:172–182. doi: 10.1016/j.tox.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 69.Harrill J.A., Hukkanen R.R., Lawson M., Martin G., Gilger B., Soldatow V., Lecluyse E.L., Budinsky R.A., Rowlands J.C., Thomas R.S. Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol. Appl. Pharmacol. 2013;272:503–518. doi: 10.1016/j.taap.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 70.Harrill J.A., Layko D., Nyska A., Hukkanen R.R., Manno R.A., Grassetti A., Lawson M., Martin G., Budinsky R.A., Rowlands J.C., Thomas R.S. Aryl hydrocarbon receptor knockout rats are insensitive to the pathological effects of repeated oral exposure to 2,3,7,8-tetrachlorodibenzo- p -dioxin: AHR-KO rats are insensitive to pathological effects of TCDD. J. Appl. Toxicol. 2016;36:802–814. doi: 10.1002/jat.3211. [DOI] [PubMed] [Google Scholar]
- 71.Teneng I., Stribinskis V., Ramos K.S. Context-specific regulation of LINE-1. Genes Cells Devoted Mol. Cell Mech. 2007;12:1101–1110. doi: 10.1111/j.1365-2443.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 72.Okudaira N., Iijima K., Koyama T., Minemoto Y., Kano S., Mimori A., Ishizaka Y. Induction of long interspersed nucleotide element-1 (L1) retrotransposition by 6-formylindolo[3,2-b]carbazole (FICZ), a tryptophan photoproduct. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18487–18492. doi: 10.1073/pnas.1001252107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler R.A., Kelley M.L., Powell W.H., Hahn M.E., Van Beneden R.J. An aryl hydrocarbon receptor (AHR) homologue from the soft-shell clam, Mya arenaria: evidence that invertebrate AHR homologues lack 2,3,7,8-tetrachlorodibenzo-p-dioxin and beta-naphthoflavone binding. Gene. 2001;278:223–234. doi: 10.1016/s0378-1119(01)00724-7. [DOI] [PubMed] [Google Scholar]
- 74.Qin H., Powell-Coffman J.A. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev. Biol. 2004;270:64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Crews S.T., Brenman J.E. Spineless provides a little backbone for dendritic morphogenesis. Genes Dev. 2006;20:2773–2778. doi: 10.1101/gad.1487706. [DOI] [PubMed] [Google Scholar]
- 76.Whitelaw M.L., McGuire J., Picard D., Gustafsson J.A., Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain S., Dolwick K.M., Schmidt J.V., Bradfield C.A. Potent transactivation domains of the Ah receptor and the Ah receptor nuclear translocator map to their carboxyl termini. J. Biol. Chem. 1994;269:31518–31524. [PubMed] [Google Scholar]
- 78.Ko H.P., Okino S.T., Ma Q., Whitlock J.P. Transactivation domains facilitate promoter occupancy for the dioxin-inducible CYP1A1 gene in vivo. Mol. Cell Biol. 1997;17:3497–3507. doi: 10.1128/mcb.17.7.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haarmann-Stemmann T., Esser C., Krutmann J. The Janus-faced role of aryl hydrocarbon receptor signaling in the skin: consequences for prevention and treatment of skin disorders. J. Invest. Dermatol. 2015 doi: 10.1038/jid.2015.285. [DOI] [PubMed] [Google Scholar]
- 80.Denison M.S., Fisher J.M., Whitlock J.P. The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J. Biol. Chem. 1988;263:17221–17224. [PubMed] [Google Scholar]
- 81.Ciolino H.P., Daschner P.J., Yeh G.C. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem. J. 1999;340(Pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- 82.Heath-Pagliuso S., Rogers W.J., Tullis K., Seidel S.D., Cenijn P.H., Brouwer A., Denison M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry (Mosc.) 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 83.Rannug A., Rannug U., Rosenkranz H.S., Winqvist L., Westerholm R., Agurell E., Grafström A.K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 84.Rannug U., Rannug A., Sjöberg U., Li H., Westerholm R., Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem. Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 85.Adachi J., Mori Y., Matsui S., Takigami H., Fujino J., Kitagawa H., Miller C.A., Kato T., Saeki K., Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 2001;276:31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 86.Miller C.A. A human aryl hydrocarbon receptor signaling pathway constructed in yeast displays additive responses to ligand mixtures. Toxicol. Appl. Pharmacol. 1999;160:297–303. doi: 10.1006/taap.1999.8769. [DOI] [PubMed] [Google Scholar]
- 87.Schaldach C.M., Riby J., Bjeldanes L.F. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry (Mosc.) 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 88.Seidel S.D., Winters G.M., Rogers W.J., Ziccardi M.H., Li V., Keser B., Denison M.S. Activation of the Ah receptor signaling pathway by prostaglandins. J. Biochem. Mol. Toxicol. 2001;15:187–196. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- 89.Sinal C.J., Bend J.R. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol. Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 90.Barouki R., Aggerbeck M., Aggerbeck L., Coumoul X. The aryl hydrocarbon receptor system. Drug Metabol. Drug Interact. 2012;27:3–8. doi: 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- 91.Panteleyev A.A., Bickers D.R. Dioxin-induced chloracne–reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 2006;15:705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 95.Beischlag T.V., Wang S., Rose D.W., Torchia J., Reisz-Porszasz S., Muhammad K., Nelson W.E., Probst M.R., Rosenfeld M.G., Hankinson O. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol. Cell Biol. 2002;22:4319–4333. doi: 10.1128/MCB.22.12.4319-4333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar M.B., Perdew G.H. Nuclear receptor coactivator SRC-1 interacts with the Q-rich subdomain of the AhR and modulates its transactivation potential. Gene Expr. 1999;8:273–286. [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar M.B., Tarpey R.W., Perdew G.H. Differential recruitment of coactivator RIP140 by Ah and estrogen receptors. Absence of a role for LXXLL motifs. J. Biol. Chem. 1999;274:22155–22164. doi: 10.1074/jbc.274.32.22155. [DOI] [PubMed] [Google Scholar]