Abstract
E75 (nelipepimut-S) is an immunogenic peptide derived from the HER2 protein. When combined with the immunoadjuvant granulocyte–macrophage colony-stimulating factor (GM-CSF), nelipepimut-S has been used as a vaccine that is capable of eliciting a robust anti-HER2 immune response. Early-phase clinical trials that enrolled women with node-positive or high-risk node-negative breast cancer who had been rendered disease free with standard of care therapy but were at risk for recurrence, demonstrated the vaccine to be safe with a suggestion of clinical benefit. Nelipepimut-S is currently being evaluated in a Phase III clinical trial. This article covers the preclinical and clinical development of nelipepimut-S.
KEYWORDS : breast cancer, cancer vaccine, E75, nelipepimut-S, peptide
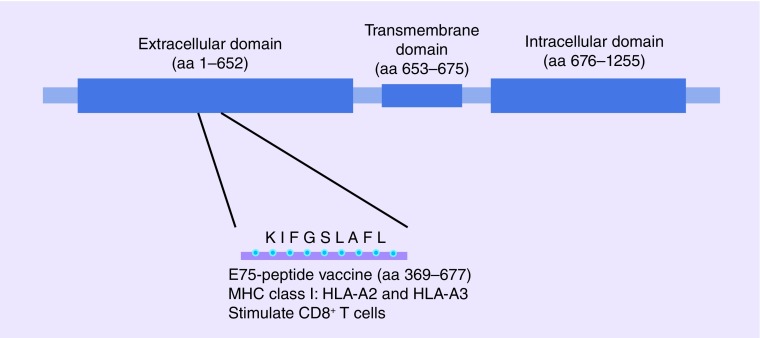
Immunotherapy has become an important focus of cancer research and drug development. Despite a history of setbacks, real clinical benefit is now being realized using multiple forms of immunotherapy in cancer patients. Cancer vaccines, a form of active immunotherapy, target immunogenic cancer-related epitopes that are distinct from normal tissue or dramatically overexpressed on malignant tissue by stimulating the immune system to recognize and eliminate tumor cells. E75 (HER2/neu 366–379, KIFGSLAFL), a nine amino acid peptide derived from the extracellular domain of the HER2 protein (Figure 1), has emerged as a target for vaccination.
Figure 1. . E75 peptide.
E75 is a nine aa peptide derived from the HER2 protein's extracellular domain.
aa: Amino acid.
E75 has been used to target cancers in several formulations, but it has been evaluated most extensively as a peptide vaccine in combination with other peptides or alone with an immunoadjuvant. Much of the early clinical work with the E75 peptide vaccines targeted patients with advanced disease, a strategy that has proven challenging due to tolerance and immunosupression. More recent work has focused on E75 peptide vaccination in breast cancer patients who are clinically disease-free but at high risk for recurrence. Currently, the E75 peptide (nelipepimut-S) combined with GM-CSF immunoadjuvant is being evaluated in a Phase III clinical trial to prevent recurrences in breast cancer patients. This article covers the preclinical development and early phase trials of the E75 peptide vaccine. Ongoing studies and future directions for trials incorporating the vaccine are also discussed.
Preclinical work
E75 emerged as a potentially important epitope from the initial preclinical work evaluating HER2 peptides for use in immunotherapy. E75 was first identified in the early 1990s as a nonapeptide from HER2 which, based on its peptide sequence, was predicted to bind to the HLA-A2 MHC type 1 molecule. Out of 19 HER2-derived peptides initially evaluated by Fisk et al. E75 alone was capable of stimulating cell lysis by four different ovarian cancer reactive cytotoxic T lymphocyte (CTL) lines [1]. This peptide, among others, was also tested in an HLA-A2 stabilization assay. E75 was noted to have high stabilizing ability, particularly compared with other HER2-derived peptides [2,3]. Additional studies confirmed that E75 represented the HLA-A2 immunodominant epitope of HER2 [4,5].
