Abstract
Background
Despite increasing opioid overdose mortality, problems persist in the availability and quality of treatment for opioid use disorder (OUD). Three FDA-approved medications (methadone, buprenorphine, and naltrexone) have high quality evidence supporting their use, but most individuals with OUD do not receive them and many experience relapse following care episodes. Developing and organizing quality measures under a unified framework such as a Cascade of Care could improve system level practice and treatment outcomes. In this context, a review was performed of existing quality measures relevant to the treatment of OUD and the literature assessing the utility of these measures in community practice.
Methods
Systematic searches of two national quality measure clearinghouses (National Quality Forum and Agency for Healthcare Research and Quality) were performed for measures that can be applied to the treatment of OUD. Measures were categorized as structural, process, or outcome measures. Second stage searches were then performed within Ovid/Medline focused on published studies investigating the feasibility, reliability, and validity of identified measures, predictors of their satisfaction, and related clinical outcomes.
Results
Seven quality measures were identified that are applicable to the treatment of OUD. All seven were process measures that assess patterns of service delivery. One recently approved measure addresses retention in medication-assisted treatment for patients with OUD. Twenty-nine published studies were identified that evaluate the quality measures, primarily focused on initiation and engagement in care for addiction treatment generally. Most measures and related studies do not specifically incorporate the evidence base for the treatment of OUD or assess patient level outcomes such as overdose.
Conclusion
Despite considerable progress, gaps exist in quality measures for OUD treatment. Development of a unified quality measurement framework such as an OUD Treatment Cascade will require further elaboration and refinement of existing measures across populations and settings. Such a framework could form the basis for applying strategies at clinical, organizational, and policy levels to expand access to quality care and reduce opioid-related mortality.
1. Introduction
In 2016, unintentional overdose fatalities exceeded 63,000 deaths, the great majority involving opioids (CDC, 2017). Overdoses frequently occur among persons who were recently discharged from detoxification programs, treatment, or criminal justice settings (Binswanger et al., 2007; Cousins, Boland, Courtney, et al., 2015; Ravndal & Amundsen, 2010; Sordo, Barrio, Bravo, et al., 2017; Strang, Mccambridge, Best, et al., 2003). Unintentional overdose death is often a consequence of untreated or improperly treated opioid use disorder (OUD), reflecting a long-standing addiction treatment gap in the United States and the difficulties patients face in accessing evidence-based care (Ghitza & Tai, 2014; Volkow, Friedan, Hyde, & Cha, 2014). Despite FDA approval of three effective medications (methadone, buprenorphine, and XR-naltrexone) shown to reduce overdose among patients with OUD (Degenhardt, Bucello, Mathers, et al., 2010; Lee, Friedmann, Kinlock, et al., 2016; Lee, Nunes, Novo, et al., 2018), there remain low rates of initiation and retention on these medications (Aletraris, Bond, & Roman, 2015; Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016; Turner, Kruszewski, & Alexander, 2015). An alarmingly low percentage - barely a fifth - of the 2.4 million individuals estimated to have OUD (SAMHSA, 2017) receive any specialty care in a given year (Saloner, 2015; Wu, Zhu, & Swartz, 2016). With only a third of those in specialty care estimated to receive one of the three FDA-approved MAT medications during a care episode, and a 6-month retention rate under 30–50% in most settings (Morgan, Shackman, Leff, Linas, & Walley, 2018; Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016; Tkacz, Severt, Cacciola, & Ruetsch, 2011), only a fraction of individuals with OUD achieve long-term remission in the US (Williams, Nunes, & Olfson, 2017).
Coincident with the intensifying opioid epidemic, there have been increasing calls for development and use of quality measures to track and improve the quality of care for behavioral health and implement policy strategies to identify and incentivize use of best practices (Pincus, Scholle, Spaeth-Rublee, Hepner, & Brown, 2016). Given the proliferation and adoption of quality measures over the past twenty years in other areas of medicine, there is much that can be learned from quality of care frameworks that have succeeded in other fields. Developing a cascade of care model to focus and inform interventions has been effective in the management of chronic health conditions including HIV (Gardner, McLees, Steiner, del Rio, & Burman, 2010), Hepatitis C (Yehia, Schranz, Umscheid, et al., 2014), and diabetes (Ali, Bullard, Gregg, et al., 2014). A more comprehensive framework for measuring and improving the health care system response to the challenge of OUD could be an important tool in reducing the harms associated with the OUD epidemic. It could, for example, guide improvement of accreditation standards for treatment programs, data collection and reporting, treatment planning and monitoring of key targets, and implementation strategies to improve outcomes and reduce opioid overdose mortality (Socias, Volkow, & Wood, 2016; Williams, Nunes, & Olfson, 2017). Perhaps most important, such a framework could quantify the current gaps in care processes for individuals with OUD and provide tools for goal setting, accountability, measurement of progress, identification of needed treatment resources, and increases in the use of guideline-consistent, evidence based care processes.
For instance, the HIV Cascade of Care framework establishes key stages through which HIV infected persons can progress (engagement in care, antiretroviral initiation, viral suppression, retention in care) to maximize health and eliminate transmission risk to others (Gardner, McLees, Steiner, del Rio, & Burman, 2010). Successful progression through each stage is dependent on satisfaction of prior stages. Adapting the cascade framework to OUD offers an informative model for organizing quality of care measurement. The model is premised on the concept that patients who achieve long-term recovery from opioids are likely to do so through a stepwise process with each step dependent on success with the prior step. It posits that patients must first engage in care in order to initiate MAT. Among those who initiate MAT successfully, efforts are then needed to retain patients in care. As an example, Belenko, Knight, Wasserman, et al. (2017) have demonstrated the utility of applying the cascade framework to juvenile justice populations with substance use to detect gaps in care and opportunities for improvement.
At the population level, effective treatment of OUD presents a series of clinical challenges that could be addressed through development of linked quality measures. Measures could systematically target key processes and outcomes for patients diagnosed with OUD or following overdose. This review includes a systematic search of national quality measure clearinghouses for measures that might be applied to the treatment of OUD, emphasizing the four stages of an OUD Treatment Cascade once patients have already been identified as having OUD: 1). Engagement in care, 2). MAT initiation, 3). Retention, and 4). Remission. A search was then performed of the literature investigating the use of these measures to assess their feasibility, reliability, importance and association with clinically meaningful outcomes. A discussion is subsequently provided on how measures could be consolidated, operationalized, and strengthened to improve outcomes for affected individuals across different settings under a unified OUD Treatment Cascade framework derived from Williams, Nunes, and Olfson (2017).
2. Methods
We performed a systematic search of two national quality measure clearinghouses containing over 3000 healthcare quality measures currently in use by healthcare organizations spanning all clinical fields. The Agency for Healthcare Research and Quality (AHRQ) is a federal agency in the Department of Health and Human Services with the mission to produce evidence to make health care safer, higher quality, more accessible, equitable, and affordable. AHRQ maintains a National Quality Measure Clearinghouse (NQMC). The National Quality Forum (NQF) is a not-for-profit, nonpartisan, multi-stakeholder membership-based organization that works “to catalyze improvements in health-care” and endorses measures developed by other parties such as the National Council on Quality Assurance (NCQA), The Joint Commission, professional associations and healthcare policy institutes such as the RAND Corporation, often supported by the Centers for Medicaid and Medicare Services (CMS). Both the AHRQ and NQF maintain comprehensive databases cataloging quality measures and their provenance (Goldman, Spaeth-Rublee, Nowels, Ramanuj, & Pincus, 2016).
