Abstract
Introduction
Both disseminated intravascular coagulation (DIC) and thrombotic microangiopathy (TMA) cause microvascular thrombosis associated with thrombocytopenia, bleeding tendency and organ failure.
Reports and discussion
The frequency of DIC is higher than that of thrombotic thrombocytopenic purpura (TTP). Many patients with TMA are diagnosed with DIC, but only about 15% of DIC patients are diagnosed with TMA. Hyperfibrinolysis is observed in most patients with DIC, and microangiopathic hemolytic anemia is observed in most patients with TMA. Markedly decreased ADAMTS13 activity, the presence of Shiga-toxin-producing Escherichia coli (STEC) and abnormality of the complement system are useful for the diagnosis of TTP, STEC-hemolytic uremic syndrome (HUS)and atypical HUS, respectively. However, there are no specific biomarkers for the diagnosis of DIC.
Conclusion
Although DIC and TMA are similar appearances, all coagulation, fibrinolysis and platelet systems are activated in DIC, and only platelets are markedly activated in TMA.
Keywords: DIC, TMA, Microvascular thrombosis, Hyperfibrinolysis, Organ failure, Microangiopathic hemolytic anemia
Background
Disseminated intravascular coagulation (DIC) [1, 2] is a serious disease that causes microvascular thrombosis associated with thrombocytopenia, a bleeding tendency and organ failure. These symptoms and laboratory data are similar to those of thrombotic microangiopathy (TMA) [3] which includes thrombotic thrombocytopenic purpura (TTP) [4, 5], Shiga-toxin-producing Escherichia coli (STEC) - hemolytic uremic syndrome (HUS) [6, 7], complement-mediated TMA (also called atypical HUS; aHUS) [7, 8] and secondary TMA [3, 9]. DIC also has several clinical subtypes, including asymptomatic type, marked bleeding type, organ failure type and complication types such as TTP or heparin-induced thrombocytopenia [10]. As the treatment of DIC [11] differs from that of TMA [4, 12], it is important to perform a differential diagnosis of DIC and TMA. The differences and similarities between DIC and TMA are reviewed in this study.
Differences in the definition and concept of DIC and TMA
The frequency of pneumonia associated DIC was reported to be about 10,000 cases per year according to the Japanese Diagnosis Procedure Combination (DPC) database [13], suggesting that DIC due to pneumonia occurs in about 70/106 populations. With the addition of other types of DIC, the frequency of all DIC is about 300/106 populations. In contrast, the frequency of TTP was reported to be 2.0/106 populations [3]. These reports suggest that the frequency of DIC in Japan is 150-fold higher than that of TTP (Fig. 1). According to the International Society of Thrombosis and Haemostasis (ISTH), DIC is an acquired syndrome characterized by the intravascular activation of coagulation with the loss of localization arising from different causes. It can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction. DIC is characterized by the generation of fibrin related markers (FRMs; soluble fibrin monomers, fibrinogen and fibrin degradation products [FDPs], D-dimers, etc.) and reflects an acquired (inflammatory) or non-inflammatory disorder of the microvasculature [1]. Regarding the definition of TMA, TMA presents with microangiopathic hemolytic anemia (MHA), including hemolytic anemia, thrombocytopenia and organ failure in the kidney, central nervous system, and other organs [3, 4]. These findings suggest that marked elevation of FRMs is required in DIC while MHA is required in TMA; the diagnosis of TTP among TMA requires a markedly decreased ADAMTS13 level [14], that of STEC-HUS requires the detection of a STEC infection [15] and that of aHUS requires the detection of abnormalities in the complement system [16].
Fig. 1.
