Abstract
Background
Metaplastic breast cancer (MBC) is characterized by chemoresistance and hematogenous spread. We sought to identify factors associated with improved MBC outcomes and increased likelihood of MBC diagnosis.
Methods
Women≥18 with Stage I–III MBC and non-MBC diagnosed 2010–2014 were identified in the National Cancer Data Base. Kaplan-Meier and multivariate Cox proportional hazards models were used to estimate associations with overall survival (OS). Multivariate logistic regression identified factors associated with MBC diagnosis.
Results
2,451 MBC and 568,057 non-MBC patients were included. 70.3% of MBC vs 11.3% of non-MBC patients were triple-negative (TN, p<0.001). 5-year OS was reduced among MBC vs non-MBC patients for the entire cohort (72.7% vs 87.5%) and among TN patients (71.1% vs 77.8%, both p<0.001). In MBC, TN (vs luminal) subtype was not associated with worse OS (HR 1.16, 95%CI 0.88–1.54, p=0.28). Compared to non-MBC patients, MBC patients were more likely to receive mastectomy (59.0% vs 44.9%), chemotherapy (74.1% vs 43.1%), and axillary dissection (ALND, 35.2% vs 32.2%, all p≤0.001). MBC patients more frequently had negative ALND (pN0) than non-MBC patients (20.0% vs 10.6%, p<0.001). Among MBC patients, chemotherapy (HR 0.69, 95%CI 0.53–0.89, p=0.004) and radiotherapy (HR 0.52, 95%CI 0.39–0.69, p<0.001) were associated with improved survival, while ALND was associated with decreased survival (HR 1.37, 95%CI 1.06–1.77, p=0.02).
Conclusions
MBC patients had worse survival than non-MBC patients, independent of receptor status, suggesting that MBC may confer an additional survival disadvantage. Multimodal therapy was associated with improved outcomes, but ALND was not and may be over-utilized in MBC.
Background
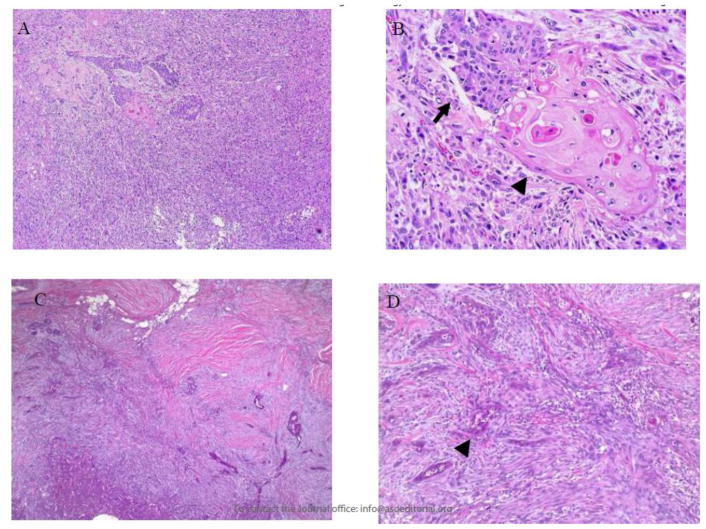
Metaplastic breast cancer (MBC) is a rare disease first recognized in 1973 but not defined as a unique histologic subtype by the World Health Organization until 2000.1 MBC is characterized by resistance to conventional systemic therapies and by hematogenous spread,2,3 in contrast to the lymphatic dissemination typically seen with invasive ductal and lobular breast carcinomas. Histologically, MBC is defined as invasive carcinoma with squamous and/or mesenchymal elements,4 which frequently include spindle cells and heterologous elements such as cartilage and bone (Figure 1). The squamous and mesenchymal components may be focal or represent most of the tumor. Because of its heterogeneity, MBC is frequently misdiagnosed or unrecognized on pathologic review.5
Figure 1. Parts A–D. Pathologic features of diagnosis of metaplastic breast carcinoma.
In metaplastic carcinoma, the mesenchymal component can be markedly atypical (spindle cells in A and B) or bland, resembling fibromatosis (spindle cells in C and D). The epithelial component can have glandular (B, arrow) and/or squamous (arrowheads in B and D) differentiation.
Given the rarity of MBC, much of the relevant literature is comprised of single-institution case series.6–10 Previous studies have demonstrated that MBC is less likely to have nodal involvement and more likely to be hormone receptor-negative (HR−), larger in size,11 higher grade,12 and have a worse prognosis compared to non-MBC. However, it is unclear whether this survival difference is driven by the high rates of HR− status typically observed among MBC cases or whether MBC itself is a poor prognostic indicator.12–14
There are no association-endorsed treatment guidelines specific to the management of MBC. In the recently revised National Comprehensive Cancer Network (NCCN) guidelines, metaplastic histology is recognized as an independent, negative prognostic indicator when it accounts for >10% of an invasive breast cancer, but no guidance for its treatment is provided.15 The 2013 St. Gallen consensus statement, which has been adopted by the European Society for Medical Oncology, recommends that MBC patients receive cytotoxic systemic therapy but mainly because it is often HR-.16,17 However, due to evidence of chemoresistance,2,18 surgery has often been the mainstay of therapy,3 and the role of multimodal therapy remains an area of active investigation. In light of its rarity, diagnostic challenges, and unclear treatment guidelines, we sought to identify treatment patterns associated with improved outcome after MBC diagnosis and clinicopathologic features associated with increased likelihood of MBC diagnosis in a contemporary population-based cohort.