Within the AHRQ and NQF databases, search terms included, “opioid use disorder,” “opioid addiction,” “heroin addiction,” “substance use disorder,” OR “substance abuse.” Measures were included in the review if they 1). Could be applied directly to the treatment of OUD, 2). Precisely defined a numerator and denominator. Measures were excluded if they 1). Addressed prevention, screening, or identification of OUD only (for instance, measures regarding high dose prescribing of opioids), 2). Were not specific to the direct treatment of OUD (for instance, screening for nicotine use among patients with OUD) or 3). Related to general quality of care for any medical condition (for instance, the percent of hospitalized patients counseled on discharge instructions).
Measures were further categorized as structural, process, or outcome measures (Donabedian, 1988; Garnick, Horgan, & Chalk, 2006) according to “measure domain” in the clearinghouses. Structural measures address the capacity of a clinical organization or system to provide effective care, such as the percentage of emergency departments with a continuously available addiction specialist or the percentage of OUD specialty treatment programs with at least one buprenorphine waivered physician. Structural measures can be incorporated into accreditation standards and recognition programs. They also often include the capacity to collect and report process and outcomes measures. Process measures assess whether effective, evidence-based care is actually being provided, such as the percent of patients who receive a urine drug screen or the percent of patients prescribed a MAT medication upon intake to specialty treatment. In some health care environments, process measures can be assessed in real time as they occur through electronic health records. Finally, outcome measures, which often require risk adjustment based on patient characteristics for comparative purposes, typically refer to patients’ clinical outcomes, such as the percentage of OUD patients initiating buprenorphine with subsequent opioid negative urines or with clinically meaningful improvements in health and quality of life (Bray et al., 2017; Jones, Vogelman, Luba, Mumtaz, & Comer, 2017).
Although the Veterans Health Administration (VHA) has promulgated many quality measures, it was not included in the primary search given that measures are inherently operationalized (“specified”) for VHA populations and settings. However, we included studies investigating use of quality measures for substance use disorders from VHA settings in the results along with other published literature that incorporated quality measures for SUDs.
In addition to a search among quality measure clearinghouses for existing measures applicable to the treatment of OUD, we performed a literature search through OVID/Medline with MeSH terms “quality indicators, healthcare,” AND “substance-related disorders” to search for studies that directly assessed the implementation, predictors, and outcomes of identified quality measures. Secondary searches were performed using backward and forward citations through Web of Science based on initial results in combination with hand search methods to identify articles pertaining to the included measures.
3. Results
3.1. Quality measures related to OUD treatment
Across the two clearinghouses, 131 measures were initially identified (31 in the NQF and 100 in the AHRQ databases). Of these 131, 12 met study criteria as applicable to the treatment of OUD. Most often, measures were excluded because they pertained to general patient satisfaction, general screening (i.e. for depression, nicotine use, access to firearms), or general improvement on rating scales (i.e. among patients with any behavioral health diagnosis); were related to the management of chronic pain; were related specifically to the care of patients with HIV; or could not be applied to treatment. Five of the 12 relevant measures were duplicates, leaving 7 unique measures (see Table 1) selected for second stage searches.
Table 1.
Quality measures related to the treatment of opioid use disorder.
| Construct | Clearinghouse | Quality measure identifying detail | Developer | Year endorsed by NQF* | Type | Specific to OUD? |
|---|---|---|---|---|---|---|
| MAT retention for 180+ days among MAT initiators | NQF clearinghouse | #3715 Continuity of pharmacy for OUD (percent with 180+ days retained on MAT among those who initiate MAT) | RAND | 2017 | Process | Yes |
| AOD service following substance-related ED visit | NQF clearinghouse | #2605 Follow up AOD service after emergency department visit for AOD (percent within 7 days and percent within 30 days) | NCQA | 2015 | Process | No |
| Referrals at discharge for inpatients with SUDs | AHRQ NQMC and NQF clearinghouse | #010148 Percent with AOD diagnosis
that receives or refuses a MAT or referral at hospital
discharge (Comparable to NQF #1664 Sub3: AOD treatment provided/offered at discharge from inpatient hospitalization) |
TJC | 2014 | Process | No |
| Referrals at discharge for inpatients with SUDs | AHRQ NQMC and NQF clearinghouse | #010149 Percent with AOD diagnosis
that receives a MAT or referral at hospital discharge (Comparable to NQF #1664 Sub3a: AOD treatment provided/offered at discharge from inpatient hospitalization) |
TJC | 2014 | Process | No |
| Initiation of AOD treatment among those with a
SUD (HEDIS Initiation measure) |
AHRQ NQMC and NQF clearinghouse | #009966 identical to: #010574
percent with initiation AOD treatment within 14 days of new SUD
diagnosis (Comparable to NQF #0004 Initiation and Engagement of AOD treatment) |
NCQA | 2009 | Process | No |
| Engagement in treatment among those with a
SUD (HEDIS engagement measure) |
AHRQ NQMC and NQF clearinghouse | #009967 identical to: #010575
Percent with engagement in AOD treatment (2+ visits) within 30
days of initiation (Comparable to NQF #0004 initiation and engagement of AOD treatment) |
NCQA | 2009 | Process | No |
| Counseling on treatment types for those with OUD | AHRQ NQMC | #004208 Percent > 18 years with current opioid addiction counseled on psychosocial and pharmacologic treatments | APA NCQA PCPI |
N/A | Process | Yes |
NQF = National Quality Forum; AOD = Alcohol or Other Drug; AHRQ NQMC = Agency for Healthcare Research and Quality National Quality Measure Clearinghouse; TJC = The Joint Commission; PCPI = Physician Consortium for Performance Improvement.
All included measures are process measures reflecting patterns of service delivery (i.e. percentage of patients with a SUD discharged from an emergency department who receive specialty care within 30 days). Two were specific to the treatment of OUD including a 2017 measure developed by RAND (continuity of MAT for OUD) tracking the percent of patients who initiate MAT that are retained on medication for a minimum of 180 days (NQF 2017). Although this measure could serve as a patient outcome, it was constructed as a process measure that could be considered to approximate clinical improvement. A second measure reflected the percent of patients with OUD counseled on the existence of available treatments. It is the only included measure to have not been endorsed by the NQF.
As of 2004, the National Committee for Quality Assurance (NCQA) has adopted two of the identified measures into the Healthcare Effectiveness Data Information Set (HEDIS). These two HEDIS measures initially originated from efforts by the Washington Circle, first conceived in 1998 by SAMHSA (Garnick, Horgan, & Chalk, 2006; Garnick, Lee, Chalk, et al., 2002; Garnick, Lee, Horgan, et al., 2009; Mccorry, Garnick, Bartlett, Cotter, & Chalk, 2000). HEDIS is the most widely used set of quality measures in the managed health care industry, used by over 90% of managed healthcare plans’ administrators to assess quality of care delivery but are often less familiar to front line clinicians (Harris et al., 2015; HEDIS, 2013). These two measures are related to SUDs generally but not specific to OUD: 1) the HEDIS Initiation measure assesses the percentage of patients who have a treatment intake within 14 days of a new SUD diagnosis, and 2) the HEDIS Engagement measure assesses the percentage of patients initiating treatment who have at least two additional alcohol or other drug (AOD) services within the 30 days following Initiation (NCQA 2017). These two measures appeared in both the AHRQ NQMC and NQF Clearinghouses. Although applicable to the treatment of OUD, these general measures do not address evaluation for or initiation of MAT. In 2017, the NCQA expanded the Initiation and Engagement measures to include receipt of MAT pharmacotherapy as a qualifying service as this is often additive to, rather than duplicative of other AOD services (Mattke, Predmore, Sloss, Wilks, & Watkins, 2017).