Subsequent independent studies confirmed the significance of E75 in tumor immunity. In a transgenic murine model, E75 was able to stimulate a HER2-specific CTL response. These HER2-specific CTL were shown to be capable of lysing tumor cells [6]. Two preclinical studies found that E75 peptide was presented on HLA-A2 in ovarian cancer cell lines and was recognized by ovarian cancer-associated CD8+ T-cell clones [2,4]. Patients with breast, ovarian, lung and colorectal cancers were found to have pre-existing immunity to E75 in a high percentage of cases [6,7]. Lymphocytes from draining lymph nodes in women with breast cancer with and without overexpression of HER2, proliferated and mounted a primarily T helper type 1 cytokine response when exposed to E75 and other HER2 peptides. Interestingly, the proliferative response was often blunted in cells from nodes with metastatic cancer compared with unaffected nodes from the same patient [8,9]. Studies also demonstrated that E75-pulsed CTLs were able to lyse HER2-expressing colon cancer and renal cell carcinoma cells ex vivo and tumor cells in vivo in transgenic mice [6,10].
After E75 was identified and characterized, work began to determine if induction of E75-specific immunity was feasible. Peripheral blood mononuclear cell-derived dentritic cells from ten healthy HLA-A2+ volunteers were stimulated with E75 and used to prime autologous peripheral blood mononuclear cells (PBMCs). Stimulated E75-specific CTL activity was achieved in half (5/10) [11,12].
Peptide-based vaccines
E75 has been used in monocyte-derived dendritic cell vaccines studied in early-phase testing in ductal carcinoma in situ, invasive breast cancer and gastric cancer; however, E75 has been evaluated most extensively as a peptide-based vaccine given alone and in combination with other immunogenic peptides and with various immunoadjuvants [4,11,13–14]. Interest in using E75 alone as a vaccine arises from the simplicity of vaccine formulation, ease of monitoring and the desire to use the immunodominant epitope alone without competition by other antigens.
• Peptide vaccines for advanced disease
Vaccination with E75 alone began with immunization of patients with metastatic breast, ovarian and colorectal cancer with 1000 µg of E75 with incomplete Freund's adjuvant. Three of four vaccinated patient responded with a detectable peptide-specific CTL response, however, these CTLs were unable to lyse HER2-expressing tumor cells ex vivo [8]. Subsequent studies evaluating this adjuvant has demonstrated peptide-specific T-cell trafficking back to sites of vaccination and ultimately deletion of these T cells due to over stimulation [15]. It is unclear if this mechanism may have impacted the results in these early patients.
Disis et al. further examined the feasibility of E75 vaccination in patients in two small trials. In one, women with breast and ovarian cancer were given six monthly intradermal inoculations of 500 µg of E75 with 125 µg of GM-CSF immunoadjuvant. They noted an immunologic response, as determined by enzyme-linked immunospot (ELISPOT) assay, in two of six vaccinated patients. The immunologic response was no longer detectable 5 months after completing the vaccination series [16]. A second small pilot study by this group evaluated the use of E75 with Flt3 ligand, a growth and differentiation factor for dendritic cells, or GM-CSF as an immunoadjuvant in men with advanced prostate cancer. Regardless of which immunoadjuvant was used, the immunologic response to vaccination was poor both in vitro, as assessed by ELISPOT assay, and in vivo, as assessed by DTH [17]. Of note, in both studies, most patients had advanced disease and received doses of E75 and immunoadjuvant less than what was later determined to be the optimal biologic dose.
This group also tested the impact of stimulating a CD4+ helper T-cell response in combination with a CD8+ T-cell response. They did this by administering a vaccine that contained longer MHC class II peptides (stimulate a CD4+ helper T-cell response) that had MHC class I peptides, including E75, embedded within the longer peptide sequences. Of 14 HLA-A2+ patients vaccinated with the E75 containing helper peptide, the majority developed demonstrable immunity to E75 after vaccination [18].
An additional peptide based vaccine Phase I trial of E75 along with other immunogenic peptides was conducted in patients with ovarian and fallopian tube cancers. Nine patients were given 100 µg of E75 with four other MHC class I peptides, an MHC class II tetanus toxid helper peptide and immunoadjuvant. The six HLA-A2+ patients were evaluated for immunologic response by ELISPOT in peripheral blood and the sentinel immunized lymph node. The vaccines were well tolerated with only grade 1 and 2 toxicity observed. Five of six patients demonstrated a robust immunologic response to E75 [19].
