Abstract
Tuberculum sella and planum sphenoidale meningiomas pose a management challenge given their intimate relationship to surrounding critical neurovascular structures. The development and advancement of expanded endoscopic transnasal surgery has provided a good surgical option that in well-selected cases, may provide several advantages over a transcranial route. These include early devascularization, complete dura and bone removal, elimination of brain retraction and enhanced visualization of the optic apparatus perforating vessels. The authors review the endoscopic transnasal approach to these tumors and discuss surgical decision-making and case selection, surgical technique and outcomes. We also discuss the expanding role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for these challenging lesions.
KEYWORDS : endonasal, endoscopic, meningioma, planum, stereotactic radiosurgery, transnasal, transphenoidal, tuberculum sella
Practice points.
The differential diagnosis for planum and tuberculum sella extra-axial lesions includes meningioma, hemangiopericytoma, dural-based metastases and inflammatory processes such as sarcoidosis and IgG-4-mediated inflammatory disease.
Thorough neuro-ophthalmological assessment including fundoscopy, objective visual field assessment and optical coherence tomography is necessary to guide treatment decisions.
Observation can be employed as an initial strategy for asymptomatic incidental lesions without documented lesion growth.
Stereotactic radiotherapy has demonstrated reasonably high rates of tumor control over 10 years.
The endoscopic transnasal approach for planum and tuberculum sella meningiomas has demonstrated high rates of vision improvement.
Planum and tuberculum sella meningiomas with extension lateral to the optic nerve are best treated via a transcranial approach.
Background
Meningiomas of the planum sphenoidale and tuberculum sella pose a significant surgical challenge due to their intimate relationship with surrounding critical neurovascular structures. These include the optic nerves and chiasm, the internal carotid arteries, the anterior cerebral and posterior communicating arteries and their perforating branches, and the infundibulum and pituitary gland. Traditionally, tumors in these locations have been exclusively resected via a transcranial microscopic route (pterional, orbitozygomatic, supraorbital or transbasal) [1–5]. More recently, with the development of expanded endoscopic transnasal approaches, there has been significant interest in removing these lesions via a transnasal route [6–9]. Regardless of surgical route, the surgical goals remain the same: preservation or restoration of visual and neurologic function while achieving safe removal of the entire tumor with a margin around the area of dural involvement if possible. In addition, the basic principles of meningioma resection apply to either approach: adequate exposure of tumor and dural tail, early devascularization, central debulking and careful extracapsular dissection. In this review article, we discuss the endoscopic transnasal approach for planum and tuberculum sella meningiomas, including the rationale, case selection, surgical technique and outcomes. We also present illustrative cases and discuss the role of upfront or adjuvant stereotactic radiation.
Establishing a rationale for the transnasal approach
The transnasal route offers several distinct advantages over the transcranial route. First, it provides direct access to the tumor and its blood supply. Thorough and early devascularization allows for a clean surgical field in which to dissect the tumor–arachnoid interface. Second, the surgical approach necessitates complete removal of bone and dura, which can sometimes be more challenging to accomplish from a transcranial approach. It is well established that recurrence rates are directly correlated with the extent of removal of involved dura and hyperostotic bone [10–12]. Thus, the endoscopic transnasal approach may have an oncologic benefit in properly selected tumors. Second, because the surgical trajectory falls directly on the tumor, the transnasal route obviates the need for brain retraction, which may have a neurological and/or neuropsychological benefit and reduce the risk of postoperative seizures. Finally, the transnasal route provides unparalleled visualization of the microvasculature of the inferior and medial aspects of the optic nerves, chiasm, pituitary gland and infundibulum and therefore, affords the opportunity for meticulous dissection of tumor with better preservation of these structures.
Case selection: transnasal versus transcranial
Selecting the optimal approach requires careful study of preoperative films to identify key anatomical or pathological factors. Based on the authors’ experience, there are a few absolute and relative contraindications for the endoscopic endonasal approach to parasellar meningiomas (Table 1). The primary pathological determinant is the lateral extent of the tumor in relation to the optic nerve (Figure 1). Tumor medial and inferior to the optic nerve is better visualized and accessed from a transnasal rather than transcranial perspective. While tumor superior to the optic nerve can be accessed by the transnasal route, there is poor visualization of the superior surface of the nerve and its microvasculature and therefore, tumor in this location is more safely removed via a transcranial approach. Tumor lateral to the optic nerve is not accessible via a transnasal route. Preoperative imaging must be carefully studied to determine the lateral extent of the tumor and dural tail. The second determinant is the extent of tumor into the sella. Tumor in the sella can be accessed via either route, but is more easily accessed via a transnasal route. One absolute determinant favoring a transnasal route is sellar tumor in the setting of a prefixed optic chiasm, which would make transcranial sellar access difficult. The third determinant is the relationship of the tumor with the anterior cerebral arteries (ACAs). In the author's experience, tumor abutting the anterior cerebral arteries can be safely approached via either approach (Figure 2). The one relative indication for a transcranial approach is involvement of the ACAs in the setting of a history of prior radiation, which may obliterate the arachnoidal plane or alter the integrity of the vasculature. An absolute indication for a transcranial route is complete ACA encasement; whereas partial ACA encasement, particularly with a spinal fluid ‘cuff’ around the vessels may still be amenable to either approach. The final absolute indication for a transcranial approach is ‘kissing’ carotids. This is an anatomical variation in which the clinoidal segments of the carotid arteries course medially and nearly or completely touch, significantly narrowing the intercarotid distance. Tumors with ventral extension to the cribiform region may present a relative indication for a transcranial approach if olfaction is normal preoperatively. Finally, heavily calcified parasellar tumors may also present a relative indication for a transcranial approach, but can be extremely challenging by any surgical approach. However, seemingly calcified tumors are sometimes psammomatous meningiomas that have a soft consistency despite their calcified appearance (Figure 2). Occasionally, giant lesions with significant extension into both the intracranial compartment and nasal cavity may require a combined staged approach using both approaches. In these cases, a transcranial route is usually performed first to decompress any compression on the brain or optic nerve(s), followed by an endoscopic transnasal approach to address the nasal component of the tumor.