3.2. Publications evaluating OUD quality measures
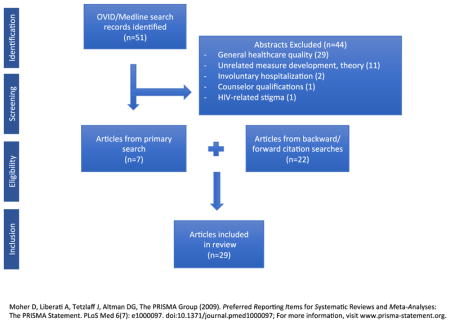
The literature search for studies pertaining to quality measures for SUD initially returned 51 articles. Among the 51, 29 were excluded as they related only to general quality improvement in healthcare or mental health (see Appendix for detail). An additional 11 were excluded as they pertained to the theoretical development or operationalization of quality indicators for addiction treatment but did not directly contribute to the development or analysis of the 7 identified quality measures in Table 1. Finally, 4 were related to involuntary hospitalization (2), counselor qualifications (1), or HIV-related stigma (1) rather than clinical management of OUD.
In sum, 7 articles were found in the primary search related to the development, use (i.e. feasibility, reliability, validity), predictors, or clinical outcomes of the included quality measures. A secondary search was performed using backward and forward search methodology from these 7 articles’ citation lists which yielded an additional 22 articles related to the included measures. Among all of the articles (N = 29), 4 related to the conceptual development of the measures (Garnick, Horgan, & Chalk, 2006; Garnick, Lee, Horgan, et al., 2009; Mccorry, Garnick, Bartlett, Cotter, & Chalk, 2000; Thomas, Garnick, Horgan, et al., 2011), 7 primarily related to feasibility, reliability, or validity of their use or specification (see Table 2), 7 primarily assessed predictors of measure satisfaction (see Table 3), and 11 primarily assessed outcomes among patients satisfying specific measures (see Table 4). However, among the 7 included measures (Table 1), only the 2 HEDIS measures of Initiation and Engagement were directly studied in the published literature. The remaining 5 measures were not assessed across any of the 29 articles, although 4 articles (Mattke, Predmore, Sloss, Wilks, & Watkins, 2017; Thomas, Garnick, Horgan, et al., 2011; Thomas, Garnick, Horgan, et al., 2013; Watkins, Paddock, Hudson, et al., 2017) pertained to expanding HEDIS Initiation and Engagement measure specification to include MAT as a qualifying treatment service or additionally investigated outcomes with continuous use of MAT as a process measure.
Table 2.
Studies evaluating use (feasibility, reliability, or validity) of quality measures applicable to OUD.
| Article | Measure | Setting | Primary outcome | Results | Specific to OUD? |
|---|---|---|---|---|---|
| Garnick, Lee, Chalk, et al., 2002 | HEDIS initiation and engagement | Administrative data among commercial MCOs | Feasibility among adult enrollees | Use of measures is feasible, meaningful, and informative. Initiation rates ranged from 26%–46%; Engagement rates were more consistent with a range of 14% to 29% | No |
| Harris, Reeder, Ellerbe, & Bowe, 2011 | HEDIS initiation and engagement | VHA | Validity of measure to correctly identify SUD treatment services through diagnosis and procedure codes | Concordance with chart review in specialty settings was high (range 92% to 98%). Concordance with chart review in non-specialty settings varied from 46% to 63%). | No |
| Garnick, Lee, Horgan, et al., 2011 | HEDIS initiation and engagement | Case studies of five states | Feasibility and implementation | Measures can be satisfactorily implemented but with mixed implications for other states. Measures reflect necessary but insufficient steps for full recovery. | No |
| Kim, Saitz, Cheng, et al., 2011 | HEDIS initiation and engagement | RCT in primary care settings | Feasibility of initiation and engagement criteria under a chronic medical disorder model | Feasible in primary care with initiation and engagement rates of 45% and 23% | No |
| Thomas, Garnick, Horgan, et al., 2013 | HEDIS initiation and engagement | National data from private health plans, VHA, and Medicaid | Feasibility of expanded measure specifications to include receipt of MAT as a qualifying service | MAT as a qualifying AOD service is feasible across systems but varies widely depending on measure specification | Yes |
| Harris et al., 2015 | HEDIS initiation and engagement | VHA | Specification validity | High concordance for residential and outpatient AOD programs (90% and 96%). Concordance in non-addiction settings ranged from 59% to 93%. | No |
| Mattke, Predmore, Sloss, Wilks, & Watkins, 2017 | HEDIS initiation and engagement | Commercial health plan claims | Feasibility of expanded measure specifications to include receipt of MAT as a qualifying AOD service | Including MAT was feasible and increased initiation rates by 2.4% (from 38.9% to 39.8%) and engagement rates by 9.9% (from 12.9% to 14%) | Yes |
VHA = Veterans Health Administration; MAT = Medication assisted treatment; RCT = Randomized Controlled Trial; AOD = Alcohol or other drug.
Table 3.
Studies investigating predictors of measure completion.
| Article | Measure | Setting | Data source | Sample size | Primary outcome | Results | Specific to OUD? |
|---|---|---|---|---|---|---|---|
| Harris & Bowe, 2008 | HEDIS Initiation and Engagement | VHA | Administrative data | N = 270,877 patients | Rates of initiation and engagement | Overall initiation and engagement rates of 29.6% and 30.1%. Patients who were female, not married, younger, and had their SUDs identified in a SUD or psychiatric specialty settings had higher rates. Engagement rates higher when treatment initiation was in outpatient settings. | No |
| Harris, Bowe, Finney, & Humphreys, 2009 | HEDIS Initiation and Engagement | VHA | Administrative data | N = 320,238 patients | Rates of initiation and engagement | Patients diagnosed with SUD in specialty settings more likely to progress to Initiation and Engagement but a significant amount of AOD care occurs outside of specialty settings in a given system, i.e. 25% of initiation and over 40% of engagement | No |
| Brown, Bennett, Li, & Bellack, 2011 | HEDIS initiation and engagement | Randomized sample of treatment-seeking patients with comorbid SMI and SUD | Multisite RCT data | N = 175 patients | Rates of initiation and engagement | Among patients with serious mental illness, males (AOR 0.46) and those with schizophrenia (AOR 0.44) had lower initiation rates. Those with current (v. recent) drug dependence (AOR 0.30) or a recent arrest (AOR 0.37) were less likely to engage | No |
| Lee, Garnick, O’Brien, et al., 2012 | HEDIS initiation and engagement | Outpatient multisite pilot study across 12 states; adolescents | Secondary data, 28 outpatient clinics | N = 2191 patients | Rates of initiation and engagement among adolescents | 76% of the sample Initiated, with 59% Engaged. Mixed race and highly truant adolescents had lower initiation rates. Latino youth were less likely to engage. | No |
| Acevedo, Garnick, Dunigan, et al., 2015 | HEDIS initiation and engagement | Public sector AOD treatment centers in 4 states | Administrative linked with criminal justice data | N = 108,654 patients | Rates of engagement with emphasis on racial disparities | Racial minorities often had lower initiation and engagement rates but there was great variation and mixed findings across the four states | No |
| Bensley, Harris, Gupta, et al., 2017 | HEDIS initiation and engagement | Patients with alcohol use disorder in the VHA | Administrative data | N = 90,879 patients | Rates of initiation and engagement | Engagement results varied with measure specification but were more likely for black patients (AOR 1.1) relative to white patients | No |
| Watkins, Ober, Lamp, et al., 2017 | HEDIS initiation and engagement | Collaborative Care intervention model in a FQHC | RCT data including EHR and pharmacy logs | N = 377 patients | Rates of initiation and engagement | Higher rates among collaborative care participants versus TAU: Initiation 31.6% vs 13.7% (AOR 3.54); Engagement, 15.5% vs 4.2% (AOR 5.89) | No |
VHA = Veterans Health Administration; AOD = Alcohol or other drug; SMI = serious mental illness; RCT = randomized controlled trial; TAU = Treatment as usual; AOR = Adjusted odds ratio; Federally Qualified Health Center; EHR = Electronic Health Records.