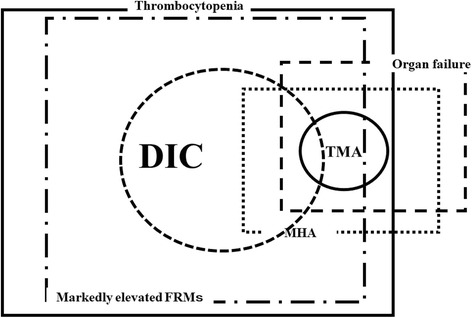
Concept of DIC and TMA. DIC, disseminated intravascular coagulation; TMA, thrombotic microangiopahy; MHA, microangiopathic hemolyitc anemia; FRMs; fibrin related markers
However, DIC has no specific marker for its diagnosis and is instead diagnosed by a scoring system using global coagulation tests. Furthermore, DIC is often associated with TMA, and TMA is often associated with DIC [17], suggesting that a differential diagnosis between DIC and TMA may be difficult.
DIC associated with TMA was observed in patients with bone marrow metastasis of solid cancer as gastric cancer, those with liver failure and those with group A streptococcal infection. In patients with DIC, bone marrow metastasis mainly causes MHA, liver failure mainly causes an increase in the von Willebrand factor/ADAMTS13 ratio, and group A streptococcal infection mainly cause massive hemolysis. However, it would be much more important to find TMA associated with DIC.
Differences and similarities in the mechanism of onset for DIC and TMA
The basic mechanism of onset for DIC is the marked activation and consumption of coagulation system followed by the activation of secondary fibrinolysis [18]. In contrast, the basic mechanism of onset for TMA is the marked activation and consumption of platelets due to several factors followed by the activation and injury of vascular endothelial cells [19, 20] (Fig. 2). Triggers of the activation of coagulation system are reported to include tissue factor (TF) [21, 22], inflammatory cytokines [23, 24] and lipopolysaccharide (LPS) [25], the activation leukocytes [26] and abnormal delivery among others. Trigger of platelet and vascular endothelial cells activation are reported to be a marked decrease in the ADAMTS13 levels in TTP [27], the detection of STEC in STEC-HUS [15] and the detection of abnormalities in the complement system in aHUS [16], along with other factors, such as transplantation, pregnancy, drugs and immune diseases, in secondary TMA [28]. Particularly marked decreases in the ADAMS13 level result in an inability to cleave ultra-large multimers of von Willebrand factor [29, 30], thereby causing platelet aggregation. Markedly fibrinolysis is frequently observed in most patients with DIC, except for some septic DIC cases [31], while markedly fibrinolysis is not observed in patients with TMA.
Fig. 2.
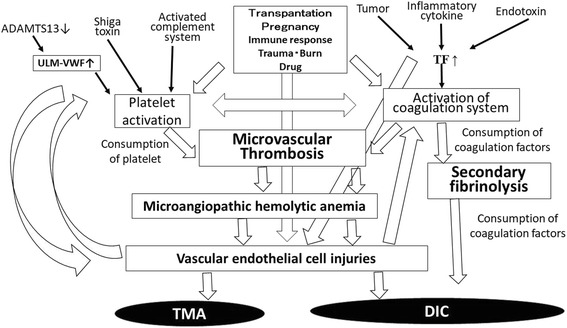
Mechanism underlying onset for DIC or TMA. DIC, disseminated intravascular coagulation; TMA, thrombotic microangiopahy; TF, tissue factor; ULM-VWF, ultra-large multimers of von Willebrand factor
Both DIC and TMA cause microvascular thrombosis, which is caused mainly by the activation of the coagulation system in DIC and by the activation of platelets and vascular endothelial cells in TMA. Several cases of TMA have been reported to be complicated with hemophilia patients treated with activated prothrombin complex concentrates (APCCs) in the clinical trial for Emicizumab [32]. As APCCs usually causes DIC but not TMA, the differential diagnosis is important in these cases [33]. Although DIC is an acquired disease, Upshaw-Schulman syndrome as familial TTP [34] and many patients with aHUS are examples of congenital TMA.