Table 1. . Anatomical and pathological factors determining optimal surgical approach.
| Transcranial | Transnasal |
|---|---|
| Absolute indication | |
| • Extension lateral to optic nerve | • Sellar extension with prefixed optic chiasm |
| • ACA encasement | |
| • Kissing carotids | |
| Relative indication | |
| • Abuts ACA with history of radiation | • Tumor inferior to ipsilateral optic nerve |
| • Ventral extension to cribiform | |
ACA: Anterior cerebral artery.
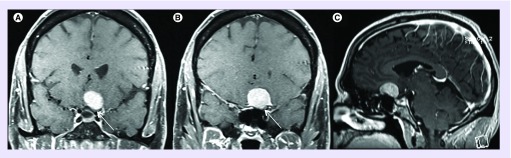
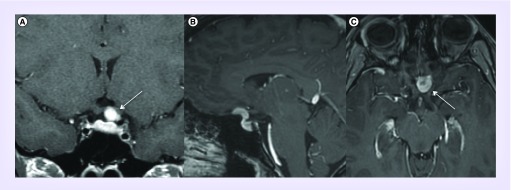
Figure 1. . Preoperative postcontrast T1 coronal (A & B) and sagittal (C) MRI demonstrating a planum meningioma.
Note: Tumor extension lateral to the optic nerve (white arrow). This tumor was resected via a left frontotemporal craniotomy with extradural clinoidectomy and optic nerve unroofing.
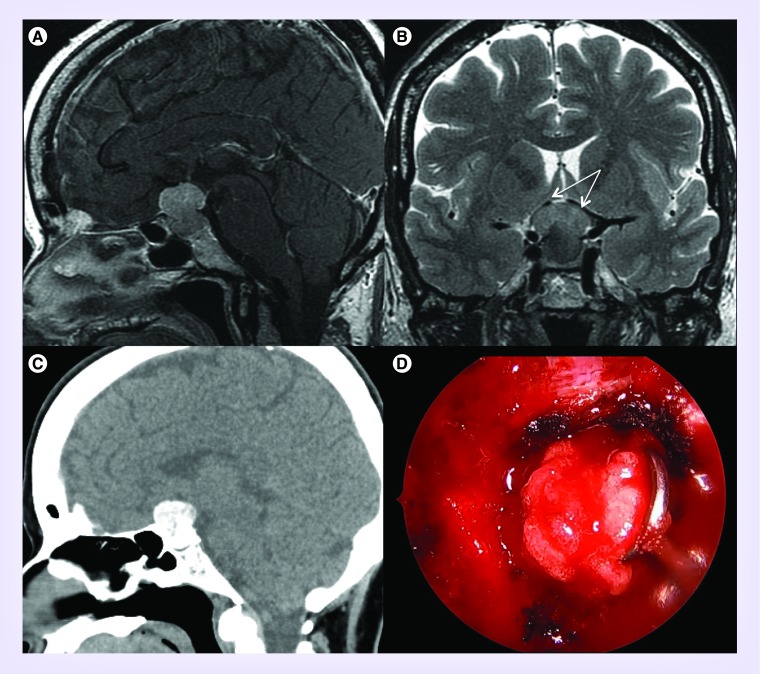
Figure 2. . Example of calcified meningioma.
Preoperative sagittal T1 with contrast (A) and coronal T2 (B) MRI demonstrating dumbbell-shaped diaphragmatic meningioma with tumor abutting and displacing bilateral anterior cererbral arteries (white arrows). (C) CT scan without contrast demonstrates significant calcifications within the lesion. (D) Intraoperative endoscopic transnasal view demonstrates mobilization of soft tumor with gritty appearance consistent with psammomatous meningioma with microcalcifications.
