Abstract
Chimeric antigen receptor modified T cells targeting CD19 and CD20 have shown activity in Phase I, II trials of patients with hematological malignancies. We conducted a systematic review and meta-analysis of all published clinical trials studying the role of efficacy as well as safety of CD-19 and CD-20 chimeric antigen receptor-T therapy for B-cell hematologic malignancies. A total of 16 studies with 195 patients were identified. The pooled analysis showed an overall response rate of 61% (118/195) with complete response of 42% (81/195) and partial response of 19% (37/195). Major adverse events were cytokine release syndrome 33%, neurotoxicity 33% and B-cell aplasia 54%. Collectively, the results indicate encouraging response in relapsed/refractory B lymphoma and leukemia, especially in acute lymphoblastic leukemia (ALL) patients.
Keywords: : chimeric antigen T cells, hematological malignancy, leukemia, lymphoma, refractory, relapse
Despite major therapeutic advances in combination chemotherapy, immunotherapy, radiation therapy, targeted therapy and stem cell transplantation, relapsed or refractory B-cell malignancies still carry poor prognosis. Therefore, promising results of adoptive cell therapy with chimeric antigen receptor (CAR) T cells suggest a feasible approach for the management of refractory or relapsing lymphoproliferative disorders [1–4] such as ALL [5–9], chronic lymphocytic leukemia (CLL) [10–12] and non-Hodgkin lymphoma (NHL) [13–15 ]. B-cell hematological malignancies express B-cell-specific tumor-associated antigens (TAA) namely CD19 and CD20, and can serve as primary targets for CAR T-cell immunotherapy [16]. In CAR T-cell immunotherapy, patients’ autologous T cells are genetically modified to express an artificial T-cell receptor known as CAR. CAR T cells are designed to effectively recognize and bind tumor cells expressing TAA and subsequently eliminate these cells by secreting perforin and granzymes [3]. Currently, three generations of CAR T cells have been constructed. The first generation CAR T cells lacks costimulatory domain, second generation has one (CD28 or 4–1BB) [17,18] and third generation has two (CD28 and 4-1BB or OX-40) costimulatory domains. The second and third generation CAR T cells have shown better responses compared with first generation CARs [8] because of effective cell division and optimal cytokine production along with prolonged T-cell expansion and sustained antitumor effects [19–21].
CAR-T-cell immunotherapy has several advantages over other available approaches. These cells have the ability to specifically recognize the targeted tumor antigens with subsequent tumor lysis. After tumor lysis, these cells may persist as memory cells, suggesting that these might be more effective than monoclonal antibodies [22]. In addition, this technique is used as a salvage or bridge therapy in many cases and does not have the limitations of HLA incompatibility posed by hematopoietic stem cell transplantation (HSCT), and even response has been observed in postallogenic stem cell (AlloSCT) relapse and blinatumomab refractory ALL patients [7]. Specificity of CAR-mediated T-cell recognition depends on expressed antibody domain, this ability is independent of major histocompatibility complex presentation and can potentially be used against any oncologic or even nononcologic target for which an antibody is available. Previously conducted reviews of anti-CD19CART therapy by XuX-J et al. [23], Zhu et al. [24] and Zhang et al. [25] have become outdated with publication of recent trials and we are including additional data for anti-CD20 CAR T immunotherapy trials. Hence, an updated analysis is warranted which utilizes the most recent data to compare the efficacy as well as safety of CD19 and CD20 CAR T therapy in ALL, CLL and NHL.
Materials & methods
The meta-analysis was designed in accordance with the principles set by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist.
Eligibility criteria
Inclusion criteria specified all clinical studies with adult patients who had B-cell malignancy (ALL, CLL and non-Hodgkin lymphoma and underwent anti-CD19 or anti-CD20 CAR T-cell therapy. We excluded ongoing clinical trials without reported outcomes, studies not reporting survival outcomes, studies with acute myeloid leukemia patients and studies not using anti-CD19 or anti-CD20 CAR T cells.
Search strategy
Literature search was performed using following electronic bibliographic databases: MEDLINE (Ovid SP and PubMed), EMBASE, The Cochrane Library (Cochrane Database of Systematic Reviews) and Cochrane Central Register of Controlled Trials (CENTRAL), Scopus and Web of Science. The initial search was not restricted to English. The searches were repeated just before the final analyses and further studies retrieved for inclusion till 25 May 2016. The bibliographies of retrieved articles and previous review articles were hand searched to obtain additional articles. Search terms and full details of the search strategy for each database are provided (refer to appendix).
Data extraction
Using the search strategy, we obtained titles and/or abstracts of retrieved studies and imported them to endnote. Two investigators independently screened the titles and abstracts; the full texts were screened if the articles met the inclusion criteria. Full text of these selected articles was obtained and evaluated by two investigators to confirm eligibility for inclusion. Data were extracted using a structured template and disagreement resolved with consensus during the process of screening and data extraction. A standardized data extraction form was used to extract the following fields: author/year, phase of the study, age, sex, study center, patient population, pre-CAR T-cell infusion HSCT, post-CAR T-cell infusion HSCT, generation of CAR T cells, receptor, CAR construct and signaling, gene transfer strategy, infused CAR T-cell dose, conditioning or lymphodepleting chemotherapy, IL-2, persistence of CAR T cells, peak blood CAR T-cell level, peak TNF, IFN-γ, peak IL-6, origin type of the CAR T cells (autologous vs donor derived/allogeneic), outcomes, survival and adverse effects.
