Abstract
The aim of this study was to investigate normal intraocular pressure (IOP) values of cattle, sheep, and goats with a rebound tonometer [TonoVet (TV)] and an applanation tonometer [Tono-Pen AVIA (TPA)] and to determine correction functions for the 2 devices. A total of 60 healthy cattle, sheep, and goats (20 of each) underwent slit-lamp biomicroscopy. Intraocular pressure (IOP) readings were taken from both eyes with the 2 different tonometers and statistically analyzed. For calibration purposes, the IOP was preset on each instrument at 5 to 60 mmHg using 5 mmHg increments in 10 bovine, 8 ovine, and 6 caprine freshly enucleated eyes. Readings were taken with both tonometers at each interval and compared to the manometrically controlled IOP (Mann-Whitney U-test, P ≤ 0.05; Bland-Altman plot, and regression analysis). The median IOP measurements (min to max) obtained with the TV were 23 mmHg (12 to 40 mmHg), 11 mmHg (7 to 20 mmHg), and 23 mmHg (9 to 37 mmHg) for cattle, sheep, and goats, respectively. Using the TPA, the median IOP measurements were 16 mmHg (8 to 27 mmHg), 10 mmHg (5 to 18 mmHg), and 13 mmHg (4 to 25 mmHg) for cattle, sheep, and goats, respectively. There were statistically significant differences between the readings taken with the TV and the TPA in all species (Wilcoxon-test, P ≤ 0.05). All measurements obtained with the TV and the TPA during the calibration procedure differed statistically significantly from the manometrically controlled IOP measurements (Mann-Whitney U-test, P ≤ 0.05). For both instruments, regression formulas were calculated to correct the measurements. Both tonometers can be used effectively to assess intraocular pressure in ruminants, using the specific regression formulas.
Résumé
L’objectif de cette étude était de mesurer la pression intraoculaire (PIO) normale chez la vache, le mouton et la chèvre à l’aide de deux tonomètres différents; le (TonoVet (TV) et le Tono-Pen AVIA (TPA) et de déterminer les fonctions de correction pour ces deux appareils. Les vingt animaux de chaque espèce ont été examinés à la lampe à fente pour être qu’ils ne présentaient aucune anomalie oculaire puis la PIO des deux yeux fut mesurée avec les deux tonomètres différents et les valeurs furent analysées statistiquement. Pour des raisons d’étalonnage la PIO fut préréglée de 5 à 60 mmHg, divisée en incréments de 5 mmHg sur 10 yeux de vaches, 8 yeux de moutons et 6 yeux de chèvres fraîchement énuclées. Pour chaque incréments de pression, les lectures furent prises avec les deux tonomètres et furent comparées à la PIO mesurée manométriquement. Chez les bovins, les ovins et les caprins la PIO médiane (min-max) obtenue avec le TV fut de 23 mmHg (12–40 mmHg), 11 mmHg (7–20 mmHg) et 23 mmHg (9–37 mmHg) et avec le TPA de 16 mmHg (8–27 mmHg), 10 mmHg (5–18 mmHg) et 13 mmHg (4–25 mmHg). Dans toutes les espèces, des différences statistiquement significatives entre les lectures prises avec la TV et la TPA furent constatées. Lors de la procédure d’étalonnage toutes les valeurs de la PIO mesurés avec le TV et le TPA furent statistiquement significativement différentes des valeurs obtenues manométriquement. Afin de corriger les mesures les formules de régression furent calculées pour les deux instruments. En tenant compte des formules de régression spécifiques les deux tonomètres peuvent être utilisés efficacement pour évaluer la pression intraoculaire chez les ruminants.
(Traduit par les auteurs)
Introduction
Normal and relatively constant intraocular pressure (IOP) is maintained by the balance of aqueous humor production and outflow. This relatively constant pressure is essential in order to maintain the shape of the eye and to ensure a stable position of the intraocular structures (1,2). Intraocular pressure (IOP) should be measured in each patient presented for ophthalmic examination, as tonometry is essential for diagnosing and monitoring uveitis and glaucoma. The most accurate method of measuring IOP is direct tonometry using a manometer, but this procedure is not practical for clinical use due to its invasiveness. In current clinical veterinary ophthalmology, applanation and rebound tonometry are the most widely used techniques for measuring IOP. Both methods indirectly determine IOP by measuring corneal tension (3).
There are many published studies that compare the different methods and available instruments for measuring IOP in various animal species. In veterinary ophthalmology, the Mackay-Marg applanation tonometer has been widely used in the past and has been shown to have good accuracy. It seems to be the most reliable device for measuring IOP in different animal species, both with and without ocular abnormalities. The accuracy and usefulness of the commercially available Tono-Pen XL, the Perkins handheld tonometer, and the TonoVet (TV) have been extensively studied in different animal species (4–15).