Endoscopic transnasal surgical technique
• Positioning
After induction of general anesthetic, the patient is positioned supine with the head in neutral position on a foam donut. Stereotactic navigation is used in all cases. Use of mask registration or alternative device can obviate the need to place the head in rigid fixation. An oral right angle endotracheal tube is used to clear the surgical space. As the transnasal procedure is a clean contaminated surgical procedure, no specific skin or nasal surgical prep is used. A small gel pad is placed underneath the thigh of the leg on the opposite side to the scrub nurse. A linear incision is marked approximately halfway between the greater trochanter of the hip and lateral epidcondyle of the knee along the intermuscular septum. After surgical prep and draping, the fascia lata is harvested with a separate set of sterile instruments and the incision is closed immediately. A first-generation cephalosporin (vancomycin is used if a penicillin allergy is present) is administered prior to skin incision. A dose of dexamethasone (10 mg) is administered for tumors compressing the optic nerve/chiasm. Routine use of lumbar drain is unnecessary.
• Nasal approach
Topical 4% cocaine or oxymetazoline soaked cotton pledgets are placed into each naris and 1% lidocaine with 1:100,000 epinephrine is injected into the nasal mucosa with particular attention to the septum on the side of planned nasoseptal flap harvest. Bilateral inferior and middle turbinates are lateralized. The natural ostium of the sphenoid sinus is identified bilaterally. A pedicled nasoseptal flap is harvested via previously described techniques [13]. Bilateral sphenoidotomies are performed with particular attention to preserving the vascular pedicle to the contralateral nasoseptal flap. A small posterior septectomy (˜10–15 mm) is made for binarial access to the sphenoid sinus. Bilateral posterior ethmoidectomies are performed to expose the planum sphenoidale. Mucosa of the sphenoid sinus is stripped to avoid postoperative mucocele formation underneath the final nasoseptal flap repair. In addition, the inferior rostrum of the sphenoid sinus and sphenoid sinus septations are drilled down to eliminate the potential for deadspace between the nasoseptal flap and sellar and clival bone.
• Bony exposure
Key anatomical landmarks including the planum sphenoidale, tuberculum sella, sella, lateral optico-carotid recesses, optic canals and clinoidal carotid protruberances are identified (Figure 3). The bone over the mid and upper sella is removed with a diamond burr and kerrison rongeurs. Next, the bone of the tuberculum sella and planum are removed. Unlike with pituitary adenomas, the bone in meningiomas is often vascular and hyperostotic. The use of a diamond burr also helps with hemostasis. The amount of planum bone removed depends on the anterior extent of the dural tail present on preoperative imaging. The degree of tumor extension into the optic canal dictates the amount of bone removed over the optic nerve (Figure 3). Note that the lateral optico-carotid recess approximates the location of the intracanalicular segment of the optic nerve. Copious irrigation is used during drilling of the optic canal to prevent any thermal injury to the nerve or its microvasculature. Finally, a key to unlocking wide access into the optic-carotid cistern is removal of the lateral strut of the tuberculum sella, which corresponds anatomically to the medial optico-carotid recess (a true recess is frequently not present) (Figure 3). During this step, stereotactic navigation and a microdoppler are useful adjuncts in locating and unroofing the carotid artery in this location just proximal to the distal dural ring (Figure 3).
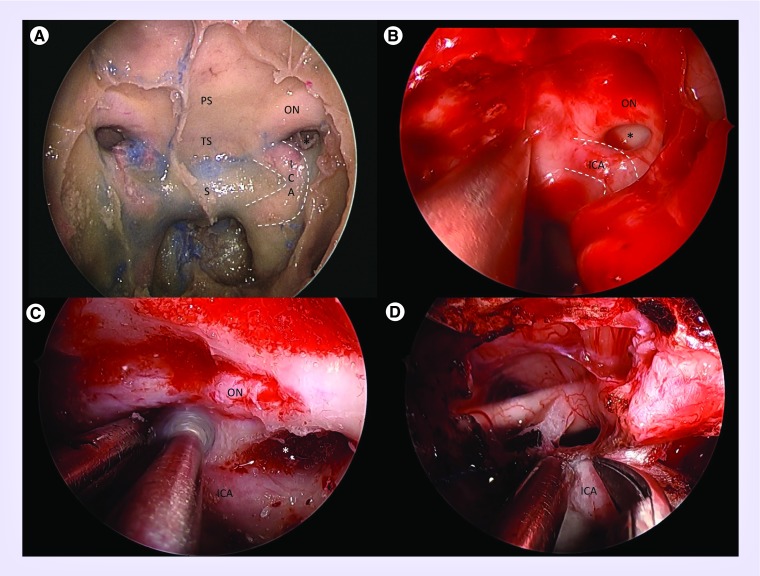
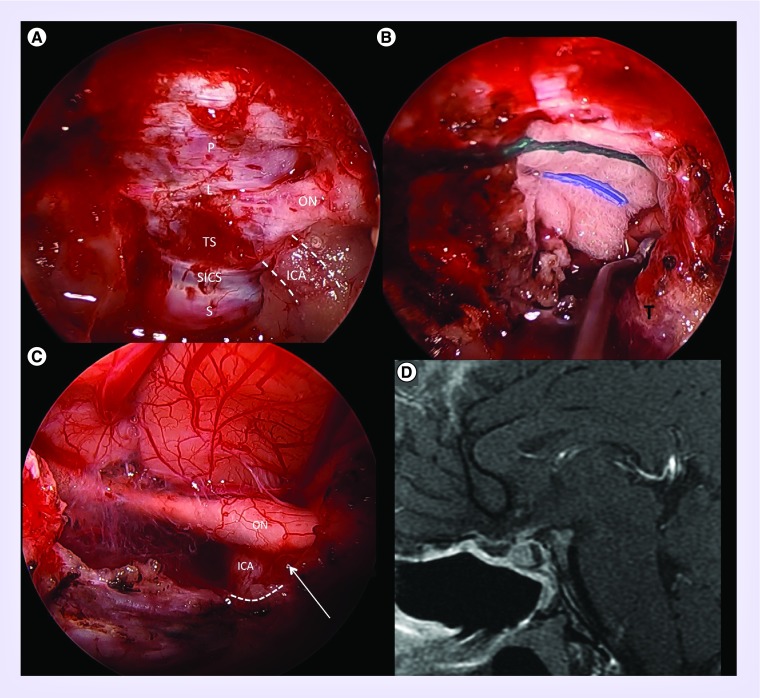
Figure 3. . Anatomy and operative exposure.
