Abstract
Marine bryozoans play an important role for the discovery of novel bioactive compounds among marine organisms. In this review, we summarize 164 new secondary metabolites including macrocyclic lactones, sterols, alkaloids, sphingolipids and so forth from 24 marine bryozoans in the last two decades. The structural features, bioactivity, structure–activity relationship, mechanism and strategies to address the resupply of these scarce secondary metabolites are discussed. The structural and bioactive diversity of the secondary metabolites from marine bryozoans indicated the possibility of using these compounds, especially bryostatin 1 (1), bryostatin analog (BA1), alkaloids (50, 53, 127–128 and 134–139), sphingolipids sulfates (148 and 149) and sulfur-containing aromatic compound (160), as the starting points for new drug discovery.
Keywords: : alkaloids, bryostatins, marine bryozoan, macrocyclic lactones, marine natural products, new drugs discovery, secondary metabolites, sphingolipids, sterols
Background
Natural products and their molecular frameworks have a long tradition as valuable starting points for new drug discovery [1]. They serve as an inspiration to discovery of new drug candidates and to illustrate the mechanisms for their complex three-dimensional architecture and exquisite biological activity. Keohane et al. surprisingly find that succinate dehydrogenase is the biological target for the natural product of promysalin, using affinity-based protein profiling [2]. A natural product-like compound was discovered as a von Hippel-Lindau mediated HIF1α interaction inhibitor with in vivo anti-angiogenic activity using structure-based virtual screening [3]. Moreover, the success of the anticancer compound cisplatin and its analogs inspired the discovery of bioactive leads from metal-based compounds. Leung and coworkers identified an enantiomeric iridum(III) metal-based compound showing potent inhibition against the Ras/Raf interaction and repression of renal cancer xenografts in vivo [4]. A novel metal-based rhodium(III) complex was identified as a new lysine-specific demethylase 1 targeting agent and epigenetic modulator [5]. Besides, an iridum(III) complex was reported as the first metal based irreversible inhibitor of bromodomain-containing protein 4, and may serve as a useful scaffold for development of more potent epigenetic agents against cancers [6]. Albada and Metzler-Nolte prepared some active synthetic antimicrobial peptides (AMPs) through rational optimizations in several generations of organometallic AMPs, and analyzed the mode of action for the typical ruthenocene derivative Rc-WRWRW-NH2. The synthetic AMPs combined with colistin and tobramycin can be used for the treatment of Pseudomonas aeruginosa infections that are associated with cystic fibrosis [7].
Marine natural products show a higher incidence of significant bioactivity and structural novelty compared with terrestrial secondary metabolites, which have led a wave for new drug discovery [8]. There are 1340 new compounds isolated from marine origin in 429 papers for 2015. Among them, nine new compounds described in five reports were from a bryozoan origin [9]. Marine bryozoans are invertebrates known from tropical to polar regions, and from the intertidal to the deep sea. Habitat-forming bryozoans are abundant and diverse in regions such as New Zealand, the Antarctica, the North Pacific around Japan, the northern Mediterranean and Adriatic, along the southern edge of the North Sea, through the English Channel and around the United Kingdom. Marine bryozoans are often colonial, benthic or epibiotic on algae, seagrass and other marine animals. Bryozoan morphology shows great variation including encrusting uni- and multilaminar colonies, branches of radially arranged zooids and erect uni- and bilaminate colonies [10]. There are more than 6000 species of marine bryozoans, which can be divided into three classes; Phylactolaemata, Stenolaemata and Gymnolaemata. Phylactolaemata is generally considered to be the most primitive class. Molecular sequence data indicate Phylactolaemata is the sister group to Gymnolaemata and Stenolaemata, which is in accordance with morphological phylogenies [11]. Gymnolaemata is the most studied class due to its ease of identification, as well as its abundance in bioactive secondary metabolites. Marine bryozoans are well known producers of bioactive compounds and an important source of marine drug leads, which has attracted researchers’ interests due to the remarkable antineoplastic activity of bryostatins discovered by Pettit et al. The secondary metabolites and their bioactivities from marine bryozoans are reported every year by the journal Natural Product Reports, within the general subject area of Marine Natural Products. Herein, we discuss the bioactivities of 164 compounds, including macrocyclic lactones, sterols, alkaloids, sphingolipids and other types of tetracyclic terpenoid lactones and sulfur-containing aromatic compounds, from 24 marine bryozoans in the orders of Cheilostomata and Ctenostomata, with the view of their potential as pharmacological probes and/or leads in drug discovery. The 164 metabolites reported since 1996 are classified as six types consisting of macrocyclic lactones, sterols, alkaloids, sphingolipids, sulfur-containing aromatic compounds and tetracyclic terpenoid lactones. These groups were based on the similarity of structural features and the numbers of metabolites identified from marine bryozoans. The structural characteristics, bioactivities and structure–activity relationship (SAR) of the related secondary metabolites are described. In particular, the synthesis, bioactivity and mechanism of action for the new drug candidate bryostatins are highlighted. In addition, strategies to solve the source and resupply of bioactive bryozoan metabolites are also discussed due to their low yield from natural origin.
Macrocyclic lactones from marine bryozoans
Discovery of macrocyclic lactones from marine bryozoans
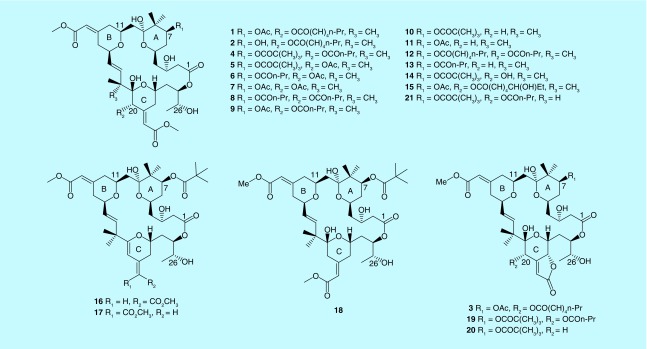
Macrocyclic lactones are a class of common compounds with striking antitumor activity in marine organisms. The size of the lactone ring is relatively large ranging from 10 to 60. Interest of studies on marine bryozoans was initiated from 1968 when Pettit et al. found potential cytotoxic constituents from Bugula neritina in California. The first macrocyclic lactone, bryostatin 1 (1), was finally identified from B. neritina by Pettit et al. in 1982, due to the limited ability at that time to isolate and elucidate trace amounts of newly active ingredients from nature [12]. After another 14 year endeavor studying macrocylic lactones from marine bryozoans, Pettit and coworkers have identified 18 analogs (bryostatins 1–18, Figure 1) and evaluated their antitumor activity [13]. During the investigation of antineoplastic constituents from B. neritina in the South China Sea, Lin et al. isolated a new macrolide bryostatin 19 showing strong cytotoxicity against the U937 cell line with an ED50 value of 3.2 nmol/l in vitro [14]. Lopanik et al. found a novel bryostatin 20 (20) that was unpalatable to fish, from the larvae of B. neritina. The result represents the first example from marine environment of a microbial symbiont producing an antipredator defense for its host [15]. Recently, four new macrocyclic lactones, bryostatin 21 (21) and 9-O-methylbryostatins 4, 16 and 17 were identified from B. neritina in the South China Sea. The novelty of bryostatin 21 is the presence of a single methyl group at C-18 compared with the previously isolated bryostatins. However, its cytotoxicity is reduced substantially. Replacement of an α hydroxyl by a methoxyl group at C-9 in bryostatins is also correlated with a loss of inhibitory activity [16]. Thus, the α methyl at C-28 and the α hydroxyl at C-9 play an important role for the potent cytotoxicities for the bryostatins. The predominant structural feature of the 24 isolated bryostatin macrolides is characterized by a 26-membered cyclic skeleton with three imbedded pyran rings, and their main differences were the substituents at C-7 and C-20. The molecular mechanism of the bryostatins antitumor activity has been their ability to selectively bind to the regulatory domain of various individual PKC isozymes within cells. Ruan and Zhu summarized the SAR of bryostatins that a 26-membered macrolactone ring is needed for good PKC binding activity, hydroxyl at C-3 with (R)-stereochemistry and a free hydroxyl at C-26 is important for a high enzyme affinity and the structure in the C7-C9 region of the A-ring is critical for the potent inhibition of tumor cells [17].
Figure 1. . Chemical structures of bryostatins (1–21) from marine bryozoans.
Total synthesis of bryostatins
Total synthesis of bryostatins is an efficient strategy to provide a reliable source of these promising compounds. To date, total synthesis of bryostatins 1, 2, 3, 7, 9 and 16 have been achieved since bryostatin 7 since the first total synthesized reported by Kageyama et al. [18]. It's worth noting that Trost et al. synthesized bryostatin 16 using a concise atom-economical and chemo-selective approach. The implementation of this strategy allows access to synthesis of various bryostatins and their analogs in the laboratory [19]. However, complicated synthetic routes to the bryostatins make these strategies difficult to adopt for commercial production. For instance, the accomplishment of total synthesis of bryostatin 1 needed 30 steps for the longest linear sequence from commercially available (R)-isobutyl lactate [20].
Simplified partial synthesis of bryostatin analogs
Since there are complicated extraction procedures and low yields of bryostatins from their natural source, and there is high cost and low commercial value of total synthesis, the simplified partial synthesis of bryostatin analogs (BA) to replace bryostatins is an attractive way to solve the source problem for bryostatins. The structural difference between bryostatin 1 and BA is the simplified substituent groups at C-7, C-9, C-13, C-20 and C-26 (Supplementary Figure 1). Wender and coworkers synthesized a number of bryostatin analogs for easy synthesis and superior clinical performance since 1980 [21]. The representative analog, termed ‘picolog’ (BA 1), exhibited superior growth inhibition of MYC-induced lymphoma in vitro compared with bryostatin 1, and it provided the first in vivo validation that the bryostatin analog, was a potential therapeutic agent to treat cancer [22]. They also designed a series of synthetically-accessible bryostatin analogs (BA 2–5), which could serve as superior drug candidates for the eradication of HIV/AIDS [23] and Chikungunya virus [24]. The designed BA retained the active groups and simplified the structure of bryostatins, which made them have more potential to be developed as new drugs.