E75 alone with GM-CSF immunoadjuvant was also tested in a dose-escalation study in patients with pretreated, measurable metastatic breast or ovarian cancer. The primary objective of the study was to determine feasibility, toxicity and immunologic response to vaccination. A total of 13 breast cancer patients and one ovarian cancer patient were enrolled. Patients received either 100, 500 or 1000 µg of E75 with 250 µg of GM-CSF immunoadjuvant intradermally weekly for 4 weeks followed by monthly for a total of ten inoculations. The vaccine series was well tolerated with only grade 1 and 2 toxicity. The majority (7/8) of tested patients demonstrated a delayed type hypersenstivitiy (DTH) response to E75 after the fifth inoculation. Vaccination induced antigen-specific CTLs that were able to lyse tumor cells expressing E75, in half (4/8) of tested patients [20].
• Peptide vaccines to prevent recurrences
The initial clinical work listed above demonstrated that the vaccine was safe and immunogenic; however, it failed to generate meaningful clinical responses in patients with metastatic disease. With the emerging idea that peptide-based vaccination could work most effectively when there is minimal disease and, therefore, minimal immune suppression, Phase I/II clinical trials were initiated in the adjuvant setting. Separate studies were conducted in patients with prostate and breast cancer. In both studies, patients had been rendered disease free after completing standard therapy, but were at risk for recurrence.
Prostate cancer
Prostate cancer has been shown to express HER2 in a high percent of cases and preclinical data indicated that stimulations of PBMCs from prostate cancer patients stimulated with E75 are capable of peptide-specific cytolytic activity [21]. A Phase I/II trial was conducted in prostate cancer patients who had undergone resection but were at high risk of recurrence by predictive modeling. Forty patients were enrolled with 21 HLA-A2+ patients vaccinated and 19 HLA-A2- patients followed as controls. Vaccinated patients were dose escalated with 100, 500 or 1000 µg of E75 with 250 µg of GM-CSF injected intradermally monthly for 4–6 months. Toxicity, immunologic response, biochemical (by monitoring prostate specific antigen) and clinical recurrences, and survival were monitored. The vaccination series, whether it was four or six inoculations, was well tolerated with only grade 1 and 2 toxicity. Five patients had their doses of GM-CSF reduced to 125 µg during the vaccination series due to large local reactions. Immune responses to vaccination were confirmed using both in vivo and in vitro assays. [22]. At a median follow-up of 58 months, there was no difference in biochemical recurrence rates (29% vaccine vs 26% control), clinical recurrence rates or overall survival [23].
Breast cancer Phase I
Along with the Phase I clinical trial in prostate cancer, Phase I trials in node negative and node positive breast cancer patients were initiated. Like the prostate cancer trial, these trials targeted patients who were clinically disease-free but at high risk of recurrence to test the hypothesis that patients with decreased disease burden and, therefore, decreased tumor-mediated suppression, are more likely to achieve clinical benefit. Run in parallel, one trial enrolled node-positive patients with the other enrolled patients who were node negative with high-risk features. All patients had completed standard surgical, chemotherapeutic and radiation therapy. Patients were allowed to be on hormonal therapy during the trial. HLA-A2+ patients were vaccinated while HLA-A2- patients were followed prospectively as controls as HLA-A2 status did not influence prognosis in previous studies. Importantly, patients with any level of HER2 expression (1+, 2+ or 3+ by immunohistochemistry [IHC]) were included in the trials. Like the prostate cancer Phase I/II trial, the vaccine was well tolerated and demonstrated encouraging immunologic results and was, therefore, transitioned to Phase II trials [24].
Breast cancer Phase II
Based on encouraging initial results, the node-positive and high-risk node-negative trials were transitioned into Phase II studies run in parallel. The trials extended follow-up out to 60 months (5 years). The trials ultimately enrolled 195 patients (100 node-positive, 95 node-negative). Six patients withdrew prior to receiving the vaccine, one was lost to follow-up, and one was excluded for nonstandard surgical therapy, leaving 187 evaluable patients.