Table 4.
Outcomes among patients satisfying quality measures.
| Article | Measure | Setting | Data source | Sample size | Primary outcome | Results | Specific to OUD? |
|---|---|---|---|---|---|---|---|
| Clinical outcomes and substance use | |||||||
| Harris, Humphreys, & Finney, 2007 | HEDIS initiation and engagement | VHA, 110 SUD treatment programs across 73 facilities | Self-administered ASI | N = 5723 patients | ASI composite drug and alcohol scores at the facility level approximately 7 months after patient intake | Patients receiving care at facilities with higher rates of Initiation has modestly greater improvements in ASI drug (but not alcohol) composite scores when adjusting for facility case-mix characteristics | No |
| Harris, Humphreys, Bowe, Tiet, & Finney, 2008 | HEDIS engagement | VHA | Administrative and survey data | N = 2789 patients | ASI composite drug, alcohol, and legal scores at patient level | Patients who engaged had statistically significant but clinically modest gains in all scores, with greater effects for alcohol and legal outcomes for patients seen in outpatient settings | No |
| Garnick, Lee, O’Brien, et al., 2012 | HEDIS engagement | Outpatient multisite pilot study across 12 states; adolescents | Secondary data, 28 outpatient clinics | N = 1491 patients | Substance use outcomes | Adolescents who engaged reported had lower risk of substance use (AOR 0.60 95% CI 0.41, 0.87), alcohol use (AOR 0.63 95% CI 0.45, 0.87), heavy alcohol use (AOR 0.53 95% CI 0.33, 0.86), or marijuana use (AOR 0.64 95% CI 0.45, 0.93) | No |
| Acevedo, Garnick, Ritter, Lundgren, & Horgan, 2016 | HEDIS engagement | AOD outpatient treatment facilities in Massachusetts | Administrative data | N = 11,591 patients | Detoxification admissions | Engaged patients had lower detoxification admission in year following index outpatient visit (HR = 0.87, p < .01) among clients in AOD treatment | No |
| Criminal justice | |||||||
| Garnick, Horgan, Lee, et al., 2007 | HEDIS initiation and engagement | Oklahoma, publicly funded outpatient treatment | Administrative data, client self-report | N = 5328 clients | Criminal justice outcomes | Engagement, but not initiation, was associated with lower risk (HR: 0.73 95% CI 0.62, 0.87) of subsequent arrest and incarceration | N |
| Garnick, Horgan, Acevedo, et al., 2014 | HEDIS engagement | Public sector AOD treatment centers in 4 states | Administrative linked with criminal justice data | N = 106,662 patients | Criminal justice outcomes | Those who engaged had significantly lower risk of any arrest in all four states studied (HR range 0.73–0.83) | No |
| Employment | |||||||
| Dunigan, Acevedo, Campbell, et al., 2014 | HEDIS Engagement | Public sector outpatient treatment in Washington state | Administrative, employment, and criminal justice data including self-report | N = 7570 patients | Employment outcomes | For clients with prior criminal justice involvement, engagement was associated with both greater employment (44.7% vs. 38.8%. p < .01) and higher wages ($12,537 vs. $11,338) in the year following treatment | No |
| Mortality | |||||||
| Watkins, Paddock, Hudson, et al., 2016 | HEDIS initiation and engagement | Cohort study of VHA patients with co-occurring disorders (COD) | Administrative data | N = 144,045 patients | 12 and 24 month mortality | Initiation associated with 15% decrease and engagement associated with 31% decrease in 12-month mortality. Increasing numbers of visits associated with further reductions in mortality. | No |
| Paddock, Hepner, Hudson, et al., 2017 | HEDIS initiation and engagement | VHA, inpatient and outpatient | Administrative data | N = 339,966 patients | 12 and 24 month mortality | AOR of 12-month mortality with initiation = 0.86, p = .001 and engagement = 0.65, p < .001; and 24-month mortality with initiation = 0.88, p = .005 and engagement = 0.78, p < .001 | No |
| Watkins, Paddock, Hudson, et al., 2017 | MAT continuity (3 months) | VHA, retrospective cohort study | Administrative data | N = 31,016 patients | 12 and 24 month mortality among patients with OUD | MAT continuity was not associated with decreased mortality however not being prescribed opioids or benzodiazepines, receipt of any psychosocial treatment, and quarterly physician visits were significantly associated with lower mortality at both 12 and 24 months | Yes |
| Patient satisfaction | |||||||
| Hepner, Paddock, Watkins, et al., 2017 | HEDIS initiation and engagement | VHA | Administrative data and phone survey | N = 2074 patients | Self-reported perceived improvement on the ECHO | Engagement, but not Initiation, was associated with perceived improvement (Coeff 0.25, p = .006) | No |
VHA = Veterans Health Administration; ASI = Addiction Severity Index; AOD = Alcohol or other drug; TAU = Treatment as usual; AOR = Adjusted odds ratio; CI = Confidence interval; ECHO = Experience of Care and Health Outcomes; HEDIS=Healthcare Effectiveness Data Information Set.
Across the 29 studies, only 4 addressed OUD specifically (Mattke, Predmore, Sloss, Wilks, & Watkins, 2017; Thomas, Garnick, Horgan, et al., 2011; Thomas, Garnick, Horgan, et al., 2013). The other 25 studies assessed measure development or outcomes for SUDs generally. Several articles indicated that there is a lack of reliable and valid quality measures developed specifically for OUD and that quality measures for SUD generally have not been specifically tested in opioid dependent populations (Garnick, Lee, Chalk, et al., 2002; Garnick, Lee, Horgan, et al., 2009; Harris, Humphreys, Bowe, Tiet, & Finney, 2008; Watkins, Paddock, Hudson, et al., 2017).
For the included measures, studies demonstrated the feasibility, reliability, and importance of the HEDIS measures (see Table 2) among managed, private, Medicaid, and Veterans Health Administration (VHA) plans (Garnick, Lee, Chalk, et al., 2002; Garnick, Lee, Horgan, et al., 2011; Thomas, Garnick, Horgan, et al., 2013), although reliability was generally found to be better in specialty settings than in non-specialty settings (Harris et al., 2015; Harris, Reeder, Ellerbe, & Bowe, 2011). However, measure specification (i.e. technical definition for population of interest and qualifying treatment criteria) produced differing results based on payers (Garnick, Lee, Horgan, et al., 2011; Thomas, Garnick, Horgan, et al., 2011) as there can be great variation across settings in patient characteristics and population-based analyses (Thomas, Garnick, Horgan, et al., 2013). Studies specific to assessing pharmacologic measures consistently showed the feasibility of measures assessing use of MAT as a performance measure in systems with robust electronic health record systems (Thomas, Garnick, Horgan, et al., 2011; Thomas, Garnick, Horgan, et al., 2013; Watkins, Paddock, Hudson, et al., 2016).
Seven studies primarily examined predictors of meeting HEDIS initiation and engagement measure criteria (see Table 3) for general addiction care (i.e. not specific to OUD). These studies found higher rates of initiation and engagement when patients were identified in specialty settings or receiving care in specialty settings (Harris & Bowe, 2008; Harris, Bowe, Finney, & Humphreys, 2009) although primary care (Kim, Saitz, Cheng, et al., 2011) and collaborative care initiatives have also shown to be successful (Watkins, Ober, Lamp, et al., 2017) at engaging patients. Other studies found that patients with greater criminal justice involvement, addiction severity, and racial/ethnic minorities often had relatively lower probabilities of treatment initiation and engagement (Acevedo, Garnick, Dunigan, et al., 2015; Brown, Bennett, Li, & Bellack, 2011; Lee, Garnick, O’Brien, et al., 2012) but this was not always observed (Bensley, Harris, Gupta, et al., 2017), reflecting variation in patient populations across settings.