Methods
Females≥18 years old diagnosed with Stage I–III breast cancer between 2010 and 2014 were identified from the National Cancer Data Base (NCDB), which captures more than 70% of all cancers diagnosed in the United States (US). To avoid potential selection bias secondary to sparse coding of HER2+ status (i.e., HER2-amplification by fluorescence in situ hybridization [FISH] and/or HER2 protein overexpression by immunohistochemistry [IHC]), only patients diagnosed in 2010 (when HER2 coding was standardized by the NCDB19) and later were included. We defined the “luminal” subtype to include cases that were ER+/PR+/HER2−, ER+/PR−/HER2−, and ER−/PR+/HER2−; the “HER2+” subtype to include cases that were ER+/PR+/HER2+, ER+/PR−/HER2+, ER−/PR+/HER2+, and ER−/PR−/HER2+; and the “triple-negative” subtype as ER−/PR−/HER2−. As axillary surgery codes were only recently captured in the NCDB, we used previously-described definitions of 1–5 nodes removed and >5 lymph nodes removed as proxies for SLNB and ALND, respectively.20,21 Patients with zero or an unknown number of examined lymph nodes; metastatic (Stage IV) or noninvasive (Stage 0) disease; absent or unknown tumor size; or with a surgical procedure coded as “none,” “local tumor destruction only,” “not otherwise specified,” or “unknown” were excluded. Patients with unknown or missing survival data were also excluded. Patients were classified as having MBC or non-MBC based on histologic codes. Chi-square and t-tests were used to compare categorical and continuous variables, respectively. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan-Meier curves were used to visualize unadjusted OS, and the log-rank test was used to test for differences between groups. The Cox proportional hazards model was used to estimate the effect of MBC versus non-MBC histology on OS after adjustment for known covariates. To estimate the effect of covariates on OS within MBC, a subgroup analysis was conducted for MBC patients.
Because MBC is often triple-negative, a sensitivity survival analysis was conducted with triple-negative breast cancer (TNBC) patients only. To account for the correlation of patients treated at the same facility, a robust sandwich covariance estimator was used for all adjusted survival models. Adjustment variables were chosen based on their association with survival, and select variables were excluded to avoid overfitting the model. We report hazard ratios (HRs) and 95% confidence intervals (CIs) with two-tailed p-values, and p<0.05 was considered significant.
A multivariate logistic regression model was used to estimate the association of socioeconomic and pre-treatment variables with the odds of being diagnosed with MBC. Odds ratios (ORs) and 95% CIs were reported for each covariate with two-tailed p<0.05 considered significant. Modeling was conducted in the generalized estimating equations framework with an exchangeable correlation structure to account for the correlation of patients treated at the same facility. Multicollinearity among the covariates included in the adjusted models was assessed by examining the variance inflation factor (VIF), tolerance, and condition index for each covariate in the full model.22
Only patients with complete data were included in each model, and effective sample sizes are included in all tables and figures. No adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Due to use of de-identified patient data, the Duke University institutional review board (IRB) granted this study exempt status.
Results
2,451 MBC patients and 568,057 non-MBC patients were identified from the NCDB (Table 1, Supplemental Figure 1). Median follow-up time was 37 months. MBC patients had higher proportions of ER−, PR−, and HER2− biomarker statuses (both individually and in combination), higher clinical (cT) and pathologic tumor (pT) stage, and less frequent nodal involvement (pathologic N [pN] stage 0 disease, all p<0.001). 70.3% of MBC patients had TNBC, compared to 11.3% of the non-MBC patients (p<0.001). Women with MBC received more extensive surgical resection, with higher proportions receiving mastectomy (59.0% vs 44.9%, p<0.001) and/or axillary lymph node dissection (ALND, 35.2% vs 32.2%, p=0.001). Among those women with cN0 disease, 22.7% of non-MBC compared to 26.4% of MBC patients received ALND. Notably, 20.0% of MBC patients who underwent ALND had a negative ALND, i.e., underwent axillary dissection but had pN0 disease, which was nearly double the proportion of non-MBC patients who had a negative ALND (10.6%, p<0.001). Higher proportions of MBC patients received adjuvant and/or neoadjuvant chemotherapy (NACT, 74.1% MBC vs 43.1% non-MBC, p<0.001). Overall, 62,115 patients underwent NACT, 12,879 (20.7%) of whom experienced nodal downstaging, i.e., change from a higher initial clinical N (cN) stage to a lower subsequent pathological N (pN) stage, though not necessarily conversion to pN0. Of the 476 patients with MBC who received NACT, 89 (18.7%) experienced nodal downstaging compared to 20.8% of the non-MBC patients who underwent NACT (p=0.25). Although overall radiation use was lower in the MBC population (52.6% MBC vs 60.9% non-MBC, p<0.001), rates of post-lumpectomy radiation (84.8% MBC vs 86.9% non-MBC, p=0.07) between the two groups were comparable. Of note, all patients were included in the analysis of demographic data, but for the remaining analyses, only patients with complete data were included to allow for adjustment for known covariates. Patient characteristics were compared between those with complete and incomplete data (Supplemental Table 1). Because of the large number of patients in our cohort, there were statistically significant differences between patients with complete and incomplete data along many dimensions including (1) race/ethnicity (79.6% vs 64.8% non-Hispanic White); (2) receptor status (74.3% vs 52.2% luminal); and receipt of (3) radiation (62.5% vs 55.7%), (4) chemotherapy (44.6% vs 39.1%), and (5) endocrine therapy (71.7% vs 58.0%, all p<0.001). While our findings should be interpreted in the context of these differences, it is unclear which of these statistically significant differences also represent clinically significant distinctions that may have impacted the extent and/or directionality of our results.
Table 1.