Bioactivity & mechanism of bryostatins
The bioactivity for bryostatins can be summarized as antitumor, enhancing memory and learning, along with immune modulatory properties. Bryostatins exhibit antitumor activities including inhibition of lymphocytic leukemia P388, histiocytic lymphoma cell U937, prostate cancer cells LNcaP, melanoma B16, reticulum cell sarcoma M5076, human leukemia HL-60, murine melanoma K1735-M2 – among others. [17,25]. The antitumor mechanisms for bryostatins are linked to the ability to selectively modulate the function of various individual PKC isozymes in cells, while the action of transient duration is the primary mechanism responsible for the unique biology of bryostatin 1. In detail, bryostatin 1 can activate the MEK/ERK path way and some transcription factors including NF-κB, AP1 and EGR1 in prostate cancer cells LNcaP at 60 min, while it fails to induce the late responses including phosphorylation of p65, translocation of cRel and RelB at 6 h and finally induces early termination of the responses in protein and RNA levels [26]. Irie et al. design a therapeutic lead (aplog-1) based on bryostatin 1's unique mechanism of activating PKCδ to suppress tumors [27]. Among the 24 macrocyclic lactones, bryostatin 7 shows the most potent binding affinity to PKC in biology, and it was also the first member of the bryostatins to be synthesized [28]. This suggests bryostatin 7 can be an effective surrogate for bryostatin 1 in the development of new drugs. Besides, bryostatins may also be used to treat Alzheimer's disease (AD), depression and traumatic brain injury due to their ability to enhance memory and learning. The mechanisms for bryostatin 1 to enhance memory and learning also involve regulation of PKC. In AD Phase IIa and expanded access trials, the ability of bryostatin 1 to elevate PKCε levels closely tracked cognitive benefits in the first 24 weeks, which suggests the potential to use bryostatin 1 as treatment of AD [29]. Bryostatin 1 is orally active in models of learning and memory. The active effect can be produced in less than 2 weeks while it is not seen with intraperitoneal administration [30]. This suggests the possible application of bryostatin 1 to treat AD in oral administration. Bryostatin 1 can also selectively activate PKCε to treat depressive behavior, immobility and impairment in spatial learning and memory [31] and it affects PKC-α and PKC-ε to protect the brain from severe neurological injury post-middle cerebral artery (post-MCAO) in rats [32]. Last, immune modulatory properties of bryostains make them potentially useful to treat cerebral infarction and HIV brain infection. The combination of exercise and bryostatin 1 administration can induce greater functional recovery than exercise alone in patients with cerebral infarction. This combination of therapy can increase the 5-HT immunoreactivity, affect 5-HT turnover and increase 5-HT concentrations in the perilesional area [33]. Bryostatin can reactivate latent viral infection in normal human astrocytes and human astrocytoma U-87 cells via activation of PKC-α and PKC-δ, and it strongly stimulates long terminal repeat promoter transcription by activating NF-κB [34]. This indicates that bryostatin may be a beneficial adjunct to treat HIV-1 brain infection. A Phase I clinical trial indicated bryostatin 1 is safe to cure HIV infection at a single dose administration, while it does not show any effect on PKC activity or inflammation biomarkers of sCD14+ and IL-6, which may be due to low plasma concentrations [35]. The above analysis for bioactivity and mechanism of bryostatins revealed this class of macrocyclic lactones is the most active secondary metabolites from marine bryozoans, and they have the greatest potential to be developed as new drug candidates.
Sterols from marine bryozoans
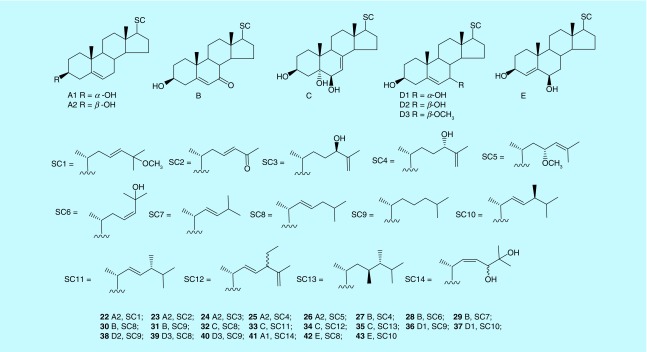
Sterols are a class of important secondary metabolites in marine organisms. Most sterols of marine origin exhibit biological activities including antitumor, antibacterial and anti-inflammatory and so forth. In addition to a basic cyclopentane parallel hydrogen-phenanthrene nucleus, the structure of ocean-sterols show more novelty in their highly oxidized skeleton and changeable side chains compared with sterols from terrestrial sources. The skeletons of sterols from marine bryozoans are mainly in the cholestane and ergostane class. From the number of hydroxyl-substituents and the position of double bonds, they mainly divided into four types of 3β-hydroxy Δ5-steroid (A2), 3β-hydroxy Δ5–7-one-steroid (B), 3β,5α,6β-trihydroxy Δ7-steroid (C) and 3β,7ζ-dihydroxy Δ5-steroid (D1-D3), and other types involving 3α-hydroxy Δ5-steroid (A1) and 3β,6β-dihydroxy Δ4-steroid (E) (Figure 2). Although the novelty and bioactivity of most bryozoan sterols are inferior compared with macrocyclic lactones, some new active ones have gradually been discovered.
Figure 2. . Sterols (22–43) from marine bryozoans (SC means side chain of sterols).
The A2 nucleus is the main type for sterols in marine bryozoans. Kerr et al. identified nine sterols with nucleus of A2 from B. neritina in California and Florida using GC–MS. The result indicates that cholesterol is the predominant sterol produced by de novo biosynthesis, while A2 with 28, 29 and 30 carbons are minor components produced by dietary origin. Besides, the C24-alkylated sterols with A2 nucleus are produced by alkylation of dietary sterols [36]. Five new sterols (22–26) were isolated from bryozoan Cryptosula pallasiana Moll, found in Huang Island of China [37,38]. Sterol 22, also identified from B. neritina [39], is characterized by a methoxyl group at C-25 and a trans-double bond between C-23 and C-24 in its side chain. Compounds 22 and 23 show moderate cytotoxicity against HL-60 cells with IC50 of 17.91 and 15.05 μg/ml, respectively. Compounds 24 and 25 were isolated for the first time from a natural source. Compound 26, which possessed a rare 23R methoxyl and a double bond at C-24, showed moderate cytotoxicity against HL-60, Hep-G2 and SGC-7901 cell lines with IC50 from 12.34–18.37 μM. The novelty of this type of sterol is based on the branched chain at C-17, and most of them show moderate cytotoxic activity. The nucleus of B is another typical of sterols discovered from marine bryozoans. Sterols 27 and 28, isolated from B. neritina, have shown cytotoxicity to HepG2, HT-29 and NCI-H460 with IC50 values in the range of 22.58–53.42 μg/ml [40]. Sterols (29–31) with B nucleus were also discovered from C. pallasiana. Compounds 29 and 30 show cytotoxicity against HL-60 cells with IC50 values of 15.12 and 14.73 μg/ml, respectively. However, sterol 31 does not show any apparent cytotoxicity, which may be connected with the loss of a trans double bond at C-22 in the side chain [37]. Besides, sterols 29–31 were also previously isolated from marine sponges Cliona copiosa and Stelodoryx chlorophylla [41,42]. Sterols with C nucleus are also featured in marine bryozoans. For example, three new sterols (32–34) with C nucleus were isolated for the first time from the marine bryozoan Myriapora truncate collected along the Mediterranean Coasts [43]. Several known sterols and a new one (35) with the same skeleton were found in the search for bioactive secondary metabolites from B. neritina in the South China Sea. Cytotoxicity evaluations of those sterols demonstrated that they are inactive constituents against tumor cell lines HepG2, NCI-H460 and SGC7901 [44]. SAR analysis suggested that the double bond at C-7 in the nucleus plays an important role in the lower cytotoxicity for this type of sterol [45]. The nucleus of 3β,7ζ-dihydroxy Δ5-steroid is a precursor of B. Three sterols with D1 nucleus (36–37) and a D2 nucleus (38) were first isolated from the marine bryozoan Biflustra grandicella collected in Huang Island of China [46]. It was shown that 3β,7ζ-dihydroxy Δ5-steroids were relatively common secondary metabolites from marine bryozoans since sterols 36 and 38 were subsequently obtained from C. pallasiana. Two new sterols (39–40) with D2 nucleus were also isolated from C. pallasiana, which displayed moderate cytotoxicity with IC50 values of 21.30 and 22.11 μg/ml to HL-60 cells [37]. Moreover, compound 40, obtained by chemical synthesis, could inhibit cholesterol acyltransferase effectively [47].
In addition to the above-mentioned four main types of sterols from marine bryozoans, there are other rare types of sterols including those with nuclei of A1 and E. One new sterol (41), possessed A1 nucleus with a unique 22Z-24ζ,25-dihydroxy side chain at C-17, was identified from B. grandicella. The sterol with the similar side chain has only been prepared by chemical synthesis [46]. Two new sterols (42–43) with nucleus of E are also discovered from B. neritina [44]. Compounds 42 and 43 showed selective growth inhibition against the HepG2 cancer cell line with IC50 values of 36.5 and 52.1 μM, respectively, while they were inactive against NCI-H460 and SGC7801 cell lines. Interestingly, sterols with E nucleus from marine bryozoans are also frequently discovered from marine microalgae, which provided possible chemical evidence that secondary metabolites found in marine bryozoans may be produced by an epizoic source, which has important ecological implications [48]. In summary, while the structural diversity of the steroidal nucleus of compounds from marine bryozoans is less extensive compared with sterols from other marine organisms such as sponges, starfishes and coral and so on, the position and stereochemistry of hydroxyl or methoxyl groups between C-23 and C-25 (sterols 26 and 41) in the side chain are novel and characteristic for marine bryozoans. As for bioactivities, studies just focused on the simple evaluation of cytotoxicity for the isolated sterols, a systematic evaluation of their biological properties should be undertaken to facilitate potential new drug discovery.