Outcome measures
The primary outcomes were overall response (OR). Secondary outcomes were complete response (CR) and partial response (PR). The other outcomes were stable disease, progressive disease, progression-free survival (PFS) and overall survival (OS). The toxicity data were analyzed for three main categories: grade 3–4 cytokine release syndrome (CRS), severe neurotoxicity and B-cell aplasia.
Statistical analysis
We performed a meta-analysis using Comprehensive Meta-analysis 3.0 using random effects model. The heterogeneity was assessed using I2values.
Subgroup analysis
We performed prespecified subgroup analysis to assess the efficacy of allogeneic CAR T-cell (donor derived) immunotherapy in ALL, CLL and NHL patients.
Results
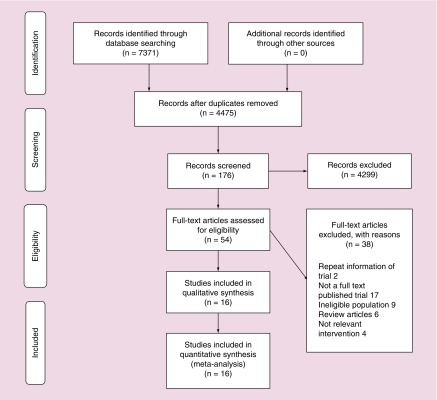
A comprehensive database search of Medline/PubMed, Scopus, Embase, Cochrane CENTRAL, Web of Science, SCI-Expanded, WOS CPCI-S retrieved 4476 citations after duplicates removed. After screening the titles, 176 studies were considered eligible for further review, and 60 potentially eligible articles were assessed for inclusion and finally 16 studies were included in the systematic review. The characteristics of the 16 included studies are summarized in Table 1. The search strategy is documented in the PRISMA flow sheet (Figure 1).
Table 1. . Chimeric antigen receptor-T therapy trials in adults with B-cell malignancy.
| Study (year) | Study center | Patient number | Patient population | Infused CART- celldose | CAR construct and signaling/(generation) | Origin type of the CAR T cells (autologous vs allogeneic) | Conditioning chemotherapy | Complete remission | Partial remission | Stable disease | Progressive disease |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brentjens et al. (2011) | MSKCC | 8 | Refractory CLL8 | 3 × 106, to 1–3 × 107 T cells/kg | scFv-CD28-CD3ζ (2nd generation) (anti-CD19) |
Autologous | Cyclophosphamide | 2 CLL | 5 CLL | ||
| Kochenderfer et al. (2012) | NCI | 8 | B-cell lymphoma: Follicular 3, CLL 4, Splenic marginal zone lymphoma 1 |
0.3 × 107 to 3.0 × 107CAR T cells/kg |
scFv-CD28-CD3ζ (2nd generation) (anti-CD19) |
Autologous | Cyclophosphamide, fludarabine | 1 CLL = 1 |
6 CLL = 2 Lym = 4 |
1 CLL = 1 |
|
| Brudno et al (2016) | NCI | 20 | 5 CLL 5DLBCL 5 ALL 5 MCL (mantle celllymphoma) |
0.4–8.2 × 106 cells/kg | scFv-CD28-CD3ζ (2nd generation) (anti-CD19) |
Allogenic | Cyclophosphamide, fludarabine | 6 CLL = 1 ALL = 4 Lym = 1 |
2 CLL = 1 Lym = 1 |
8 CLL = 1 Lym = 7 |
4 CLL = 2 ALL = 1 Lym = 1 |
| Kochenderfer (2015) | NCI | 13 | 5-DLBCL 4-PMBCL, 1-NHL 3-chronic lymphocytic leukemia |
1–5 × 106 cells/kg | scFv-CD28-CD3ζ (2nd generation) (anti-CD19) |
Autologous | Bendamustine 1 Bendamustine/Rituximab 1 Pentostatin/Cyclophosphamide1 | 7 CLL = 2 Lym = 5 |
3 CLL = 1 Lym = 2 |
1 Lymp | |
| Kalos et al. (2011) | UPENN | 3 | Chemotherapy- resistantCLL | 1.1–5.8 × 109 cells/kg | scFv-CD-137- CD3ζ (2nd generation) (anti-CD19) |
Autologous | Cyclophosphamide, Fludarabine, Vincristine Etoposide, methotrexate, cytarabine and adriamycin | 2 CLL = 2 |
1 CLL = 1 |
||
| Maude et al. (2014) | UPENN | 5 | Relapsed ALL | 0.76 × 106 to 20.6 × 106 CTL019 cells/kg |
scFv-CD-137- CD3ζ (2nd generation) (anti-CD19) |
Autologous | Fludarabine/cyclophosphamide 3, pentostatin/cyclophosphamide 5, bendamustine 6 | 5 ALL = 5 |
|||
| Porter et al. (2015) | UPENN | 14 | Refractory CLL | 0.08–1.4 × 108 cells/m2 | scFv-4-1BB-CD3ζ (2nd generation) (anti-CD 19) |
Autologous | Fludarabine/cyclophosphamide 3, pentostatin/cyclophosphamide 5, bendamustine 6 | 4 | 4 CLL = 4 |
6 | |
| Cruz et al. (2013) | BCM | 8 | Relapsed B-ALL, 1 Pre-BALL2 B-ALL1 B-CLL4 |