Demographic and Clinicopathologic Characteristics of Metaplastic and non-Metaplastic Breast Cancer Patients, National Cancer Data Base, 2010–2014
| All Patients (N=570,508) | Metaplastic (N=2,451) | Non-Metaplastic (N=568,057) | P-Value | |
|---|---|---|---|---|
| Age – Median, years (IQR) | 61 (51 – 70) | 62 (52 – 72) | 61 (51 – 70) | <0.001 |
| Follow-up–Median, months (95% CI) | 37.3 (37.3–37.4) | 37.0 (36.3–38.0) | 37.3 (37.3–37.4) | NS |
| Race/Ethnicity | <0.001 | |||
| Non-Hispanic White | 432,990 (75.9%) | 1,724 (70.3%) | 431,266 (75.9%) | |
| Non-Hispanic Black | 60,082 (10.5%) | 409 (16.7%) | 59,673 (10.5%) | |
| Hispanic | 30,292 (5.3%) | 137 (5.6%) | 30,155 (5.3%) | |
| Other | 22,773 (4%) | 80 (3.3%) | 22,693 (4%) | |
| Distance Traveled, miles - Median (IQR) | 8.9 (4.3 – 18.5) | 9.0 (4.2 – 19.1) | 8.9 (4.3 – 18.5) | 0.08 |
| Charlson/Deyo Comorbidity Score | <0.001 | |||
| 0 | 477,013 (83.6%) | 1,976 (80.6%) | 475,037 (83.6%) | |
| 1 | 76,852 (13.5%) | 372 (15.2%) | 76,480 (13.5%) | |
| ≥2 | 16,643 (2.9%) | 103 (4.2%) | 16,540 (2.9%) | |
| Income Level | <0.001 | |||
| <$30,000 | 59,140 (10.4%) | 301 (12.3%) | 58,839 (10.4%) | |
| $30,000–$34,999 | 86,654 (15.2%) | 409 (16.7%) | 86,245 (15.2%) | |
| $35,000–$45,999 | 148,048 (26%) | 647 (26.4%) | 14,7401 (25.9%) | |
| ≥$46,000 | 258,218 (45.3%) | 1,017 (41.5%) | 257,201 (45.3%) | |
| Insurance Status | <0.001 | |||
| Private | 301,066 (52.8%) | 1,149 (46.9%) | 299,917 (52.8%) | |
| Medicaid | 36,789 (6.4%) | 179 (7.3%) | 36,610 (6.4%) | |
| Medicare | 206,811 (36.3%) | 999 (40.8%) | 205,812 (36.2%) | |
| Other Government | 5,822 (1%) | 17 (0.7%) | 5,805 (1%) | |
| Not Insured | 11,486 (2%) | 49 (2%) | 11,437 (2%) | |
| Education Level | 0.004 | |||
| ≤71% High School Graduation Rate | 77,611 (13.6%) | 382 (15.6%) | 77,229 (13.6%) | |
| 71.1%–80% High School Graduation Rate | 116,014 (20.3%) | 518 (21.1%) | 115,496 (20.3%) | |
| 80.1%–86% High School Graduation Rate | 127,577 (22.4%) | 555 (22.6%) | 127,022 (22.4%) | |
| >86% High School Graduation Rate | 230,778 (40.5%) | 919 (37.5%) | 229,859 (40.5%) | |
| County Type | 0.51 | |||
| Metro | 472,612 (82.8%) | 2,022 (82.5%) | 470,590 (82.8%) | |
| Urban | 73,832 (12.9%) | 330 (13.5%) | 73,502 (12.9%) | |
| Rural | 9,560 (1.7%) | 47 (1.9%) | 9,513 (1.7%) | |
| Facility Type | <0.001 | |||
| Academic | 175,865 (30.8%) | 870 (35.5%) | 174,995 (30.8%) | |
| Integrated Network | 61,531 (10.8%) | 272 (11.1%) | 61,259 (10.8%) | |
| Comprehensive | 273,616 (48%) | 1,057 (43.1%) | 272,559 (48%) | |
| Community | 59,496 (10.4%) | 252 (10.3%) | 59,244 (10.4%) | |
| Facility Location | <0.001 | |||
| Midwest | 144,665 (25.4%) | 687 (28%) | 143,978 (25.3%) | |
| Northeast | 119,939 (21%) | 509 (20.8%) | 119,430 (21%) | |
| South | 205,418 (36%) | 899 (36.7%) | 204,519 (36%) | |
| West | 100,486 (17.6%) | 356 (14.5%) | 100,130 (17.6%) | |
| Receptor Group | <0.001 | |||
| TNBC | 65,852 (11.5%) | 1,724 (70.3%) | 64,128 (11.3%) | |
| HER2+ | 73,091 (12.8%) | 118 (4.8%) | 72,973 (12.8%) | |
| Luminal | 392,111 (68.7%) | 469 (19.1%) | 391,642 (68.9%) | |
| Clinical T Stage | <0.001 | |||
| 0 | 1,095 (0.2%) | 4 (0.2%) | 1,091 (0.2%) | |
| 1 | 334,321 (58.6%) | 697 (28.4%) | 333,624 (58.7%) | |
| 2 | 138,095 (24.2%) | 1,108 (45.2%) | 136,987 (24.1%) | |
| 3 | 23,910 (4.2%) | 284 (11.6%) | 23,626 (4.2%) | |
| 4 | 10,462 (1.8%) | 133 (5.4%) | 10,329 (1.8%) | |
| X | 35,987 (6.3%) | 146 (6%) | 35,841 (6.3%) | |
| Clinical N Stage | 0.42 | |||
| 0 | 444,088 (77.8%) | 1,923 (78.5%) | 442,165 (77.8%) | |
| 1 | 65,172 (11.4%) | 265 (10.8%) | 64,907 (11.4%) | |
| 2 | 11,293 (2%) | 58 (2.4%) | 11,235 (2%) | |
| 3 | 5,419 (0.9%) | 19 (0.8%) | 5,400 (1%) | |
| X | 29,870 (5.2%) | 122 (5%) | 29,748 (5.2%) | |
| Pathologic T Stage | <0.001 | |||
| 0 | 9,822 (1.7%) | 36 (1.5%) | 9,786 (1.7%) | |
| 1 | 366,396 (64.2%) | 754 (30.8%) | 365,642 (64.4%) | |
| 2 | 148,609 (26%) | 1,165 (47.5%) | 147,444 (26%) | |
| 3 | 22,461 (3.9%) | 333 (13.6%) | 22,128 (3.9%) | |
| 4 | 6,275 (1.1%) | 102 (4.2%) | 6,173 (1.1%) | |