The toxicities from the primary vaccine series were generally mild and well tolerated. Local toxicities were all grade 1 (83.3%) and grade 2 (16.7%). Similarly, systemic toxicities were mild with only two grade 3 and no grade 4 or 5 toxicities observed [25].
Of the 187 evaluable patients, 108 HLA-A2/3+ patients were vaccinated while 79 HLA-A2/A3- patients were followed as controls. The vaccine and control groups were well matched for demographic and prognostic features except that vaccinated patients were more likely to be hormone receptor negative. After 5 years, disease-free survival (DFS) for vaccinated patients was 89.7% compared with 80.2% for control patients (p = 0.08), giving the vaccine a 48% reduction in relative risk of recurrence [25]. While it is important to recognize that this trial did not demonstrate a statistically significant difference between the vaccine and control groups, it did confirm the vaccine to be safe and showed evidence of potential efficacy which needs to be confirmed in a subsequent Phase III trial that is appropriately powered to answer the question of clinical benefit. Such a trial is currently ongoing as will be discussed further below.
Optimal biologic dose
As previously mentioned, the node-positive and high-risk node-negative breast cancer trials began as dose-escalation/schedule optimization trials. Patients were inoculated with between 100 and 1000 µg of peptide along with 125 or 250 µg of GM-CSF monthly for four or six inoculations total. The vaccine series produced manageable local and systemic toxicity without any grade 4 toxicity or dose-limiting toxicities observed [24]. The maximum dose tested, 1000 µg of E75 with 250 µg of GM-CSF for six monthly inoculations, was found to have higher rates of immunologic response without significantly greater toxicity compared with other dosing groups, and was thus determined to be the optimal biologic dose (OBD) [26]. In the 37 vaccinated patients who received the OBD, a DFS rate of 94.6% was noted versus 80.2% in unvaccinated control patients (p = 0.05). Although the differences were not statistically significant, it should be noted that, compared with control patients, optimally dosed patients were more likely to have high-grade (p = 0.30), and estrogen receptor (ER)-negative tumors (p = 0.25). They were also more likely to have node-positive disease (p = 0.16), and consistent with this, the optimally dosed patients were also more likely to have received chemotherapy (p = 0.03) compared with control patients.
Booster inoculations
During the conduct of the Phase II breast cancer trials, two modifications to the protocol were made based on observations of trial patients. One modification came as an increasing number of recurrences were noted in the vaccine group corresponding with waning immunity a year or more after completion of the vaccine series. The planned 24-month landmark analysis demonstrated an improvement in DFS, the primary end point, in the vaccine group compared with control group (94.3 vs 86.8%; p = 0.08). However, over time, late recurrences were observed in the vaccine group corresponding with decreased immunity measured by E75-specific T cells. Therefore, a booster inoculation program was instituted. For previously vaccinated patients, booster inoculations were offered but not mandated. For patients that enrolled on the trial after the booster inoculations were started, boosters were included as part of their trial participation. Booster inoculations were administered every 6 months. A total of 53 (49.0%) of the vaccinated patients received booster inoculations. The booster inoculations were well tolerated and effective in maintaining measurable E75-specific immunity [27]. Local and systemic toxicities were similar to those seen during the primary vaccination series. Delayed urticarial reactions occurred in four patients 1–2 weeks after receiving booster inoculations [25]. This is a unique toxicity that has also been seen in other peptide-based cancer vaccines and is due to the GM-CSF immunoadjuvant [28]. In total, 21 vaccinated patients received their initial booster inoculation within 6 months of completion of the primary vaccine series and were thus considered optimally boosted. Optimally boosted patients were well matched to control patients with respect to clinicopathologic characteristics with the exception that boosted patients were more likely to be ER negative and progesterone receptor negative therefore less likely to receive hormonal therapy. The DFS rate at 5 years for optimally boosted patients was 95.2% (p = 0.11 vs control patients; 80.2% at 5 years).
