Abstract
All cancer patients experience distress from the diagnosis, the effects of the disease or the treatment. Clinically significant distress decreases overall quality of life and the recognition of distress with prompt intervention is essential. The National Comprehensive Cancer Network distress thermometer (NCCN-DT) is a validated measuring tool that has been utilized in the primary brain tumor population to detect psychologic distress thereby provoking a referral process to the appropriate support system. Brain tumor patients commonly reported emotional and physical distress encompassing: fatigue, fears, memory and concentration and worry. More research is needed to identify the stressors of all primary brain tumor patients and their caretakers and integrate appropriate interventions to improve health-related quality of life in both groups.
KEYWORDS : brain, distress, psychosocial
Practice points.
Up to 74% of primary brain tumor participants in previous studies experience some form of distress throughout their disease trajectory.
The National Comprehensive Cancer Network distress thermometer (NCCN-DT) is a validated screening tool used to detect psychologic distress in cancer patients.
Risk factors for cancer patients with increased stress are: female gender, living alone, having children, lower income, longer duration of illness, younger age, a history of psychiatric disorders, a substance or physical/sexual abuse history, or comorbid illnesses.
Fatigue is the most troubling symptom, especially in high grade glioma patients and has found to be predictive of poorer overall survival and decreased health-related quality of life (HRQOL).
Half of all glioblastoma long-term survivors have cognitive dysfunction which has an impact on their HRQOL.
Pediatric primary brain tumor patients have higher rates of physical dysfunction, more psychologic distress, depression, fatigue, insomnia and daytime sleepiness.
Caregivers also experience distress with feelings of helplessness, worry, anxiety, depression, fatigue, lack of concentration and insomnia.
Routine use of the NCCN-DT at the clinic visit is needed to aid in appropriate interventions such as mental health care, social work and spiritual services in order to improve HRQOL.
All cancer patients experience distress from the diagnosis, the effects of the disease or the treatment. Recognition of distress with prompt intervention is essential in order to improve health-related quality of life (HRQOL). In a large cross sectional analysis of 2776 cancer patients, lung patients had the highest level of distress followed by pancreatic, Hodgkin's lymphoma and brain, of which brain was 2.9% of the population [1]. Up to 47% of cancer patients report significant levels of distress along with 40% having mood disorders [1–3].
Fatigue, pain, anxiety and depression are the most frequently reported cancer symptoms which can be compounded by existing psychologic conditions. Having a psychiatric history, a substance or physical/sexual abuse history, or comorbid illnesses with uncontrolled symptoms are all risk factors for increased stress. Even though younger patients have better progression-free survival, these patients, along with those that are female, live alone, have children, have lower incomes and longer duration of illness also have an increased risk of distress [1,4–7].
Studies report that upwards of 74% of brain tumor patients experience distress at some point during their disease [3]. Based on the Central Brain Tumor Registry of the United States Statistical Report, over 23,000 cases of high grade brain tumors were predicted for 2015 with the 5-year survival of glioblastoma being 5% [8,9]. These individuals have numerous responsibilities including familial, social and economic, all of which are greatly impacted by the diagnosis of a brain tumor. These patients have to cope with changes in physical and cognitive capacity, and personality and mood fluctuations which impact the quality of life of the patient, their family and their community.
Younger aged individuals diagnosed with brain tumors have more life altering changes in their interpersonal and social relationships such as raising and supporting a family, and continuing with employment and social engagements. Since low grade brain gliomas (LGG) usually occur at a younger age, it is not surprising that studies found more distress in this population. In a 2006 study by Keir et al., more patients with LGG experienced elevated levels of stress compared with their high grade glioma (HGG) counterparts [10] which was confirmed in a 2012 prospective analysis comparing high and low grade brain tumor patients before and after radiation [11]. In fact, they had significantly elevated post-traumatic stress syndrome and distress with decreased HRQOL [11].
The management of brain cancer is not only to stabilize tumor growth and prolong life but also to preserve the physical, cognitive and emotional state of the patient. Primary brain tumor (PBT) patients encounter many stigmas throughout their disease trajectory including disfigurement and alopecia after surgery and radiation, loss of economy due to decreased cognition, loss of functional status with motor and sensory deficits and loss of individual freedoms such as driving due to epilepsy and behavioral issues. The social stigma of having a brain tumor can lead to depression and social isolation. Patients may experience discrimination in the work place because of decreased cognitive and functional status, the need for time off due to treatment and its related effects and the expectation of imminent death [12,13]. Compared to newly diagnosed patients, those with recurrent disease have more difficulties involving motor dysfunction, speech deficits, weakness and incontinence [14]. Symptom burden and prevalence of distress is high in PBT patients with overall lower HRQOL compared with other cancers [3] while recurrent HGG patients fared worse than those with localized cancer but similar to those of advanced ovarian, lung and metastatic cancers [14].