Among the published studies evaluating outcomes (n = 11) after meeting HEDIS Initiation and Engagement measures (see Table 4), these two measures have been consistently linked to clinical improvements such as reduced drug and alcohol use and risk of detoxification readmission (Acevedo, Garnick, Ritter, Lundgren, & Horgan, 2016; Garnick, Lee, O’Brien, et al., 2012; Harris, Humphreys, Bowe, Tiet, & Finney, 2008; Harris, Humphreys, & Finney, 2007). Similar associations have been reported with regard to improved criminal justice outcomes (Garnick, Horgan, Acevedo, et al., 2014; Garnick, Horgan, Lee, et al., 2007), employment outcomes (Dunigan, Acevedo, Campbell, et al., 2014), and patient perceptions of care (Hepner, Paddock, Watkins, et al., 2017). Despite statistical significance, however, the strength of these associations was often clinically modest. More recent studies have further reported that the HEDIS Initiation and Engagement measures are associated with reduced risk of mortality (Paddock, Hepner, Hudson, et al., 2017; Watkins, Paddock, Hudson, et al., 2016). However, a study in the VHA setting found that continuous receipt of MAT was not associated with lower mortality (Watkins, Paddock, Hudson, et al., 2017).
4. Discussion
Despite the recent promulgation of thousands of quality measures across the healthcare landscape, we found only a few measures related to SUDs that can be applied to the treatment of OUD (n = 7). Among the seven identified unique measures (Table 1), all are process measures that reflect patterns of service delivery. Most do not specifically incorporate the evidence-base for the treatment of OUD or assess patient level outcomes such as overdose. A secondary literature search for articles investigating the development, use, predictors, and outcomes of these measures produced 29 publications. Findings are mostly limited to the evaluation of HEDIS Initiation and Engagement measures for SUDs generally (rather than being specific to OUD) and show modest but consistent beneficial outcomes for patients who engage in care, including reduced mortality in some studies. The scarcity of measures specific to OUD is problematic given the large and growing impact of OUD on health and mortality outcomes. Since performance on generic measures of care processes for SUD is not specifically informative of system performance in addressing OUD (i.e., it is possible to do well on these measures by providing excellent treatment for other SUDs but poor treatment for OUD) there is a need for stewardship of OUD-specific quality measures.
There is a critical opportunity to organize, coordinate, and expand existing quality measures to effectively engage patients with OUD in specialty care. Mounting evidence demonstrates OUD is a chronic disorder requiring ongoing treatment and has unique risks (i.e. sudden death with relapse) and treatment pathways (3 pharmacological options with differing induction strategies) distinguishing it from other SUDs (Kampman & Jarvis, 2015; USDHHS, 2016; Strang, Mccambridge, Best, et al., 2003; Amato, Davoli, Perucci, et al., 2005). Effective treatment of OUD presents a series of clinical challenges; development of quality measures provides opportunities to systematically target several points of intervention, and could be informed by goal setting and tracking of progress at each stage with feasible, reliable, and valid measures under a unified framework. The proposed OUD Treatment Cascade model encompassing four key stages for patients identified with OUD, 1). Treatment engagement, 2). MAT initiation, 3). Retention, and 4). Remission could build on existing measures to enhance patient outcomes (Williams, Nunes, & Olfson, 2017). Coordinated measure development at structural, process, and outcome levels would likely be most impactful.
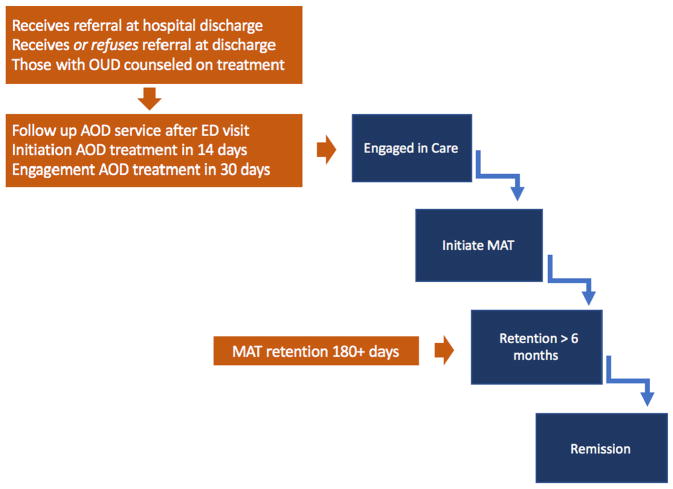
Fig. 1 depicts the currently available measures (from Table 1) mapped onto the proposed OUD Treatment Cascade framework, illustrating gaps in measurement and opportunities for measure development. Fig. 1 highlights how current measures mostly address early stages of care engagement rather than progression through the full Cascade. While the existing NCQA HEDIS Initiation and Engagement measures are used by virtually all managed care plans (Harris et al., 2015; HEDIS, 2013) and provide a basis for comparisons across insured populations through the analysis of claims data, they do not offer the level of detail needed to track individual patient progress through OUD Treatment Cascade stages. Most existing measures address whether patients receive AOD services in general, but do not track patients with OUD as they progress through an episode of care with evidence based treatment. For instance, unlike that for other SUDs, the gold standard treatment for OUD emphasizes use of MAT without a predefined length of treatment. Although some patients may receive high quality care for other SUDs that does not involve MAT, the evidence does not currently support this as a first line approach for OUD. Additionally, studies such as Watkins, Paddock, Hudson, et al., 2017 have demonstrated the limitations of general SUD measure reliability for OUD and pitfalls of their misapplication to OUD, limiting clinical validity. For example, in many systems, the initiation of MAT may reflect underlying addiction severity requiring risk adjustment as a confounder for outcomes such as mortality in addition to serving as a process measure approximating quality care.
Fig. 1.
Existing SUD quality measures and applicability to an OUD treatment cascade.
In addition to developing measures under a unified framework to assess success along an OUD Treatment Cascade, greater specification of measures could help to define populations that would serve as the denominator for whom quality measures are targeted. A distinction between acute presentations (such as overdoses or emergency department visits) and the engagement of patients with OUD identified via routine screening and clinical management for population-based targeting is key. This distinction highlights the opportunity acute presentations offer for engaging patients with untreated OUD in specialty care and initiating a MAT medication (D’Onofrio et al., 2015). For instance, the current measure assessing receipt of a follow up AOD service after an ED visit could be applied to patients who acutely present with an overdose. The measure assessing initiation of AOD treatment within 14 days of a new OUD diagnosis or those with OUD counseled on available treatments can be applied to patients identified with OUD in primary care settings. Currently, many individuals with OUD who overdose or experience complications (such as Hepatitis C, HIV infection, or abscesses) receive acute services, but are not effectively engaged in care to treat their underlying OUD (Frazier, Cochran, et al., 2017; Larochelle, Liebschutz, Zhang, Ross-Dengan, & Wharam, 2016).