| X | 4,881 (0.9%) | 21 (0.9%) | 4,860 (0.9%) | |
| Pathologic N Stage | <0.001 | |||
| 0 | 396,801 (69.6%) | 1,947 (79.4%) | 394,854 (69.5%) | |
| 1 | 115,550 (20.3%) | 337 (13.7%) | 115,213 (20.3%) | |
| 2 | 30,681 (5.4%) | 79 (3.2%) | 30,602 (5.4%) | |
| 3 | 14,796 (2.6%) | 29 (1.2%) | 14,767 (2.6%) | |
| X | 3,412 (0.6%) | 20 (0.8%) | 3,392 (0.6%) | |
| Tumor Size (cm) – Median (IQR) | 1.6 (1 – 2.5) | 3 (2 – 4.5) | 1.6 (1 – 2.5) | <0.001 |
| Grade | <0.001 | |||
| 1 | 125,026 (21.9%) | 51 (2.1%) | 124,975 (22%) | |
| 2 | 237,486 (41.6%) | 286 (11.7%) | 237,200 (41.8%) | |
| 3 | 170,576 (29.9%) | 1,768 (72.1%) | 168,808 (29.7%) | |
| Breast Surgery | <0.001 | |||
| Lumpectomy | 314,237 (55.1%) | 1,005 (41%) | 313,232 (55.1%) | |
| Mastectomy | 256,271 (44.9%) | 1,446 (59%) | 254,825 (44.9%) | |
| Diagnosis Timing | <0.001 | |||
| At Surgery | 50,029 (8.8%) | 307 (12.5%) | 49,722 (8.8%) | |
| Prior to Surgery | 513,799 (90.1%) | 2,121 (86.5%) | 511,678 (90.1%) | |
| Axillary Surgery | 0.001 | |||
| ALND | 183,517 (32.2%) | 863 (35.2%) | 182,654 (32.2%) | |
| SLNB | 386,991 (67.8%) | 1,588 (64.8%) | 385,403 (67.8%) | |
| Axillary Surgery + LN Status | <0.001 | |||
| ALND + No Positive LNs | 60,604 (10.6%) | 490 (20%) | 60,114 (10.6%) | |
| SLNB + No Positive LNs | 340,011 (59.6%) | 1,491 (60.8%) | 338,520 (59.6%) | |
| ALND + Positive LNs | 122,674 (21.5%) | 371 (15.1%) | 122,303 (21.5%) | |
| SLNB + Positive LNs | 46,668 (8.2%) | 96 (3.9%) | 46,572 (8.2%) | |
| Treatment with Radiation | <0.001 | |||
| Yes | 347,004 (60.8%) | 1,290 (52.6%) | 345,714 (60.9%) | |
| No | 218,911 (38.4%) | 1,135 (46.3%) | 217,776 (38.3%) | |
| Treatment with Radiation after Lumpectomya | 273,093 (86.9%) | 852 (84.8%) | 272,241 (86.9%) | 0.07 |
| Treatment with Chemotherapy | <0.001 | |||
| Yes | 246,547 (43.2%) | 1,816 (74.1%) | 244,731 (43.1%) | |
| No | 311,373 (54.6%) | 611 (24.9%) | 310,762 (54.7%) | |
| Chemotherapy Type | <0.001 | |||
| Adjuvant Only | 184,172 (32.3%) | 1,340 (54.7%) | 182,832 (32.2%) | |
| Neoadjuvant +/− Adjuvant | 62,115 (10.9%) | 476 (19.4%) | 61,639 (10.9%) | |
| No Chemotherapy | 311,373 (54.6%) | 611 (24.9%) | 310,762 (54.7%) | |
| Treatment with Endocrine Therapy | ||||
| Yes – Out of All Patients | 389,214 (68.2%) | 372 (15.2%) | 388,842 (68.5%) | <0.001 |
| No – Out of All Patients | 164,799 (28.9%) | 2,000 (81.6%) | 162,799 (28.7%) | |
| Yes – Out of HR+ Patients* | 383,744 (81.6%) | 326 (61.3%) | 383,418 (81.7%) | <0.001 |
| No – Out of HR+ Patients* | 73,237 (15.6%) | 188 (35.3%) | 73,049 (15.6%) | |
| # LNs Examined - Median (IQR) | 3 (2 – 8) | 4 (2 – 9) | 3 (2 – 8) | 0.01 |
| # Positive LNs - Median (IQR) | 0 (0 – 1) | 0 (0 – 0) | 0 (0 – 1) | <0.001 |
| LN Status | <0.001 | |||
| Any Positive LNs | 169,342 (29.7%) | 467 (19.1%) | 168,875 (29.7%) | |
| No Positive LNs | 400,615 (70.2%) | 1,981 (80.8%) | 398,634 (70.2%) |
Out of all patients who underwent lumpectomy.
Out of all patients who were ER+ and/or PR+.
ALND, axillary lymph node dissection; CI, confidence interval; IQR, interquartile range; LN, lymph node; SLNB, sentinel lymph node biopsy
All possible values are included for each covariate; some columns may not add to 100% due to the incomplete data.
Univariate analysis revealed demographic differences between MBC and non-MBC patients (Table 1). Women with MBC were more often non-Hispanic Black (16.7% vs 10.5%, p<0.001), had Charlson-Deyo (CD) comorbidity scores≥1 (19.4% vs 16.4%, p<0.001), had an annual income of <$35K (29.0% vs 25.6%, p<0.001), used government insurance (48.8% vs 43.7%, p<0.001), and resided in areas with lower educational attainment, that is, areas in which rates of high-school graduation were ≤80% (36.7% vs 33.9%, p=0.004). MBC patients were more likely to be treated at academic centers (35.5% vs 30.8%, p<0.001). Finally, based on examination of the temporal relationships between dates of diagnosis and first surgery, MBC patients as compared to non-MBC patients were more often diagnosed at the time of first surgery rather than preoperatively (12.5% vs 8.8%, p<0.001).