Inclusion of HLA-A3+ patients
The second modification was to increase the potentially eligible patients for vaccination to include HLA-A3+ patients. Based on preclinical data confirming that E75 is bound by the HLA-A3 molecule, E75 was thought to be clinically effective in HLA-A3+ patients in addition to HLA-A2+ patients [7]. Adding HLA-A3+ patients potentially expands the vaccine's applicability from 40–50% of the US population (HLA-A2+ alone) to as much as 60–75% [29]. Because of this, HLA-A3+ were included in the vaccine group while HLA-A2/A3- patients continued to be followed as controls. During the conduct of the trial, 13 HLA-A3+ patients were vaccinated and demonstrated similar toxicity profiles, immunologic response by DTH, and 5-year DFS rate (92.3%) compared with HLA-A2+ vaccinated patients [30]. While this small number of HLA-A3+ patients limits the conclusions that can be drawn, taken together with the preclinical data, this does support the inclusion of HLA-A3+ patients in subsequent studies evaluating the E75 vaccine.
HER2 status
As previously stated, an important aspect of this trial is that it included patients with any level of HER2 expression (IHC 1+, 2+, or 3+). HER2, in certain tumors, can be a substantial driver of malignant behavior. In breast cancer, approximately 20% of cancers overexpress HER2, and if left untreated, have a worse prognosis than HER2-nonoverexpressing breast cancers. However, with the advent of trastuzumab and other HER2-targeted therapies, the prognosis for HER2-overexpressing tumors is similar or better than nonoverexpressing tumors. This, however, leaves patients with HER2-nonoverexpressing tumors and, in particular, triple-negative breast cancer, with a need for further treatment options. In these trials, DFS in patients with HER2 nonoverexpressing tumors (IHC 1+ or 2+) was 88.1% in vaccinated patients compared with 77.5% in well-matched controls (p = 0.16). Part of the effectiveness of active immunotherapy with E75 in HER2 low-expressing tumors may be due to decreased immune tolerance due to increased antigen exposure and lower MHC class I surface expression associated with higher HER2 expression levels [31]. This suggests that E75 may have a role in preventing recurrence in this at-need patient population [32].
Table 1 summarizes key findings from the trials conducted to date evaluating the E75 peptide as a component of a vaccine.
Table 1. . Summary of clinical trials evaluating the E75 peptide as a component of a vaccine strategy.
| Study (year) | Phase | Population (n) of patients enrolled | Key findings | Ref. |
|---|---|---|---|---|
| Zaks et al. (1998) | I | Metastatic ovarian (2), colorectal (1), breast (1) cancers | Vaccination with E75 peptide combined with incomplete Freund's adjuvant produced E75-specific CTL responses; however, in ex vivo assays, these CTL failed to react to HER2+ tumor cells | [8] |
| Knutson et al. (2002) | I | Stage III + IV breast (4) and ovarian (2) cancer | Vaccination with E75 peptide combined with the adjuvant GM-CSF stimulated E75-specific CTL responses that diminished with time | [16] |
| McNeel et al. (2003) | I | Advanced prostate cancer (20) | Vaccination with the E75 peptide combined with the adjuvant Flt3 ligand resulted in a poor peptide-specific immune response suggesting Flt3 ligand may not be an effective immunoadjuvant | [17] |
| Disis et al. (2002) | I | Stage III + IV breast, ovarian and NSCLC (64; 14 given E75 containing helper peptides) | Evaluated a strategy of stimulating a CD4+ helper T-cell response in addition to a CD8 response by administering a vaccine comprised of longer MHC class II peptides that had MHC class I peptides, including E75, embedded within the longer sequences. HLA-A2+ patients developed demonstrable immunity to E75 after vaccination | [18] |
| Chianese-Bullock et al. (2008) | I | Stage II–IV ovarian cancer (9) | Multipeptide vaccination produced high rates of immunologic response; however, the majority of patients experienced disease progression. | [19] |
| Murray et al. (2002) | I | Metastatic ovarian (1) and breast (13) cancer | Vaccination with E75 + GM-CSF resulted in immunologic responses to E75 in most patients; however, all patients experienced disease progression | [20] |
| Gates et al. (2009) | I/II | Clinically disease-free prostate cancer (vaccinated 21, control 19) | One of the early studies evaluating the E75 + GM-CSF vaccine in the adjuvant setting administered to disease-free patients. There was a high rate of in vivo and ex vivo immunologic response to vaccination and suggestion of potential clinical benefit if the vaccination series was completed prior to biochemical recurrence | [22,23] |
| Mittendorf et al. (2014) | I/II | Clinically disease-free breast cancer (vaccinated 100, control 95) | Early-phase trials evaluating E75 + GM-CSF in the adjuvant setting in node-positive and high-risk node-negative breast cancer vaccination. Trials confirmed the vaccine to be safe and capable of stimulating an antigen-specific immune response. Trials provided data that informed the design of an ongoing Phase III registration trial: optimal dose of the peptide (1000 µg) was confirmed | [24–27,30] |
| Booster inoculations were shown to be effective in maintaining antigen-specific immunity | ||||
| Robust immune responses were observed in patients with tumors expressing low levels of the HER2 protein (immunohistochemistry 1+ or 2+) | ||||
CTL: Cytotoxic T lymphocyte; GM-CSF: Granulocyte–macrophage colony-stimulating factor; NSCLC: Non-small-cell lung cancer..