The primary objective of this review is to provide a brief introductory overview of the distress experienced by all PBT patients. The role of the NCCN-DT will also be discussed along with its proposed routine use in the clinical setting. Primary stressors experienced by these patients will be highlighted with a report of some treatments previously utilized. New insights into complementary therapies will be addressed along with suggestions that may improve HRQOL.
A literature search was performed using PubMed with the following terms: cancer, glioma, brain, psychosocial, distress and NCCN, which yielded free full text review articles and original studies in English.
Measuring distress using NCCN-DT
The National Comprehensive Cancer Network has issued a distress management tool for cancer patients. The National Comprehensive Cancer Network Distress Thermometer (NCCN-DT) is a validated measuring tool used to detect psychologic distress in cancer patients. The patient's level of distress is represented by a thermometer on a scale of 0 (no distress) to 10 (extreme distress) along with a 39 item checklist asking yes or no questions regarding practical, family, emotional, spiritual and physical problems experienced by the patient in the past week. As per the 2015 NCCN guidelines for distress management, along with previous studies assessing brain tumor patients [6,15], a patient scoring 4 or higher should be evaluated and then referred to mental health, social work or chaplain services. An analysis of 42 studies pooling over 14,000 patients found the sensitivity of the DT to be 81% and specificity 72% at a cut off of 4 [16] but other studies have identified a cut off score of ≥6 with a sensitivity ranging from 88 to 100% and a specificity of ≥53%; thereby proposing a cut off score of ≥6 [17].
Regardless of the DT cut off score, PBT patients experience distress throughout their disease trajectory and report many concerns. In two different studies on distress, Keir et al. reported 52 and 63% of the PBT participants had elevated stress utilizing the NCCN-DT and Perceived Stress Scale, respectively (Table 1) [10,15]. Goebel and colleagues found that 73.6% of PBT patients had clinically significant distress with cut off scores of ≥4 and 48.4% of patients with cut offs ≥6 [9,15]. Emotional and physical distress were the two most commonly reported domains encompassing the problems of fatigue, fears, depression, memory and concentration and worry [9,18]. Whether it is the fear and emotional distress experienced by the patient or the physical manifestations of the disease, newly diagnosed PBT patients reported more concerns than those who were further along in their disease course [9,15,19]. On the other hand, Kvale et al. reported a lower distress score in glioblastoma (GBM) patients, with concerns of getting around, memory/concentration and insurance/financial [20]. A low distress score, and a statistically significant inverse relationship between the total distress score and HRQOL was also seen in a recent study of 798 PBT patients [18,20]. In the latter two studies, the NCCN-DT was used routinely during the clinic visit. These lower distress scores could possibly be related to the usage of the NCCN-DT survey and timely interventions. On the other hand, individuals may not have accurately assessed their true level of distress or ignored their problems. In these situations, obtaining the input of the caregiver and comparing it with the patient would be informative.
Table 1. . Research studies focusing on the primary brain tumor population.
| Study (year) | Subjects | Distress tool | Cut off score | n | Mean distress score (standard deviation) | Elevated% distressed | # Concerns | Ref. |
|---|---|---|---|---|---|---|---|---|
| Keir et al. (2006) | PBTP | PSS-10 | M 12.1; F 13.7 | 60 | M 13.19 (SD = 6.41); F 17.21 (SD = 7.28) | 63% | [10] | |
| Keir et al. (2008) | PBTP | NCCN-DT | ≥4 | 75 | 52% | 5.8 | [15] | |
| Kvale et al. (2009) | GBM patients | NCCN-DT | ≥4 | 50 | 2.15 (SD = 2.66) | 28% | [20] | |
| Goebel et al. (2011) | PBTP + spouses | NCCN-DT | ≥5 | 26 pairs | Patient: 6 (SD = 2.6) | 73% | [19] | |
| Partner: 6.58 (SD = 2) | 85% | |||||||
| Goebel et al. (2011) | PBTP | NCCN-DT | ≥6 | 159 | 5.51 (SD = 2.86) | 48% (74% with cutoff ≥4) | 6.86 (SD = 4.79) | [9] |
| Goebel and Mehdorn (2011) | PBTP | NCCN-DT | ≥6 | 150 | 5.73 (SD = 2.97) | 6.28 | [17] | |
| Trad et al. (2015) | PBTP (new and recurrent) + caregiver | NCCN-DT | ≥4 | 108 patients | Newly diagnosed patients: 3.15 (SD = 2.2) | 38% newly diagnosed | [21] | |
| Recurrent patients: 6.49 (SD = 2.61) | 75% recurrent | |||||||
PBTP: Primary brain tumor patients.