1.5–4.5 × 107 cells/m2, and 1.2 × 108 cells/m2 | scFv-CD28-CD3ζ (2nd generation) or scFv-CD3ζ (1st generation) (Anti-CD19) |
Allogenic | NA | 3 Pre-B ALL = 2 Pre-ALL = 1 |
1 CLL | 1 CLL | 3 CLL = 2 Pre-B-ALL = 1 |
| Savoldo et al. (2011) | BCM | 6 | Relapsed or refractoryNHL 1SLL 2 Follicular 3 DLBCL |
1–2 × 108 cells/m2 | scFv-CD28-CD3ζ (2nd generation) or scFv-CD3ζ (1st generation) (anti-CD19) |
Autologous | NA | 4 Lym = 4 |
2 Lym = 2 |
||
| Jensen et al. (2010) | COH | 4 | Relapsed diffuse large cell lymphoma (DLCL)2 Relapsed follicular lymphoma (FL)2 |
1 × 108 – 2 × 109 cells/m2 | scFv-CD3ζ (1st generation) (anti-CD 19 and anti-CD 20) | Autologous | HSCT/ Fludarabine Rituximab3 |
2 Lym | 2 Lym | ||
| Wang et al. (2014) | Chinese PLA General Hospital |
7 | Relapsed or refractory CD20 + DLBCL | NR | scFv-CD-137- CD3ζ (2nd generation) (Anti-CD 20) |
Autologous | Cyclophosphamide, Vincristine, Doxorubicin, Etoposide and Carboplatin cytarabine. | 1 Lym = 1 |
3 Lym | 1 Lym | 1 Lym |
| Dai et al. (2015) | Chinese PLA General hospital | 9 | Relapsed and refractory ALL | 1 × 106 – 1.27 × 107 cells/kg | scFv-CD-137- CD3ζ (2nd generation) (anti-CD 19) |
Autologous/a llogenic (n = 2) | C-MOAD2, none7 | 2 ALL | 2 ALL | 3 ALL | |
| Till et al. (2012) | Fred hutchins on cancer research center, seattle, WA | 4 | MCL 3 FL 1 |
1 × 108 – 3.3 × 109 cells/m2 |
scFv-CD-137-CD- 28-CD3ζ (3rd generation) (Anti-CD20) |
Autologous | Rituximab3, fenretinide2, CHOP4, fludarabine1, bortezomib | 1 Lym = 1 |
2 Lym = 2 |
||
| Till et al. (2008) | Fred Hutchins on Cancer Research Center, Seattle, WA | 7 | FL 7 | 108/m2 – 3.3 × 109 cells/m2 | CD3ζ (1st generation) (anti-CD20) |
Autologous | Cyclophosphamide, vincristine, and prednisone (CVP) 4 FND = fldarabine, mitoxantrone and dexamethasone2 None2 131I-tositumomab 1 |
2 Lym | 1 Lym | 4 Lym | |
| Turtle (2015) | Fred Hutchins on Cancer Research Center, Seattle, WA | 34 | CLL 6 DLBCL18 FL6 MCL4 |
2 × 105 – 2 × 107 cells/kg | scFv, CD-137, CD3ζ (anti-CD19) | Autologous | Cyclophosphamide +/- etoposide or cyclophosphamide andfludarabine. |
9 CLL = 3 Lym = 6 |
9 CLL = 1 Lym = 8 |
11 CLL = 2 Lym = 9 |
|
| Park (2015) | MSKCC | 45 | Relapse/refractory ALL |
1 × 106 to 3 × 106 cells/kg | scFv-CD28-CD3ζ (2nd generation) (anti-CD 19) |
Autologous | Cyclophosphamide or cyclophosphamide+ fludarabine. | 37 | |||
ALL: Acute lymphoblastic leukemia; BCM: Baylor College of Medicine; CLL: Chronic lymphocytic leukemia; COH: City of hope; HSCT: Hematopoietic stem cell transplantation; MSKCC: Memorial Sloan-Kettering Cancer Center; NCI: National Cancer Institute; scFv: Single-chain variable fragment.
Figure 1. . PRISMA flow sheet.
All studies were published from 2008 to 2016 [6–15,26–31]. All trials were conducted at seven centers, six centers in the USA: University of Pennsylvania, Memorial Sloan-Kettering Cancer Center, The Fred Hutchinson Cancer Research Center, the National Cancer Institute, Baylor College of Medicine, City of Hope and one center in China: Chinese PLA General Hospital.
A total of 16 studies with 195 patients were included in the systematic review. The included trials consisted of patient with following malignancies; ALL 68 (35%), CLL 47 (24%), NHL80 (41%). The pooled analysis showed an OR of 61% (118/195) with CR of 42% (81/195) and PR of 19% (37/195). Stable disease was seen in 11% of the patients and disease progression was seen in 22% of the patients. OS and PFS were not consistently reported in all the studies but 6-month OS was as high as 90% in Brudno et al. study while highest 6-month PFS of 67% was reported by Maude et al.