Discussion
Based on initial Phase I/II data in breast cancer, E75 (now licensed to Galena Biopharma; nelipepimut-S) with GM-CSF immunoadjuvant is currently being assessed in an ongoing Phase III clinical trial. The randomized Phase III (PRESENT trial, NCT01479244) enrolled HLA-A2+/A3+, node-positive, HER2 1+ or 2+ breast cancer patients who were clinically disease-free after completion of standard therapy. Importantly, all vaccinated patients will be optimally dosed and receive booster inoculations as part of their treatment. The primary end point, DFS, will be assessed at 3 years after enrollment. The 750 patient trial completed enrollment in April 2015.
Additionally, nelipepimut-S is undergoing investigations in two Phase II trials in combination with trastuzumab. In both trials, clinically disease-free, node-positive or high-risk node-negative, breast cancer patients are randomized to trastuzumab given concurrently with E75 and GM-CSF or trastuzumab with GM-CSF alone with a primary outcome of DFS. One trial is enrolling HER2 IHC 1+ or 2+ breast cancer patients (NCT01570036) while the other, HER2 overexpressing breast cancer patients (NCT02297698). There is preclinical and early clinical evidence that suggests that targeting HER2 with trastuzumab works synergistically with active immunotherapy [33,34]. Trastuzumab binding leads to increased internalization and proteolytic degradation of HER2 molecules. This, in turn, leads to increased HER2 peptide presentation on MHC receptors and greater susceptibility to HER2-targeted vaccination.
Having shown the vaccine to potentially be effective in the setting of secondary prevention, there is interest in further exploring whether it could be effective as a primary prevention agent. As an initial step to address this question, a clinical trial evaluating E75 + GM-CSF in patients with ductal carcinoma in situ (NCT023636582) is set to begin accrual in the spring of 2016. In this randomized, Phase II study, patients diagnosed with DCIS and found to be HLA-A2+ will be randomized to E75 + GM-CSF versus GM-CSF alone. Patients will receive three inoculations prior to surgery then complete the final three inoculations of the primary vaccination series in the postoperative setting. The trials primary end point is to evaluate the generation of E75-specific CTL with several secondary end points to include looking for evidence of epitope spreading to other tumor antigens in the peripheral blood as well as evaluating the T-cell infiltrate in the resected surgical specimen.
In addition to these efforts, there is future interest in testing the efficacy of E75 peptide vaccination for patients with more advanced cancer in combination with immune checkpoint blockade, regulator T-cell depletion and/or novel immunoadjuvants.
Conclusion
Multiple trials have consistently demonstrated that the E75 peptide vaccine is safe and capable of stimulating a strong antitumor immune response. E75 (nelipepimut-S) with GM-CSF immunoadjuvant has demonstrated encouraging Phase II clinical trial results and is currently being tested in a Phase III trial to prevent breast cancer recurrences in patients with node-positive HER2 1+ or 2+ breast cancer who have been rendered disease free with standard of care therapy. Should E75 prove effective in late-stage testing, E75 may have a role in breast cancer treatment and will serve as proof of principle for peptide vaccination in preventing recurrences in cancer patients.
EXECUTIVE SUMMARY.
Preclinical work
E75, a nonapeptide derived from the extracellular domain of the HER2 protein, has been identified as the protein's HLA-A2 immunodominant epitope.