To accurately measure pressure, each type of tonometer must be calibrated for use with different animal species as ocular anatomy, e.g., corneal thickness and curvature, corneal and scleral rigidity, and viscosity of tear film, varies among the species. Calibration curves are therefore necessary for the different tonometers. Although several studies are available in dogs, cats, horses, cows, sheep, rats, mice, and chinchillas (3,11,15–18), there are no calibration studies for the TV and newly designed Tono-Pen AVIA (TPA) in domestic ruminants.
Depending on the instrument used, the mean IOP reported for cattle, sheep, and goats ranged from 15 to 28 mmHg, 11 to 14 mmHg, and 11 to 12 mmHg, respectively (Table I) (5,6,12,19–22). Although the incidence of glaucoma in cattle is very low, even less than 1%, various ocular diseases (inflammatory or neoplastic conditions) can lead to alterations in production of aqueous humor and outflow, which results in an increased or decreased IOP (23–25). Ocular hypertension has been experimentally induced in cattle and sheep using topical application of corticosteroids (21,26).
Table I.
Published values for intraocular pressure (IOP) in ruminants using different tonometers.
| Study | Species and breed | Number of animals (N) | Mean IOP ± SD (mmHg) | Tonometer |
|---|---|---|---|---|
| Gum et al, 1998 (12) | Cattle (Holstein-Fresian, Jersey) | 32 | 27.5 ± 4.8 | Mackay-Marg |
| 27 | 28.2 ± 4.6 | |||
| 27 | 26.9 ± 6.7 | Tono-Pen XL | ||
| Kotani, 1993 (19) | Cattle | 23.4 ± 5.9 | Mackay-Marg | |
| Andrade et al, 2011 (5) | Horses and cattle | 10 | 18.8 ± 1.7 | Perkins |
| Tofflemire et al, 2015 (20) | Cattle calves (Holstein) | 33 | 15.2 ± 5.2 | TonoVet |
| Gerometta et al, 2009 (21) | Sheep (Corriedale) | 18 | 10.6 ± 1.4 | Perkins |
| Ribeiro et al, 2014 (22) | Sheep (Santa Ines) | 10 | OS 12.70 ± 1.09 | Tono-Pen XL |
| OD 13.90 ± 0.84 | ||||
| Broadwater et al, 2007 (6) | Goats (Pygmy) | 10 | 11.8 ± 1.5 | TonoVet |
| 10.8 ± 1.7 | Tono-Pen XL |
SD — standard deviation; OS — oculus sinister; OD — oculus dexter.
To the authors’ knowledge, no studies are currently available that compare applanation and rebound tonometry or calibration curves for use of the TV or the TPA in ruminants. The aim of this study was to investigate normal IOP values in cattle, sheep, and goats using a rebound tonometer (TonoVet) and an applanation tonometer (Tono-Pen AVIA) and to determine regression formulas for the 2 devices.
Materials and methods
Patient examination
A total of 60 healthy, privately owned cattle, sheep, and goats (20 of each) was examined individually at the owner’s request as part of a general health check. All procedures were conducted according to the guidelines of the Association for Research in Vision and Ophthalmology (ARVO). The animals were examined in their normal environment to keep the stress level as low as possible. The cows were restrained in their feed fence with the head fixed with a halter to either the right or left side to avoid any pressure on the jugular vein. All sheep were positioned on their backside as routinely done for shearing and their heads were gently held straight ahead. Goats were kept in a standing position with their heads restrained by their horns, looking straight ahead.
All 60 animals underwent slit-lamp biomicroscopy of the anterior segments of both eyes using a Kowa-SL 15 (Kowa, Tokyo, Japan). The intraocular pressure of each eye was initially measured with the TonoVet (Tiolat Oy, Helsinki, Finland) using the “d” setting. After rebound tonometry, 2 drops of the local anesthetic agent oxy-buprocaine hydrochloride (Novesine 0.4%; OmniVision, Puchheim, Germany) were applied to both eyes. Thirty seconds after instillation of this medication, the Tono-Pen AVIA (Reichert Technologies, Depew, New York, USA) was used to take IOP readings from each eye. Triplicate readings were taken for each eye with both devices and then readings were averaged. The eye to evaluate first was selected randomly. Care was taken to open the eyes without applying any pressure on the globe.
Manometric examination
Before the manometric examination, the manometer (D D-890; ATP Messtechnik, Ettenheim, Germany) was checked by the Bureau of Standards of the Federal States of Berlin and Brandenburg, Germany.
Ten bovine, 8 ovine, and 6 caprine freshly enucleated eyes from slaughtered animals were used for the ex-vivo measurements. After enucleation, the eyes were immersed in a 0.9% sodium chloride (NaCl) solution and stored at room temperature. All measurements were conducted within 6 h after enucleation.