Alkaloids from marine bryozoans
Alkaloids are the major components of marine bryozoans. Most alkaloids in marine bryozoans are rich in halogen substitution, which is a characteristic functional group for marine natural products. Alkaloids in marine bryozoans also show unique structural diversity and bioactive diversity, which increases the possibility to develop them as pharmacological leads in the new drug discovery process. Herein, we give a systematic summary of structural diversity and bioactive diversity for alkaloids from bryozoans in recent years.
β-Phenylethylamine alkaloids
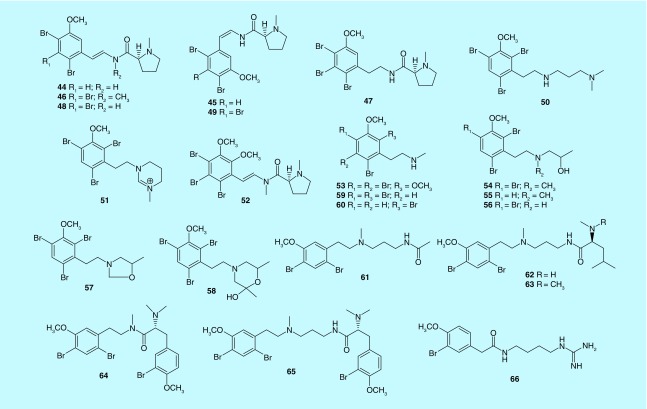
In general, marine bryozoans of the genus Amathia from different geographic areas contain a large number of bromine-containing alkaloids with β-phenylethylamine skeleton (Figure 3). The possible biosynthetic pathway of this type of alkaloids is derived from a processor of 2-(2,4-dibromo-5-methoxyphenyl) ethanamine, with related amino acids followed by introduction of a double bond, or methyl, methoxyl or bromine substituents [49]. Five brominated β-phenylethylamine and proline related alkaloids, amathamides A–F (44–49), are discovered from A. wilsoni in the Australian island of Tasmania [50,51]. The structures of these alkaloids differ from each other by the degree of bromination and methylation, as well as the presence or stereochemistry of a double bond. Two other bryozoans, A. tortusa and A. convoluta in Tasmania were also abundant in this type of alkaloids. Convolutamines I (50) and J (51), two new β-phenylethylamine alkaloids, were identified in the discovery of new antitrypanosomal leads from A. tortusa in Tasmania. Compounds 50 and 51 were shown to be active toward the parasite Trypanosoma brucei with IC50 values of 1.1 and 13.7 μM, respectively. They also exhibit cytotoxicity against HEK293 with IC50 of 22.0 and 41.0 μM [52]. Interestingly, β-phenylethylamine alkaloids from the bryozoan A. convoluta show special structural and bioactive diversity in different geographic areas. Amathamide G (52) and convolutamine H (53), possess a fully substituted aromatic ring, and have been isolated from A. convoluta in Tasmania [53,54]. Compound 53 (LD99 = 0.2 μg/ml) is a more potent nematocide against Haemonchus contortus than the commercial anthelmintic, levamisole (LD99 = 1.6 μg/ml). In fact, convolutamines A–G (54–60) are typical brominated β-phenylethylamine alkaloids, which were first discovered from A. convoluta in Florida [55,56]. Convolutamines A (54), B (55) and D (56) exhibit cytotoxic activity against P388 with IC50 values of 10.6, 4.8 and 8.6 μg/ml, respectively. Convolutamine F (59) exhibits activity against KB, KB/VJ-300 and U937 cells. It also exhibits inhibitory effects for cell division of fertilized sea urchin eggs. Five novel β-phenylethylamine alkaloids, volutamides A–E (61–65), were identified from the temperate Atlantic bryozoan A. convoluta. The antifeedant bioactive results show that this type of alkaloids may serve as chemical defenses against diverse generalist predators, and against fouling by killing the larvae of competing invertebrates [57]. A new β-phenylethylamine alkaloid, securidine A (66), was obtained from the cold water marine bryozoan Securiflustra securifrons in west Spitzbergen. However, compound 66 shows no significant cytotoxic, antibacterial and antidiabetic activity. It also shows no inhibition of biofilm formation by Staphylococcus epidermidis at 100 μM [58].
Figure 3. . β-Phenylethylamine alkaloids (44–66) from marine bryozoans.
Indole alkaloids
Many alkaloids in marine bryozoans could be classified as indole alkaloids. Marine bryozoans Amathia alternate, Zoobotryon verticillatum and Cryptosula pallasiana contain simple indole alkaloids. Secondary metabolites of marine bryozoans Chartella papyracea, Securiflustra securifrons and Hincksinoflustra denticulate provide relatively complex polycyclic indole structures. Moreover, Flustra foliacea is a special marine bryozoan, which contains both simple indole alkaloids and complex polycyclic indole alkaloids.
Simple indole alkaloids
Four new bromotryptamine peptides with a simple indole ring, alternatamides A–D (67–70) (Supplementary Figure 2), were isolated from the marine bryozoan A. alternate, collected along the Atlantic coast of the USA off of northern Carolina [59]. We can conclude from the structure of the four indole alkaloids that they are amides from indole ethylamine and valine, along with methylation and bromination. Compounds 67–69 show modest antibacterial activities against several Gram-positive bacteria with minimum inhibitory concentration (MIC) values ranging from 4 to 32 μg/ml, while they were not active against Gram-negative bacteria. A new brominated indole alkaloid (71) and two known brominated indole alkaloids (72–73) were identified from bryozoan Z. verticillatum in the southern Atlantic coast of Spain. Compounds 72 and 73 were also found in the same bryozoan collected in CA, USA [49]. In the search of antitumor secondary metabolites from marine bryozoan, three known simple indole alkaloids (74–76) were found from C. pallasiana in Huang Island of China [60].
Polycyclic indole alkaloids
The bryozoan Family Flustridae (Cheilostomata order) is the primary source of polycyclic indole alkaloids. Four of these bryozoans, Chartella papyracea, Securiflustra securifrons, Hincksinoflustra denticulate and Flustra foliacea, are the most common source for this type of alkaloids (Supplementary Figure 3). Chartellines A–C (77–79) and chartellamides A–B (80–81), discovered from C. papyracea in the Roscoff region of France, are the first examples of polycyclic indole alkaloids in marine bryozoans. Chartelline A (77) was inactive in antimicrobial assays and also inactive against leukemia cells in the NCI's test [49]. Seven halogenated indole-imidazole alkaloids with novel polycyclic skeletons, securamines A–G (82–88), were identified from S. securifrons in North Sea off the Danish west coast. Interestingly, two macrocyclic alkaloids, securine A (89) and securine B (90) can be in equilibrium with securamine A (82) and B (83), respectively, when they are dissolved in DMSO. Further studies indicate that the securines could act as precursors for chartellines as well as for securamines [61,62]. A novel pentacyclic tribromo alkaloid, hinckdentine A (91), was identified from H. denticulate in Tasmania by single crystal x-ray, and it has subsequently been fully synthesized [63].
In contrast to the above three marine bryozoans, Flustra foliacea is a commonly studied bryozoan due to its ease of identification and worldwide distribution. The secondary metabolites of F. foliacea vary from the different geographical locations of this species. Sharp et al. reviewed the secondary metabolites of F. foliacea using an ecological perspective [64]. The typical example is the discovery of flustramines with broad spectrum of antibacterial [65], nonspecific voltage-sensitive potassium channel blocking [66] and subtype-specific nicotinic acetylcholine receptor blocking activities [67]. In the terms of indol alkaloids, both simple ones and polycyclic ones can be found in this species. Recently, a new polycyclic indole alkaloid (92) and three new simple bromotryptamine alkaloids (93–95), were isolated from F. foliacea in the North Sea of Steingrund [66]. Compound 95 shows significant cytotoxic activity against HCT-116 with IC50 of 5.8 μM [68]. Eleven new polycyclic indole alkaloids, flustramine F-P (96–106), were discovered from F. foliacea in the Bay of Fundy of Canada. Flustramine F (96), I (99) and L (102) showed a broad spectrum of antimicrobial activities. A slow interconversion can occur from flustramine L (102) to H (98) and N (104) after years of storage [69].
γ-Lactam alkaloids
Except for β-phenylethylamine alkaloids from the genus Amathia, γ-Lactam alkaloids are also discovered commonly in this genus (Supplementary Figure 4). Convolutamides A–F (107–112) were identified from the Floridian bryozoan A. convoluta. They were characterized as an N-acyl-γ-lactam moiety with a dibromophenol group. Convolutamides A (107) and B (108) show cytotoxic activity against L1210 with IC50 values of 4.8 and 2.8 μg/ml, respectively [70]. In addition, six new γ-lactam alkaloids, amathaspiramides A–F (113–118) were identified from A. wilsoni in New Zealand. Compounds 113–115 and 117 were assessed for their bioactivities [71]. The result shows that 117 exhibits strong antiviral activity against Polio virus Type 1. Compounds 113 and 117 show moderate cytotoxicity against BSC-1 cells, and mild antibacterial activity against the Gram-positive bacterium Bacillus subtilis and the fungus Trichophyton mentagrophytes, while compounds 114 and 115 were inactive constituents based on these bioactivities. This indicates the pyrrole ring is important for the biological activity of this type of alkaloid. The replacement of it with a γ-lactam may reduce their bioactivities.
Pyrrole alkaloids
Pyrrole alkaloids from marine bryozoans can be classified as single pyrrole, dipyrroles and tetrapyrroles based on the number of pyrrole rings (Supplementary Figure 5). Most of the amathamides, flustramines and amathaspiramides mentioned above contain just a single pyrrole ring. Two single pyrrole alkaloids (119–120) were isolated from C. pallasiana in Huang Island of China. Compounds 119–120 were isolated from marine bryozoans for the first time, while they have been discovered frequently in marine sponges [60]. A dipyrrole, tambjamine A (121), previously reported from the tropical bryozoan Sessibugula translucens, has been found in the Antarctic bryozoan Bugula longissima [72]. Four new dipyrroles, tambjamines G–J (122–125), were identified from Bugula dentate in Tasmania of Australia. It has been reported that B. dentate can protect themselves through delivering those toxic dipyrroles [73]. An antimicrobial blue pigment (126) possessing the tetrapyrroles skeleton has been isolated from B. dentate in the Gulf of Sagami of Japan. Compound 126 is ascribed to the dark blue color for B. dentate [74].