Difference in the diagnosis between DIC and TMA
As there is no gold standard for diagnosing DIC and no specific biomarker that clearly diagnoses DIC, the differential diagnosis between DIC and TMA is difficult. Four diagnostic criteria for DIC has been established by the Japanese Ministry of Health, Labor and Welfare [35], ISTH [1], Japanese Association for Acute Medicine [36] and the Japanese Society on Thrombosis and Hemostasis (JSTH) [37, 38]. These diagnostic criteria use a similar scoring system based on global coagulation tests (GLTs) such as the platelet count, prothrombin time (PT), FRMs (Table 1). Therefore, there are no significant differences in the usefulness among various diagnostic criteria for DIC [39]. The diagnosis of TMA is based on the presence of hemolytic anemia (hemoglobin < 10 g/dl), thrombocytopenia (12 × 109/ml) and organ failure. TMA patients with ADAMTS13 < 10%, those with STEC and those with abnormalities in the complement system can be easily diagnosed with TTP, STEC-HUS and aHUS, respectively (Table 2) [4, 40, 41]. However, markedly decreased ADAMTS13 levels have been reported in severe sepsis patients without TTP [42, 43], suggesting that platelet activation due to decreased ADAMTS13 might be observed in DIC patients with severe sepsis. The diagnosis of other TMA aside from DIC with hemolysis is difficult. Most patients with TMA can be diagnosed using several DIC diagnostic criteria to have DIC, but only 10%–15% of DIC patients can be diagnosed to have TMA (Fig. 2).
Table 1.
Diagnostic criteria for infectious DIC
| ISTH | P | JSTH | P | JMHLW | P | JAAM | P | |
|---|---|---|---|---|---|---|---|---|
| PLT (× 103/μl) | 100 ≧ > 50 | 1 | 120 ≧ > 80 | 1 | 120 ≧ > 80 | 1 | 120 ≧ > 8.0 | 1 |
| 50 ≧ | 2 | 80 ≧ > 50 | 2 | 80 ≧ > 50 | 2 | |||
| 50 ≧ | 3 | 50 ≧ | 3 | 80 ≧ | 3 | |||
| Reduction of PLT | 30% | 1* | 30% | 1* | ||||
| 50% | 3* | |||||||
| Prothrombin time ratio or Prolongation (s) | 3 ≦ < 6.0 | 1 | 1.25 ≦ < 1.67 | 1 | 1.25 ≦ < 1.67 | 1 | 1.2 ≦ | 1 |
| 6 ≦ | 2 | 1.67 ≦ | 2 | 1.67 ≦ | 2 | |||
| Fibrinogen (g/L) | 1.0 ≧ | 1 | 1.5 ≧ > 1.0 | 1 | ||||
| 1.0 ≧ | 2 | |||||||
| Fibrin related markers, FDP (μg/ml) | 10 ≦ < 20 | 1 | 10 ≦ < 20 | 1 | 10 ≦ < 25 | 1 | ||
| Increase | 2 | 20 ≦ < 40 | 2 | 20 ≦ < 40 | 2 | |||
| Markedly increase | 3 | 40 ≦ | 3 | 40 ≦ | 3 | 25 ≦ | 3 | |
| Antithrombin | < 70% | 1 | ||||||
| TAT or SF | 2 fold higher of NR | |||||||
| Underlying diseases | Positive | 1 | ||||||
| Bleeding | Positive | 1 | ||||||
| OF due to thrombosis | Positive | 1 | ||||||
| SIRS | Positive | 1 | ||||||
| DIC | 5 ≦ | 5 ≦ | 7 ≦ | 4 ≦ | ||||
ISTH International Society of Thrombosis and Haemostasis, JSTH Japanese Society of Thrombosis and Hemostasis, JMHLW Japanese Ministry of Health, Labor and Welfare, JAAM Japanese Association for Acute Medicine, PLT platelet count, FDP fibrinogen and fibrin degradation products, TAT thrombin antithrombin complex, SF soluble fibrin, SIRS systemic inflammatory response syndrome, DIC disseminated intravascular coagulation
*PLT and reduction of PLT pointes should be within 3 points
Table 2.