For the manometric experiments, the enucleated eyes were placed on a bed of modeling material on top of a plastic cup to avoid any pressure or movement during the examination. The eyes were cannulated through the sclera into the vitreous cavity with a 23-gauge needle. The needle was connected to the manometer and to a saline solution reservoir via a 3-way stopcock. An open system was used for all measurements. The IOP was sequentially increased from 5 to 60 mmHg in increments of 5 mmHg by adjusting the height of the saline reservoir. Minimal changes in the manometrically controlled IOP (± 0.1 mmHg) were tolerated, but higher differences were corrected immediately. There was no leakage of fluid observed around the needles during any measurements.
The rebound tonometer was always used first and the applanation tonometer second. To keep the cornea moist throughout the whole examination, 4 to 5 drops of saline were applied to its surface before each measurement. For each interval, 6 consecutive measurements were taken with both devices. In each measurement series, the lowest and the highest value were excluded from statistical analysis. All values were compared to the manometrically controlled IOP.
Statistical analysis
The statistical software R (version 3.1.0) was used for statistical analysis. Data were tested for a normal distribution with the Shapiro-Wilk test. The Wilcoxon test was conducted to detect differences between the measurements of the TV and the TPA for the right and left eyes of living animals and for comparison with the manometric results at each IOP level. For evaluation of differences between the right and left eye measurements, the Mann-Whitney U-test was used. Linear regression analysis was carried out and a regression formula was calculated to correct the results obtained from the TV and the TPA. The level of significance of all comparisons was set at P ≤ 0.05.
Results
Clinical tonometry
A total of 120 eyes from 60 healthy domestic ruminants (20 cows, 20 sheep, and 20 goats) was examined. All cattle were Holstein-Fresian dairy cows with a median age of 4 y (2 to 10 y). The sheep were German heaths from a hobby breeder. Eleven animals were female and 9 were male, both with a median age of 0.5 y old (0.5 to 12 y). The goats were all female Toggenburgers and were kept for milk production and reproduction. They were a median age of 6.5 y (2 to 14 y). Based on the ophthalmic examination, all animals were free of ocular disease.
The obtained values were not normally distributed. The results of the intraocular pressure measurements [median (min to max), mean ± standard deviation (SD), and P-values] are listed in Table II. There were statistically significant differences between the measurements obtained with the TV and the TPA in all 3 species, as shown in Table II. Differences between the left and right eyes were statistically significant for the measurements using the TV in cattle and goats.
Table II.
Results of intraocular pressure measurements [median, min to max, mean ± standard deviation (SD)] and corresponding P-values in cattle, sheep, and goats using the TonoVet and Tono-Pen AVIA.
| Eye | TonoVet Median (min to max) |
Tono-Pen AVIA Median (min to max) |
TonoVet Mean ± SD |
Tono-Pen AVIA Mean ± SD |
|
|---|---|---|---|---|---|
| Cattle | OD | 23 (15 to 37) | 15.5 (8 to 27) | 23.9 ± 5.0 | 15.5 ± 3.9 |
| 22 (12 to 40) | 16 (9 to 25) | 21.5 ± 6.3 | 15.6 ± 4.3 | ||
| P-value | OS* | < 0.000 | |||
| OD* | < 0.000 | ||||
| Sheep | OS | 11 (8 to 20) | 10 (5 to 18) | 12.7 ± 3.0 | 9.8 ± 2.7 |
| OD | 10.5 (7 to 20) | 10 (6 to 18) | 11.7 ± 3.3 | 10.5 ± 2.4 | |
| P-value | OS* | < 0.000 | |||
| OD* | 0.009 | ||||
| Goats | OS | 22 (9 to 34) | 13 (4 to 25) | 21.6 ± 5.4 | 13.0 ± 4.3 |
| OD | 24 (11 to 37) | 13 (6 to 25) | 24.3 ± 5.6 | 14.1 ± 4.6 | |
| P-value | OS* | < 0.000 | |||
| OD* | < 0.000 |
Significant difference (Wilcoxon test, P ≤ 0.05).
OS — oculus sinister; OD — oculus dexter.