Other types of alkaloids
In addition to the above-mentioned alkaloids, other types of alkaloids include purine, pyridine, indolizine, quinoline, isoquinoline, quinolinone, quinone methide, β-carboline and 2,6-naphthyridine. Blackman et al. reviewed the purine alkaloids from marine bryozoans before 1996, while no new ones are found later. Herein, we mainly summarize the new pyridine, indolizine, quinoline, isoquinoline, quinolinone, quinone methide, β-carboline and 2,6-naphthyridine alkaloids (Figure 4), as well as their bioactivities from marine bryozoans after 1996.
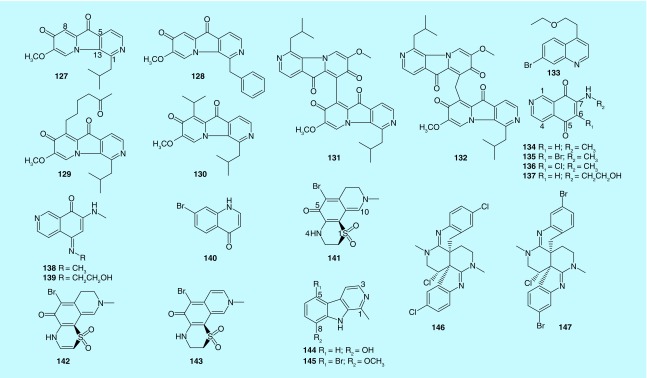
Figure 4. . Indolizine, quinoline, isoquinoline, quinone methide, β-carboline and 2,6-naphthyridine alkaloids (127–147) from marine bryozoans.
Six novel alkaloids with a pyridine ring in parallel with an indolizine skeleton, pterocellins A–F (127–132), were identified from Pterocella vesiculosa in the Hen and Chicken Islands to the north of New Zealand [75,76]. Pterocellins A (127) and B (128) show strong cytotoxicity against P388 cells with IC50 values of 477 and 323 ng/ml, respectively, while pterocellin D (130) displays modest activity with an IC50 value of 4773 ng/ml, and pterocellins C (129), E (131) and F (132) were essentially inactive with IC50 values greater than 6250 ng/ml. Similarly, 127 shows strong antiviral activity and cytotoxicity against BSC-1 cells, while 129, 131 and 132 were inactive. Besides, 127 and 128 inhibit two bacteria, Escherichia coli and Pseudomonas aeruginosa, and three fungi, Trichophyton mentagrophytesm, Candida albicans and Cladisporum resinae, while 129–132 are inactive. The SAR of those pterocellins demonstrates that the H-8 may be crucial to the bioactivity of this type of alkaloids. Furthermore, pterocellin A exhibits strong cytotoxicity against Hela cells with an IC50 of 886 ng/ml. The possible mechanism is that pterocellin A is an inducer of apoptosis in Hela cells via mitochondria related processes [77].
Wulff et al. first discovered a new naturally occurring bromo-substituted quinoline alkaloid (133) from F. foliacea [78]. Subsequently, three isoquinolintriones were isolated from Biflustra perfragilis, which have been reviewed by Blackman et al. [49]. Recently, six new alkaloids with an isoquinoline 5,8-dione skeleton, calibugulones A–F (134–139), were identified from Caulibugula intermis in the South Pacific off Palau. All of the isolates displayed significant inhibition against the IC-2WT murine tumor cells in vitro with IC50 from 0.03 to 1.67 μg/ml. The SAR of those caulibugulones demonstrates that the halogen substitution at C-6 is not an important determinant of cytotoxicity, while substitution of an ethyl alcohol at either the C-7 or C-5 nitrogen resulted in a five- to tenfold reduction in cytotoxicity [79]. Besides, a new natural product, 7-bromoquinolin-4(1H)-one (140), was isolated from bryozoan C. pallasiana, and it was inactive to HL-60 cells [80]. Furthermore, euthyroideones A–C (141–143), which possess a unique heterocyclic ring system that contains brominated quinone methide, sulfone and amine groups, were discovered from the bryozoan Euthyroides episcopalis in Fiordland of New Zealand. Euthyroideone B (142) shows modest cytotoxicity against BSC-1 cells, while 141 and 143 were inactive [81].
β-Carboline alkaloids have been reported in the marine bryozoans Costaticella hastata, Orthoscuticella ventricosa and Cribricellina cribraria [49]. Recently, two new β-carboline alkaloids (144–145) were identified from bryozoans C. cribraria and Pterocella vesiculosa, respectively [82,83]. Compound 144, named 8-hydroxyharman, shows relatively weak cytotoxicity against P388 cells with an IC50 more than 12,500 ng/ml. Compound 145 is a 5-bromo-8-methoxy-1-methyl-β-carboline, which shows relatively moderate cytotoxicity against P388 cells with an IC50 of 5089 ng/ml. It also shows inhibition against the Gram-positive bacteria C. albicans, B. subtilis and T. mentagrophytes with minimum inhibitory doses of 2–5 μg/ml. The SAR indicates that the substituent of a vinyl group at C-1 or bromine at C-5 plays an important role for the cytotoxicity against P388. Furthermore, the antimicrobial activity can be increased by a bromine substituent at C-5, but decreased by an additional 8-methoxy substituent. Interestingly, two novel heterocyclic alkaloids, caulamidine A (146) and B (147), possessed the 2,6-naphthyridine core and fused by a dihydroindole-derived and tetrahydroquinoline-derived systems, were isolated from bryozoan Caulibugula intermis and elucidated by the new computer-assisted structural tools. Compounds 146 and 147 appear antimalarial activity against Plasmodium falciparum with IC50 values from 8.3–12.9 μM. Besides, they also show modest cytotoxicity in NCI-60 cell screen with a single dose of 40 μM [84].
In summary, the features of affluent alkaloids types, polythalogen substitutions, unique heterocyclic ring systems, along with accidental sulfone substitutions outline the chemiodiversity for alkaloids from marine bryozoans. The frequent substitutions of one or more bromines in alkaloids increase the possibility to discover novel compounds from marine bryozoans. Especially, the unique heterocyclic ring systems in polycyclic indole alkaloids, such as hinckdentine A (91), attract chemists to synthesize it and its derivatives to find pharmacological leads. As for their bioactivities, β-phenylethylamine alkaloids 50 and 53 show significant insecticidal activity against T. brucei (LC50 = 1.1 μM) and H. contortus (LD99 = 0.2 μg/ml), respectively. Pyridine paralleled indolizine alkaloids of pterocellins A (127) and B (128) show significant cytotoxicity against P388 cells (IC50 is 477 and 323 ng/ml respectively). Isoquinoline 5,8-dione alkaloids of calibugulones A-F (134–139) display significant inhibition (IC50 = 0.03–1.67 μg/ml) against IC-2WT murine tumor cells. These indicate the potential to develop those alkaloids as pharmacological leads in the discovery of new drugs.
Sphingolipids from marine bryozoans
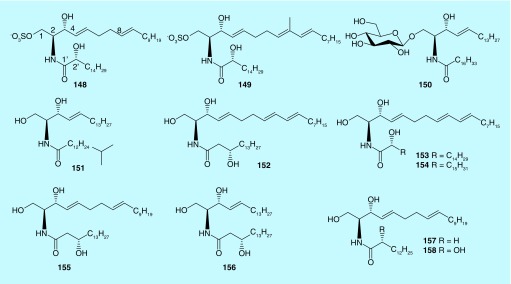
Sphingolipids, comprised by a sphingoid base (LCB) with a long fatty acid base (FAB) through an amide bond, are divided into two groups of ceramides and cerebrosides based on whether glycosylated. Sphingolipids are common secondary metabolites for terrestrial plants and marine invertebrates. The bioactivities of sphingolipids include cytotoxicity, antibacteria, immune modulation – among others. Similar to moderate cytotoxic sterols form bryozoans, researches on sphingolipids from marine bryozoans are also limited in discovery of new compounds and evaluation of their cytotoxicity (Figure 5). However, two sulfates of ceramides (148–149), identified from bryozoan Watersipora cucullata, show significant inhibition against human topoisomerase I with IC50 values of 0.4 and 0.2 μM, respectively. This indicates those ceramide sulfates can be developed as inhibitors for topoisomerase I to cure antitumor [85]. Marine bryozoan B. neritina is affluent in sphingolipids [86]. Seven new sphingolipids, compounds (150 and 151) and neritinaceramides A–E (152–156), were isolated from this species in the South China Sea [87,88]. Compound 151 possessed a rare branched methyl fragment of [-CH(CH3)2] in its FAB. Compounds 152, 155 and 156, characterized at a C-3′S hydroxyl group in their FAB fragment, were novel structural feature in sphingolipids. Besides, the rare structural feature of 4E,8E,10E-triene skeleton in the LCB for compounds 152–154, was first discovered from marine bryozoans. Neritinaceramides A-E (152–156) exhibit moderate cytotoxicity against HepG2 and SGC7901 cells with a range of IC50 values from 47.3 to 58.1 μM, while they show inactive to NCI-H460 cells. Besides, two new ceramides (157–158), possessing 14 carbons in the FAB, were identified from C. pallasiana [38]. They are also displayed moderate cytotoxicity against HL-60, Hep-G2 and SGC-7901 with the IC50 values from 21.13 to 32.36 μM. The SAR analysis demonstrates that the presence of the trans double bond between C-4 and C-5 in the vicinity of their polar head, the category of the sugar moieties at C-1 in the LCB and the additional hydroxyl group at position C-2′ or C-4, are important for their cytotoxicity.
Figure 5. . Sphingolipids (148–158) from marine bryozoans.
Other secondary metabolites from marine bryozoans
The changeable marine climate and the different geographical location of bryozoans provide the possible complexity and novelty of secondary metabolites for marine bryozoans. Although the novel structures found from bryozoans are relatively little compared with other marine organisms, the strong antitumor activity of bryostatins and the novelty of alkaloids from bryozoans still attract researchers’ attention. In addition to the above introduced structural types, the secondary metabolites from marine bryozoans are also including triterpenoids and sulfur-containing aromatic compounds, and so forth (Figure 6). Some of them show drug development value due to their significant bioactivities.