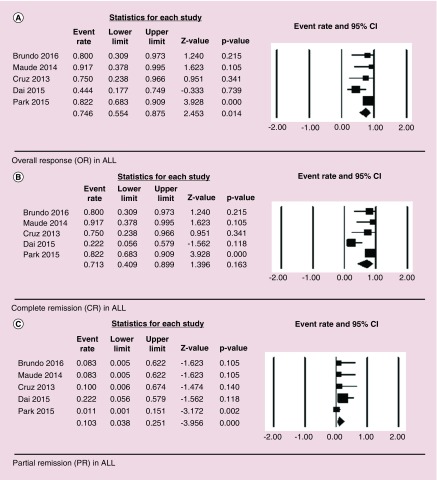
For ALL, OR of 78% (53/68) was observed with HR of 0.75 (95% CI: 0.55–0.88, p = 0.014), CR of 75% (51/68) was observed with HR of 0.71 (95% CI: 0.41–0.90, p = 0.163) and PR of 3% (2/68) was observed with HR of 0.103 (95% CI: of 0.04–0.25, p = 0.00). There was significant heterogeneity among the studies with I2 of 32.26, Q = 5.91 and p = 0.014 (Figure 2).
Figure 2. . Forest plot showing overall response (OR – Figure 2A), complete remission (CR – Figure 2B), and partial remission (PR – Figure 2C) in acute lymphoblastic leukemia (ALL) patients.
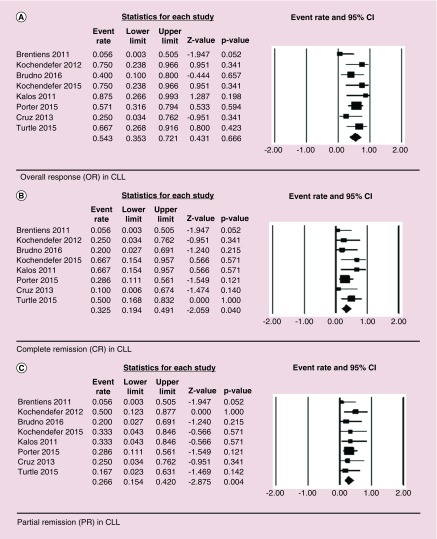
For CLL, OR of 51% (24/47) was observed with HR of 0.54 (95% CI: 0.35–0.72, p = 0.67), CR of 28% (13/47) was observed with HR of 0.33 (95% CI: 0.19–0.49, p = 0.04) and PR of 23% (11/47) was observed with HR of 0.27 (95% CI: 0.15–0.42, p = 0.004). There was no significant heterogeneity among the studies with I2 of 21.74, Q = 8.94 and p = 0.67 (Figure 3).
Figure 3. . Forest plot showing overall response (OR – Figure 3A), complete remission (CR – Figure 3B), and partial remission (PR – Figure 3C) in chronic lymphocytic leukemia (CLL) patients.
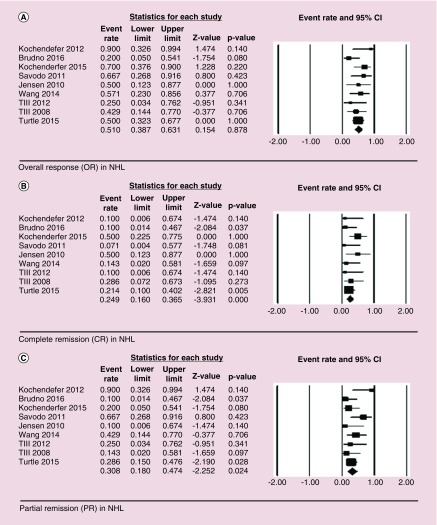
For NHL, OR 51% (41/80) was observed with HR of 0.51 (95% CI: of 0.39–0.63, p = 0.88), CR of 21% (17/80) was observed with HR of 0.25 (95% CI: 0.16–0.37, p = 0.00) and PR of 30% (24/80) was observed with HR of 0.31 (95% CI: being 0.18–0.47, p = 0.02). No significant heterogeneity was observed among the studies with I2 of 6.55, Q = 8.56 and p = 0.88 (Figure 4).
Figure 4. . Forest plot showing overall response (OR – Figure 4A), complete remission (CR – Figure 4B), and partial remission (PR – Figure 4C) in non-Hodgkin lymphoma (NHL) patients.
A total of 34 patients were treated with donor-derived CAR T cells in these four studies 6, 26, 28 [32], with an OR of 41% (CR = 10/34, PR = 4/34) with the longest CR being 30 months in a patient of CLL. A total of 17 patients received HSCT (10 ALL, 1 CLL, 6 NHL) before CAR T-cell infusion and seven ALL patients received HSCT after CAR T-cell infusion.
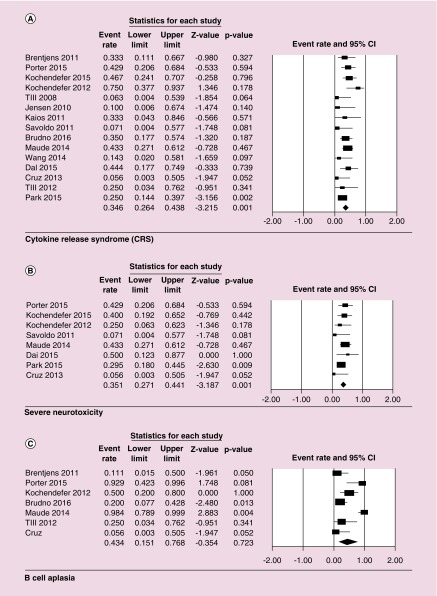
Major reported side effects were CRS, neurotoxicity and B-cell aplasia (Table 2). Data for CRS were available for 180 patients, 33% (60 patients) of which developed grade 3–4 CRS with HR being 0.37 (95% CI: 0.26–0.44, p = 0.001). Neurotoxicity data were reported for a total of 129 patients with 33% (42 patients) developing severe neurotoxicity with HR of 0.35 (95% CI: 0.27–0.44; p = 0.001). For B-cell aplasia data were reported only for 85 patients, 46 (54%) of which developed B-cell aplasia with HR of 0.43 (95% CI: 0.15–0.77; p = 0.72) (Figure 5).