Manometric examination
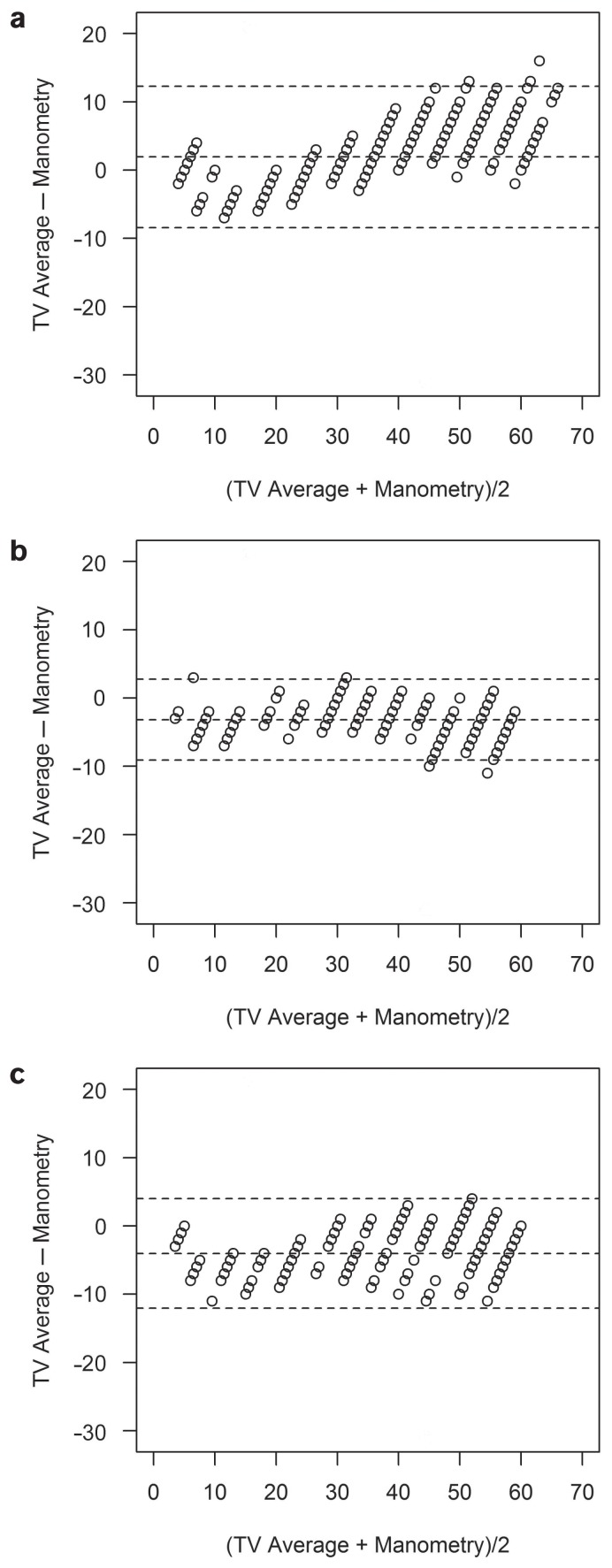
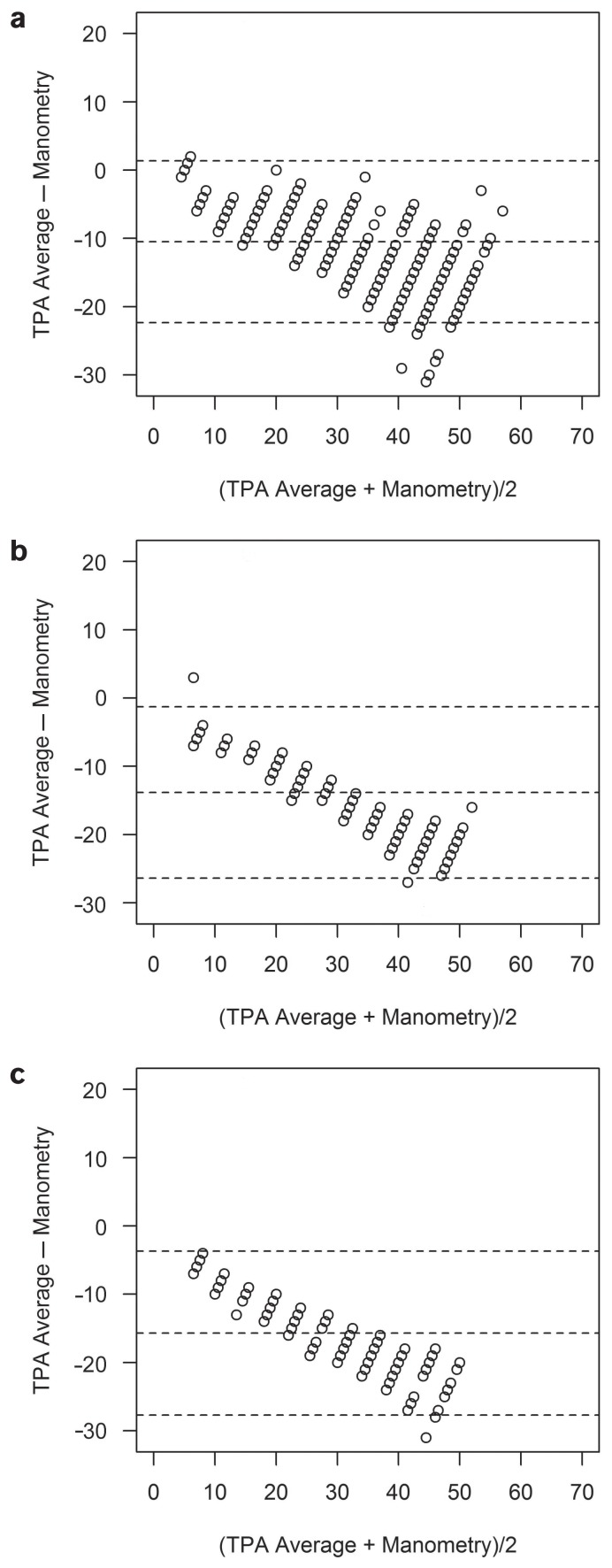
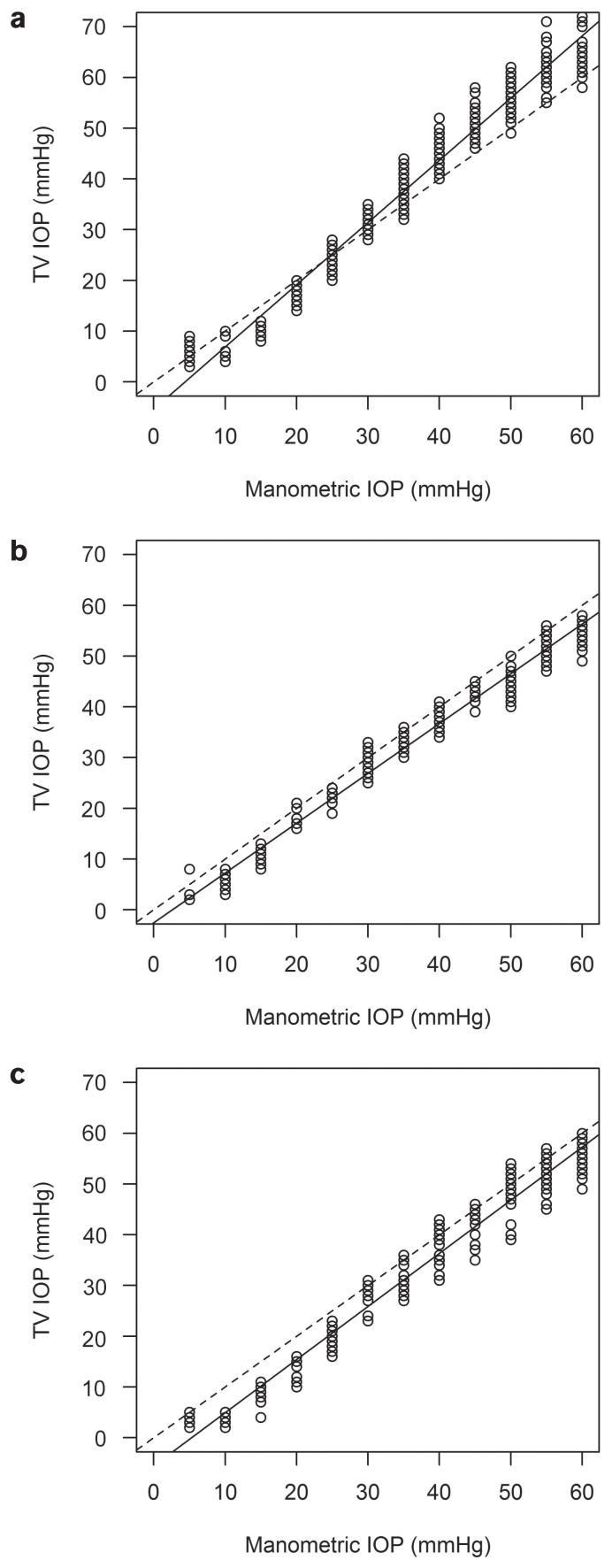
All results from the measurements with the TV and the TPA, except for 1 IOP level in sheep, differed significantly from the manometrically set pressure level. In cattle, the TV underestimated the manometrically determined pressure by 5 to 25 mmHg and overestimated it by 30 to 60 mmHg. The TPA underestimated the pressure throughout the whole range of IOP levels (Figures 1a, 2a, 3a, and 4a). In sheep and goats, both instruments underestimated the manometric pressure by 5 to 60 mmHg (Figures 1b, c; 2b, c; 3b, c; and 4b, c). To correct the measured results, regression formulas were calculated for the 2 tonometers for all 3 species (Table III).
Figure 1.
Bland-Altman plots for cattle (a), sheep (b), and goats (c) showing that the TonoVet tends to underestimate and then overestimate the true intraocular pressure (mmHg).
Figure 2.
Bland-Altman plots for cattle (a), sheep (b), and goats (c) showing that the Tono-Pen AVIA underestimates the true intraocular pressure (mmHg) over the whole pressure range.
Figure 3.
Regression analysis for the TonoVet in cattle (a), sheep (b), and goats (c). Calculated regression line (solid line) and ideal regression line (interrupted line).
Figure 4.
Regression analysis for the Tono-Pen AVIA in cattle (a), sheep (b), and goats (c). Calculated regression line (solid line) and ideal regression line (interrupted line).
Table III.