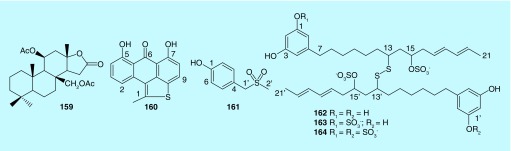
Figure 6. . Triterpenoid (159) and sulfur-containing aromatic compounds (160–164) from marine bryozoans.
A novel tetracyclic terpenoid lactone, murrayanolide (159), possessing an unusual C21 skeleton was identified from bryozoan Dendrobeania murrayana in the east coast of Canada. Compound 159, the first C21 tetracyclic terpenoid lactone from bryozoans, exhibits 54% inhibition against metalloprotease collagenase IV at a dose of 25 μg/ml [89]. A sulfur-containing aromatic compound, bryoabthrathiophene (160) was isolated from bryozoan Wateripora subtorquata in Tsutsumi Island of Japan. Compound 160 exhibits significant antiangiogenic activity with IC50 of 0.005 μM on bFGF-induced proliferation of BAEC (Bovine aorta endothelial cell), indicating it can be useful for the development of novel antiangiogenic agent [90]. Besides, a new natural compound, p-methylsulfonylmethyl-phenol (161), was found from bryozoan C. pallasiana, while it was inactive to HL-60 cells [80]. Furthermore, three new disulfides, Pentaporins A–C (162–164), were discovered from Mediterranean bryozoan Pentapora fascialis. Compounds 162–164 show anthelmintic activity against Trichinella spiralis. The sulfate ester groups are responsible for the anthelmintic activity [91].
Strategies to solve source & resupply for bioactive bryozoan metabolites
Based on the above analysis, the structural diversity and bioactive diversity of secondary metabolites from marine bryozoans pour the desires to discover pharmacological leads from this marine organism. Its needed to mention that bryostatin 1 (1) and bryostatin 4 (4) have entered Phase II clinical trials in the US for the treatment of cancer, AD, effects of stroke and HIV [25,29,35]. However, the yield of this type of macrocyclic lactones from bryozoans is low. It depends on the geographical site, time of year and depths of the collection. For example, 1000 kg of damp B. neritina collected from gulf in Mexico gave 306 mg of bryostatin 4, while an approximately equal amount collection from the South China Sea gave 970 mg of bryostatin 4. The low and uncertain yield of bryostatins made them hard to be used in clinic. Fortunately, cultivation of bryozoans is an effective way to solve the source and resupply for bioactive bryozoan metabolites. Compared with collection bryozoans from nature, cultivation of bryozoans can provide a controlled environment to avoid regional difference about the yield of bryostatins. Bugula neritina can commonly be cultured in the laboratory since its abundance and the larvae ease to be collected and induced to adhere to a surface [92]. The company of CalBioMarine Technologies from California has grown B. neritina into the sea after their initial colonization by the larvae on plastic plates. The way to cultivate bryozoans solved the problem of the changeable marine climate, while it not solves the fact that the low yield of bryostatins. In fact, bryostatins were first isolated from B. neritina, and later found in marine bryozoan Amathia convolute and other marine organisms Lissodendoryx isodictyalis and Aplidium californium [13]. Recent studies have proved that bryostatins were actually produced by an uncultured symbiotic bacterium Endobugula sertula from B. neritina. Thus, heterologous expression of the putative bryostatin polyketide synthase gene cluster from E. sertula, with the help of cultured B. neritina in laboratory, provided new eyesight to produce the bioactive bryozoan metabolites in large enough quantities for further development of them into pharmaceutical [93].
Conclusion
In this review, the bioactivities of 164 compounds isolated from 24 marine bryozoans including two orders of Cheilostomata and Ctenostomata are discussed. The bioactive secondary metabolites of 11 bryozoan species are studied for the first time since 1996 (Table 1). Bryozoan B. neritina is still a focus to produce new bioactive compounds, followed by A. wilsoni, A. convolute, C. pallasiana, F. foliacea, P. vesiculosa and S. securifron. Alkaloids including β-phenylethylamine, indole, γ-lactam, pyrrole and other types of pyridine, indolizine, quinoline, isoquinoline, quinolinone, quinone methide, β-carboline and 2,6-naphthyridine, make up the major types of chemical structures for bryozoans. The rests are the sterols, sphingolipids, macrocyclic lactones, triterpenoids and sulfur-containing aromatic compounds – among others. Antitumor activity is the most important bioactivity for these discovered secondary metabolites from bryozoans (Table 2). Macrocyclic lactones and some types of alkaloids appear excellent antitumor activity, while most sterols and sphingolipids exhibit moderate cytotoxicity. Interestingly, alkaloids show variety of biological activities including antitrypanosomal, antitumor, nematicidal, antifeedant, antibacterial, antiviral, antipredator and antimalarial activities, as well as the inhibitory effects for cell division. Besides, the inhibitory effect for human DNA topoisomerase I of sphingolipids, inhibition for metalloprotease of tetracyclic terpenoid lactone, along with the antiangiogenic and anthelmintic activities of sulfur-containing aromatic compounds, demonstrate the diversity of bioactivities for secondary metabolites of marine bryozoans. Finally, we use the LC50, IC50 or LD99 value near to 1 or below 1 μg/ml (μM) for a certain biological target samples or cells as the evaluation standard, to summarize the potential pharmacologic leads or drug candidates for the 164 secondary metabolites from marine bryozoans. The results show macrocyclic lactones, such as bryostatin 1 (1) and bryostatin analog (BA1), can be developed as antitumor, enhancing memory and learning, and immune modulating related agents. β-Phenylethylamine alkaloids (50 and 53) can be developed as insecticidal agents. Pyridine paralleled indolizine alkaloids of pterocellins A (127) and B (128), isoquinoline 5,8-dione alkaloids of calibugulones A–F (134–139) and sphingolipids sulfates (148 and 149) can be developed as antitumor agents. Sulfur-containing aromatic compound, bryoabthrathiophene (160), can be developed as antiangiogenic agent. Although various pharmacological leads have been discovered from marine bryozoans, no one has been successfully approved as new drugs. The most promising bryostatin 1 is still hard to progress to Phase III clinical trials as antitumor agent. Except the drawbacks of low productivity for bryostatin 1, its toxic side effects of myalgia, fatigue, local phlebitis, thrombocytopenia, nausea and vomiting during clinical studies need to pay attention [25,94]. Compared with successful development of bretuximab vedotin as antibody–drug conjugate in its safe treatment of Hodgkin lymphoma [95,96], the strategies to selective deliver cancer drugs to tumor cells, while avoid to influence the nontarget cells can be considered to reduce the toxic side effects in the development of these pharmacological leads.
Table 1. . Summary of secondary metabolites from marine bryozoans.
| Bryozoans | Order | Structures | Compound type | Ref. |
|---|---|---|---|---|
| Amathia wilsoni | Ctenostomata | 44–49, 113–118 | Alkaloids | [50,51,71] |
| Amathia tortusa† | Ctenostomata | 50, 51 | Alkaloids | [52] |
| Amathia convoluta | Ctenostomata | 52–65, 107–112 | Alkaloids | [53–57,70] |
| Amathia alternate | Ctenostomata | 67–70 | Alkaloids | [59] |
| Biflustra grandicella† | Cheilostomata | 36–38, 41 | Sterols | [46] |
| Bugula dentate | Cheilostomata | 122–126 | Alkaloids | [73,74] |
| Bugula longissima† | Cheilostomata | 121 | Alkaloids | [72] |
| Bugula neritina | Cheilostomata | 1–22, 27, 28, 35, 42, 43, 150–156 | Macrocyclic lactones, sterols, sphingolipids | [12–17,36,39,40,44,45,48,87,88] |
| Caulibugula intermis† | Cheilostomata | 134–139, 146,147 | Alkaloids | [79] |
| Chartella papyracea | Cheilostomata | 77–81 | Alkaloids | [49] |
| Cribricellina cribraria | Cheilostomata | 144 | Alkaloids | [82] |
| Cryptosula pallasiana† | Cheilostomata | 22–26, 29–31, 39, 40, 74–76, 119,120, 140, 157,158, 161 | Sterols, alkaloids, sphingolipids | [37,38,60,80] |
| Dendrobeania murrayana | Cheilostomata | 159 | Terpenoids | [89] |
| Euthyroides episcopalis† | Cheilostomata | 141–143 | Alkaloids | [81] |
| Flustra foliacea | Cheilostomata | 92–104, 133 | Alkaloids | [66–69,78] |
| Hincksinoflustra denticulate | Cheilostomata | 91 | Alkaloids | [63] |
| Myriapora truncate | Cheilostomata | 32–34 | Sterols | [43] |
| Pentapora fascialis† | Cheilostomata | 160–162 | Aromatic compounds | [91] |
| Pterocella vesiculosa† | Cheilostomata | 127–132, 145 | Alkaloids | [75–77,83] |
| Securiflustra securifrons† | Cheilostomata | 66, 82–90 | Alkaloids | [58,61,62] |
| Sessibugula translucens | Cheilostomata | 121 | Alkaloids | [72] |
| Watersipora cucullata† | Cheilostomata | 148–149 | Alkaloids | [85] |
| Wateripora subtorquata† | Cheilostomata | 160 | Aromatic compounds | [90] |
| Zoobotryon verticillatum | Ctenostomata | 71–73 | Alkaloids | [49] |
†Secondary metabolites for marine bryozoans studied for the first time since 1996.