Table 2. . Major side effects frequencies.
| Study (year) | CRS incidence and severity | Neurotoxicities | B-cell aplasia |
|---|---|---|---|
| Brentjens et al. (2011) | Fever 8/9 grade 1-3 including neutropenic fever, rigors and chill 5/9, Neutropenia 4/9 grade 3-4 (1/4 without fever), hypotension 3/9 grade 3-5, renal failure 1/9 grade 5, dyspnea 1/9 | Not reported | 1/9 patients |
| Porter et al. (2015) | CRS 9/14 with ≥ grade 1 (grade 1-2, n = 3; grade 3-4, n = 6; ICU admission, n = 4) | 6/14, including ≤ grade 2 hallucinations, confusion, delirium (n = 5) and grade 4 confusion (n = 1) | 4/4 patients receiving CR, 2/4 PR patients |
| Kochenderfer (2015) | Fever 12/15, hypotension 4/15, dyspnea 1/15 | 6/15, including confusion, obtundation, aphasia, encephalopathy | Not reported |
| Kochenderfer et al. (2012) | Fever 1/8, hypotension 4/8, capillary leak syndrome 3/8, acute renal failure 3/8, dyspnea 1/8 | 2/8 patients, obtundation | 4/8 patients |
| Davila et al. (2014) | Neutropenia 12/16 (11 associated with fever and grade 3, while one without fever and grade 4), hypotension 6/16 grade 3, chills 1/16 | 6/16 grade 3-4 obtundation | Not reported |
| Till et al. (2008) | Fever 1/7 (grade 2), chills 2/7 (grade 1), flu like symptoms 1/7 (grade 2), dyspnea 1/7 | Not reported | Not reported |
| Jensen et al. (2010) | Fever 2/4, rigors 2/4, lymphopenia 3/4 | Not reported | Not reported |
| Kalos et al. (2011) | Fever 2/3, rigors 2/3, hypotension 1/3 (1/3 develop anemia, leukocytosis, thrombocytopenia), dyspnea 1/3 | Not reported | Not reported |
| Savoldo et al. (2011) | NO | Not reported | NO |
| Kochenderfer et al. (2013) | Fever 2/10, hypotension 2/10, neutropenia and anemia 1/10, dyspnea 1/10 | Not reported | 03-Oct |
| Maude et al. (2014) | CRS in 30/30 (8/30 severe CRS, hypotension in 8, febrile neutropenia in 22) | 13/30 delirium to encephalopathy with one or more of following: aphasia, confusion, hallucination | 30/30 |
| Wang et al. (2014) | CRS 4/7 (all 4 fever, fatigue 2/4, diaphoresis, hypotension1/4 but that was due to hemorrhage of alimentary tract), dyspnea 2/7 | Not reported | Not reported |
| Dai et al. (2015) | CRS 4/9 (fever 4/4, dyspnea 2/4, capillary leak syndrome 1/4, oliguria 2/4), dyspnea 2/4 | 2/4 (Facial paralysis and headache, insomnia & irritability in 1 pt, while in other Numbness and stiffness of lower limbs and abdominal skin) | Not reported |
| Cruz et al. (2013) | NO | Not reported | NO |
| Till et al. (2012) | Fever 1/4 (grade 2), Grade 1 rigor and chills 1/4, flu-like syndrome 1/4, dyspnea 2/4, while orthostatic hypotension 1/4 (grade 2), hypoxemia 1/4 (grade 3) | Not reported | 1/4 developed cytopenia |
CRS: Cytokine-release syndrome.
Figure 5. . Forest plot of adverse events as an effect of CAR T cell therapy, cytokine-release syndrome (CRS; A), severe neurotoxicity (B), and B-cell aplasia (C).
The CARS construction and signaling domains are described in Table 1. Gene transfer was done using retrovirus in seven studies or lentivirus in five studies and electroporation in three studies. Lymphodepleting conditioning therapies included chemotherapy. The most commonly used lymphodepleting drugs were cyclophosphamide (Cy) and fludarabine. Other drugs used were vincristine, doxorubicin, etoposide and carboplatin, rituximab and cytosinearabinoside.
Discussion
Our systematic review of relevant literature showed highly favorable outcomes in adult aggressive B-cell malignancy patients who were treated with engineered CAR T cells. The effectiveness of therapy is variable depending on the type of B-cell malignancy, notably for ALL, CAR T therapy outcome is superior when compared with outcomes for B-cell lymphoma patients by a significant margin. Over the last few years, considerable advances were made in optimizing the structure and signaling potency of CAR T cells, which is translating into better clinical efficacy. Variables which can impact the outcomes include the specific construct technique, subpopulation of cells used to generate CART, biology and severity of targeted hematological malignancy. The results for R/R ALL patients (n = 68) treated with CAR T cells at different centers showed a dramatic complete remission rate of above 80% in this difficult to treat population. Maude et al. reported CR rate as high as 90% in 30 pediatric and adult patients [7]. There are important differences in the CAR T design, lymphodepleting strategy and inclusion criteria among various studies and these important variables must be considered while deriving inference about efficacy, and outcomes of individual trials.