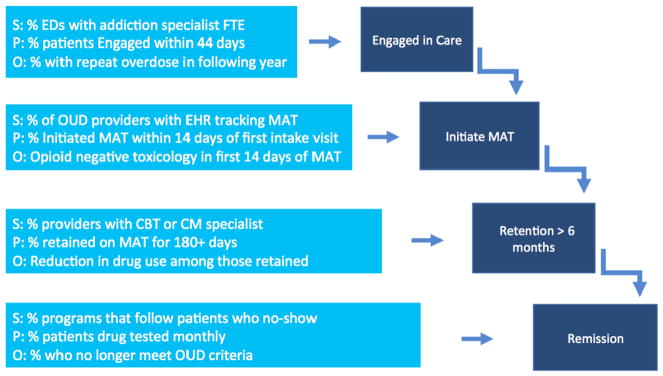
Fig. 2 models candidate measure concepts for each of the four proposed stages of an OUD Treatment Cascade at the structural, process, and outcome levels for populations of patients with OUD with acute presentations to emergency rooms, such as for a medically treated overdose. The figure is intended to illustrate the opportunity to develop interlocking measures to monitor patient progression through an OUD Treatment Cascade and identify key stages where patients may struggle. Given that measure development is often a lengthy process and must be specified for each population, researchers and policymakers should consider prioritizing measures that are likely to be the most feasible and have the greatest impact.
Fig. 2.
Candidate quality measure concepts for an OUD treatment cascade at structural, process, and outcome levels for patients treated for overdose.
Many interventions and services for responding to the epidemic (e.g. prescription drug monitoring programs and prescriber guidelines) address prevention and risk management but do not specifically facilitate progression into specialty AOD treatment for individuals who have been identified as having OUD. Greater emphasis on actively reaching and evaluating such individuals in emergency, acute, and criminal justice settings could improve rates of MAT initiation, especially for patients otherwise ambivalent about seeking treatment. Our hope is that this framework will motivate development of quality measures to better assess whether specific interventions more effectively engage individuals identified with OUD into evidence-based specialty care or, for instance, promote initiation of MAT. These are suggestions for measure development that require refinement over time to demonstrate an empiric basis of feasibility, reliability, and clinical validity to be endorsed and used by the field.
Following approval of the ASAM Standards of Care for the Addiction Specialist Physician in 2013 (ASAM, 2014), an ASAM Performance Measures Panel operationalized the standards into three candidate SUD performance measures. Most notably, one measure was tested to assess the percent of patients diagnosed with OUD in a given year who receive a prescription for MAT at least once in the same year (using administrative claims) (Harris, Weisner, Chalk, et al., 2016). This OUD MAT measure has been evaluated in both VHA (Harris, Weisner, Chalk, et al., 2016) and commercially insured (Thomas, Ritter, Harris, et al., 2018) populations demonstrating feasibility. Further stewardship of this measure would relate to the MAT initiation stage of the OUD Treatment Cascade presented in Fig. 2. As currently specified, this measure does not reference timeliness of MAT initiation following intake for a given care episode.
Because the drug treatment system has historically operated outside of the general healthcare system, policymakers and administrators have faced complex administrative challenges to integrate addiction treatment into the modern healthcare system with integrated EHR capabilities for continuous reporting and quality improvement (Friedmann, Saitz, & Samet, 2003). Currently, over a third of treatment programs still do not accept insurance of any kind (Andrews, Abraham, Grogan, et al., 2015) making the use of HEDIS measures (originally developed to be valid using claims data alone) difficult to apply to these settings. State agencies that oversee the funding and regulation of each state’s specialty addiction facilities have opportunities to help their state’s treatment programs qualify for insurance reimbursement and incentivize them to adopt adequate data collection and reporting systems to maintain licensing and accreditation (Buck, 2011).
One strategy for improving patient outcomes along the OUD Treatment Cascade may involve substance use disorder treatment providers (SAMHSA-accredited and private providers) reorienting systems to track all patients who enter care for OUD, especially those who discontinue medication treatment or stop appearing for appointments and have presumably relapsed to active use and are at greatest risk of overdose (Barrett, Li, Spaeth-Rublee, & Pincus, 2017; Strang, Mccambridge, Best, et al., 2003; Williams, Nunes, & Olfson, 2017). Intensive case management with patient navigators and peer counselors can assist programs in tracking patients outside of clinical settings. Information about individuals who “fall off” the Cascade may motivate efforts to design interventions to improve outcomes over time (Chalk & Mark, 2017).
There are several limitations to the use of quality measures which qualify their applicability to an OUD Treatment Cascade. Foremost, quality measures can pose risks of regulatory overreach and potential downsides of electronic health records and data monitoring (Schuster, Onorato, & Meltzer, 2017). Although a given measure in isolation may be feasible, reliable, and clinically valid, in concert with the large number of other measures, it may prove burdensome and ultimately hamper effective clinical practice. Additionally, any well-intended measure may inadvertently incentivize counterproductive behavior by providers such as “denominator management,” whereby rates of accurate diagnosis are artificially suppressed to game the system. We have attempted to address these concerns by proposing a unified framework that conceptualizes interlocking measures to capture synergisms and avoid redundancy. Effective implementation strategies would benefit from monitoring for unintended applications. Additionally, many specialty addiction treatment providers still lack data collection and reporting systems, impeding their ability to participate in measure assessment or eligibility for insurance reimbursement. An impetus for further measure development concerns the lack of access to evidence based treatment that many patients continue to face across a treatment landscape notorious for practice variation amid a persistent gap between the science and practice of addiction treatment.
5. Limitations
The current review has some limitations. First, we limited the current study to an investigation of quality measures contained in major national clearinghouses. As a result, measures in other healthcare systems such as the VHA, general outcome measures used in clinical trials, or professional society practice guidelines such as those from the American Society of Addiction Medicine (e.g. ASAM, 2014) or American Psychiatric Association were outside of our scope. These additional levers for improving OUD management warrant further study. Given increasing rates of mortality among individuals with OUD despite FDA approval of three highly effective pharmacotherapies, we decided to focus on existing measures that could be applied to the treatment of OUD rather than to prevention or identification in earlier stages of an OUD Cascade of Care. Second, the literature review was conducted by the lead author (ARW) and results may have differed with a group consensus process to screen articles. Finally, despite the increasing prominence of quality measures across the healthcare landscape, we did not limit our review of measures or published studies to those developed specifically for OUD as so few (e.g. two measures, according to our findings) would have been available for assessment.
6. Conclusion
Development and strategic application of coordinated performance measures at key stages may offer opportunities to maximize the use of evidence-based treatment and assess and improve outcomes across settings and populations. With adoption of an OUD Treatment Cascade as an organizing conceptual framework, quality measures could be developed and tested systematically and iteratively refined to help maximize care outcomes. An OUD Treatment Cascade framework could improve treatment program accreditation standards, data collection and reporting, monitoring of key targets, and enhance outcomes. Developing quality measures to identify which patients struggle at which stages of the Cascade could also target clinical and policy interventions to help federal and state efforts improve patient outcomes.
Acknowledgments
Funding provided by NIDA grant K23DA044342-01 (Williams) and the Christopher D. Smithers Foundation, Inc.
Appendix A
Footnotes
Conflicts of interest
Dr. Nunes received medication or software for research studies from Alkermes and Reckitt-Benckiser. Dr. Bisaga received medication, extended-release naltrexone, for NIH funded research studies from Alkermes. Dr. Friedmann reports having received study medication in-kind from Alkermes and served on their Scientific Advisory Board. He also received support for training and travel from Braeburn, and served as an expert consultant in legal proceedings for Endo Pharmaceuticals. All remaining authors report no financial relationships with commercial interests.