Table 2. . Bioactivities of the typical compounds from marine bryozoans.
| Compound types | Typical compounds | Biological activities | Ref. |
|---|---|---|---|
| Macrocyclic lactones | 1–18 | Antitumor activity Enhancing memory and learning Immune modulation |
[12,13,17,25–35] |
| 20 | Antipredator defense | [15] | |
| 19, 21 | Antitumor activity | [14,16] | |
| Sterols | 22, 23, 26–30, 39, 40, 42, 43 | Antitumor activity | [37,38,40,44] |
| 40 | Inhibitory effects for cholesterol acyltransferase | [47] | |
| Alkaloids | 50, 51 | Antitrypanosomal activity | [52] |
| 50, 51, 54–56, 59, 95, 107,108, 113, 117, 127,128, 130, 134–139, 142, 144–147 | Antitumor activity | [52,55,56,68,70,71,75–77,79,81–84] | |
| 53 | Nematicidal activity | [54] | |
| 59 | Inhibitory effects for cell division | [55,56] | |
| 61–65 | Antifeedant activity | [57] | |
| 67–69, 96, 99, 102, 113, 117, 126–128, 145 | Antibacterial activity | [59,69,71,74–76,82,83] | |
| 117, 127 | Antiviral activity | [71,75,76] | |
| 122 | Antipredator defense | [73] | |
| 146,147 | Antimalarial activity | [84] | |
| Sphingolipids | 148,149 | Inhibitory effects for human DNA topoisomerase I | [86] |
| 152–158 | Antitumor activity | [38] | |
| Tetracyclic terpenoid lactone | 159 | Inhibitory effects for metalloprotease collagenase IV | [89] |
| Sulfur-containing aromatic compounds | 160 | Antiangiogenic activity | [90] |
| 162–164 | Anthelmintic activity | [91] | |
Future perspective
Summarily, in the last two decades, although relatively little research has been undertaken into the secondary metabolites of bryozoans comparing with other marine invertebrates, bryozoans have proven to be an excellent source for novel and bioactive compounds. However, the low productivity with complicated structural feature for bryozoans’ metabolites is a bottleneck to discover pharmacologic leads from marine bryozoans. Fortunately, there are evidences to verify that some of bioactive secondary metabolites are actually produced by co-epiphyte microorganisms of marine bryozoans. It provides possibility to engineering produce bioactive secondary metabolites for further systematic evaluation of their bioactivities. In our view, like the success to develop bryostatin 1 as new drug candidate, more and more novel lead compounds can be discovered from marine bryozoans and their co-epiphyte microorganisms in the progress of new drug discovery. Furthermore, the success of antibody–drug conjugate in reducing drug's toxic side-effects provides potential for the development of these lead compounds as new drugs.
Executive summary.
Background
Natural products serve as an inspiration to discovery of new drug candidates. Marine bryozoans are well known producers of bioactive secondary metabolites and important marine drug sources.
Macrocyclic lactones from marine bryozoans
The structure features, bioactivities, structure–activity relationship (SAR) and mechanisms are summarized for 21 bryostatins (1–21) and three derivatives from marine bryozoans.
The 26-membered macrolactone ring, hydroxyl at C-3 with (R)-stereochemistry, free hydroxyl at C-26 and the structure in the C7-C9 region of the A-ring are critical to bryostatins for their potent inhibition of tumor cells.
The significant bioactivity including antitumor, enhancing memory and learning, and immune modulatory properties for bryostatins inspire researcher focused on total synthesis and synthesis bryostatin analogs for new drug candidates.
Sterols from marine bryozoans
Sterols with moderate cytotoxicity from bryozoans can be divided into four types of 3β-hydroxy Δ5-steroid, 3β-hydroxy Δ5–7-one-steroid, 3β,5α,6β-trihydroxy Δ7-steroid and 3β,7ζ-dihydroxy Δ5-steroid, and other types involving 3α-hydroxy Δ5-steroid and 3β,6β-dihydroxy Δ4-steroid.
The SAR for sterols indicates the double bond at C-7 and missing trans double bond at C-22 can decrease their cytotoxicity.
Alkaloids from marine bryozoans
Alkaloids are the major components for bryozoans. They can be divided into four main types of β-phenylethylamine alkaloids, indole alkaloids, γ-lactam alkaloids and pyrrole alkaloids. Besides, other types of alkaloids including pyridine, indolizine, quinoline, isoquinoline, quinolinone, quinone methide, β-carboline and 2,6-naphthyridine are also summarized.
Alkaloids from marine bryozoans show variety of biological activities including antitrypanosomal, antitumor, nematicidal, antifeedant, antibacterial, antiviral, antipredator and antimalarial activities, as well as inhibitory effects for cell division.
Alkaloids (50 and 53) can be developed as insecticidal agents. Alkaloids (127, 128 and 134–139) have potential to be developed as antitumor agents.
Sphingolipids from marine bryozoans
Sphingolipids from bryozoans with moderate cytotoxicity are discussed. Sphingolipids sulfates (148 and 149) can be developed as antitumor agents.
The SAR analysis demonstrates the trans double bond between C-4 and C-5, the category of the sugar moieties at C-1 and the hydroxyl at C-2′ or C-4, are important for their cytotoxicity.
Other secondary metabolites from marine bryozoans
Inhibitory effects for metalloprotease of tetracyclic terpenoid lactone, as well as the antiangiogenic and anthelmintic activities of sulfur-containing aromatic compounds are reviewed from bryozoans. Bryoabthrathiophene (160) can be developed as antiangiogenic agent.
Strategies to solve the source & resupply for bioactive bryozoan metabolites
Cultivation of bryozoans is an effective way to solve the source and resupply for bioactive bryozoan metabolites.
Supplementary Material
Footnotes
Supplementary data
FS: See online at: https://www.future-science.com/doi/10.4155/fmc-2018-0012
Author contributions
As first author and corresponding author, XR Tian organized and wrote the whole manuscript. Co-first author, HF Tang gave the idea, financial support and careful revision of the manuscript. XL Tian, JJ Hu and LL Huang searched the related references, drew the related figures and tables and gave suggestions to improve the manuscript. KR Gustafson edited and corrected the final version of the manuscript.
Financial and competing interests disclosure
This work was financially supported by National Key R&D Program of China (2017YFD0201105), National Natural Science Foundation of China (no. 31201551 and no. 81473132), and Natural Science Foundation of Shaanxi Province, China (no. 2017JM3015). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8(6):531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 2.Keohane CE, Steele AD, Fetzer C, et al. Promysalin elicits species-selective inhibition of pseudomonasaeruginosa by targeting succinate dehydrogenase. J. Am. Chem. Soc. 2018;140(5):1774–1782. doi: 10.1021/jacs.7b11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Wang W, Chen L, et al. Discovery of a VHL and HIF1α interaction inhibitor with in vivo angiogenic activity via structure based virtual screening. Chem. Commun. 2016;52(87):12837–12840. doi: 10.1039/c6cc04938a. [DOI] [PubMed] [Google Scholar]
- 4.Liu LJ, Wang W, Huang SY, et al. Inhibition of the Ras/Raf interaction and repression of renal cancer xenografts in vivo by an enantiomeric iridium(III) metal-based compound. Chem. Sci. 2017;8(7):4756–4763. doi: 10.1039/c7sc00311k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Wang W, Liang JX, et al. A rhodium(III)-based inhibitor of lysine-specific histone demethylase 1 as an epigenetic modulator in prostate cancer cells. J. Med. Chem. 2017;60(6):2597–2603. doi: 10.1021/acs.jmedchem.7b00133. [DOI] [PubMed] [Google Scholar]
- 6.Zhong HJ, Lu L, Leung KH, et al. An iridium(III)-based irreversible protein–protein interaction inhibitor of BRD4 as a potent anticancer agent. Chem. Sci. 2015;6(10):5400–5408. doi: 10.1039/c5sc02321a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albada B, Metzler-Nolte N. Highly potent antibacterial organometallic peptide conjugates. Acc. Chem. Res. 2017;50(10):2510–2518. doi: 10.1021/acs.accounts.7b00282. [DOI] [PubMed] [Google Scholar]
- 8.Montaser R, Luesch H. Marine natural products: a new wave of drugs? Future Med. Chem. 2011;3(12):1475–1489. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2017;34(3):235–294. doi: 10.1039/c6np00124f. [DOI] [PubMed] [Google Scholar]
- 10.Wood ACL, Probert PK, Rowden AA, Smith AM. Complex habitat generated by marine bryozoans: a review of its distribution, structure, diversity, threats and conservation. Aquatic Conserv.: Mar. Freshw. Ecosyst. 2012;22(4):547–563. [Google Scholar]
- 11.Taylor PD, Waeschenbach A. Phylogeny and diversification of bryozoans. Palaeontology. 2015;58(4):585–599. [Google Scholar]
- 12.Pettit GR, Herald CL, Doubek DL, et al. Isolation and structure of bryostatin1. J. Am. Chem. Soc. 1982;104(24):6846–6848. [Google Scholar]; • The first bioactive macrocyclic lactone, bryostatin 1, was identified from Bugula neritina.
- 13.Mutter R, Wills M. Chemistry and clinical biology of the bryostatins. Bioorg. Med. Chem. 2000;8(8):1841–1860. doi: 10.1016/s0968-0896(00)00150-4. [DOI] [PubMed] [Google Scholar]; • Review for the chemistry and clinical biology of bryostatins 1–18.
- 14.Lin H, Liu G, Yi Y, Yao X, Wu H. Studies on antineoplastic constituents from marine bryozoan Bugula neritina inhabiting South China Sea: isolation and structural elucidation of a novel macrolide. Acad. J. Sec. Mil. Med. Univ. 2004;25(5):473–478. [Google Scholar]
- 15.Lopanik N, Gustafson KR, Lindquist N. Structure of bryostatin 20: a symbiont-produced chemical defense for larvae of the host bryozoan, Bugula neritina . J. Nat. Prod. 2004;67(8):1412–1414. doi: 10.1021/np040007k. [DOI] [PubMed] [Google Scholar]
- 16.Yu HB, Yang F, Li YY, Gan JH, Jiao WH, Lin HW. Cytotoxic bryostatin derivatives from the South China Sea broyozoan Bugula neritina . J. Nat. Prod. 2015;78(5):1169–1173. doi: 10.1021/acs.jnatprod.5b00081. [DOI] [PubMed] [Google Scholar]
- 17.Ruan BF, Zhu HL. The chemistry and biology of the bryostatins: potential PKC inhibitors in clinical development. Curr. Med. Chem. 2012;19(16):2652–2664. doi: 10.2174/092986712800493020. [DOI] [PubMed] [Google Scholar]; • Review for the mechanism of bryostatins.