Prognosis for a post-transplant ALL relapse patient is typically dismal, a significant number (46%) of patients in these CAR T studies had a prior history of alloSCT. Davilla et al. reported successful salvage (CR 88%) and patient were able to proceed to allogeneic stem cell transplantation [6,29]. Even patients with extramedullary R/R B ALL responded as reported by Dai et al. (OS 56%) [29]. Durable remissions were observed in CAR T studies but only half of the salvaged patients ultimately bridged to alloSCT in the National Cancer Institute and Memorial Sloan Kettering cancer center groups [6,33]. Maude et al., (2014) study showed sustained remission with a 6-month event-free survival rate, OS rate of 67 and 78% respectively [7]. B-ALL remains a challenge because disease relapse can happen even after CD19 directed T-cell therapy, which is possible through various escape and resistance mechanisms. Optimized CAR T design, simultaneous multiple antigen targeting, improvement in gene transfer technologies, optimal CD4/CD8 ratio and subsequent CAR T-cell infusions may prevent relapse of ALL by boosting long term engineered T-cell persistence especially if a patient is not eligible to receive AlloSCT.
Other than ALL population, CAR T cells showed a clinically significant response among patients with CLL and aggressive B-cell lymphoma patients. Patients (n = 47) with advanced, refractory and high risk CLL, 28% achieved CR and 23% showed PR with CAR T therapy. Some of treated patients received allogeneic rather than autologous CAR T cells. Porter et al., study about relapsed and refractory CLL patients (n = 14) who received CD19 expressing CAR T cells; the overall response rate in these patients was 57%. CD28 costimulatory domain containing CD19 CAR T cells reported clinically significant positive responses in CLL patients. Second and third generation CD19 CAR T cells showed encouraging clinical outcomes in relapsed refractory lymphoma. Jensen et al. [14], Savoldo et al. [27], and Cruz et al. [28], used first generation CAR T cells and reported decreased immune activation, limited efficacy and short duration of persistence. Second generation CAR T cells, which include co-stimulation domains such as CD28, CD137, or 4–1BB showed superior results when compared with first generation of these cells. After combining results from all R/R NHL studies, CR rates were 21%, and 30% patients were able to achieve PR. A direct comparison of efficacy and T cell persistence of CD19 targeting versus CD20 targeting CAR T cells is missing, with decades of experience with rituximab antibody targeting CD20 antigen and extensive data on use of Blinatumomab (CD19), question about superiority of targeting CD20 versus CD19 needs to be addressed in prospective trials.
Relapsed disease after failure of AlloSCT is a major therapeutic challenge and data on safety and efficacy of donor driven CAR T cells is still evolving. Clinical trials using autologous CD19-targeted T cells as well as allogenic CD19-targeted T cells have shown efficacy against B cell malignancies. Ghosh et al. in their elegant mouse model of donor derived CAR T cells, demonstrated alloreactive T cells expressing CD28-costimulated CD19 CARs experienced enhanced stimulation which led to their clonal deletion, showed loss of effector function, diminished proliferation and decreased occurrence of GVHD, while other CAR T cells retained graft versus malignancy effect. They also showed first-generation and 4–1BB-costimulated CAR T cells led to increased occurrence of GVHD [34]. Anwer et al., in their systematic review of 74 patients where donor origin CAR T cells were used, summarized results with conclusion that donor origin CAR T cells have strong graft versus leukemia effect but without significant incidence of GVHD [35]. Question about the efficacy and safety of donor derived CD19 CAR T cells in post-AlloSCT relapse was specifically addressed in this review [6,28,29,32] and reported rates for GVHD (5.4%) were low. Role of donor derived CAR T cells collected from original donor or from patient with mixed chimerism for MRD eradication before or after AlloSCT is an open area for further study. Short-term outcomes in patients who cannot proceed to AlloSCT after CAR T immunotherapy are comparable to patients who proceed to AlloSCT. A unique approach utilizing donor derived CD19 virus-specific cytotoxic T cells (VSTs) was used by Cruz et al. in their study with eight patients, and these VSTs do not exhibit alloreactivity, hence they successfully postulated no flare of GVHD with VSTs with strong potential for graft versus leukemia effect [28].
Lymphodepleting chemotherapy given before the CAR T cells have shown to enhance responses by eradicating native regulatory T cells and eliminating other competing immune cells [36]. Among various other factors, antitumor efficacy of first generation of CAR T cells may have been limited by ineffective lymphodepletion as well as lack of co-stimulation [36]. As a proof of principle, in an early study of refractory CLL patients with bulky lymphadenopathy who were treated with CAR T cells, no objective responses were observed [36]. Four subsequent patients received lymphodepletion with Cy followed by CAR T infusion, these patients showed positive responses with one patient exhibiting marked reduction of peripheral adenopathy and two others with stable disease [36]. Adequate lymphodepletion may prevent transgene rejection, as observed by the Seattle group in adults with B-ALL and B-NHL [37–41]. Higher CAR T-cell levels were seen in adults with B-ALL receiving a combination of fludarabine in addition to Cy (Flu + Cy) versus Cy alone at 28 days following CAR-T-cell infusion. A trend toward improved disease free survival was also observed in the cohort receiving Flu + Cy. Similarly, in other trial with B-NHL patients, significantly higher peak CAR T-cell levels were seen with Flu + Cy preconditioning compared with Cy alone. Zhang et al. [25] reported significant association of lymphodepletion therapy with clinical efficacy and prognosis. Brudno et al. [32] showed higher OR were linked with higher levels of CAR T cells among patients achieving CR or PR. In addition to conditioning chemotherapy, other strategies like using central memory T cells for CAR T construct may help cells to persist for long duration with possibility to multiply overtime [42,43] in contrast to effector T origin CAR T cells with limited proliferative capacity [44]. In addition, the administration of interleukin or the insertion of IL-12 and IL-15 genes in CD19 specific CAR T cells has resulted in sustained antitumor effects because of longer persistence and retention of central memory function [45,46].