Stressors in primary brain tumor patients
Physical functioning greatly impacts HRQOL scores, where fatigue, sleep disturbances, headaches, seizures and cognitive dysfunction are the most common symptoms [9,15,18,20,22–23]. For all PBT patients, fatigue is considered their most troubling problem [15,18] more so in HGG patients [4,24]. Fatigue has been found to be predictive of poor overall survival [4] with decreases in HRQOL [25,26]. In fact, the incidence of fatigue has been reported in 89–94% of recurrent HGG patients and is usually the most frequently reported symptom with cancer patients, in general. Antiepileptics, chemotherapy, radiation and tumor progression can all cause fatigue. Fatigue can be present before treatment and may increase after chemoradiation which can affect performance status and HRQOL [4,22,27]. Reversible causes of fatigue should be investigated such as vitamin deficiencies, hypothyroidism and low testosterone levels and treatment should be initiated. Pharmacologic treatments for fatigue have been studied and medical management with low-dose naltrexone, modafinil and armodafinil did not demonstrate significant improvement in PBT patients’ fatigue, but previous research has revealed exercise may improve fatigue [28–31].
Mood disorders can include: depression, anxiety, mania and even psychosis depending on tumor location, usage of medications (e.g., corticosteroids and antiepileptic drugs), and situational life changes. Emotional concerns play an important role in cancer patients’ overall HRQOL especially since 20–50% of cancer patients have a comorbid mental disorder [19,32–33]. Cancer patients, with a predisposition or history of emotional problems, have a 2.6-times greater chance of dying than those without emotional problems [34]. Depression has been noted in up to 95% of brain tumor patients whereas anxiety is reported up to 60% [35,36]. GBM patients reported more depression and more interference with activities and intimate relationships than those with other cancers [37,38]. Although depression has been shown to be an independent predictor of HRQOL, with an association with reduced physical function and cognitive impairment and a possible decrease in overall survival, only approximately 60% of patients receive antidepressants [39–42]. Neuropsychological assessments with possible pharmacologic intervention maybe needed to provide the optimal treatment, because high levels of depression could decrease motivation, treatment decision capacity and increase mortality. Corticosteroid use and sleep disorders have also been associated with depression in the brain tumor population [43]. Since some individuals are against psychologic care, an assessment and intervention of these other factors could be beneficial [11]. HRQOL could also be improved by encouraging the patient to strengthen social and intimate relationships, by addressing emotional and sexual concerns, which are inadequately addressed at the clinic visit [44,45].
Cognitive dysfunction, which has been found in half of all glioblastoma long-term survivors (LTS), has been shown to impact HRQOL [46]. Both chemotherapy and radiation have been noted to worsen cognition especially if present at baseline [47,48]. Verbal memory, spatial memory, attention and problem solving skills are the most commonly affected cognitive domains that are caused by radiation [49–52]. It has been reported that total dose, volume and fraction doses >2 Gy of radiation impact toxicity in brain tumor patients [53], while other studies show that tumor localization and volume have greater impact on cognition rather than radiation exposure [54]. Cortical atrophy, leukoaraiosis and ischemic events are all risk factors for cognitive decline which may lead to dementia and death. Decreased cognition has been correlated with deterioration in functional independence while verbal memory has been prognosticated with survival [22,55–56]. All PBT patients are burdened with cognitive impairment related to the tumor itself, the location of the tumor, seizures or treatment effects [35,57]. Antiepileptic medications, along with frequent seizure burden and mood disturbances can worsen cognitive decline in addition to the long-term effects of radiation [35,42,57]. In a study assessing neurocognitive functioning before and after radiation, comparing low and HGG patients, all patients scored below average on verbal fluency and mental flexibility, but short term memory recall and digit span scores improved in LGG and HGG patients, respectively [11], proving that certain cognitive domains can improve over time providing timely assessment and interventions.
Given the preponderance of research with radiation and its effects on cognitive dysfunction, studies are ongoing, focusing on hippocampal sparing in radiotherapy planning, or decreasing inflammation by repurposing medications in order to reduce neurocognitive impairment [49,58–60]. Utilizing puzzles and brain games have been advocated while donepezil, methylphenidate and modafinil were studied to improve cognition and fatigue in glioma patients. A recent pilot study examining the use of donepezil revealed an improvement in baseline cognitive test scores especially attention, psychomotor speed and visual memory along with an improvement in HRQOL [61]. Increased motivation and stamina along with cognition were seen in glioma patients using methylphenidate [62] but there was no significant improvement with modafinil [30].