- 18.Kageyama M, Tamura T, Nantz MH, et al. Synthesis of bryostatin 7. J. Am. Chem. Soc. 1990;112(20):7407–7408. [Google Scholar]
- 19.Trost BM, Dong G. Total synthesis of bryostatin 16 using atom-economical and chemoselective approaches. Nature. 2008;456(7221):485–488. doi: 10.1038/nature07543. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important approach that can be used to synthesize various bryostatins and their analogs in the laboratory.
- 20.Keck GE, Poudel YB, Cummins TJ, Rudra A, Covel JA. Total synthesis of bryostatin 1. J. Am. Chem. Soc. 2011;133(4):744–747. doi: 10.1021/ja110198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wender PA, Baryza JL, Hilinski MK, et al. Beyond natural products: synthetic analogues of bryostatin 1. In: Huang Z, editor. Drug Discovery Research: New Frontiers in the Post-Genomic Era. Wiley; NJ, USA: 2007. pp. 127–162. [Google Scholar]; • A review for partial synthesis of bryostatin analogs in the progress of new drug discovery.
- 22.DeChristopher BA, Fan AC, Felsher DW, Wender PA. “Picolog,” a synthetically-available bryostatin analog, inhibits growth of MYC-induced lymphoma in vivo . Oncotarget. 2012;3(1):58–66. doi: 10.18632/oncotarget.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro . Nat. Chem. 2012;4(9):705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staveness D, Abdelnabi R, Near KE, et al. Inhibition of chikungunya virus-induced cell death by salicylate-derived bryostatin analogues provides additional evidence for a PKC-independent pathway. J. Nat. Prod. 2016;79(4):680–684. doi: 10.1021/acs.jnatprod.5b01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollára P, Rajchard J, Balounováb Z, Pazourek J. Marine natural products: bryostatins in preclinical and clinical studies. Pharma. Biol. 2014;52(2):237–242. doi: 10.3109/13880209.2013.804100. [DOI] [PubMed] [Google Scholar]
- 26.Kedei N, Michalowski AM, Blumberg PM. Transient duration of action is the primary mechanism responsible for the unique biology of bryostatin 1. Cancer Res. 2014;74(Suppl. 19):3195. [Google Scholar]
- 27.Irie K, Yanagita RC, Nakagawa Y. Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism of protein kinase Cδ. Med. Res. Rev. 2012;32(2):518–535. doi: 10.1002/med.20220. [DOI] [PubMed] [Google Scholar]
- 28.Kedei N, Lewin NE, Géczy T, et al. Biological profile of the less lipophilic and synthetically more accessible bryostatin 7 closely resembles that of bryostatin 1. ACS Chem. Biol. 2013;8(4):767–777. doi: 10.1021/cb300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson TJ, Sun M-K, Lim C, et al. Bryostatin effects on cognitive function and PKCε in Alzheimer's disease Phase IIa and expanded access trials. J. Alzheimer's Dis. 2017;58(2):521–535. doi: 10.3233/JAD-170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrott LM, Jackson K, Yi P, et al. Acute oral bryostatin-1 administration improves learning deficits in the APP/PS1 transgenic mouse model of Alzheimer's disease. Curr. Alzheimer Res. 2015;12(1):22–31. doi: 10.2174/1567205012666141218141904. [DOI] [PubMed] [Google Scholar]
- 31.Alkon DL, Hongpaisan J, Sun MK. Effects of chronic bryostatin-1 on treatment-resistant depression in rats. Euro. J. Pharmacol. 2017;807:71–74. doi: 10.1016/j.ejphar.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Tan Z, Turner RC, Leon RL, et al. Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke. 2013;44(12):3490–3497. doi: 10.1161/STROKEAHA.113.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizutani K, Sonoda S, Wakita H, et al. Effects of exercise and bryostatin-1 on serotonin dynamics after cerebral infarction. Neruroreport. 2016;27(9):659–664. doi: 10.1097/WNR.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 34.Díaz L, Martínez-Bonet M, Sánchez J, et al. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-κB-dependent mechanism. Sci. Rep. 2015;5:12442. doi: 10.1038/srep12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutiérrez C, Serrano-Villar S, Madrid-Elena N, et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. Aids. 2016;30(9):1385–1392. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 36.Kerr RG, Vicchiarelli R, Kerr SS. Identification and biosynthetic origins of sterols in the marine bryozoan Bugula neritina . J. Nat. Prod. 1999;62(3):468–470. doi: 10.1021/np9804740. [DOI] [PubMed] [Google Scholar]
- 37.Tian XR, Tang HF, Li YS, et al. New cytotoxic oxygenated sterols from the marine bryozoan Cryptpsula pallasiana . Mar. Drugs. 2011;9(2):162–183. doi: 10.3390/md9020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian XR, Gao YQ, Tian XL, et al. New cytotoxic secondary metabolites from marine bryozoan Cryptosula pallasiana . Mar. Drugs. 2017;15(4):120. doi: 10.3390/md15040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Wang Z, Zhang H, et al. Steroids from the marine bryozoan Bugula neritina . Chem. Nat. Comp. 2010;46(2):390–392. [Google Scholar]
- 40.Yang F, Zhang HJ, Chen JT, et al. New cytotoxic oxygenated sterols from marine bryozoan Bugula neritina . Nat. Prod. Res. 2011;25(16):1505–1511. doi: 10.1080/14786410903211235. [DOI] [PubMed] [Google Scholar]
- 41.Notaro G, Piccialli V, Sica D. New steroidal hydroxyketones and closely related diols from the marine sponge Cliona copiosa . J. Nat. Prod. 1992;55(11):1588–1594. [Google Scholar]
- 42.Riccardis F, Minale L, Iorizzi M, Debitus C, Lévi C. Marine sterols. Side-chain-oxygenated sterols. Possibly of abiotic origin, from the New Caledonian sponge Stelodoryx chlorophylla . J. Nat. Prod. 1993;56(2):282–287. [Google Scholar]
- 43.Cafieri F, Fattorusso E, Gavagnin M, Santacroce C. 3β,5α,6β-trihydroxysterols from the Mediterranean bryozoan Myriapora truncata . J. Nat. Prod. 1985;48:944–947. [Google Scholar]
- 44.Tian XR, Tang HF, Li YS, et al. New 3β, 6β-dihydroxy and 3β,5α,6β-trihydroxy sterols from marine bryozoan Bugula neritina in South China Sea and their cytotoxicity. Phytochem. Lett. 2014;9:1–6. [Google Scholar]
- 45.Liu TF, Lu X, Tang H, et al. 3β,5α,6β-Oxygenated sterols form the South China Sea gorgonian Muriceopsis flavida and their tumor cell growth inhibitory activity and apoptosis-inducing function. Steroids. 2013;78(1):108–114. doi: 10.1016/j.steroids.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Yang F, Zhang HJ, Liu XF, Chen WS, Tang HF, Lin HW. Oxygenated steroids from marine bryozoan Biflustra grandicella . Biochem. Syst. Ecol. 2009;37(5):686–689. [Google Scholar]
- 47.Harte RA, Yeaman SJ, McElhinney J, Suckling CJ, Jackson B, Suckling KE. Effects of novel synthetic sterol probes on enzymes of cholesterol metabolism in cell-free and cellular systems. Chem. Phys. Lipids. 1996;83(1):45–59. doi: 10.1016/0009-3084(96)02593-5. [DOI] [PubMed] [Google Scholar]
- 48.Tian XR, Tang HF, Li YS, Lin HW, Ma N, Zhang W. Sterols from marine bryozoan Bugula neritina . Biochem. Syst. Ecol. 2010;38(3):435–437. [Google Scholar]
- 49.Blackman AJ, Walls JT. Bryozoan secondary metabolites and their chemical ecology. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Elsevier; Amsterdam, The Netherlands: 1995. pp. 73–112. [Google Scholar]; • Review for the secondary metabolites and their chemical ecology from marine bryozoans before 1996.