(A) Cadaveric dissection demonstrating endoscopic transnasal transphenoidal exposure and anatomy. Bony anatomy includes the sella (S), tuberculum sella (TS), planum sphenoidale (PS) and lateral opticocarotid recess (*), the latter of which separates the optic nerve (ON) and internal carotid artery (ICA; outlined in dashed white lines). (B) Similar intraoperative view demonstrating left opticocarotid recess (*) with surrounding ON and ICA (dashed white lines). (C) The left optic nerve has been unroofed and the lateral tubercular strut is being drilled to maximize exposure of the opticocarotid region. (D) Intraoperative anatomy after resection of tubeculum sella meningioma demonstrating removal of the last portion of dura abutting the distal dural ring of the ICA.
• Tumor resection
Tumor resection follows the same general principles of microsurgery and meningioma resection. The dura is coagulated generously to devascularize the meningioma. The authors prefer to limit the dural opening to just over the central aspect of the tumor and begin aggressive internal debulking via suction, microscissors, curettes, ultrasonic aspirator or micro-debrider as needed depending on the tumor consistency. Limiting the dural opening prior to debulking preserves the tumor attachment to the dura, which can limit transmission of dissection forces to surrounding critical neurological structures, such as the optic system, during debulking. After internal debulking, the dura is incised circumferentially around the area of tumor involvement allowing access to the intradural surface of the tumor. Finally, bimanual extracapsular arachnoid dissection is performed under direct endoscopic visualization and the remainder of the tumor is removed en bloc or in large piecemeal fashion depending on the tumor consistency. Particular attention is directed toward careful dissection and prudent judgment around the optic nerve and its microvasculature, the anterior cerebral arteries superiorly and the posterior communicating arteries laterally.
• Reconstruction
Measurements of the dural defect are taken and used to create an appropriately sized bilayer fascia lata inlay/onlay ‘button’ graft as we have previously described [14]. Sutures are placed at the four corners of the two layers and at a distance to approximate the size of the dural defect in order to hold the two layers against the dura in a stable configuration. The larger inlay is tucked underneath the dural defect and the onlay is placed over the defect (Figure 4). The ‘button’ graft doubles the surface area for dural healing and can conform to irregular and multiplanar defects. When a reasonable seal has been obtained, the graft will transmit normal dural pulsations. The nasoseptal flap is then placed over the defect and a cottonoid is used to guide the surface of the flap down over the cranial base defect and maximize surface contact by eliminating any dead space underneath the flap. Finally, polyethylene glycol (PEG) hydrogel glue is applied to secure the edges of the nasoseptal flap, followed by an absorbable packing to reinforce the center of the flap. After the middle turbinates are medialized, absorbable packing is placed in the middle meatus and absorbable gelatin film is placed between the inferior turbinate and septum to avoid scarring and nasal obstruction. Postoperatively, the head of bed is kept elevated and straining is avoided. The patients are encouraged to ambulate as soon as possible.