MBC patients had worse unadjusted OS compared to non-MBC patients (see Supplemental Figure 2), and this survival difference persisted in multivariate analysis (HR 1.45, 95% CI 1.29–1.64, p<0.001, Supplemental Table 2). Within the MBC-only cohort (Table 2), radiation therapy (HR 0.52, 95% CI 0.39–0.69) and chemotherapy (HR 0.69, 95% CI 0.53–0.89) continued to be associated with improved OS, while increasing comorbidity score, higher pT and pN stages, higher grade, and ALND continued to be associated with worse OS, similar to trends seen in the overall cohort (Supplemental Table 2). There was an overall association between receptor status and OS (p=0.03). However, when compared to luminal disease as a reference, OS did not differ significantly for TNBC or HER2+ disease. As was seen for the entire cohort, no survival differences were observed between those who received mastectomy vs lumpectomy (HR 0.79, 95% CI 0.58–1.08, p=0.14).
Table 2.
Adjusted Overall Survival – Metaplastic Patients Only (N=1,845)
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Age (Years) | 1.018 (1.009 – 1.027) | <0.001 | <0.001 |
| Race/Ethnicity | 0.60 | ||
| Non-Hispanic White | REF | ||
| Non-Hispanic Black | 0.854 (0.625 – 1.168) | 0.32 | |
| Non-Hispanic Other | 0.894 (0.512 – 1.562) | 0.70 | |
| Hispanic | 0.768 (0.472 – 1.249) | 0.29 | |
| Facility Type | 0.42 | ||
| Academic | REF | ||
| Integrated Network | 0.992 (0.696 – 1.415) | 0.97 | |
| Comprehensive | 0.859 (0.679 – 1.087) | 0.21 | |
| Community | 0.769 (0.518 – 1.141) | 0.19 | |
| Charlson/Deyo Comorbidity Score | 0.05 | ||
| 0 | REF | ||
| 1 | 1.244 (0.943 – 1.641) | 0.12 | |
| ≥2 | 1.688 (1.039 – 2.74) | 0.03 | |
| Pathologic T Stage | <0.001 | ||
| 1 | REF | ||
| 0 | 1.745 (0.455 – 6.693) | 0.42 | |
| 2 | 2.254 (1.594 – 3.187) | <0.001 | |
| 3 | 5.355 (3.611 – 7.941) | <0.001 | |
| 4 | 8.284 (5.19 – 13.224) | <0.001 | |
| X | 3.266 (0.909 – 11.728) | 0.07 | |
| Pathologic N Stage | <0.001 | ||
| 0 | REF | ||
| 1 | 1.423 (1.068 – 1.895) | 0.02 | |
| 2 | 1.972 (1.193 – 3.258) | 0.008 | |
| 3 | 4.362 (2.759 – 6.895) | <0.001 | |
| X | 0.915 (0.3 – 2.794) | 0.88 | |
| Grade | 0.03 | ||
| 1 | REF | ||
| 2 | 2.554 (0.642 – 10.155) | 0.18 | |
| 3 | 3.7 (0.961 – 14.238) | 0.06 | |
| Receptor Status | 0.03 | ||
| Luminal | REF | ||
| HER2+ | 0.520 (0.262 – 1.032) | 0.06 | |
| TNBC | 1.164 (0.882 – 1.536) | 0.28 | |
| Breast Surgery | 0.14 | ||
| Lumpectomy | REF | ||
| Mastectomy | 0.793 (0.581 – 1.081) | 0.14 | |
| Axillary Surgery | 0.02 | ||
| SLNB | REF | ||
| ALND | 1.370 (1.059 – 1.773) | 0.02 | |
| Radiation Therapy | <0.001 | ||
| No | REF | ||
| Yes | 0.518 (0.389 – 0.689) | <0.001 | |
| Chemotherapy | 0.004 | ||
| No | REF | ||
| Yes | 0.689 (0.534 – 0.888) | 0.004 |
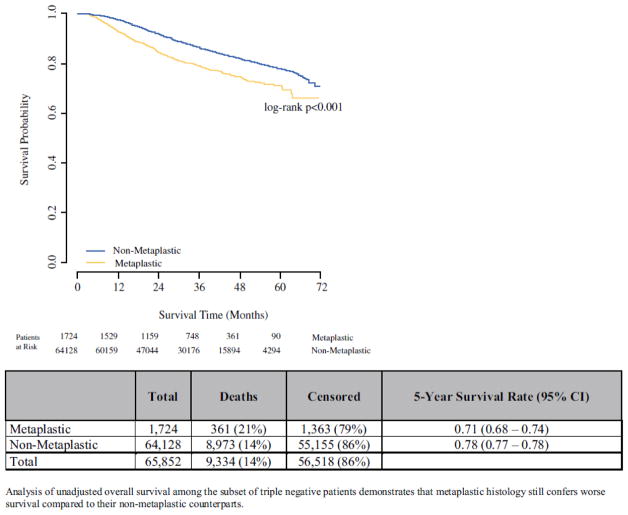
Given the high rates of triple-negative disease among MBC, we performed a sensitivity survival analysis examining the association between MBC and OS among TNBC patients only. Triple-negative MBC had worse unadjusted OS compared to triple-negative non-MBC (Figure 2). This difference persisted after adjustment for covariates (HR 1.49, 95% CI 1.31–1.68, p<0.001, Supplemental Table 3).
Figure 2.