Distress in long-term survivors
Cancer survivorship focuses on all aspects of the cancer patients’ experience from diagnosis to death. Up to 43% of cancer survivors continue to experience stress throughout their disease trajectory, but for PBT patients, fear of recurrence could increase stress [63]. In previous studies, there was no significant difference in the PSS scores between long versus short term PBT survivors although there were fewer concerns in the LTS group [23]. A survival study consisting of HGG patients demonstrated that LTS experienced improvement in physical, social and emotional functioning throughout their disease trajectory where the short term survivors developed more general health issues and reported a more compromised HROL [64]. While tumor recurrence [64,65] can decrease HRQOL and increase distress [21], there is a possibility of improvement in all aspects of functioning in the LTS population with proper intervention.
Surviving a childhood brain cancer is more difficult than surviving other childhood cancers. In fact, childhood brain cancer survivors have worse HRQOL outcomes than other cancer survivors and their healthy peers [66,67]. Education, employment, social functioning and fertility are all impacted in the pediatric cancer survivor. When compared with their siblings, these patients are 80% more likely to report cognitive defects, five-times greater to report functional impairment and twice as likely to report emotional distress [68]. Pediatric PBT patients have higher rates of physical dysfunction, more psychologic distress, depression, fatigue, insomnia and daytime sleepiness along with decreased social skills and interactions with their peers [69–73]. Survivors of pediatric PBTs experience more long-term adverse events compared with other cancers [74]. Endocrine, metabolic, cardiac and neurologic sequelae from the cancer or treatments need to be addressed to maintain overall health. Even though there are special education programs for those who have cognitive and emotional disabilities [69,75], interventions or support groups that help to develop social skills and friendships in order to decrease depression, anxiety and improve quality of life are needed.
Caregiver distress
Since PBT patients have high rates of stress, anxiety and depression and decreased HRQOL [20,26], it is reasonable to assume their caregivers also experience distress. Psychosocial distress has been reported in up to 85% of PBT caregivers with mean distress scores higher than the cancer patients’ [19,76]. Caregivers experience feelings of helplessness in caring for their partner and distress over their partner's suffering which contribute to increased anxiety, depression, insomnia, agitation, fatigue and decreased concentration [19]. Since caregiver distress is under recognized, more research is needed to properly evaluate caregiver burden and facilitate interventions to help improve their HRQOL.
Increased stress is also found in parents and siblings of children with PBTs [77,78]. Parents have to contend with the uncertainty of tumor recurrence and worrying about the physical, psychological and emotional complications in their child's daily life [67]. Parents need to balance the time and attention given to all their children and each other, cope with their own daily activities and the social stigma of having a special child, and deal with any financial hardships which may arise. Since survivors rely more on their family [67,79], familial interventions have shown efficacy in reduction of distress for them; but more support is needed for the family of these survivors.
Conclusion & future perspective
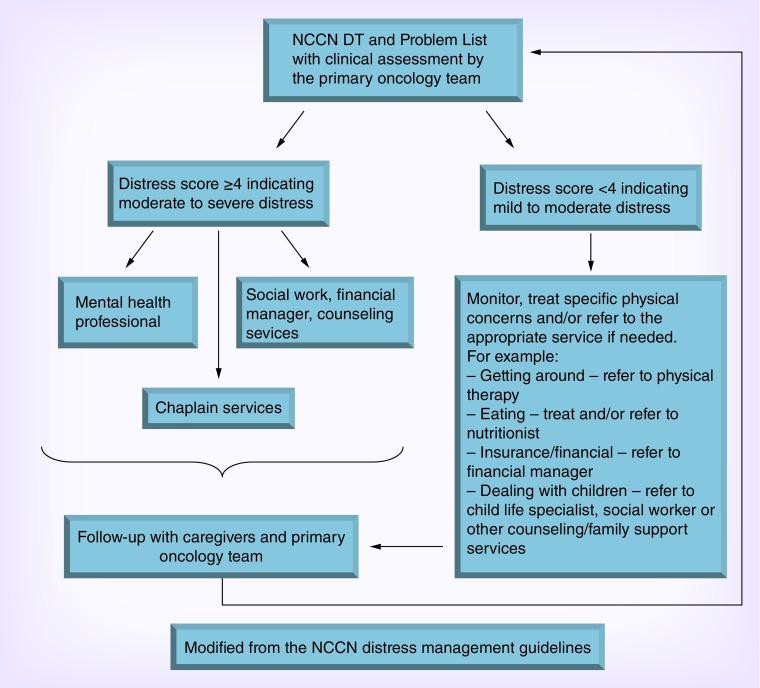
PBT patients experience high levels of distress throughout their disease trajectory, therefore, screening is the first step in assessing distress (Figure 1). Once clinically significant distress is discovered, the patient is referred to the appropriate person to provide support, that is, neuropsychologist, social worker, chaplain, etc. Even though this multidisciplinary approach is used in many cancer centers, patients may not utilize these services. In actuality, almost half of all patients that were identified in a cross sectional analysis of over 2000 cancer patients suffering from significant distress levels, did not use psychosocial resources, nor did they intend to use them [1]. Therefore, other interventions are needed to enable the patients to empower themselves and improve their HRQOL.