| STEC-HUS | aHUS | TTP | TMA | |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 10.0 ≧ | 10.0 ≧ | Hemolysis | 10.0 ≧ |
| Platelet (× 104/μl) | 15.0 ≧ | 15.0 ≧ | Thrombocytopenia | 15.0 ≧ |
| Ogan failure | Renal failure Creatinine ≧1.5 folds of the standard | Renal failure Creatinine ≧1.5 folds of the standard | Neurological symptoms | ? |
| Laboratory finding | Detection of STEC | Genetic abnormality in the complement system | ADAMTS13 < 10% | ? |
TMA thrombotic microangiopathy, aHUS atypical hemolytic uremic syndrome, TTP thrombotic thrombocytopenic purpura, STEC Shiga toxin-producing Escherichia coli
Differences and similarities between DIC and TMA
The differences and similarities between DIC and TMA are described in Table 3. Among clinical symptoms, bleeding and organ failure are frequently observed in patients with DIC as well as those with TMA, but lung or cardiovascular failures is more frequently observed only in patients with DIC [44], while renal or central nervous system failure is more frequently observed in patients with TMA [3]. Hypotension as organ failure is observed in many patients with DIC, while hypertension tend to be observed in patients with TMA [45]. Hypertension may be caused by acute kidney injury or arterial occlusion. Anemia is also more frequently observed in patients with TMA [20, 46] than in those with DIC. Red blood cell fragmentation may be caused by microvascular thrombosis on the arterial side which has a high blood pressure, but not on the venous side (Fig. 3). Among laboratory data, thrombocytopenia is observed in both DIC and TMA. A decreased hemoglobin level and increased levels of creatinine, total bilirubin and LDH are observed in most patients with TMA, but these abnormalities are observed in only 15% of patients with DIC. A prolonged PT and decreased AT and albumin levels are frequently (but not always) observed in patients with DIC. Markedly elevated FRMs are observed in most patients with DIC. As markedly fibrinolysis may dissolve microthromboses in patients with DIC but not in those with TMA, thrombosis of DIC is not usually detected on autopsy. Therefore, elevated FRMs and decreased platelet counts are the most useful markers for DIC [1].
Table 3.
Differences and similarities between TMA and DIC
| Severe DIC | Severe TMA | ||
|---|---|---|---|
| Symptoms | Organ failure | Often (Lung, Kidney, Shock) | Usually (Kidney, CNS) |
| Bleeding and bleeding tendency | Frequent | Frequent | |
| Blood pressure | Low | High | |
| Hematuria | Sometimes | Frequent | |
| Anemia | Often | Usually | |
| Laboratory data | Platelet count | Low | Low |
| Hemoglobin | Often low | Low | |
| Fibrin related markers | Markedly high | Slightly high | |
| Prothrombin time | Often prolong | Normal | |
| Antithrombin | Often low | Normal | |
| Albumin | Often low | Normal | |
| Creatinine | Often high | High | |
| Total bilirubin, LDH | Often high | High | |
| Treatments | Supportive therapy | Recommended | Recommended |
| Blood transfusion (RBC, FFP) | Recommended | Recommended, | |
| Blood transfusion (PC) | Recommended | Not recommended | |
| Anticoagulant | Recommended (Japan) | Not mentioned | |
| PE/FFP | Not mentioned | Recommended | |
| Special treatment | AT, rhTM (Japan) | Hemodialysis (HUS), Eculizumab (aHUS), Rituximab (TTP) |
Fig. 3.