Unadjusted overall survival: TNBC patients only
To identify a population of patients for whom there should be a higher suspicion of MBC, multivariate logistic regression analysis was performed (Table 3). All VIFs were <10, all tolerance values were >0.1, and all condition indices were <30, thus demonstrating no significant collinearity between covariates (Supplemental Tables 4 and 5). Sociodemographic variables such as race, income, and insurance status were not predictors of MBC diagnosis. However, biologic features including higher cT stage (cT4 vs cT1: OR 6.18, p<0.001), lower cN stage (cN1 vs cN0: OR 0.38, p<0.001), HER2+ status (HER2+ vs luminal: OR 1.42, p=0.002), triple-negative status (TNBC vs luminal: OR 20.7, p<0.001), and intraoperative diagnosis (diagnosis during vs prior to first surgery: OR 1.42, p<0.001) were all predictive of MBC diagnosis.
Table 3.
Adjusted Logistic Model for Diagnosis of Metaplastic Breast Cancer (N=450,616)
| OR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Receptor Group | <0.001 | ||
| Luminal | REF | ||
| HER2+ | 1.424 (1.141 – 1.777) | 0.002 | |
| TNBC | 20.712 (18.222 – 23.543) | <0.001 | |
| Clinical T Stage | <0.001 | ||
| 1 | REF | ||
| 0 | 1.945 (0.721 – 5.25) | 0.19 | |
| 2 | 2.998 (2.688 – 3.345) | <0.001 | |
| 3 | 5.456 (4.58 – 6.499) | <0.001 | |
| 4 | 6.183 (4.996 – 7.652) | <0.001 | |
| X | 2.08 (1.662 – 2.602) | <0.001 | |
| Clinical N Stage | <0.001 | ||
| 0 | REF | ||
| 1 | 0.378 (0.322 – 0.445) | <0.001 | |
| 2 | 0.37 (0.273 – 0.5) | <0.001 | |
| 3 | 0.234 (0.142 – 0.388) | <0.001 | |
| X | 0.731 (0.582 – 0.917) | 0.007 | |
| Diagnosis Timing | <0.001 | ||
| Prior to First Surgery | REF | ||
| During First Surgery | 1.416 (1.22 – 1.644) | <0.001 |
Model also adjusted for age, race/ethnicity, distance traveled to treating facility, county type, facility type, facility location, insurance status, income level, education level, and Charlson-Deyo comorbidity score.
Discussion
In our study, the largest contemporary analysis of MBC to include complete receptor status information, we confirm that metaplastic histology confers a worse prognosis, even after adjusting for receptor status, and that hormone receptor-positive (HR+) and HER2+ breast cancer collectively represent a not insignificant proportion (23.9%) of MBC. Furthermore, we delineate treatment factors associated with improved outcomes.
MBC accounts for less than 1% of all breast cancers,23 and the rarity of this disease is reflected in the relative paucity of published MBC research. A retrospective review from 2011 found only 19 published articles specific to MBC, 18 of which were case reports or series.24 There have only been four database studies (Supplemental Table 6) in the past decade looking specifically at MBC, which confirmed the worse prognosis of MBC compared to non-MBC but could not evaluate the full influence of receptor status including HER2.11,12,14,25
We found that sociodemographic factors including race, income, education, and insurance status were associated with MBC diagnosis in univariate analysis, but these associations did not persist in multivariate modeling. Although this could be interpreted as the effect of collinearity, our analysis demonstrated that no significant collinearity existed. Of note, several of these socioeconomic characteristics are also associated with increased incidence of and worse outcomes following diagnosis of other aggressive forms of breast cancer including TNBC and p53-mutated tumors, indicating that these factors may represent non-biologic stressors that contribute to the development of aggressive disease subtypes.26,27 Although further investigation is needed to delineate epidemiologic and genetic risk factors for MBC, it is clear from our analysis that triple-negative receptor status is the strongest predictor of MBC. Thus, in TNBC cases involving any clinicopathologic ambiguity, specialized pathologic evaluation may be warranted given the challenges of making a histological diagnosis of MBC and the prognostic implications for failing to do so. This approach would parallel existing recommendations such as those prompting repeat HER2 testing of the surgical specimen following a demonstration of grade 3 disease. Epidermal growth factor receptor (EGFR, i.e., HER1) is amplified in approximately 70% of MBC; thus, IHC stains such as antibodies specific for the L858R and delE746-A750 EGFR mutations may also be helpful in distinguishing MBC from other malignancies.28
Despite worse outcomes and therapeutic challenges, there are currently no MBC-specific treatment guidelines in the US. Previous studies have yielded conflicting results regarding the effect of breast surgery type on OS.15,29 In our study, MBC patients had higher rates of mastectomy than non-MBC patients, but type of surgery was not associated with a survival difference. Concordant with previous studies, a significant proportion of MBC patients presented with node-negative disease, which is in line with its sarcomatoid phenotype and its tendency towards hematogenous rather than lymphatic spread.3,30 However, despite this typical presentation, we observed higher rates of ALND, and, more importantly, higher rates of both ALND in cN0 patients (26.4% vs 22.7%) and negative ALND (i.e., ALND without pathological evidence of nodal involvement in the surgical specimen) in MBC patients compared to non-MBC patients (20.0% vs 10.6%). As there is variable coding regarding chemotherapy sequence in NCDB, the influence of post-NACT clinical downstaging on the decision to perform ALND cannot be evaluated in the current study. However, the high rate of ALND suggests that, whether NACT was administered or not, surgeons may commit to performing ALND regardless of nodal response to NACT. Together, these findings should prompt further investigation regarding the utility of ALND with or without NACT in MBC, whose biology likely predisposes against nodal metastatic spread.