Toxic side effects of CAR T therapy include CRS, tumor lysis syndrome, B-cell aplasia and central nervous system toxicity. B-cell aplasia detected by peripheral blood flow cytometry, which is an ‘on target/off tumor’ side effect, can serve as a useful indirect marker to assess the persistence of anti-CD19 or CD20 CAR T cells. B-cell depletion has inherent issues with concerns about safety due to secondary infections, however it is postulated that risk of infection can be mitigated at least to some extent with Immunoglobulin G replacement in cases of hypogammaglobinemia and subclass gammaglobulin deficiency [9,12,13]. This adverse effect potentially can be avoided with the use highly specific TAA to design CAR T cells. Data on threshold to give Immunoglobulin G replacement therapy in this scenario is emerging and for the time being oncologists may have to rely on institutional practices in place for replacement on the similar lines as for treatment of hypogammaglobinemia in post-AlloSCT recipients. Activated T cells produces several proinflmmatory cytokines, including IL-6, TNF-α and IFN-γ and cause CRS with symptoms like fever, hypotension, hypoxia and multiorgan failure. C-reactive protein level can indicate the severity of CRS [6] but confirmation of its predictive accuracy is being investigated [7,47]. The clinical and laboratory findings of CRS mimics macrophage activation syndrome [5]. Tumor burden at the time of CAR T-cell infusion correlate directly with severity of CRS [7,48]. Therefore, it is imperative to used salvage and preconditioning regimens to decrease tumor burden before CAR T infusion if possible. Tocilizumab, an IL-6inhibitor, is used successfully to control severity of CRS and antibody does not interfere with the effectiveness of adoptive cell therapy [5]. Judicious use of low dose steroids (up to 20 mg dexamethasone per day) have been proposed to manage lower grade CRS [5,6,48]. As understanding about CRS evolved over time, many earlier trials did not report information about this side effect in a consistent manner. Our review highlights the need to adhere to a uniformly accepted grading scale in the clinical trials as well as in the clinical practice setting. CAR T toxicity related neurological signs and symptoms range from seizures to dysphasia, delirium and death. This type of toxicity seems self-limited, in most cases it is without adverse sequelae but recently two unexpected deaths were reported in JCAR015 Phase II ROCKET trial of an anti-CD19 CAR T-cell therapy. It is assumed that some of these neurologic signs occur due to T-cell–mediated CNS inflammation and edema rather than direct toxic effect on CNS tissues [6]. Exact understanding about pathophysiology of CNS related symptoms is evolving and requires further investigation [6,7,29,49]. Delayed toxicities of CAR T cells are not known and long term follow-up data are evolving.
Strengths & weaknesses
This review is time critical as it is highlighting the efficacy data for adult patients along with summary of efficacy and toxicity profile. This manuscript can help to strengthen the case, for the extension of CAR T therapy approval to include adults in addition to children and younger adults for which FDA recently gave approval for CD19 directed CAR T therapy. Our review includes additional trials and have highest number of patients (195) reported so far as compared with the previous three published systematic reviews. CD20 CAR studies were included in addition to CD19 CAR, having decades of experience with Rituximab alone or combination therapy and few years of Blinatumomab use experience, this is a valid question to explore unique properties of CD20 targeting CAR T cells. The current data does not compare the clinical efficacy of CD19 versus CD20 CAR T, this manuscript may trigger the interest to explore the superiority of one construct and CD target over the other in a subset of patients and may help to overcome tumor escape mechanisms. Our analysis was limited due to the nature of early phase studies. Number of patients in these studies are small, with no long-term efficacy and safety data in general. There is significant heterogeneity observed in the trials using CAR T cells for hematological malignancies. Before drawing inferences from these studies, caution is required due to variability in the biology of various malignancies treated in included trials, difference in use of chemotherapy type, dose, nature of lymphodepleting therapies, different CAR T constructs, different inclusion and exclusion criteria across studies.
Future respective
Moving forward, to increase the efficacy and safety of CAR T-cell therapy, it is very important to optimize each step of the procedure involved with generation of CAR T cells, gene transfer technique, disease specific lymphodepleting conditioning regimens, selection of lymphoid population to achieve longer survival and self-renewal of CAR T cells in vivo along with minimizing the risk by better prediction, anticipation, prompt recognition and early management of toxicities.
Practice points.
Chimeric antigen receptor (CAR) T-cell therapy is a very promising treatment option for refractory and relapsed hematological malignancies and in near future, its role will evolve not only for hematological malignancies but also for other disease such as rheumatological disorders and solid organ malignancies.