Figure 1. . Algorithm for NCCN-DT and Problem List.
Data taken from the NCCN distress management guidelines version.
The majority of PBT patients believe that stress could be reduced by using stress reduction techniques [10]. Most PBT patients were interested in participating in exercise, massage, meditation, yoga and coping skills [10]. Studies utilizing yoga, mindfulness and meditation programs, and exercise have led to improved relaxation, anxiety, fatigue, depression and overall distress [80–84]. In fact, exercise has been implicated in increased survival in recurrent glioma patients [81]. First, research is needed in assessing the validity of these interventions on improving distress in the PBT patient and then, information about these approaches needs to be available to both providers and patients.
As pediatric and adult PBT patients’ survival increases, their needs due to tumor and treatment sequelae will increase. Even though up to 74% of PBT patients experience distress, it has been noted that the highest amount is usually seen in the newly diagnosed population [9,17,19,23]. PBT patients experience different forms of distress, whether it is physical or emotional, at different time periods which could also be impacted by disease recurrence [21,85]. Practitioners should utilize the problem list of the NCCN-DT, at the routine clinic visit, to provide timely and appropriate treatments which may decrease the chances of clinically significant distress occurring. Prospective analyses in the PBT population would be beneficial to determine timely and useful interventions to help patients cope with their burdens and ever changing condition and ultimately to improve HRQOL. Two studies utilizing routine distress screening in glioma and lung cancer patients reported an improvement in patients’ physical and psychologic problems [86,87], but a larger study for PBT patients is warranted. In order to truly assess the patient, an investigation of caregiver burden should also be included. A quick tool, such as the NCCN-DT, for measuring caregiver distress is necessary, along with psychosocial interventions for this population. Further research is needed in order to mitigate the stressors such as fatigue, depression and cognitive dysfunction, along with self-help therapies, such as exercise and mediation in the PBT population. Open dialog about distress is essential among the provider, caregiver and patient as this may prove beneficial in the patient accepting care. In order to achieve improvement in HRQOL, more psychosocial support is needed for both the patient and the caregiver.
Acknowledgements
The authors thank W Gentry for her excellent secretarial support in the development of this manuscript.
Footnotes
Financial & competing interests disclosure
K Peters has served on advisory boards for Agios and Novocure. She has research support with Agios, EISAI, Genentech, Merck and VBL. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br. J. Cancer. 2004;90(12):2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Large study recognizing that cancer patients have high levels of distress and are not utilizing the resources available.
- 2.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 3.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J. Neurooncol. 2006;76(3):283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]; •• A study showing increased fatigue is an independent predictor of overall survival.
- 5.Buckner JC, Schomberg PJ, McGinnis WL, et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92(2):420–433. doi: 10.1002/1097-0142(20010715)92:2<420::aid-cncr1338>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Network NCC. Distress Management. NCCN Version 3 2015 (11/16/15) 2015. www.nccn.org/professionals/physician_gls/pdf/distress.pdf
- 7.Holland JC, Bultz BD National Comprehensive Cancer Network. The NCCN guideline for distress management: a case for making distress the sixth vital sign. J. Natl Compr. Canc. Netw. 2007;5(1):3–7. [PubMed] [Google Scholar]
- 8.Ostrom QT, Gittleman H, Liao P, et al. 19th Annual Scientific Meeting of the Society for Neuro-Oncology. Miami, FL, USA: 13–16 November 2014. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Presented at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goebel S, Stark AM, Kaup L, Von Harscher M, Mehdorn HM. Distress in patients with newly diagnosed brain tumours. Psychooncology. 2011;20(6):623–630. doi: 10.1002/pon.1958. [DOI] [PubMed] [Google Scholar]; •• A study utilizing the NCCN DT with novel cut off score perioperatively in primary intracranial tumor patients.