E75-stimulated cytotoxic T lymphocytes (CTLs) are capable of lysing HER2-expressing tumor cells in vitro.
In vivo experiments in a transgenic mouse model showed that E75 is capable of stimulating an antigen-specific CTL response.
Patients with breast, ovarian, lung and colorectal cancers have been found to have pre-existing immunity to E75 confirming that it is a naturally processed and presented epitope.
Peptide vaccines for advanced disease
Initial studies combining E75 with the immunoadjuvant incomplete Freund's adjuvant showed that vaccinated patients generated a peptide-specific CTL response, however, the CTL were not able to lyse HER2-expressing cells ex vivo.
Subsequent studies combining E75 with the immunoadjuvant granulocyte–macrophage colony-stimulating factor (GM-CSF) showed this formulation to be safe and capable of stimulating an immunologic response, however, in the majority of trials evaluating this vaccine, the immune response was not sustained.
Early-phase trials enrolling patients with metastatic disease did not report efficacy end points.
Peptide vaccines for secondary prevention
The metastatic microenvironment is characterized by immunosuppressive cells and cytokines. The ability of a single epitope peptide vaccine to have therapeutic efficacy in this setting is limited. Therefore, subsequent clinical trials evaluating E75 + GM-CSF evaluated a potential role for the vaccine administered in the adjuvant setting to prevent disease recurrence.
A Phase I/II trial that enrolled 40 prostate cancer patients confirmed the E75 + GM-CSF vaccine to be capable of stimulating in vivo and in vitro immune responses however after a median follow-up of 58 months, there was no difference in biochemical or clinical recurrence rates.
Phase I/II trials that enrolled 187 evaluable node-positive and high-risk node-negative breast cancer patients confirmed the vaccine to be safe and capable of stimulating an antigen-specific immune response. The 5-year disease-free survival rate for vaccinated patients was 89.7 versus 80.2% for control patients (p = 0.08). For patients receiving the optimal dose of the vaccine, the 5-year disease-free survival rate was 94.6%.
Based on encouraging data from the early phase trials, the E75 + GM-CSF vaccine is now being evaluated in a Phase III registration trial that completed randomization in April 2015.
Ongoing Phase II trials are evaluating combination immunotherapy with the E75 + GM-CSF vaccine and the monoclonal antibody trastuzumab.
Footnotes
Disclosure
In addition to the peer review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the author at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusions.
Financial & competing interests disclosure
GE Peoples has inventor rights to E75. This vaccine has been licensed for commercial development. He is entitled to financial proceeds associated with this license per Federal policy. GE Peoples also consults in the development of the vaccine. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 1995;181(6):2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Report detailing the identification and initial preclinical evaluation of the E75 epitope as an immunogenic peptide.
- 2.Fisk B, Anderson BW, Gravitt KR, et al. Identification of naturally processed human ovarian peptides recognized by tumor-associated CD8+ cytotoxic T lymphocytes. Cancer Res. 1997;57(1):87–93. [PubMed] [Google Scholar]
- 3.Fisk B, Savary C, Hudson JM, et al. Changes in an HER-2 peptide upregulating HLA-A2 expression affect both conformational epitopes and CTL recognition: implications for optimization of antigen presentation and tumor-specific CTL induction. J. Immunother. Emphasis Tumor Immunol. 1995;18(4):197–209. doi: 10.1097/00002371-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kono K, Takahashi A, Sugai H, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin. Cancer Res. 2002;8(11):3394–3400. [PubMed] [Google Scholar]
- 5.Kawashima I, Hudson SJ, Tsai V, et al. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum. Immunol. 1998;59(1):1–14. doi: 10.1016/s0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 6.Lustgarten J, Theobald M, Labadie C, et al. Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD.8. Hum. Immunol. 1997;52(2):109–118. doi: 10.1016/S0198-8859(96)00292-3. [DOI] [PubMed] [Google Scholar]
- 7.Sotiropoulou PA, Perez SA, Iliopoulou EG, et al. Cytotoxic T-cell precursor frequencies to HER-2 (369 – 377) in patients with HER-2/neu-positive epithelial tumours. Br. J. Cancer. 2003;89(6):1055–1061. doi: 10.1038/sj.bjc.6601244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58(21):4902–4908. [PubMed] [Google Scholar]; • Initial study of the E75 peptide as a component of a vaccine that showed that the epitope was capable of stimulating an antigen-specific immune response.