Calculated regression formulas and corresponding r2 values for 2 tonometers used in cattle, sheep, and goats to correct measured values.
| TonoVet | Tono-Pen AVIA | |
|---|---|---|
| Cattle | y = 1.226× – 5.392 | y = 0.7141× – 0.7864 |
| r2 | 0.98 | 0.92 |
| Sheep | y = 0.9816× – 2.5601 | y = 0.6337× – 1.1840 |
| r2 | 0.98 | 0.98 |
| Goats | y = 1.047× – 5.0551 | y = 0.6476× – 3.0905 |
| r2 | 0.97 | 0.97 |
Discussion
An adequate interpretation of IOP values requires reference values for the particular measuring device and the relevant species. Tonometers therefore need to be calibrated for use in different species, as ocular anatomy varies among different animal species.
Our IOP readings in cattle using the TV were almost consistent with the values obtained in a previous study using the Mackay-Marg tonometer (19) and were slightly lower than those reported in a study using the Mackay-Marg tonometer and the Tono-Pen XL (12). The values measured with the TPA in our study were quite similar to those reported in a study using the Perkins handheld tonometer (5). Another study using the Perkins tonometer found a mean IOP (± SD) of 10.6 ± 1.4 mmHg in sheep, which is quite similar to our measurements taken with the TPA (21). Our measurements with the TV revealed slightly higher results, which were comparable to those from a recent study using the Tono-Pen XL (22).
Only 1 study using the Tono-Pen XL and the TV established reference values for IOP measurements in goats (6). The results in that study were quite similar to the values we obtained with the TPA. We found significantly higher values for the IOP in goats measured with the TV. This may be due to differences in breeds, different fixation methods, or diurnal variation. All the cattle and goats in our study were females, but gender is not reported to have a significant influence on IOP in several other species (6,27,28).
In contrast to previous studies in which no differences were detected between the left and right eyes (6,12,14,20,22), we found statistically significant differences between the left and right eyes in cattle and goats, but only for the measurements made with the TV. As the eye measured first was always randomly selected, the order of measuring cannot account for this phenomenon. As this difference between eyes was only detectable in cattle and goats and only with the use of the TV, an examiner-related cause seems unlikely. The reason for these statistically significant differences remains unknown. The difference between eyes is only up to 2 mmHg, however, and may not be of any clinical importance. This difference may no longer be significant in a study with a larger number of animals.
The TV offers 3 settings (“h” for horse, “d” for dog, and “p” for other species) for evaluating the intraocular pressure in different species to account for various globe sizes and anatomic variations. In the clinical part of our study, the “h” setting was initially used in cows (n = 10), as it was assumed that the bovine globe was most similar to the equine globe. As readings without error were obtained in only 2/10 animals, the “d” setting was tried and evaluable readings were obtained in 10/10 animals. Similar issues were discussed in another study, in which it was stated that the most accurate setting for use in cattle is unclear (20). The manometric results of our study showed that the “d” setting can be used for tonometry in cattle, sheep, and goats.
Although glaucoma is rare in ruminants, tonometry is still an important part of the ophthalmic examination. Both instruments (the TV and the TPA) used in this study are handheld devices that can be easily transported and used in mobile food animal practice. Measurements made with the TV appeared to be harder to obtain in cows due to the difficulty of holding their heads in an appropriate position. The TV needs to be held in a perpendicular position to the cornea with the tip parallel to the ground, whereas the TPA can be used independently of the head position.
Comparing the 2 tonometers, we found statistically significant differences in all 3 species. We always measured with the rebound tonometer (TonoVet) first because the measurements can be taken without the use of a topical anesthetic agent. Some authors have reported that a tonographic effect of a rebound tonometer is unlikely, assuming that the order of tonometer application does not affect the IOP results when using a rebound tonometer before an applanation tonometer (11,29).
Another study showed that the Tono-Pen significantly underestimated the pressure in normal, healthy cat eyes in-vivo compared to the Mackay-Marg tonometer (10). Interestingly, with regard to the order in which the instruments are used, different values were obtained with the Tono-Pen when used after the Mackay-Marg tonometer. Furthermore, this study found that both instruments tended to underestimate the pressure in open and closed in-vitro systems in cat eyes compared to direct manometry (10). No significant differences were found between the 2 different applanation tonometers in studies of in-vivo measurements using either the Mackay-Marg or the Tono-Pen XL in horses and cows, respectively (9,12).
Compared with manometry in freshly enucleated eyes, all values from both tonometers, except for 1 using the TPA in sheep eyes, differed significantly from the manometrically set IOP. We excluded any tonographic effect, at least for the manometric study, by immediately adjusting the saline reservoir if pressure changes exceeded 0.1 mmHg.
In general, there were different results regarding the over- or underestimation of rebound tonometers. The underestimation of IOP by 5 to 25 mmHg and overestimation by 30 to 60 mmHg in cattle eyes using the TV in our study is consistent with a previous study in cats (30). In sheep and goat eyes, we found an overall underestimation with the TV. Another study found a good agreement for the TV in enucleated dog eyes throughout the whole pressure range (5 to 80 mmHg), whereas in enucleated horse eyes, the TV significantly underestimated the IOP for pressures greater than 70 mmHg (31). Another study in horses found that the TV tended to slightly overestimate IOP in the clinically relevant pressure range from 10 to 60 mmHg, while underestimating the true IOP for pressure values greater than 70 mmHg (18).