Radiation and chemotherapy were associated with improved OS, in contrast to historical evidence of MBC resistance to nonsurgical therapy.2,3 We excluded endocrine therapy from multivariate modeling for the TNBC- and MBC-only analyses due to low utilization in these TNBC-dominant populations. NCDB has insufficient granularity to directly compare chemotherapeutic regimens, but previous studies have demonstrated a relative susceptibility to taxane-based chemotherapy and to a regimen consisting of liposomal doxorubicin, bevacizumab, and everolimus.31 Further studies are needed to elucidate the optimal chemotherapeutic regimen and sequence.
Certain targeted therapies may also be leveraged against MBC. Recent studies have demonstrated frequent overexpression of programmed death ligand 1 (PD-L1) in MBC,32 prompting interest in combining checkpoint inhibitors with conventional chemotherapy.33 There is also emerging evidence that subsets of MBC harbor somatic mutations in the PI3K, mTOR, and EGFR pathways34,35 and have demonstrated favorable responses to corresponding targeted therapies.36 Application of such novel therapeutics warrants investigation in MBC, an orphan disease with a poor prognosis and limited therapeutic strategies.
Limitations
The limitations of our study are inherent to retrospective analyses of the NCDB. We selected for MBC based on histology codes, but neither the subtype of metaplastic disease nor a central pathologic review of tissue samples was available in this database. The lack of central pathologic review may have artificially increased the number of HR+ MBC cases reported in this study compared to prior studies. However, the impact of these potentially misclassified cases on our results and conclusions is mitigated by our including a subgroup analysis restricted to TNBC. The sparse coding of HER2 status before 2009 limited the analysis to patients diagnosed in 2010 and later. Given the unreliable use of axillary surgery codes in the NCDB, we used previously described definitions of 1–5 nodes removed and >5 lymph nodes removed as proxies for SLNB and ALND, respectively, though we recognize that lymph node yield may bely the true intent of the operation, and in some hands, SLNB may yield more lymph nodes than ALND.20,21 Finally, the absence of recurrence data in the NCDB limited evaluation of the long-term effects of both locoregional and systemic treatments. Our study paves the way for further institutional analyses, which would allow for central pathologic review and evaluation of long-term, cancer-specific outcomes.
Conclusions
In summary, our findings corroborate previous studies demonstrating low likelihood of lymphatic involvement in MBC despite otherwise aggressive disease biology. Importantly, our findings show that despite previous reports of chemoresistance, multimodal therapy is associated with improved outcomes in patients with MBC. Also, by identifying characteristics associated with MBC, we highlight opportunities to heighten suspicion for and improve the rigor of diagnosis for this condition. Finally, the findings from our study point towards the possibility of a more tailored, potentially less morbid therapeutic approach to MBC that may involve less extensive surgical resection and a larger role for multimodal therapy. Further research into the optimal sequence and composition of therapy is needed and should ultimately be incorporated into association-endorsed guidelines for clinical practice.
Supplementary Material
Supplemental Figure 1. CONSORT diagram of included and excluded patients
Supplemental Figure 2. Unadjusted Overall Survival
Supplemental Table 1. Demographic and Clinicopathologic Characteristics of Patients with Complete and Incomplete Data
Supplemental Table 2. Adjusted Overall Survival – Entire Cohort (N=427,512)
Supplemental Table 3. Adjusted Overall Survival – Triple-Negative Patients (N=54,236)
Supplemental Table 4. Variance Inflation Factors and Tolerance for Each Covariate
Supplemental Table 5. Condition Indices for Each Component
Supplemental Table 6. Previous Retrospective Database Studies of MBC
Synopsis.
MBC patients had worse survival than non-MBC patients, a difference not associated with frequency of triple-negative subtype. MBC patients had more axillary dissections despite having less node-positive disease. Despite purported chemoresistance, multimodal therapy was associated with improved survival in MBC.
Acknowledgments
Portions of this study’s findings were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, 2–6 June 2017, at which time Dr. Ong received an ASCO Conquer Cancer Foundation Merit Award for this work. The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding: Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 5KL2TR001115 (PI: Boulware). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None
Author Contributions: OMF provided overall supervision of the study. CTO, BMC, and OMF designed the study and wrote the manuscript. SMT designed and conducted the statistical analysis, interpreted the results, generated the tables and figures for the manuscript, and reviewed and revised the manuscript. AH contributed original images and annotations of the pathologic diagnosis of disease. RAG, JKP, LHR, JF, TH, and ESH gave conceptual advice and edited the manuscript.