References
- Acevedo A, Garnick D, Ritter G, Lundgren L, Horgan C. Admissions to detoxification after treatment: Does engagement make a difference? Substance Abuse. 2016;37(2):364–371. doi: 10.1080/08897077.2015.1080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo A, Garnick DW, Dunigan R, et al. Performance measures and racial/ ethnic disparities in the treatment of substance use disorders. Journal of Studies on Alcohol and Drugs. 2015;76:57–67. [PMC free article] [PubMed] [Google Scholar]
- Aletraris L, Bond EM, Roman PM. Adoption of injectable naltrexone in US substance use disorder treatment programs. Journal of Studies on Alcohol and Drugs. 2015;1:143–151. [PMC free article] [PubMed] [Google Scholar]
- Ali MK, Bullard KM, Gregg EW, et al. A cascade of care for diabetes in the United States: Visualizing the gaps. Annals of Internal Medicine. 2014;161(10):681–689. doi: 10.7326/M14-0019. [DOI] [PubMed] [Google Scholar]
- Amato L, Davoli M, Perucci CA, et al. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28(4):321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Andrews C, Abraham A, Grogan CM, et al. Despite resources from the ACA, most states do little to help addiction treatment programs implement care reform. Health Affairs. 2015;34(5):828–835. doi: 10.1377/hlthaff.2014.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASAM. The ASAM performance measures for the addiction specialist physician. Chevy Chase, MD: The American Society for Addiction Medicine; 2014. [Google Scholar]
- Barrett J, Li M, Spaeth-Rublee B, Pincus HA. Value-based payment as part of a broader strategy to address opioid addiction crisis. Health affairs blog. 2017 Dec 1; [Google Scholar]
- Belenko S, Knight D, Wasserman GA, et al. The Juvenile Justice Behavioral Health Services Cascade: A new framework for measuring unmet substance use treatment services needs among adolescent offenders. Journal of Substance Abuse Treatment. 2017;74:80–91. doi: 10.1016/j.jsat.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley KM, Harris AHS, Gupta S, et al. Racial/ethnic disparities in initiation of and engagement with addiction treatment among patients with alcohol use disorders in the veterans health administration. Journal of Substance Abuse Treatment. 2017;73:27–34. doi: 10.1016/j.jsat.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison–A high risk of death for former inmates. The New England Journal of Medicine. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JW, Aden B, Eggman AA, Hellerstein L, Wittenberg E, Nosyk B, … Shackman BR. Quality of life as an outcome of opioid use disorder treatment: A systematic review. Journal of Substance Abuse Treatment. 2017;76:88–93. doi: 10.1016/j.jsat.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bennett ME, Li L, Bellack AS. Predictors of initiation and engagement in substance abuse treatment among individuals with co-occurring serious mental illness and substance use disorders. Addictive Behaviors. 2011 May;36(5):439–447. doi: 10.1016/j.addbeh.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JA. The looming expansion of public substance abuse treatment under the affordable care act. Health Affairs. 2011;30(8):1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Vital Statistics System. Provisional counts of drug overdose deaths as of 8/6/2017. National Center for Health Statistics, Centers for Disease Control and Prevention; 2017. [Google Scholar]
- Chalk M, Mark T. Health Affairs, Health Policy Lab. 2017. Jun, Deploying the cascade of care framework to address the opioid epidemic means taking a closer look at quality measures. 212017) [Google Scholar]
- Cousins G, Boland F, Courtney B, et al. Risk of mortality on and off methadone substitution treatment in primary care: A national cohort study. Addiction. 2015;111:73–82. doi: 10.1111/add.13087. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction. 2010;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;260(12):1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, et al. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: A randomized clinical trial. JAMA. 2015;313(16):1636–1644. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunigan R, Acevedo A, Campbell K, et al. Engagement in outpatient substance abuse treatment and employment outcomes. The Journal of Behavioral Health Services & Research. 2014 Jan;41(1) doi: 10.1007/s11414-013-9334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W, Cochran LC, et al. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA. 2017;318(8):750–752. doi: 10.1001/jama.2017.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Saitz R, Samet JH. Principles of addiction medicine. Chevy Chase, Maryland: ASAM; 2003. Linking addiction treatment with other medical and psychiatric treatment systems; pp. 497–507. [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. HIV/AIDS; 2011. 2010;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Horgan CM, Acevedo A, et al. Criminal justice outcomes after engagement in outpatient substance abuse treatment. Journal of Substance Abuse Treatment. 2014 March ;46(3):295–305. doi: 10.1016/j.jsat.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Horgan CM, Chalk M. Performance measures for alcohol and other drug services. Alcohol Research & Health. 2006;29(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Horgan CM, Lee MT, et al. Are Washington circle performance measures associated with decreased criminal activity following treatment? Journal of Substance Abuse Treatment. 2007 Dec;33(4):341–352. doi: 10.1016/j.jsat.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Lee MT, Chalk M, et al. Establishing the feasibility of performance measures for alcohol and other drugs. Journal of Substance Abuse Treatment. 2002;23:375–385. doi: 10.1016/s0740-5472(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Garnick DW, Lee MT, Horgan CM, et al. Adapting Washington circle performance measures for public sector substance abuse treatment systems. Journal of Substance Abuse Treatment. 2009 Apr;36(3):265–277. doi: 10.1016/j.jsat.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Lee MT, Horgan CM, et al. Lessons from five states: Public sector use of the Washington circle performance measures. Journal of Substance Abuse Treatment. 2011;40:241–254. doi: 10.1016/j.jsat.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, Lee MT, O’Brien P, et al. The Washington circle engagement performance measures’ association with adolescent treatment outcomes. Drug and Alcohol Dependence. 2012 August ;124(3) doi: 10.1016/j.drugalcdep.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Tai B. Challenges and opportunities for integrating preventive substance-use-care services in primary care through the affordable care act. Journal of Health Care for the Poor and Underserved. 2014;25(10):36–45. doi: 10.1353/hpu.2014.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman ML, Spaeth-Rublee B, Nowels AD, Ramanuj PP, Pincus HA. Quality measures at the interface of behavioral health and primary care. Current Psychiatry Reports. 2016;18(39):1–8. doi: 10.1007/s11920-016-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AHS, Bowe T. Predictors of initiation and engagement in VA substance use disorder (SUD) treatment. Psychological Services. 2008;5(3):228–238. [Google Scholar]
- Harris AHS, Bowe T, Finney JW, Humphreys K. HEDIS initiation and engagement quality measures of substance use disorder care: Impact of setting and health care specialty. Population Health Management. 2009;12(4):191–196. doi: 10.1089/pop.2008.0028. 14. [DOI] [PubMed] [Google Scholar]
- Harris AHS, Ellerbe L, Phelps TE, Finney JW, Bowe T, Gupta S, … Trafton J. Examining the specification validity of the HEDIS quality measures for substance use disorders. Journal of Substance Abuse Treatment. 2015 Jun;53:16–21. doi: 10.1016/j.jsat.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Harris AHS, Humphreys K, Bowe T, Tiet Q, Finney JW. Does meeting the HEDIS substance abuse treatment engagement criterion predict patient outcomes? The Journal of Behavioral Health Services & Research. 2008;37(1):25–39. doi: 10.1007/s11414-008-9142-2. [DOI] [PubMed] [Google Scholar]