The overall underestimation that occurred when using the TPA in our study is consistent with the findings for applanation tonometers in dogs and cats (11,15). In a calibration study, the TonoPen XL underestimated the true IOP in cows and sheep mainly at high settings (15). In another study, 2 applanation tonometers (the Mackay-Marg and the Tono-Pen) also underestimated IOP significantly compared to direct manometry in enucleated cat eyes in open and closed in-vitro systems (10). In equine eyes, neither the Mackay-Marg nor the Tono-Pen calculated IOP accurately compared to the manometric measurements (9). The Tono-Pen consistently overestimated IOP at lower pressure levels and underestimated IOP at higher pressure levels compared to manometric measurements in the previously mentioned study in cow and sheep eyes (15), whereas other studies in different species found that overall, the true IOP was underestimated (10,11,15).
In all 3 species, the rebound tonometer (TonoVet) provided more accurate results for IOP than the applanation tonometer (Tono-Pen AVIA), which is in agreement with another recent study in cat eyes (8). In contrast to our study, however, the IOP values measured in this study using the TV were consistently slightly higher than the manometrically controlled IOP in the cat eyes (8).
Although we found high r2 values in most cases, it must be recognized that there is a significant difference between the manometrically set IOP and the measured IOP with both tonometers, which is in accordance with a previous study (11). We calculated regression formulas to correct the measured values in order to obtain reliable values. For daily clinical use, a simple correction factor would have been more suitable.
The limitations of our study were the small number of subjects and the fact that only one breed of each species was examined. Furthermore, we did not measure the central corneal thickness, which could be a possible source of error (32,33).
In conclusion, our study established additional reference values for measuring IOP in ruminants. Our results show the importance of calibrating every tonometer for each species. The same type of tonometer should always be used for surveillance of clinical patients. It should also be remembered that applanation tonometers, such as the Tono-Pen AVIA, tend to underestimate the true IOP, especially at higher pressure levels. While the TonoVet (TV) offered much more reliable results, however, it was more difficult to use with cows. In general, both tonometers, TonoVet (TV) and Tono-Pen AVIA (TPA), can be used effectively to assess intraocular pressure in ruminants, using the specific regression formulas.
Acknowledgment
The authors thank Florian Peche (BSc) for support with the statistical analysis of this study.
References
- 1.Samuelson DA. Ophthalmic anatomy. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2013. pp. 39–170. [Google Scholar]
- 2.Gum GG, MacKay EO. Physiology of the eye. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2013. pp. 171–207. [Google Scholar]
- 3.Featherstone HJ, Heinrich CL. Ophthalmic examination and diagnostics, Part 1: The eye examination and diagnostic procedures. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2013. pp. 533–613. [Google Scholar]
- 4.Andrade SF, Cremonezi T, Zachi CA, et al. Evaluation of the Perkins handheld applanation tonometer in the measurement of intraocular pressure in dogs and cats. Vet Ophthamol. 2009;12:277–284. doi: 10.1111/j.1463-5224.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- 5.Andrade SF, Kupper DS, Pinho LF, et al. Evaluation of the Perkins handheld applanation tonometer in horses and cattle. J Vet Sci. 2011;12:171–176. doi: 10.4142/jvs.2011.12.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broadwater JJ, Schorling JJ, Herring IP, Pickett JP. Ophthalmic examination findings in adult pygmy goats (Capra hircus) Vet Ophthalmol. 2007;10:269–273. doi: 10.1111/j.1463-5224.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 7.Priehs DR, Gum GG, Whitley RD, Moore LE. Evaluation of three applanation tonometers in dogs. Am J Vet Res. 1990;51:1547–1550. [PubMed] [Google Scholar]
- 8.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet® rebound tonometer in normal and glaucomatous cats. Vet Ophthalmol. 2013;16:111–118. doi: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller PE, Pickett JP, Majors LJ. Evaluation of two applanation tonometers in horses. Am J Vet Res. 1990;51:935–937. [PubMed] [Google Scholar]
- 10.Miller PE, Pickett JP, Majors LJ, Kurzman ID. Evaluation of two applanation tonometers in cats. Am J Vet Res. 1991;52:1917–1921. [PubMed] [Google Scholar]