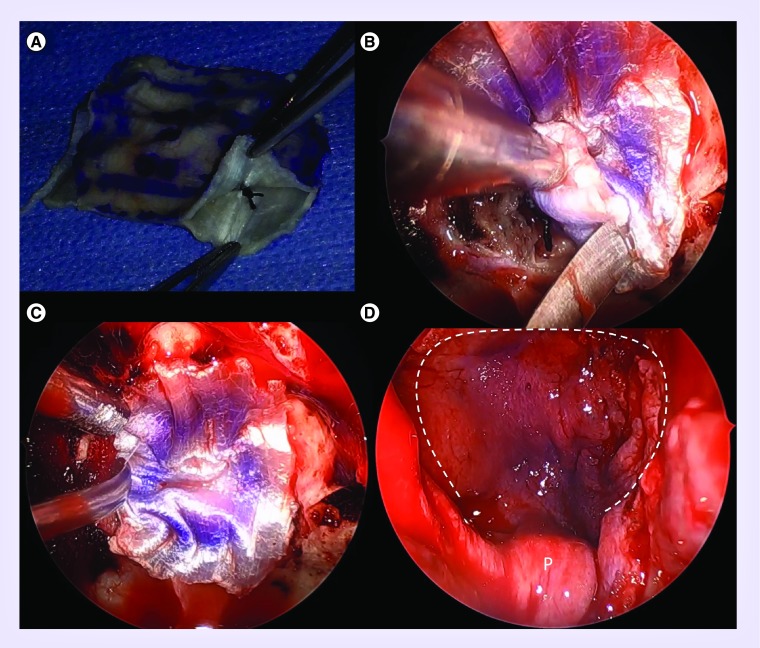
Figure 4. . Skull base reconstruction.
(A) Fascia lata inlay/onlay ‘button’ graft. The two layers are sutured together. Four sutures are placed to approximate the size of the dural defect. (B) Intraoperative view after inlay has been tucked into the subdural space, with button graft suture abutting the dural defect. (C) The onlay portion of the button graft is laid epidurally. (D) The vascularized nasoseptal flap (white dashed line) is placed over the button graft while ensuring the pedicle (P) is not twisted or kinked.
Significant practice variations exist in materials and techniques for dural reconstruction. Materials used include autologous (e.g., fascia lata, fat graft) and synthetic (e.g., collagen matrix, acellcular dermis) and may be fashioned as inlay (i.e., subdural) and/or onlay (i.e., epidural). In addition, some centers may use a rigid buttress using local septal bone or synthetic materials (e.g., porous polyethylene) to help create a watertight seal. Regardless of dural reconstructive technique, most centers agree that a vascularized nasoseptal flap should be used. In addition to reconstructive techniques, there is considerable variation in antibiotic use ranging from 24 h of a first-generation cephalosporin to an extended course of broad-spectrum antibiotics. Future studies are needed to determine the optimal practice.
Case illustrations
• Case #1
A 26-year-old female initially presented to another physician with galactorrhea 4 years ago and was found to have an elevated prolactin level. She had an MRI demonstrating a Rathke's cleft cyst. She was briefly treated with cabergoline and follow-up prolactin levels were normal after stopping the medication. A follow-up MRI 4 years later demonstrated a new 13 × 12 × 6 mm tuberculum sella meningioma with compression of the left optic nerve (Figure 5). She had some subjective visual aura-type symptoms, but objective testing revealed normal vision with full visual fields. Because of her young age and documented growth over 4 years, she was offered surgical resection. She underwent an endoscopic transnasal transtuberculum transplanum approach for resection of the meningioma. Wide exposure was obtained by unroofing the left optic canal and removing the lateral tubercular strut to expose the internal carotid artery (Figure 6). Gross total resection of the tumor was obtained. Reconstruction was performed using fascia lata button reconstruction and nasoseptal flap as described in the surgical technique section above. Pathology revealed WHO grade I meningioma with Ki-67 index of 4.8%. Patient had transient nonspecific vision blurriness in the left eye postoperatively, which resolved in follow-up. She had no endocrinopathy before or after surgery.
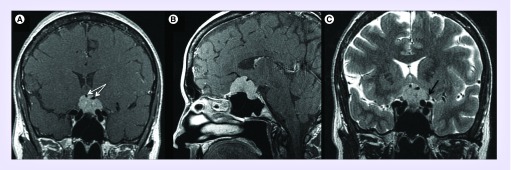
Figure 5. . Preoperative coronal (A), sagittal (B), axial (C) MRI T1 with gadolinium sequences, demonstrating a 13 × 12 × 6 mm tuberculum sella meningioma with compression of the left optic nerve (white arrow).
Figure 6. . Intraoperative views.
(A) Exposure after bone removal demonstrating planum (P), limbus (L), dura under tuberculum sella (TS), superior intercavernous sinus (SICS) and dura of the sella (S). Because the tumor was left eccentric and compressing the left optic nerve, bony decompression of the left optic nerve (ON) and exposure of the distal clinoidal internal carotid artery (ICA; white dash lines) were performed. (B) Tumor (T) dissection off the left optic nerve. (C) Gross total resection with visualization of the left optic nerve (ON), ophthalmic segment internal carotid artery (ICA) and take off of the ophthalmic artery (white arrow). Note dura was resected to the distal dural ring (white dotted line) for maximal dural resection. (D) A 3-month postoperative MRI demonstrating gross total resection and vascularized nasoseptal flap well apposed to the anterior skull base.