- 10.Keir ST, Guill AB, Carter KE, Friedman HS. Stress and intervention preferences of patients with brain tumors. Support. Care Cancer. 2006;14(12):1213–1219. doi: 10.1007/s00520-006-0087-9. [DOI] [PubMed] [Google Scholar]; •A study showing primary brain tumor patient's preference for stress relief interventions.
- 11.Kangas M, Tate RL, Williams JR, Smee RI. The effects of radiotherapy on psychosocial and cognitive functioning in adults with a primary brain tumor: a prospective evaluation. Neuro Oncol. 2012;14(12):1485–1502. doi: 10.1093/neuonc/nos244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda M, Eakin EG, Bailey L, Walker D, Troy K. Supportive care needs of people with brain tumours and their carers. Support. Care Cancer. 2006;14(11):1094–1103. doi: 10.1007/s00520-006-0074-1. [DOI] [PubMed] [Google Scholar]
- 13.Kiasuwa Mbengi R, Otter R, Mortelmans K, et al. Barriers and opportunities for return-to-work of cancer survivors: time for action-rapid review and expert consultation. Syst. Rev. 2016;5(1):35. doi: 10.1186/s13643-016-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2000;2(4):221–228. doi: 10.1093/neuonc/2.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, Friedman HS. Screening for distress in patients with brain cancer using the NCCN's rapid screening measure. Psychooncology. 2008;17(6):621–625. doi: 10.1002/pon.1271. [DOI] [PubMed] [Google Scholar]; ••One of the first studies documenting stress in the primary brain tumor population utilizing the NCCN distress thermometer.
- 16.Ma X, Zhang J, Zhong W, et al. The diagnostic role of a short screening tool – the distress thermometer: a meta-analysis. Support. Care Cancer. 2014;22(7):1741–1755. doi: 10.1007/s00520-014-2143-1. [DOI] [PubMed] [Google Scholar]
- 17.Goebel S, Mehdorn HM. Measurement of psychological distress in patients with intracranial tumours: the NCCN distress thermometer. J. Neurooncol. 2011;104(1):357–364. doi: 10.1007/s11060-010-0501-5. [DOI] [PubMed] [Google Scholar]; ••A study validating a new cut off score of the NCCN DT compared to HADS.
- 18.Randazzo DM, McSherry F, Herndon JE, et al. 2015 American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA: 29 May–2 June (2015). Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. Presented at. [Google Scholar]
- 19.Goebel S, Von Harscher M, Mehdorn HM. Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support. Care Cancer. 2011;19(11):1797–1805. doi: 10.1007/s00520-010-1021-8. [DOI] [PubMed] [Google Scholar]
- 20.Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support. Care Cancer. 2009;17(7):793–799. doi: 10.1007/s00520-008-0551-9. [DOI] [PubMed] [Google Scholar]
- 21.Trad W, Koh ES, Daher M, et al. Screening for psychological distress in adult primary brain tumor patients and caregivers: considerations for cancer care coordination. Front. Oncol. 2015;5:203. doi: 10.3389/fonc.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osoba D, Aaronson NK, Muller M, et al. Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J. Neurooncol. 1997;34(3):263–278. doi: 10.1023/a:1005790632126. [DOI] [PubMed] [Google Scholar]
- 23.Keir ST, Swartz JJ, Friedman HS. Stress and long-term survivors of brain cancer. Support. Care Cancer. 2007;15(12):1423–1428. doi: 10.1007/s00520-007-0292-1. [DOI] [PubMed] [Google Scholar]
- 24.Klein M, Engelberts NH, Van Der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann. Neurol. 2003;54(4):514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 25.Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncol. Nurs. Forum. 1999;26(5):921–925. [PubMed] [Google Scholar]
- 26.Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support. Care Cancer. 2006;14(12):1205–1212. doi: 10.1007/s00520-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 27.Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J. Clin. Oncol. 2001;19(20):4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- 28.Page BR, Shaw EG, Lu L, et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro Oncol. 2015;17(10):1393–1401. doi: 10.1093/neuonc/nov084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters KB, West MJ, Hornsby WE, et al. Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. J. Neurooncol. 2014;120(3):499–506. doi: 10.1007/s11060-014-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boele FW, Douw L, De Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. doi: 10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012;8:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psychooncology. 2002;11(6):479–494. doi: 10.1002/pon.614. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 34.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94(10):2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 35.Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. doi: 10.1215/15228517-2008-093. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A review comparing the HRQOL of short and long-term survivors of PBTs.