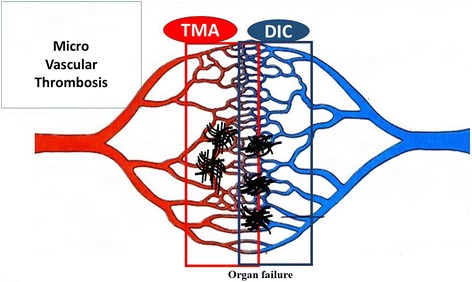
Microvascular thrombosis in DIC and TMA. DIC, disseminated intravascular coagulation; TMA, thrombotic microangiopahy
In Japan, regarding the treatment of DIC and TMA, platelet transfusion is contraindicated for TMA [4], while anticoagulant therapy for DIC, but not for TMA, is recommended [10, 11]. Anti-fibrinolytic therapy is recommended for DIC patients with hyperfibrinolysis. Plasma exchange is recommended in most some cases of TMA such as TTP [47], but not for DIC. Antithrombin concentrate [48] and recombinant thrombomodulin [49] for DIC are frequently used in Japan, while eculizumab [50] has proven effective for compliment mediated TMA, such as aHUS, and rituximab [51] is effective for TTP in patients with a high titer of inhibitor for ADAMTS13.
Conclusion
DIC and TMA are similar appearances, however, all coagulation, fibrinolysis and platelet systems are activated in DIC, and only platelets are markedly activated in TMA. As treatment is different between DIC and TMA, differential diagnosis between DIC and TMA is important.
Acknowledgements
None
Funding
This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Availability of data and materials
None
Abbreviations
- aHUS
atypical HUS
- DIC
Disseminated intravascular coagulation
- DPC
Diagnosis Procedure Combination
- FDP
fibrinogen and fibrin degradation products
- FRMs
fibrin related markers
- GLTs
global coagulation tests
- HUS
Hemolytic uremic syndrome
- ISTH
International Society of Thrombosis and Haemostasis
- JSTH
Japanese Society on Thrombosis and Hemostasis
- MHA
microangiopathic hemolytic anemia
- PT
prothrombin time
- STEC
Shiga- toxin-producing Escherichia coli
- TMA
thrombotic microangiopathy
- TTP
thrombotic thrombocytopenic purpura
Authors’ contributions
WH fully wrote this manuscript. MT, SK and IH reviewed these references. KN, IT, and MM discussed and gave the suggestions for this manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Human Ethics Review Committee of the Mie University School of Medicine, and a signed consent form was obtained from each subject. This study was faithfully carried out in accordance with the principles of the Declaration of Helsinki.
Consent for publication
A signed consent form was obtained from each subject.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hideo Wada, Phone: 81-59-232-1111, Email: wadahide@clin.medic.mie-u.ac.jp.
Takeshi Matsumoto, Email: matsutak@clin.medic.mie-u.ac.jp.
Kei Suzuki, Email: keis@clin.medic.mie-u.ac.jp.
Hiroshi Imai, Email: hi119@clin.medic.mie-u.ac.jp.
Naoyuki Katayama, Email: n-kata@clin.medic.mie-u.ac.jp.
Toshiaki Iba, Email: toshiiba@juntendo.ac.jp.
Masanori Matsumoto, Email: mmatsumo@naramed-u.ac.jp.