• Case #2
A 52-year-old healthy female presented with several months of left eye blurriness. On examination, she was found to have a left-sided superotemporal arcuate field cut, visual acuity 20/30, relative afferent pupillary defect and decreased color vision (3/11 Ishihara color plates). Vision in the right eye was normal. MRI revealed a 2.8 × 2.4 × 1.7 cm homogenously enhancing tumor based on the planum sphenoidale and tuberculum sella with significant compression of the left optic nerve and partial encasement of the anterior cerebral arteries (Figure 7). Both transcranial and endoscopic transnasal options were discussed with the patient. Because of 270 encasement of the anterior cerebral arteries, a transcranial route was considered, but the patient had strong preference to attempt resection from a transnasal route.
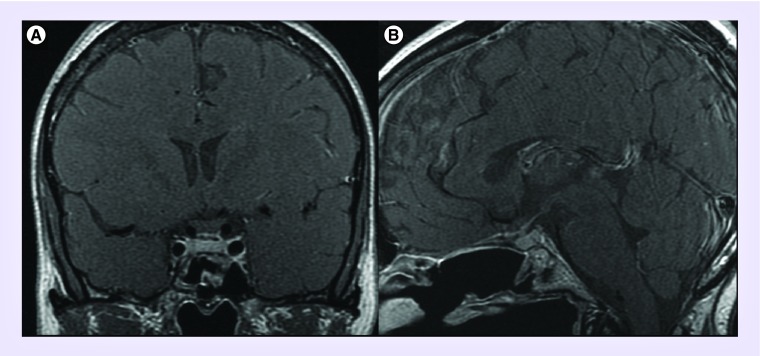
Figure 7. . Preoperative coronal (A) and sagittal (B) MRI, T1 with gadolinium and coronal T2 (C) demonstrating 2.8 × 2.4 × 1.7 cm planum and tuberculum sella meningioma.
Note: 270 encasement of anterior cerebral arteries (white arrows), extension superior and lateral to left internal carotid artery and significant compression of left optic nerve (black arrow).
She underwent an endoscopic endonasal transplanum, transtuberculum approach for resection of the lesion with fascia lata button dural reconstruction and nasoseptal flap. Gross total resection was achieved without complications (Figure 8). Pathology revealed a WHO grade I meningioma with Ki-67 index 2.4%. In postoperative follow-up, her vision in the left eye normalized and her right eye vision remained intact. She was symptomatically hypocortisolemic postoperatively and put on steroid replacement, but was eventually taken off steroids after retesting 6 weeks after surgery. The vision remains normal and there is no evidence of residual or recurrent tumor after 18 months follow-up.
Figure 8. . Postoperative MRI with coronal (A) and sagittal (B) T1 with gadolinium sequences, demonstrating gross total resection, re-expansion of the pituitary gland and nasoseptal flap reconstruction.
Surgical outcomes
Given the relatively more recent use of the endoscopic transnasal approach for planum sphenoidale and tuberculum sella meningiomas, there is currently a paucity of data on surgical outcomes. A systematic review in 2012 captured all endoscopic cases reported up until 2010 [15]. A total of 93 patients were included in the endoscopic transnasal cohort. In these early cases, gross total resection was achieved in 74.7% of cases. Visual outcome was improved in 69.1%, stable in 18.2% and worse in 12.7% [15]. The incidence of postoperative cerebrospinal fluid (CSF) leak was high (21.3%). In this report, the benefits of the microsurgical transcranial approach included higher rates of gross total resection (84.1 vs 74.7%; p = 0.04) and lower risk of CSF leak (4.3 vs 21.3%; p < 0.001). Risk of meningitis was similar (0.2 open vs 0% endoscopic) [15]. A recent large case series of 75 patients undergoing endoscopic endonasal resection of suprasellar meningiomas was published in 2014 [7]. Among 56 patients with preoperative visual disturbance and postoperative follow-up, 85.7% demonstrated improvement in vision after surgery and only two patients (3.6%) experienced worsening vision. Gross total resection was achieved in 76%. Postoperative CSF leaks were reported in 25.3% of patients, but decreased significantly after the introduction of vascularized nasoseptal flaps.
Stereotactic radiotherapy
Conventional external beam radiotherapy of parasellar meningiomas can achieve favorable local control of tumor. Though it is well tolerated, it may cause hypopituitarism, neurocognitive side effects or the development of secondary brain tumors [16]. The development of stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (FSRT) has allowed the treatment of patients with greater conformality, less complications and potential better tumor control than conventional radiotherapy. Radiation generally does not reduce the meningioma size quickly and often not at all, therefore it is most appropriate for patients with minimal or no neurological symptoms from compression. With increasing utilization and improved imaging quality, more small and asymptomatic parasellar meningiomas are being diagnosed. This has afforded the option of employing stereotactic radiation as an upfront definitive treatment in select cases of progressively enlarging tumors before they become symptomatic. In addition, stereotactic radiation is commonly used as an adjuvant therapy after surgical debulking or for recurrent tumors after initial gross total resection [17].