- 36.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J. Nurs. Scholarsh. 2007;39(1):61–67. doi: 10.1111/j.1547-5069.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 37.Edelstein K, Coate L, Massey C, Jewitt NC, Mason WP, Devins GM. Illness intrusiveness and subjective well-being in patients with glioblastoma. J. Neurooncol. 2016;126(1):127–135. doi: 10.1007/s11060-015-1943-6. [DOI] [PubMed] [Google Scholar]
- 38.Devins GM, Bezjak A, Mah K, Loblaw DA, Gotowiec AP. Context moderates illness-induced lifestyle disruptions across life domains: a test of the illness intrusiveness theoretical framework in six common cancers. Psychooncology. 2006;15(3):221–233. doi: 10.1002/pon.940. [DOI] [PubMed] [Google Scholar]
- 39.Mainio A, Tuunanen S, Hakko H, Niemela A, Koivukangas J, Rasanen P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur. Arch. Psychiatry. Clin. Neurosci. 2006;256(8):516–521. doi: 10.1007/s00406-006-0674-2. [DOI] [PubMed] [Google Scholar]
- 40.Litofsky NS, Farace E, Anderson F, Jr, Meyers CA, Huang W, Laws ER., Jr Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–366. doi: 10.1227/01.neu.0000103450.94724.a2. discussion 366–357. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J. Neurooncol. 2002;57(1):41–49. doi: 10.1023/a:1015728825642. [DOI] [PubMed] [Google Scholar]
- 42.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J. Natl Cancer Inst. 2011;103(1):61–76. doi: 10.1093/jnci/djq458. [DOI] [PubMed] [Google Scholar]; •A review associating depression with decreased HRQOL.
- 43.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230–238. doi: 10.1002/pon.562. [DOI] [PubMed] [Google Scholar]
- 44.Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin. Oncol. Nurs. 2008;24(2):71–79. doi: 10.1016/j.soncn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Hughes MK. Sexual dysfunction and brain tumors: why address it? Neuro Oncol. 2015;17(4):483–484. doi: 10.1093/neuonc/nov016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott JN, Rewcastle NB, Brasher PM, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann. Neurol. 1999;46(2):183–188. [PubMed] [Google Scholar]
- 47.Iconomou G, Mega V, Koutras A, Iconomou AV, Kalofonos HP. Prospective assessment of emotional distress, cognitive function, and quality of life in patients with cancer treated with chemotherapy. Cancer. 2004;101(2):404–411. doi: 10.1002/cncr.20385. [DOI] [PubMed] [Google Scholar]
- 48.Phillips KA, Bernhard J. Adjuvant breast cancer treatment and cognitive function: current knowledge and research directions. J. Natl Cancer Inst. 2003;95(3):190–197. doi: 10.1093/jnci/95.3.190. [DOI] [PubMed] [Google Scholar]
- 49.Greene-Schloesser D, Robbins ME. 17th Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. Washington, DC, USA: 15–18 November 2012. Radiation-induced cognitive impairment-from bench to bedside. Presented at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 51.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 52.Chambers SK, Grassi L, Hyde MK, Holland J, Dunn J. Integrating psychosocial care into neuro-oncology: challenges and strategies. Front. Oncol. 2015;5:41. doi: 10.3389/fonc.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. doi: 10.1016/s0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 54.Bodensohn R, Corradini S, Ganswindt U, et al. A prospective study on neurocognitive effects after primary radiotherapy in high-grade glioma patients. Int. J. Clin. Oncol. 2015 doi: 10.1007/s10147-015-0941-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J. Clin. Oncol. 2000;18(3):646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 56.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5(2):89–95. doi: 10.1215/S1522-8517-02-00026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen C, Bao WM, Yang BJ, et al. Cognitive deficits in patients with brain tumor. Chin. Med. J. (Engl). 2012;125(14):2610–2617. [PubMed] [Google Scholar]
- 58.Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother. Oncol. 2010;97(3):370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazda T, Jancalek R, Pospisil P, et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat. Oncol. 2014;9:139. doi: 10.1186/1748-717X-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 61.Correa DD, Kryza-Lacombe M, Baser RE, Beal K, DeAngelis LM. Cognitive effects of donepezil therapy in patients with brain tumors: a pilot study. J. Neurooncol. 2016;127(2):313–319. doi: 10.1007/s11060-015-2035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J. Clin. Oncol. 1998;16(7):2522–2527. doi: 10.1200/JCO.1998.16.7.2522. [DOI] [PubMed] [Google Scholar]
- 63.Vachon M. Psychosocial distress and coping after cancer treatment. How clinicians can assess distress and which interventions are appropriate – what we know and what we don't. Am. J. Nurs. 2006;106(3 Suppl.):26–31. doi: 10.1097/00000446-200603003-00011. [DOI] [PubMed] [Google Scholar]
- 64.Bosma I, Reijneveld JC, Douw L, et al. Health-related quality of life of long-term high-grade glioma survivors. Neuro Oncol. 2009;11(1):51–58. doi: 10.1215/15228517-2008-049. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A study comparing the HRQOL of short and long-term high grade glioma (HGG) survivors.