References
- 1.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific subcommittee on disseminated intravascular coagulation (DIC) of the international society on thrombosis and Haemostasis (ISTH): towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 2.Wada H, Matsumoto T, Yamashita Y, Hatada T. Disseminated intravascular coagulation: testing and diagnosis. Clin Chim Acta. 2014;436C:130–134. doi: 10.1016/j.cca.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Wada H, Matsumoto T, Yamashita Y. Natural history of thrombotic thrombocytopenic Purpura and hemolytic uremic syndrome. Semin Thromb Hemost. 2014;40:866–873. doi: 10.1055/s-0034-1395154. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto M, Fujimura Y, Wada H, Kokame K, Miyakawa Y, Ueda Y, Higasa S, Moriki T, Yagi H, Miyata T, Murata M. For TTP group of blood coagulation abnormalities research team, research on rare and intractable disease supported by health, labour, and welfare sciences research grants.: Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. 2017;106:3–15. doi: 10.1007/s12185-017-2264-7. [DOI] [PubMed] [Google Scholar]
- 5.South K, Lane DA. ADAMTS-13 and von Willebrand factor: a dynamic duo. J Thromb Haemost. 2017; in press [DOI] [PMC free article] [PubMed]
- 6.Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. Semin Immunopathol. 2014;36:399–420. doi: 10.1007/s00281-014-0416-x. [DOI] [PubMed] [Google Scholar]
- 7.Jokiranta TS. HUS and atypical HUS. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen YM. Clinical evaluation of thrombotic microangiopathy: identification of patients with suspected atypical hemolytic uremic syndrome. Thromb J. 2016;14(Suppl 1):19. doi: 10.1186/s12959-016-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epperla N, Hemauer K, Hamadani M, Friedman KD, Kreuziger LB. Impact of treatment and outcomes for patients with posttransplant drug-associated thrombotic microangiopathy. Transfusion. 2017;57:2775–2781. doi: 10.1111/trf.14263. [DOI] [PubMed] [Google Scholar]
- 10.Wada H, Asakura H, Okamoto K, Iba T, Uchiyama T, Kawasugi K, Koga S, Mayumi T, Koike K, Gando S, Kushimoto S, Seki Y, Madoiwa S, Maruyama I, Yoshioka A. Japanese Society of Thrombosis Hemostasis/DIC subcommittee: expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6–11. doi: 10.1016/j.thromres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, Kim HK, Nielsen JD, Dempfle CE, Levi M, Toh CH. The scientific standardization committee on DIC of the international society on thrombosis Haemostasis.: guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11:761–767. doi: 10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- 12.Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, Cheung B, Machin SJ. British Committee for Standards in Haematology: guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- 13.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12:1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 14.Yoshii Y, Fujimura Y, Bennett CL, Isonishi A, Kurumatani N, Matsumoto M. Implementation of a rapid assay of ADAMTS13 activity was associated with improved 30-day survival rate in patients with acquired primary thrombotic thrombocytopenic purpura who received platelet transfusions. Transfusion. 2017;57:2045–2053. doi: 10.1111/trf.14152. [DOI] [PubMed] [Google Scholar]
- 15.Grisaru S, Xie J, Samuel S, Hartling L, Tarr PI, Schnadower D, Freedman SB, Alberta Provincial Pediatric Enteric Infection Team Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with Shiga toxin-producing Escherichia coli: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:68–76. doi: 10.1001/jamapediatrics.2016.2952. [DOI] [PubMed] [Google Scholar]