There is a paucity of data on long-term outcomes after SRS for meningiomas near the anterior optic pathway. In a series of 972 patients with meningiomas in diverse locations treated with gamma knife radiosurgery (GKRS), the reported tumor control rates were 93% at 5 years and 87% at both 10 and 15 years [18]. Patients in this series received a median tumor margin dose of 13 Gy [18]. A multicenter study from the North American Gamma Knife Consortium presented 763 cases of sellar and parasellar meningiomas treated by GKRS [17]. In total, 355 (50.7%) patients had surgery prior to GKRS. The median tumor margin dose was 13 Gy and maximum dose to the optic apparatus varied between 8 and 12 Gy. Median follow-up after GKRS was 66.7 months. Tumor progression was seen in 74 (9.8%) patients. Actuarial progression-free survival rates at 3, 5, 8 and 10 years were 98, 95, 88 and 82% respectively. However, new or worsening cranial nerve deficits were observed in 9.6% of patients. Optic (3%) and trigeminal (4.7%) nerves were the most commonly affected. Out of the patients with new cranial nerve deficits, 75.5% did not have an increase in tumor volume. Functional improvements in cranial nerves, especially in trigeminal and abducens nerves, were observed in 34% of patients with pre-existing deficits. In the follow-up provided, there were no radiation-induced neoplasms, symptomatic carotid artery occlusions or treatment-related strokes reported.
The primary concern of single-fraction stereotactic radiosurgery (SRS) for anterior cranial base meningiomas is the radiation tolerance of the anterior visual pathway and potential for delayed endocrinopathy. Early studies suggested that the risk of radiation-induced optic neuropathy (RION) is increased if the radiation doses to the optic nerves and chiasm exceeds 8 Gy, but more recent studies have shown that radiation doses of 10–12 Gy to small volumes of the optic apparatus are well tolerated and have a low risk of RION [19]. If there is a conflict between adequate tumor dose and protection of the visual pathway, one may either lower the dose to the entire tumor or lower the dose to the portion of the tumor adjacent to the visual pathway [20]. In a large series of radiosurgery for parasellar meningiomas, factors associated with better tumor control included higher tumor margin dose and smaller tumor volume. In addition, risk factors for cranial neuropathy included larger pretreatment tumor volume, tumor adjacent to optic apparatus, higher tumor margin dose, tumor progression and longer follow-up [20].
Fractionated stereotactic radiotherapy has the radiation dose distribution advantages of SRS and radiobiological advantages of conventional fractionated radiotherapy. FSRT is particularly suitable for lesions located in or near critical anatomic structures, like the optic apparatus [16]. The tolerance of the optic apparatus, brainstem and other cranial nerves to FSRT is higher than the radiation dose needed to treat the meningioma. Thus, FSRT should be routinely used for meningiomas for which treatment with SRS would result in unacceptable risk of severe long-term damage. Moreover, normal tissue tolerance also limits SRS targets with a maximum diameter of 3–4 cm. Tumors exceeding the limits of SRS can be effectively and safely treated with FSRT. The results of FSRT appear encouraging in regards to both local control and morbidity. A series of 136 patients with skull base meningiomas treated with FSRT demonstrated an overall progression-free survival of 96.9% after 3 years, 93.8% after 5 years and 91.5% after 10 years [21]. Another series of 318 patients with intracranial meningiomas treated with FSRT demonstrated local control, overall survival and cause-specific survival at 5 years of 92.9, 88.7 and 97.2%, and at 10 years they were 87.5, 74.1 and 97.2%, respectively. There were no new neurologic deficits or treatment-related mortalities encountered [22]. These favorable results warrant long-term follow-up and prospective studies.
With the advancements in radiotherapy techniques, over the last two decades, there has been a shift in approach from aggressive radical resection to maximal safe resection. Therefore, if there are components of tumor that are significantly adherent or scarred to critical neurovascular structures (e.g., internal carotid artery), small residual tumor may be left and either observed for growth or treated upfront with various radiotherapy modalities.
Conclusion
The endoscopic transnasal approach to planum sphenoidale and tuberculum sella meningiomas may offer several advantages in well-selected cases including early devascularization, complete dura and bone removal, elimination of brain retraction and enhanced visualization of the optic apparatus perforating vessels. In order to select the optimal surgical approach, preoperative imaging must be carefully evaluated with particular attention directed toward the relationship of the tumor to the optic nerve, internal carotid arteries, anterior cerebral arteries and extension into the sella, High rates of gross total resection and vision improvement can be achieved with this approach. Finally, upfront or adjuvant stereotactic radiotherapy can provide good tumor control in well-selected cases.
Future perspective
The introduction of the endoscopic transnasal approach has significantly altered our surgical decision-making of planum and tuberculum sella meningiomas. Initial criticism of this approach was that while visualization was superior, the ability to effectively perform expanded approaches through the nose was limited. However, over the last decade, there have been significant technological advancements in instrumentation to improve our ability to work in small corridors. Future advancements in technology, particularly in the area of surgical robotics may expand our maneuverability in the transnasal approach. In addition, additional surgical experience and accompanying literature will allow us to determine the optimal instrumentation, reconstructive techniques and perioperative care for these patients. Finally, further advancements in the genetic analysis of meningiomas may open up the possibility of targeted molecular therapy for difficult or recurrent tumors.