- Harris AHS, Humphreys K, Finney JW. Veterans affairs facility performance on Washington circle indicators and casemix-adjusted effectiveness. Journal of Substance Abuse Treatment. 2007;33:333–339. doi: 10.1016/j.jsat.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Harris AHS, Reeder RN, Ellerbe LS, Bowe TS. Validation of the treatment identification strategy of HEDIS addiction quality measures: Concordance with medical record review. BMC Health Services Research. 2011 doi: 10.1186/1472-6963-11-73. http://www.biomedcentral.com/1472-6963/11/73. [DOI] [PMC free article] [PubMed]
- Harris AHS, Weisner CM, Chalk M, et al. Specifying and pilot testing quality measures for the American Society of Addiction Medicine’s Standards of Care. Journal of Addiction Medicine. 2016;10:148–155. doi: 10.1097/ADM.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDIS®. Technical specifications for health plans. Vol. 2. Washington, CA: National Committee for Quality Assurance; 2013. [Google Scholar]
- Hepner KA, Paddock SM, Watkins KE, et al. Association between quality measures and perceptions of care among patients with substance use disorders. Psychiatric Services. 2017;68:1150–1156. doi: 10.1176/appi.ps.201600484. doi: https://doi.org/10.1176/appi.ps.201600484. [DOI] [PubMed] [Google Scholar]
- Jones JD, Vogelman JS, Luba R, Mumtaz M, Comer SD. Chronic pain and opioid abuse: Factors associated with health-related quality of life. The American Journal on Addictions. 2017;26:815–821. doi: 10.1111/ajad.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of Addiction Medicine. 2015;9(5):358–367. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Saitz R, Cheng DM, et al. Initiation and engagement in chronic disease management care for substance dependence. Drug and Alcohol Dependence. 2011 May 1 ;115(0):80–86. doi: 10.1016/j.drugalcdep.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Liebschutz JM, Zhang F, Ross-Dengan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose, a cohort study. Annals of Internal Medicine. 2016;164(1):1–9. doi: 10.7326/M15-0038. [DOI] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. NEJM. 2016;374:1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicenter, open-label, randomize controlled trial. Lancet. 2018;391(10118):309–318. doi: 10.1016/S0140-6736(17)32812-X. published online November 14, 2017 https://doi.org/10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Garnick DW, O’Brien PL, et al. Adolescent treatment initiation and engagement in an evidence-based practice initiative. Journal of Substance Abuse Treatment. 2012;42(4):346–355. doi: 10.1016/j.jsat.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattke S, Predmore Z, Sloss E, Wilks A, Watkins KE. Evidence for misspecification of a nationally used quality measure for substance use treatment. Journal for Healthcare Quality. 2017:1–8. doi: 10.1097/JHQ.0000000000000106. [DOI] [PubMed]
- Mccorry F, Garnick DW, Bartlett J, Cotter F, Chalk M. Developing performance measures for alcohol and other drug services in managed care plans. The Joint Commission Journal on Quality Improvement. 2000 Nov;26(11):633–643. doi: 10.1016/s1070-3241(00)26054-9. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Shackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine/naloxone utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment. 2018 Jul 3;85:90–96. doi: 10.1016/j.jsat.2017.07.001. pii: S0740-5472(16)30413-5 https://doi.org/10.1016/j.jsat.2017.07.001 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Quality Forum Behavioral Health. Draft Report for Comment 2017. 2016–2017. [Google Scholar]
- Paddock SM, Hepner KA, Hudson T, et al. Association between process-based quality indicators and mortality for patients with substance use disorders. Journal of Studies on Alcohol and Drugs. 2017 Jul;:588–596. doi: 10.15288/jsad.2017.78.588. [DOI] [PMC free article] [PubMed]
- Pincus HA, Scholle SH, Spaeth-Rublee B, Hepner KA, Brown J. Quality measures for mental health and substance use: Gaps, opportunities, and challenges. Health Affairs. 2016;35(6):1000–1008. doi: 10.1377/hlthaff.2016.0027. [DOI] [PubMed] [Google Scholar]
- Ravndal E, Amundsen EJ. Mortality among drug users after discharge from inpatient treatment: An 8-year prospective study. Drug and Alcohol Dependence. 2010;108(1–2):65–69. doi: 10.1016/j.drugalcdep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Saloner B. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013 during the last decade, non-medical use. JAMA. 2015;314(14):1515–1517. doi: 10.1001/jama.2015.10345. [DOI] [PubMed] [Google Scholar]
- SAMHSA Substance Abuse and Mental Health Services Administration, National Survey of Substance Abuse Treatment Services (N-SSATS) Data on Substance Abuse Treatment Facilities. BHSIS Series S-88, HHS Publication No. (SMA) 17-5031. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017. 2015. [Google Scholar]
- Schuster MA, Onorato SE, Meltzer DO. Measuring the cost of quality measurement a missing link in quality strategy. JAMA. 2017;318(13):1219–1220. doi: 10.1001/jama.2017.11525. [DOI] [PubMed] [Google Scholar]
- Socias EM, Volkow N, Wood E. Adopting the ‘Cascade of Care’ framework: An opportunity to close the implementation gap in addiction care? Addiction. 2016 doi: 10.1111/add.13479. [DOI] [PMC free article] [PubMed]
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357(j1550):1–14. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Mccambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326:959–960. doi: 10.1136/bmj.326.7396.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Garnick DW, Horgan CM, et al. Advancing performance measures for use of medication in substance abuse treatment. Journal of Substance Abuse Treatment. 2011 Jan;40(1):35–43. doi: 10.1016/j.jsat.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Garnick DW, Horgan CM, et al. Establishing the feasibility of measuring performance in use of addiction pharmacotherapy. Journal of Substance Abuse Treatment. 2013;45:11–18. doi: 10.1016/j.jsat.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Ritter GA, Harris AHS, et al. Applying American Society of Addiction Medicine performance measures in commercial health insurance and services data. Journal of Addiction Medicine. 2018 Mar 29; doi: 10.1097/ADM.0000000000000408. http://dx.doi.org/10.1097/ADM.0000000000000408 (Epub ahead of print) [DOI] [PubMed]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases. 2016;35(1):22–35. doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J, Severt J, Cacciola J, Ruetsch C. Compliance with buprenorphine medication-assisted treatment and relapse to opioid use. The American Journal on Addictions. 2011;21:55–62. doi: 10.1111/j.1521-0391.2011.00186.x. [DOI] [PubMed] [Google Scholar]
- Turner L, Kruszewski SP, Alexander GC. Trends in the use of buprenorphine by office-based physicians in the United States, 2003–2013. The American Journal on Addictions. 2015;24:24–29. doi: 10.1111/ajad.12174. [DOI] [PubMed] [Google Scholar]
- USDHHS, U.S. Department of Health and Human Services (HHS) Office of the Surgeon General, Facing Addiction in America: The surgeon general’s report on alcohol, drugs, and health. Chapter 4. Washington, DC: HHS21; Nov, 2016. [PubMed] [Google Scholar]
- Volkow ND, Friedan TR, Hyde PS, Cha SS. Medication-assisted therapies-tackling the opioid-overdose epidemic. The New England Journal of Medicine. 2014;370(22):2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Ober AJ, Lamp K, et al. Collaborative care for opioid and alcohol use disorders in primary care: The SUMMIT randomized clinical trial. JAMA Internal Medicine. 2017;177(10):1480–1488. doi: 10.1001/jamainternmed.2017.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paddock SM, Hudson TJ, et al. Association between quality measures and mortality in individuals with co-occurring mental health and substance use disorders. Journal of Substance Abuse Treatment. 2016;69:1–8. doi: 10.1016/j.jsat.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paddock SM, Hudson TJ, et al. Association between process measures and mortality in individuals with opioid use disorders. Drug and Alcohol Dependence. 2017;177:307–314. doi: 10.1016/j.drugalcdep.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Nunes EV, Olfson M. To battle the opioid overdose epidemic, deploy the “Cascade of Care” model. Health affairs blog. 2017 Mar 13; [Google Scholar]
- Wu L, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Schranz A, Umscheid CA, et al. The treatment cascade for chronic hepatitis C virus infection in the United States: A systematic review and meta-analysis. PLoS ONE. 2014;9(7):e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]