- 16.Berger BE. The alternative pathway of complement and the evolving clinical-pathophysiological Spectrum of atypical hemolytic uremic syndrome. Am J Med Sci. 2016;352:177–190. doi: 10.1016/j.amjms.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Schwameis M, Schörgenhofer C, Assinger A, Steiner MM, Jilma B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost. 2015;113:708–718. doi: 10.1160/TH14-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada H. Disseminated intravascular coagulation. Clin Chim Acta. 2004;344:13–21. doi: 10.1016/j.cccn.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita Y, Naitoh K, Wada H, Ikejiri M, Mastumoto T, Ohishi K, Hosaka Y, Nishikawa M, Katayama N. Elevated plasma levels of soluble platelet glycoprotein VI (GPVI) in patients with thrombotic microangiopathy. Thromb Res. 2014;133(3):440–444. doi: 10.1016/j.thromres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Ito-Habe N, Wada H, Matsumoto T, Ohishi K, Toyoda H, Ishikawa E, Nomura S, Komada Y, Ito M, Nobori T, Katayama N. Elevated Von Willebrand factor propeptide for the diagnosis of thrombotic microangiopathy and for predicting a poor outcome. Int J Hematol. 2011;93:47–52. doi: 10.1007/s12185-010-0732-4. [DOI] [PubMed] [Google Scholar]
- 21.Sase T, Wada H, Nishioka J, abe Y, Gabazza EC, Shiku H, Suzuki K, Nakamura S, Nobori T. Measurement of tissue factor messenger RNA levels in leukocytes from patients in hypercoagulable state caused by several underlying diseases. Thromb Haemost. 2003;89:660–665. doi: 10.1055/s-0037-1613572. [DOI] [PubMed] [Google Scholar]
- 22.Sase T, Wada H, Kamikura Y, Kaneko T, Abe Y, Nishioka J, Nobori T, Shiku H. Tissue factor messenger RNA levels in leukocytes compared with tissue factor antigens in plasma from patients in hypercoagulable state caused by various diseases. Thromb Haemost. 2004;92:132–139. doi: 10.1160/TH03-08-0535. [DOI] [PubMed] [Google Scholar]
- 23.Wada H, Tamaki S, Tanigawa M, Takagi M, Deguchi A, Mori Y, Katayama N, Yamamoto T, Deguchi K, Shirakawa S. Plasma level of IL-1β in disseminated intravascular coagulation. Thrombo Haemost. 1991;65:364–368. [PubMed] [Google Scholar]
- 24.Wada H, Ohiwa M, Kaneko T, Tamaki S, Tanigawa M, Takagi M, Mori M, Shirakawa M. Plasma level of tumor necrosis factor in disseminated intravascular coagulation. Am J Hematol. 1991;37:147–151. doi: 10.1002/ajh.2830370302. [DOI] [PubMed] [Google Scholar]
- 25.Duburcq T, Tournoys A, Gnemmi V, Hubert T, Gmyr V, Pattou F, Jourdain M. Impact of obesity on endotoxin-induced disseminated intravascular coagulation. Shock. 2015;44:341–347. doi: 10.1097/SHK.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Kaneko T, Wada H, Kobayashi T, Abe Y, Nobori T, Shiku H, Stearns-Kurosawa DJ, Kurosawa S. Proteinase 3 expression on neutrophil membranes from patients with infectious disease. Shock. 2006;26:128–133. doi: 10.1097/01.shk.0000223122.11147.5a. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Wada H, Nishioka J, Yamamoto M, Matsumoto T, Tamaru T, Nomura S, Masuya M, Mori Y, Nakatani K, Nishikawa M, Katayama N, Nobori T. ADAMTS13 related markers and Von Willebrand factor in plasma from patients with thrombotic Microangipathy (TMA) Thromb Res. 2008;121:849–854. doi: 10.1016/j.thromres.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Franchini M, Montagnana M, Targher G, Lippi G. Reduced von Willebrand factor-cleaving protease levels in secondary thrombotic microangiopathies and other diseases. Semin Thromb Hemost. 2007;33:787–797. doi: 10.1055/s-2007-1000365. [DOI] [PubMed] [Google Scholar]
- 29.Ruggeri ZM, Zimmerman TS. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981;57:1140–1143. [PubMed] [Google Scholar]
- 30.Arya M, Anvari B, Romo GM, Cruz MA, Dong JF, McIntire LV, Moake JL, Lopez JA. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99:3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 31.Wada T, Gando S, Maekaw K, Katabami K, Sageshima H, Hayakawa M, Sawamura A. Disseminated intravascular coagulation with increased fibrinolysis during the early phase of isolated traumatic brain injury. Crit Care. 2017;21:219. doi: 10.1186/s13054-017-1808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, Santagostino E, Kruse-Jarres R, Negrier C, Kessler C, et al. Emicizumab prophylaxis in hemophilia a with inhibitors. N Engl J Med. 2017;377:809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 33.Wada H, Matsumoto T, Katayama N. Emicizumab prophylaxis in hemophilia a with inhibitors. N Engl J Med. 2017;377:2193–2194. doi: 10.1056/NEJMc1712683. [DOI] [PubMed] [Google Scholar]