- 50.Blackman AJ, Matthews DJ. Amathamide alkaloids from the marine bryozoan Amathia wilsoni Kirkpatrick. Heterocycles. 1985;23(11):2829–2833. [Google Scholar]
- 51.Blackman AJ, Green RD, Blackman AJ, Green RD. Further amathamide alkaloids from the bryozoan Amathia wilsoni . Aust. J. Chem. 1987;40(10):1655–1662. [Google Scholar]
- 52.Davis RA, Sykes M, Avery VM, Camp D, Quinn RJ. Convolutamines I and J, antitrypanosomal alkaloids from the bryozoan Amathia tortusa . Bioorg. Med. Chem. 2011;19(22):6615–6619. doi: 10.1016/j.bmc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Blackman AJ, Eldershaw TPD, Garland SM. Alkaloids from two further Amathia bryozoan species. Aust. J. Chem. 1993;46(3):401–405. [Google Scholar]
- 54.Narkowicz CK, Blackman AJ, Lacey E, Gill JH, Heiland K. Convolutindole A and Convolutamine H, new nematocidal brominated alkaloids from the marine bryozoan Amathia convoluta . J. Nat. Prod. 2002;65(6):938–941. doi: 10.1021/np010574x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Kamano Y, Kizu H, Itokawa H, Pettit GR, Herald CL. Convolutamines A-E, novel β-phenylethylamine alkaloids from marine bryozoan Amathia convoluta . Chem. Lett. 1995;26(21):2771–2774. [Google Scholar]
- 56.Kamano Y, Kotake A, Hashima H, et al. Three new alkaloids, convolutamines F and G, and convolutamydine E, from the Floridian marine bryozoan Amathia convolute . Collect. Czech. Chem. Commun. 1999;30(45):1147–1153. [Google Scholar]
- 57.Montanari AM, Fenical W, Lindquist N, Lee AY, Clardy J. Volutamides A-E, halogenated alkaloids with antifeedant properties from the Atlantic bryozoan Amathia convoluta . Tetrahedron. 1996;52(15):5371–5380. [Google Scholar]
- 58.Michael P, Hansen KØ, Isaksson J, Andersen JH, Hansen E. A novel brominated alkaloid securidine A, isolated from the marine bryozoan Securiflustra securifrons . Molecules. 2017;22(7):1236. doi: 10.3390/molecules22071236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee NK, Fenical W, Lindquist N. Alternatamides A–D: new bromtryptamine peptide antibiotics from the atlantic marine bryozoan Amathia alternata . J. Nat. Prod. 1997;60(7):697–699. doi: 10.1021/np970042+. [DOI] [PubMed] [Google Scholar]
- 60.Tian XR, Tang HF, Li YS, et al. Alkaloids from marine bryozoan Cryptpsula pallasiana . Biochem. Syst. Ecol. 2010;38(6):1250–1252. [Google Scholar]
- 61.Rahbaek L, Anthoni U, Christophersen C, Nielson PH, Petersen BO. Marine alkaloids. 18. Securamines and securines, halogenated indole-imidazole alkaloids from the marine bryozoan Securiflustra securifrons . J. Org. Chem. 1996;61(3):887–889. [Google Scholar]
- 62.Rahbk L, Christophersen C. Marine alkaloids. 19. Three new alkaloids, securamine E–G, from the marine bryozoans Securiflustra Securifrons . J. Nat. Prod. 1997;60(2):175–177. [Google Scholar]
- 63.Higuchi K, Sato Y, Tsuchimochi M, Suqiura K, Hatori M, Kawasaki T. First total synthesis of hinckdentine A. Org. Lett. 2009;11(1):197–199. doi: 10.1021/ol802394n. [DOI] [PubMed] [Google Scholar]
- 64.Sharp JH, Winson MK, Porter JS. Bryozoan metabolites: an ecological perspective. Nat. Prod. Rep. 2007;24(4):659–673. doi: 10.1039/b617546e. [DOI] [PubMed] [Google Scholar]
- 65.Holst PB, Anthoni U, Christophersen C, Nielsen PH. Marine alkaloids, 15. Two alkaloids, flustramine E and debromoflustramine B, from the marine bryozoan Flustra foliacea . J. Nat. Prod. 1994;57(7):997–1000. doi: 10.1021/np50109a020. [DOI] [PubMed] [Google Scholar]
- 66.Peters L, König GM, Terlau H, Wright AD. Four new bromotryptamine derivatives from the marine bryozoan Flustra foliacea . J. Nat. Prod. 2002;65(11):1633–1637. doi: 10.1021/np0105984. [DOI] [PubMed] [Google Scholar]
- 67.Peters L, Wright AD, Kehraus S, Gündisch D, Tilotta MC, König GM. Prenylated indole alkaloids from Flustra foliacea with subtype specific binding on NAChRs. Planta Med. 2004;70(10):883–886. doi: 10.1055/s-2004-832610. [DOI] [PubMed] [Google Scholar]
- 68.Lysek N, Rachor E, Lindel T. Isolation and structure elucidation of deformlflustrabromine from the North Sea bryozoans Flustra foliacea . Z. Naturforsch. 2002;57(12):1056–1061. doi: 10.1515/znc-2002-11-1218. [DOI] [PubMed] [Google Scholar]
- 69.Rochfort SJ, Moore S, Craft C, Martin NH, Van Wagoner RM, Wright JL. Further studies on the chemistry of the Flustra alkaloids from the bryozoan Flustra foliacea . J. Nat. Prod. 2009;72(10):1773–1781. doi: 10.1021/np900282j. [DOI] [PubMed] [Google Scholar]
- 70.Zhang HP, Shigemori H, Ishibashi M, et al. Convolutamides A–F, novel γ-lactam alkaloids from the marine bryozoan Amathia convoluta . Tetrahedron. 1994;50(34):10201–10206. [Google Scholar]
- 71.Morris BD, Prinsep MR. Amathaspiramides A–F, novel brominated alkaloids from the marine bryozoan Amathia wilsoni . J. Nat. Prod. 1999;62(5):688–693. doi: 10.1021/np980410p. [DOI] [PubMed] [Google Scholar]
- 72.Lebar MD, Heimbegner JL, Baker BJ. Cold-water marine natural products. Nat. Prod. Rep. 2007;24(4):774–797. doi: 10.1039/b516240h. [DOI] [PubMed] [Google Scholar]
- 73.Blackman AJ, Li C. New tambjamine alkaloids from the marine bryozoan Bugula dentata . Aust. J. Chem. 1994;47(8):1625–1629. [Google Scholar]
- 74.Matsunaga S, Fusetani N, Hashimoto K. Bioactive marine metabolites. VIII. Isolation of an antimicrobial blue pigment from the bryozoans Bugula dentata . Experientia. 1986;42(1):84. [Google Scholar]
- 75.Yao B, Prinsep MR, Nicholson BK, Gordon DP. The pterocellins, novel bioactive alkaloids from the marine bryozoan Pterocella vesiculosa . J. Nat. Prod. 2003;66(8):1074–1077. doi: 10.1021/np030104y. [DOI] [PubMed] [Google Scholar]
- 76.Prinsep MR. Further pterocellins from the New Zealand marine bryozoan Pterocella vesiculosa . J. Nat. Prod. 2008;71(1):134–136. doi: 10.1021/np070255r. [DOI] [PubMed] [Google Scholar]
- 77.Wang AT, Prinsep MR, Martinus R. Pterocellin A isolated from marine bryozoan Pterocella vesiculosa is cytotoxic to human HeLa cells via mitochondrial apoptotic processes. Springerplus. 2016;5(1):742. doi: 10.1186/s40064-016-2397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wulff P, Carlé JS, Christophersen C. Marine alkaloids 6. The first naturally occurring bromo-substituted quinoline from Flustra foliacea . Comp. Biochem. Physiol., B: Biochem. Mol. Biol. 1982;71(3):525–526. [Google Scholar]
- 79.Milanowski DJ, Gustafson KR, Kelley JA, McMahon JB. Caulibugulones A–F, novel cytotoxic isoquinoline quinones and iminoquinones from the marine bryozoan Caulibugula intermis . J. Nat. Prod. 2004;67(1):70–73. doi: 10.1021/np030378l. [DOI] [PubMed] [Google Scholar]
- 80.Tian XR, Tang HF, Li YS, et al. Studies on the chemical constituents from marine bryozoan Cryptosula pallasiana . Rec. Nat. Prod. 2015;9(4):628–632. [Google Scholar]
- 81.Morris BD, Prinsep MR. The euthyroideones, novel brominated quinone methides from the New Zealand marine bryozoan Euthyroides episcopalis . J. Org. Chem. 1998;63(25):9545–9547. [Google Scholar]
- 82.Harwood DT, Urban S, Blunt JW, Munro MH. β-Carbolines from the New Zealand bryozoan, Cribricellina cribraria . Nat. Prod. Res. 2003;17(1):15–19. doi: 10.1080/1057563021000001063. [DOI] [PubMed] [Google Scholar]
- 83.Till M, Prinsep MR. 5-Bromo-8-methoxy-1-methyl-beta-carboline, an alkaloid from the New Zealand marine bryozoan Pterocella vesiculosa . J. Nat. Prod. 2009;72(4):796–798. doi: 10.1021/np8007655. [DOI] [PubMed] [Google Scholar]
- 84.Milanowski DJ, Oku N, Cartner LK, et al. Unequivocal determination of caulamidines A and B: application and validation of new tools in the structure elucidation tool box. Chem. Sci. 2018;9:307–314. doi: 10.1039/c7sc01996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ojika M, Yoshino G, Sakagami Y. Novel ceramide 1-sulfates, potent DNA topoisomerase I inhibitors isolated from the bryozoan Watersipora cucullata . Tetrahedron Lett. 1997;38(24):4235–4238. [Google Scholar]
- 86.Zhou G, Huang M, Shi J. Ceramides and cerebrosides from Bugula neritina . Chin. J. Mar. Drugs. 2005;24(6):37–40. [Google Scholar]
- 87.Tian XR, Tang HF, Li YS, et al. Ceramides and cerebrosides from the marine bryozoan Bugula neritina inbating South China Sea. J. Asian Nat. Prod. Res. 2009;11(12):1005–1012. doi: 10.1080/10286020903207050. [DOI] [PubMed] [Google Scholar]
- 88.Tian XR, Tang HF, Feng JT, et al. Neritinaceramides A–E, new ceramides from the marine bryozoan Bugula neritina inhibiting South China Sea and their cytotoxicity. Mar. Drugs. 2014;12(4):1987–2003. doi: 10.3390/md12041987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu CM, Wright JLC. Murrayanolide, an unusual C21 tetracyclic terpenoid lactone from the marine bryozoan Dendrobeania murrayana . J. Nat. Prod. 1995;58(12):1978–1982. doi: 10.1021/np50126a033. [DOI] [PubMed] [Google Scholar]
- 90.Jeong SJ, Higuchi R, Miyamoto T, Ono M, Kuwano M, Mawatari SE. Bryoanthrathiophene, a new antiangiogenic constituent from the bryozoan Watersipora subtorquata (d'Orbigny, 1852) J. Nat. Prod. 2002;65(9):1344–1345. doi: 10.1021/np010577+. [DOI] [PubMed] [Google Scholar]
- 91.Eisenbarth S, Gehling M, Harder A, Steffan B. Pentaporins A, B and C: disulfides from the marine bryozoan Pentapora fascialis . Tetrahedron. 2002;58(42):8461–8464. [Google Scholar]
- 92.Davidson SK, Haygood MG. Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont ‘Candidatus Endobugula sertula’. Biol. Bull. 1999;196(3):273–280. doi: 10.2307/1542952. [DOI] [PubMed] [Google Scholar]; • An example to cultivate bryozoans in laboratory to produce bioactive secondary metabolites.
- 93.Sudek S, Lopanik NB, Waggoner LE, et al. Identification of the putative bryostatin polyketide synthase gene cluster from ‘Candidatus Endobugula sertula’, the uncultivated microbial symbiont of the marine bryozoan Bugula neritina . J. Nat. Prod. 2007;70(1):67–74. doi: 10.1021/np060361d. [DOI] [PubMed] [Google Scholar]
- 94.Schwartsmann G, da Rocha AB, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2(4):221–225. doi: 10.1016/s1470-2045(00)00292-8. [DOI] [PubMed] [Google Scholar]
- 95.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30(7):631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 96.Diamantis N, Banerji U. Antibody–drug conjugates – an emerging class of cancer treatment. Br. J. Cancer. 2016;114(4):362–367. doi: 10.1038/bjc.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.