References
- 1.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. World Health Organization; 2000. [Google Scholar]
- 2.Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999:10. doi: 10.1023/a:1008329910362. [DOI] [PubMed] [Google Scholar]
- 3.Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, Kontogianni P, Fotopoulos G. Management and Outcomes in Metaplastic Breast Cancer. Clinical breast cancer. 2016;16(6):437–443. doi: 10.1016/j.clbc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Huvos AG, Lucas JC, Jr, Foote FW., Jr Metaplastic breast carcinoma. Rare form of mammary cancer. N Y State J Med. 1973;73(9):1078–1082. [PubMed] [Google Scholar]
- 5.Brenner RJ, Turner RR, Schiller V, Arndt RD, Giuliano A. Metaplastic carcinoma of the breast. Cancer. 1998;82(6):1082–1087. [PubMed] [Google Scholar]
- 6.Dave G, Cosmatos H, Do T, Lodin K, Varshney D. Metaplastic carcinoma of the breast: a retrospective review. International journal of radiation oncology, biology, physics. 2006:64. doi: 10.1016/j.ijrobp.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012:65. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 8.Aydiner A, Sen F, Tambas M, et al. Metaplastic breast carcinoma versus triple-negative breast cancer: survival and response to treatment. Medicine. 2015;94(52):e2341. doi: 10.1097/MD.0000000000002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai H-W, Tseng L-M, Chang T-W, et al. The prognostic significance of metaplastic carcinoma of the breast (MCB)–a case controlled comparison study with infiltrating ductal carcinoma. The Breast. 2013;22(5):968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Park HS, Park S, Kim JH, et al. Clinicopathologic features and outcomes of metaplastic breast carcinoma: comparison with invasive ductal carcinoma of the breast. Yonsei medical journal. 2010;51(6):864–869. doi: 10.3349/ymj.2010.51.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National cancer data Base. Annals of surgical oncology. 2007:14. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RA, Guye ML, Luu T, Lai LL. Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Annals of surgical oncology. 2015;22(1):24–31. doi: 10.1245/s10434-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 13.Barquet-Muñoz SA, Villarreal-Colin SP, Herrera-Montalvo LA, et al. Metaplastic breast cancer: a comparison between the most common histologies with poor immunohistochemistry factors. BMC Cancer. 2015;15(1):75. doi: 10.1186/s12885-015-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright GP, Davis AT, Koehler TJ, Melnik MK, Chung MH. Hormone receptor status does not affect prognosis in metaplastic breast cancer: a population-based analysis with comparison to infiltrating ductal and lobular carcinomas. Annals of surgical oncology. 2014;21(11):3497–3503. doi: 10.1245/s10434-014-3782-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Jung SY, Ro JY, et al. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65(5):441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of Oncology. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology. 2013;24(suppl_6):vi7–vi23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 18.Abouharb S, Moulder S. Metaplastic breast cancer: clinical overview and molecular aberrations for potential targeted therapy. Current oncology reports. 2015;17(3):431. doi: 10.1007/s11912-014-0431-z. [DOI] [PubMed] [Google Scholar]
- 19.(NAACCR) NAAoCCR. NAACCR 2010 Implementation Guidelines and Recommendations. 2010 https://20tqtx36s1la18rvn82wcmpn-wpengine.netdna-ssl.com/wp-content/uploads/2016/11/2010-Implementation-Guidelines-and-Recommendations_Revised-June-2010.pdf.
- 20.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(18):2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 22.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. 5. McGraw-Hill Irwin; 2005. [Google Scholar]
- 23.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast cancer research and treatment. 2007;101(3):349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 24.Toumi Z, Bullen C, Tang AC, Dalal N, Ellenbogen S. Metaplastic breast carcinoma: a case report and systematic review of the literature. Pathol Int. 2011;61(10):582–588. doi: 10.1111/j.1440-1827.2011.02698.x. [DOI] [PubMed] [Google Scholar]
- 25.Tseng WH, Martínez SR. Metaplastic breast cancer: to radiate or not to radiate? Annals of surgical oncology. 2011:18. doi: 10.1245/s10434-010-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker L, Quinlan PR, Patten N, et al. p53 mutation, deprivation and poor prognosis in primary breast cancer. Br J Cancer. 2010;102(4):719–726. doi: 10.1038/sj.bjc.6605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dookeran KA, Dignam JJ, Ferrer K, Sekosan M, McCaskill-Stevens W, Gehlert S. p53 as a Marker of Prognosis in African-American Women with Breast Cancer. Annals of Surgical Oncology. 2010;17(5):1398–1405. doi: 10.1245/s10434-009-0889-3. [DOI] [PubMed] [Google Scholar]
- 28.Rungta S, Kleer CG. Metaplastic carcinomas of the breast: Diagnostic challenges and new translational insights. Archives of pathology & laboratory medicine. 2012;136(8):896–900. doi: 10.5858/arpa.2012-0166-CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dave G, Cosmatos H, Do T, Lodin K, Varshney D. Metaplastic carcinoma of the breast: a retrospective review. Int J Radiat Oncol Biol Phys. 2006;64(3):771–775. doi: 10.1016/j.ijrobp.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Leddy R, Irshad A, Rumboldt T, Cluver A, Campbell A, Ackerman S. Review of Metaplastic Carcinoma of the Breast: Imaging Findings and Pathologic Features. Journal of Clinical Imaging Science. 2012;2(1):21–21. doi: 10.4103/2156-7514.95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basho RK, Gilcrease M, Murthy RK, et al. Targeting the pi3k/akt/mtor pathway for the treatment of mesenchymal triple-negative breast cancer: Evidence from a phase 1 trial of mtor inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncology. 2017;3(4):509–515. doi: 10.1001/jamaoncol.2016.5281. [DOI] [PubMed] [Google Scholar]
- 32.Joneja U, Vranic S, Swensen J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J Clin Pathol. 2017;70(3):255–259. doi: 10.1136/jclinpath-2016-203874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams S. Dramatic response of metaplastic breast cancer to chemo-immunotherapy. npj Breast Cancer. 2017;3(1):8. doi: 10.1038/s41523-017-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng CKY, Piscuoglio S, Geyer FC, et al. The Landscape of Somatic Genetic Alterations in Metaplastic Breast Carcinomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(14):3859–3870. doi: 10.1158/1078-0432.CCR-16-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz TL, Mogal H, Papageorgiou C, Veerapong J, Hsueh EC. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Experimental hematology & oncology. 2013;2(1):31. doi: 10.1186/2162-3619-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulder S, Helgason T, Janku F, et al. Inhibition of the phosphoinositide 3-kinase pathway for the treatment of patients with metastatic metaplastic breast cancer. Ann Oncol. 2015;26(7):1346–1352. doi: 10.1093/annonc/mdv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CONSORT diagram of included and excluded patients
Supplemental Figure 2. Unadjusted Overall Survival
Supplemental Table 1. Demographic and Clinicopathologic Characteristics of Patients with Complete and Incomplete Data
Supplemental Table 2. Adjusted Overall Survival – Entire Cohort (N=427,512)
Supplemental Table 3. Adjusted Overall Survival – Triple-Negative Patients (N=54,236)
Supplemental Table 4. Variance Inflation Factors and Tolerance for Each Covariate
Supplemental Table 5. Condition Indices for Each Component
Supplemental Table 6. Previous Retrospective Database Studies of MBC