Footnotes
Financial & competing interests disclosure
JJ Evans receives royalties from Mizuho for the development of surgical instruments and also is a consultant with Stryker for new technology. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this special report.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bassiouni H, Asgari S, Stolke D. Tuberculum sellae meningiomas: functional outcome in a consecutive series treated microsurgically. Surg. Neurol. 2006;66(1):37–44. doi: 10.1016/j.surneu.2005.11.059. discussion 44–35. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Muzumdar D, Desai KI. Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients. Neurosurgery. 2002;51(6):1358–1363. discussion 1363–1354. [PubMed] [Google Scholar]
- 3.Nakamura M, Roser F, Struck M, Vorkapic P, Samii M. Tuberculum sellae meningiomas: clinical outcome considering different surgical approaches. Neurosurgery. 2006;59(5):1019–1028. doi: 10.1227/01.NEU.0000245600.92322.06. discussion 1028–1019. [DOI] [PubMed] [Google Scholar]
- 4.Schick U, Hassler W. Surgical management of tuberculum sellae meningiomas: involvement of the optic canal and visual outcome. J. Neurol. Neurosurg. Psychiatry. 2005;76(7):977–983. doi: 10.1136/jnnp.2004.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatemi N, Dusick JR, De Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64(5 Suppl. 2):269–284. doi: 10.1227/01.NEU.0000327857.22221.53. discussion 284–266. [DOI] [PubMed] [Google Scholar]
- 6.De Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2008;62(6 Suppl. 3):1192–1201. doi: 10.1227/01.neu.0000333785.04435.2c. [DOI] [PubMed] [Google Scholar]
- 7.Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients. J. Neurosurg. 2014;120(6):1326–1339. doi: 10.3171/2014.2.JNS13767. [DOI] [PubMed] [Google Scholar]; •• Largest endoscopic endonasal surgical outcomes series.
- 8.Liu JK, Christiano LD, Patel SK, Tubbs RS, Eloy JA. Surgical nuances for removal of tuberculum sellae meningiomas with optic canal involvement using the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg. Focus. 2011;30(5):E2. doi: 10.3171/2011.3.FOCUS115. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Lu XJ, Ji WY, et al. Visual outcome after extended endoscopic endonasal transsphenoidal surgery for tuberculum sellae meningiomas. World Neurosurg. 2010;73(6):694–700. doi: 10.1016/j.wneu.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson Grade I resection as the goal: clinical article. J. Neurosurg. 2012;117(6):999–1006. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]
- 11.Morokoff AP, Zauberman J, Black PM. Surgery for convexity meningiomas. Neurosurgery. 2008;63(3):427–433. doi: 10.1227/01.NEU.0000310692.80289.28. discussion 433–424. [DOI] [PubMed] [Google Scholar]
- 12.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry. 1957;20(1):22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Original paper describing extent of resection with long-term recurrence rates.
- 13.Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 14.Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120(5):876–880. doi: 10.1002/lary.20861. [DOI] [PubMed] [Google Scholar]; • Description of fascia lata ‘button’ technique for dural reconstruction in endoscopic endonasal approaches.
- 15.Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012;77(5–6):713–724. doi: 10.1016/j.wneu.2011.08.025. [DOI] [PubMed] [Google Scholar]; •• Systematic review of transcranial versus transnasal planum and tuberculum sella meningioma resection.
- 16.Conti A, Pontoriero A, Midili F, et al. CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. SpringerPlus. 2015;4:37. doi: 10.1186/s40064-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan JP, Starke RM, Kano H, et al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J. Neurosurg. 2014;120(6):1268–1277. doi: 10.3171/2014.2.JNS13139. [DOI] [PubMed] [Google Scholar]; • Large multicenter study reporting tumor control rates after radiosurgery for parasellar meningioma.
- 18.Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53–58. doi: 10.1227/01.NEU.0000311061.72626.0D. discussion 58–60. [DOI] [PubMed] [Google Scholar]
- 19.Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2013;87(3):524–527. doi: 10.1016/j.ijrobp.2013.06.2047. [DOI] [PubMed] [Google Scholar]
- 20.Williams BJ, Yen CP, Starke RM, et al. Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival. J. Neurosurg. 2011;114(6):1571–1577. doi: 10.3171/2011.1.JNS091939. [DOI] [PubMed] [Google Scholar]
- 21.Kaul D, Budach V, Misch M, Wiener E, Exner S, Badakhshi H. Meningioma of the skull base: long-term outcome after image-guided stereotactic radiotherapy. Cancer Radiother. 2014;18(8):730–735. doi: 10.1016/j.canrad.2014.07.159. [DOI] [PubMed] [Google Scholar]
- 22.Fokas E, Henzel M, Surber G, Hamm K, Engenhart-Cabillic R. Stereotactic radiation therapy for benign meningioma: long-term outcome in 318 patients. Int. J. Radiat. Oncol. Biol. Phys. 2014;89(3):569–575. doi: 10.1016/j.ijrobp.2014.02.042. [DOI] [PubMed] [Google Scholar]