- 65.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J. Neurol. Neurosurg. Psychiatry. 2005;76(4):562–568. doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macartney G, Harrison MB, VanDenKerkhof E, Stacey D, McCarthy P. Quality of life and symptoms in pediatric brain tumor survivors: a systematic review. J. Pediatr. Oncol. Nurs. 2014;31(2):65–77. doi: 10.1177/1043454213520191. [DOI] [PubMed] [Google Scholar]
- 67.Woodgate RL, Tailor K, Yanofsky R, Vanan MI. Childhood brain cancer and its psychosocial impact on survivors and their parents: a qualitative thematic synthesis. Eur. J. Oncol. Nurs. 2016;20:140–149. doi: 10.1016/j.ejon.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 69.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009;27(14):2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS) Sleep. 2008;31(2):271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol. Biomarkers Prev. 2008;17(2):435–446. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 72.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 2004;22(6):999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 73.Zhou ES, Manley PE, Marcus KJ, Recklitis CJ. Medical and psychosocial correlates of insomnia symptoms in adult survivors of pediatric brain tumors. J. Pediatr. Psychol. 2015 doi: 10.1093/jpepsy/jsv071. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann. Intern. Med. 2005;143(9):639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 75.Prevatt FF, Heffer RW, Lowe PA. A review of school reintegration programs for children with cancer. J. Sch. Psychology. 2000;38(5):447–467. [Google Scholar]
- 76.Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients – observations from a pilot study. Support. Care Cancer. 2006;14(12):1258–1261. doi: 10.1007/s00520-006-0090-1. [DOI] [PubMed] [Google Scholar]
- 77.Witt WP, Litzelman K, Wisk LE, et al. Stress-mediated quality of life outcomes in parents of childhood cancer and brain tumor survivors: a case–control study. Qual. Life Res. 2010;19(7):995–1005. doi: 10.1007/s11136-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peterson CC, Drotar D. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: review of research, clinical evidence, and future directions. Clin. Child Psychol. Psychiatry. 2006;11(3):349–366. doi: 10.1177/1359104506064980. [DOI] [PubMed] [Google Scholar]
- 79.Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I'll show them: the social construction of (in)competence in survivors of childhood brain tumors. J. Pediatr. Oncol. Nurs. 2008;25(3):164–174. doi: 10.1177/1043454208315547. [DOI] [PubMed] [Google Scholar]
- 80.McCall M, McDonald M, Thorne S, Ward A, Heneghan C. Yoga for health-related quality of life in adult cancer: a randomized controlled feasibility study. Evid. Based Complement. Alternat. Med. 2015;2015:12. doi: 10.1155/2015/816820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruden E, Reardon DA, Coan AD, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J. Clin. Oncol. 2011;29(21):2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levin GT, Greenwood KM, Singh F, Tsoi D, Newton RU. Exercise improves physical function and mental health of brain cancer survivors: two exploratory case studies. Integr. Cancer Ther. 2015 doi: 10.1177/1534735415600068. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 2007;21(8):1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Monti DA, Kash KM, Kunkel EJ, et al. Changes in cerebral blood flow and anxiety associated with an 8-week mindfulness programme in women with breast cancer. Stress Health. 2012;28(5):397–407. doi: 10.1002/smi.2470. [DOI] [PubMed] [Google Scholar]
- 85.Rooney AG, McNamara S, Mackinnon M, et al. The frequency, longitudinal course, clinical associations, and causes of emotional distress during primary treatment of cerebral glioma. Neuro Oncol. 2013;15(5):635–643. doi: 10.1093/neuonc/not009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watson L, Groff S, Tamagawa R, et al. Evaluating the impact of provincial implementation of screening for distress on quality of life, symptom reports, and psychosocial well-being in patients with cancer. J. Natl Compr. Canc. Netw. 2016;14(2):164–172. doi: 10.6004/jnccn.2016.0019. [DOI] [PubMed] [Google Scholar]
- 87.Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems – secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880–1888. doi: 10.1002/pon.3223. [DOI] [PubMed] [Google Scholar]