- 34.Fujimura Y, Matsumoto M, Isonishi A, Yagi H, Kokame K, Soejima K, Murata M, Miyata T. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J Thromb Haemost. 2011;9:283–301. doi: 10.1111/j.1538-7836.2011.04341.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the research committee on DIC in Japan. Bibl Haematol. 1983;49:265–275. doi: 10.1159/000408467. [DOI] [PubMed] [Google Scholar]
- 36.Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) study group: natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36:145–150. doi: 10.1097/01.CCM.0000295317.97245.2D. [DOI] [PubMed] [Google Scholar]
- 37.Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, Wada H. DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis: Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016;14:42. doi: 10.1186/s12959-016-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, Asakura H. DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis: The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017;15:17. doi: 10.1186/s12959-017-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemitsu T, Wada H, Hatada T, Ohmori Y, Ishikura K, Takeda T, Sugiyama T, Yamada N, Maruyama K, Katayama N, Isaji S, Shimpo H, Kusunoki M, Nobori T. Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thromb Haemost. 2011;105:40–44. doi: 10.1160/TH10-05-0293. [DOI] [PubMed] [Google Scholar]
- 40.Terano C, Ishikura K, Hamada R, Yoshida Y, Kubota W, Okuda Y, Shinozuka S, Harada R, Iyoda S, Fujimura Y, Hamasaki Y, Hataya H, Honda M. Practical issues in using eculizumab for children with atypical hemolytic uremic syndrome in the acute phase: a review of 4 patients. Nephrology (Carlton). 2017; in press [DOI] [PubMed]
- 41.Kato H, Nangaku M, Hataya H, Sawai T, Ashida A, Fujimaru R, Hidaka Y, Kaname S, Maruyama S, Yasuda T, Yoshida Y, Ito S, Hattori M, Miyakawa Y, Fujimura Y, Okada H, Kagami S. Joint Committee for the Revision of clinical guides of atypical hemolytic uremic syndrome in Japan: clinical guides for atypical hemolytic uremic syndrome in Japan. Pediatr Int. 2016;58:549–555. doi: 10.1111/ped.13044. [DOI] [PubMed] [Google Scholar]
- 42.Ono T, Mimuro J, Madoiwa S, Soejima K, Kashiwakura Y, Ishiwata A, Takano K, Ohmori T, Sakata Y. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107:528–534. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 43.Habe K, Wada H, Ito-Habe N, Hatada T, Matsumoto T, Ohishi K, Maruyama K, Imai H, Mizutani H, Nobori T. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb Res. 2012;129:598–602. doi: 10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Wada H, Matsumoto T, Hatada T. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. Expert Rev Hematol. 2012;5:643–652. doi: 10.1586/ehm.12.57. [DOI] [PubMed] [Google Scholar]
- 45.Rafiq A, Tariq H, Abbas N, Shenoy R. Atypical hemolytic-uremic syndrome: a case report and literature review. Am J Case Rep. 2015;16:109–114. doi: 10.12659/AJCR.895098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito N, Wada H, Matsumoto M, Fujimura Y, Murata M, Izuno T, Sugita M, Ikeda Y. National questionnaire survey of TMA. Int J Hematol. 2009;90:328–335. doi: 10.1007/s12185-009-0421-3. [DOI] [PubMed] [Google Scholar]
- 47.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA, Group CAS Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 48.Iba T, Gando S, Saitoh D, Wada H, Di Nisio M, Thachil J. Antithrombin supplementation and risk of bleeding in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2016;145:46–50. doi: 10.1016/j.thromres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, Sanui M, Takimoto K, Mayumi T, Ono K. Japan septic disseminated intravascular coagulation (JSEPTIC DIC) study group: recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115:1157–1166. doi: 10.1160/TH15-12-0987. [DOI] [PubMed] [Google Scholar]
- 50.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 51.George JN, Woodson RD, Kiss JE, Kojouri K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic purpura: a proposed study of the transfusion medicine/hemostasis clinical trials network with a systematic review of rituximab therapy for immune-mediated disorders. J Clin Apheresis. 2006;21:49–56. doi: 10.1002/jca.20091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None