- 11.Görig C, Coenen RT, Stades FC, Djajadiningrat-Laanen SC, Boevé MH. Comparison of the use of new handheld tonometers and established applanation tonometers in dogs. Am J Vet Res. 2006;67:134–144. doi: 10.2460/ajvr.67.1.134. [DOI] [PubMed] [Google Scholar]
- 12.Gum GG, Gelatt KN, Miller DN, MacKay EO. Intraocular pressure in normal dairy cattle. Vet Ophthalmol. 1998;1:159–161. doi: 10.1046/j.1463-5224.1998.00017.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalesnykas G, Uusitalo H. Comparison of simultaneous readings of intraocular pressure in rabbits using Perkins handheld, Tono-Pen XL, and TonoVet tonometers. Graefes Arch Clin Exp Ophthalmol. 2007;245:761–762. doi: 10.1007/s00417-006-0470-8. [DOI] [PubMed] [Google Scholar]
- 14.Leiva M, Naranjo C, Peña MT. Comparison of the rebound tonometer (ICare) to the applanation tonometer (Tonopen XL) in normotensive dogs. Vet Ophthalmol. 2006;9:17–21. doi: 10.1111/j.1463-5224.2005.00429.x. [DOI] [PubMed] [Google Scholar]
- 15.Passaglia CL, Guo X, Chen J, Troy JB. Tono-Pen XL calibration curves for cats, cows and sheep. Vet Ophthalmol. 2004;7:261–264. doi: 10.1111/j.1463-5224.2004.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- 17.Snyder KC, Lewin AC, Mans C, McLellan GJ. Tonometer validation and intraocular pressure reference values in the normal chinchilla (Chinchilla lanigera) Vet Ophthalmol. 2017 doi: 10.1111/vop.12468. [DOI] [PubMed] [Google Scholar]
- 18.Güse J. Intraokulare Druckmessung am Pferdeauge mittels Tonovet® versus in-vitro Manometrie [dissertation] Hannover, Germany: University of Veterinary Medicine Hannover, Foundation; 2008. [Google Scholar]
- 19.Kotani T. Which are the optimal tonometers for different animal species? Anim Eye Res. 1993;12:55–61. [Google Scholar]
- 20.Tofflemire KL, Whitley EM, Gould SA, et al. Schirmer tear test I and rebound tonometry findings in healthy calves. Vet Ophthalmol. 2015;18:147–151. doi: 10.1111/vop.12165. [DOI] [PubMed] [Google Scholar]
- 21.Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Invest Ophthalmol Vis Sci. 2009;50:669–673. doi: 10.1167/iovs.08-2410. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro AP, Crivelaro RM, Teixeira PP, et al. Effects of different mydriatics on intraocular pressure, pupil diameter, and ruminal and intestinal motility in healthy sheep. Vet Ophthalmol. 2014;17:397–402. doi: 10.1111/vop.12121. [DOI] [PubMed] [Google Scholar]
- 23.Mertel L, Cammarata G, Magni R, Brooks D, Samuelson D. Clinical and pathological study of a cow with chronic glaucoma. Vet Comp Ophthalmol. 1996;6:18–26. [Google Scholar]
- 24.Sarma B, Pathak SC, Saikia J. Incidence of eye diseases in bovine in Assam, India. Indian Vet J. 1990;14:98–101. [Google Scholar]
- 25.Pearce J, Moore C. Food animal ophthalmology. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2013. pp. 1610–1674. [Google Scholar]
- 26.Gerometta R, Podos SM, Candia OA, et al. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004;122:1492–1497. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- 27.Willis AM, Anderson DE, Gemensky AJ, Wilkie DA, Silveira F. Evaluation of intraocular pressure in eyes of clinically normal llamas and alpacas. Am J Vet Res. 2000;61:1542–1544. doi: 10.2460/ajvr.2000.61.1542. [DOI] [PubMed] [Google Scholar]
- 28.Gelatt KN, MacKay EO. Distribution of intraocular pressure in dogs. Vet Ophthalmol. 1998;1:109–114. doi: 10.1046/j.1463-5224.1998.00024.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldblum D, Kontiola AI, Mittag T, Chen B, Danias J. Noninvasive determination of intraocular pressure in the rat eye. Comparison of an electronic tonometer (TonoPen), and a rebound (impact probe) tonometer. Graefes Arch Clin Exp Ophthalmol. 2002;240:942–946. doi: 10.1007/s00417-002-0571-y. [DOI] [PubMed] [Google Scholar]
- 30.Rusanen E, Florin M, Hässig M, Speiss BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Vet Ophthalmol. 2010;13:31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 31.Knollinger AM, La Croix NC, Barrett PM, Miller PE. Evaluation of a rebound tonometer for measuring intraocular pressure in dogs and horses. J Am Vet Med Assoc. 2005;227:244–248. doi: 10.2460/javma.2005.227.244. [DOI] [PubMed] [Google Scholar]
- 32.Goldmann H, Schmidt T. [Applanation tonometry]. Ophthalmologica. 1957;134:221–242. doi: 10.1159/000303213. [Article in German] [DOI] [PubMed] [Google Scholar]
- 33.Goldmann H, Schmidt T. [Further contribution to applanation tonometry]. Ophthalmologica. 1961;141:441–456. doi: 10.1159/000304099. [Article in German] [DOI] [PubMed] [Google Scholar]