The durable responses of CAR T-cell therapy will likely help integrate its use into standard treatment protocols, front line or as salvage personalized anticancer therapy, immunotherapy, targeted therapy and as an integral part of allogenic stem cell transplantation to reduce relapse risk.
Several higher risk groups with refractory disease such as those with overall poor prognosis, or those with relapsed disease after allogenic stem cell (AlloSCT) can potentially achieve complete or partial remission by utilizing CAR T mediated targeted antitumor activity.
To enhance the safety and effectiveness of this therapy, further optimization is required in specific antigens identification, CAR T signaling, T-cell subtypes selection, optimal preconditioning and multitarget strategies to overcome tumor escape.
Adoptive CAR T cells can serve as a salvage, preemptive, prophylactic or bridge strategy to AlloSCT. An effective CAR T therapy has the potential to substitute for AlloSCT in the treatment of at least a subset of patients with hematopoietic malignancies such as ALL, CLL, lymphoma and plasma cell disorders.
Acknowledgements
We thank all patients, their families, investigators and collaborators for participation in the research studies and their contribution towards science and discovery.
Footnotes
Financial & competing interests disclosure
This work was supported by grant P30 CA023074 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Eshhar Z. Adoptive cancer immunotherapy using genetically engineered designer T-cells: first steps into the clinic. Curr. Opin. Mol. Ther. 2010;12(1):55–63. [PubMed] [Google Scholar]
- 2.Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J. Biomed. Biotechnol. 2010:956304. doi: 10.1155/2010/956304. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol. Rev. 2014;257(1):14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 4.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6(224):224ra225–224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10(5):267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7(303):303ra139–303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang WY, Han QW, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin. Immunol. 2014;155(2):160–175. doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Quintás-Cardama A, Wierda W, O'brien S. Investigational immunotherapeutics for B-cell malignancies. J. Clin. Oncol. 2010;28(5):884–892. doi: 10.1200/JCO.2009.22.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 19.Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J. Immunol. 2001;167(11):6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 20.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRζ chain. J. Immunol. 2004;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Zhong X-S, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI 3 kinase/AKT/Bcl-X L activation and CD8+ T cell–mediated tumor eradication. Mol. Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 23.Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of Phase I clinical trials. Leuk. Lymphoma. 2013;54(2):255–260. doi: 10.3109/10428194.2012.715350. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Tan Y, Ou R, et al. Anti-CD19 chimeric antigen receptor-modified T cells for B-cell malignancies: a systematic review of efficacy and safety in clinical trials. Eur. J. Haematol. 2015;96(4):389–396. doi: 10.1111/ejh.12602. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Cao L, Xie J, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in Phase I clinical trials: a meta-analysis. Oncotarget. 2015;6(32):33961–33971. doi: 10.18632/oncotarget.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2014;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz CRY, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a Phase I study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai H, Zhang W, Li X, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016;34(10):1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DW, Stetler-Stevenson M, Sabatino M, et al. Intent-to-treat results of a Phase I trial of CD19 chimeric antigen receptor engineered T cells using a consistent treatment regimen reveals a 67% complete response rate in relapsed, refractory acute lymphoblastic leukemia. Blood. 2014;124(21):381–384. [Google Scholar]
- 34.Ghosh A, Smith M, James SE, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med. 2017;23(2):242–249. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anwer F, Shaukat AA, Zahid U, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9(2):123–130. doi: 10.2217/imt-2016-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brentjens RJ, Riviere I, Park J, et al. Lymphodepletion and tumor burden govern clinical responses in patients with B-cell malignancies treated with autologous, CD19-targeted T cells. J. Clin. Oncol. 2011;29(15):683–690. [Google Scholar]
- 37.Kochenderfer JN, Somerville R, Lu L, et al. Anti-CD19 CAR T cells administered after low-dose chemotherapy can induce remissions of chemotherapy-refractory diffuse large B-cell lymphoma. Blood. 2014;124(21):550. [Google Scholar]
- 38.Till BG, Jensen MC, Qian X, et al. Phase I trial results testing adoptively transferred T cells expressing CD20-specific chimeric antigen receptors containing CD28 and CD137 costimulatory domains in patients with mantle cell lymphoma and indolent lymphoma. Blood. 2010;116(21):3940–3950. [Google Scholar]
- 39.Turtle CJ. Chimeric antigen receptor modified T cell therapy for B cell malignancies. Int. J. Hematol. 2014;99(2):132–140. doi: 10.1007/s12185-013-1490-x. [DOI] [PubMed] [Google Scholar]
- 40.Turtle CJ, Maloney DG, Shank DM, et al. A Phase I/II clinical trial of immunotherapy for CD19+ B cell malignancies with defined composition of CD4+ and CD8+ central memory T cells lentivirally engineered to express a CD19-specific chimeric antigen receptor. Mol. Ther. 2014;22:S296. [Google Scholar]
- 41.Turtle CJ, Sommermeyer D, Berger C, et al. Therapy of B cell malignancies with CD19-specific chimeric antigen receptor-modified t cells of defined subset composition. Blood. 2014;124(21):384. doi: 10.1097/PPO.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118(1):294. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl Acad. Sci. USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J. Immunother. 2012;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegram HJ, Purdon TJ, Van Leeuwen DG, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29(2):415–422. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer journal (Sudbury, Mass) 2014;20(2):119. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a Phase I dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]