- 9.Kuerer HM, Peoples GE, Sahin AA, et al. Axillary lymph node cellular immune response to HER-2/neu peptides in patients with carcinoma of the breast. J. Interferon Cytokine Res. 2002;22(5):583–592. doi: 10.1089/10799900252982061. [DOI] [PubMed] [Google Scholar]
- 10.Brossart P, Stuhler G, Flad T, et al. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 1998;58(4):732–736. [PubMed] [Google Scholar]
- 11.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96(9):3102–3108. [PubMed] [Google Scholar]
- 12.Anderson BW, Peoples GE, Murray JL, Gillogly MA, Gershenson DM, Ioannides CG. Peptide priming of cytolytic activity to HER-2 epitope 369–377 in healthy individuals. Clin. Cancer Res. 2000;6(11):4192–4200. [PubMed] [Google Scholar]
- 13.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 14.Fracol M, Xu S, Mick R, et al. Response to HER-2 pulsed DC1 vaccines is predicted by both HER-2 and estrogen receptor expression in DCIS. Ann. Surg. Oncol. 2013;20(10):3233–3239. doi: 10.1245/s10434-013-3119-y. [DOI] [PubMed] [Google Scholar]
- 15.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin. Cancer Res. 2002;8(5):1014–1018. [PubMed] [Google Scholar]
- 17.McNeel DG, Knutson KL, Schiffman K, Davis DR, Caron D, Disis ML. Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J. Clin. Immunol. 2003;23(1):62–72. doi: 10.1023/a:1021904432489. [DOI] [PubMed] [Google Scholar]
- 18.Disis ML. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J. Clin. Oncol. 2002;20(11):2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 19.Chianese-Bullock KA, Irvin WP, Jr, Petroni GR, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J. Immunother. 2008;31(4):420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- 20.Murray JL, Gillogly ME, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin. Cancer Res. 2002;8(11):3407–3418. [PubMed] [Google Scholar]
- 21.Woll MM, Hueman MT, Ryan GB, et al. Preclinical testing of a peptide-based, HER2/neu vaccine for prostate cancer. Int. J. Oncol. 2004;25(6):1769–1780. [PubMed] [Google Scholar]
- 22.Hueman MT, Dehqanzada ZA, Novak TE, et al. Phase I clinical trial of a HER-2/neu peptide (E75) vaccine for the prevention of prostate-specific antigen recurrence in high-risk prostate cancer patients. Clin. Cancer Res. 2005;11(20):7470–7479. doi: 10.1158/1078-0432.CCR-05-0235. [DOI] [PubMed] [Google Scholar]
- 23.Gates JD, Carmichael MG, Benavides LC, et al. Longterm followup assessment of a HER2/neu peptide (E75) vaccine for prevention of recurrence in high-risk prostate cancer patients. J. Am. Coll. Surg. 2009;208(2):193–201. doi: 10.1016/j.jamcollsurg.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol. 2005;23(30):7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the Phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014;25(9):1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Final 5-year analysis of the early-phase trials evaluating the E75 + GM-CSF vaccine. These data support the conduct of the ongoing Phase III trial.
- 26.Holmes JP, Gates JD, Benavides LC, et al. Optimal dose and schedule of an HER-2/neu (E75) peptide vaccine to prevent breast cancer recurrence: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2008;113(7):1666–1675. doi: 10.1002/cncr.23772. [DOI] [PubMed] [Google Scholar]
- 27.Holmes JP, Clifton GT, Patil R, et al. Use of booster inoculations to sustain the clinical effect of an adjuvant breast cancer vaccine: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2011;117(3):463–471. doi: 10.1002/cncr.25586. [DOI] [PubMed] [Google Scholar]
- 28.Trappey AF, Berry JS, Vreeland TJ, et al. Risk factors for development of delayed urticarial reactions in the Phase II trial of HER2 peptide vaccines plus GM-CSF versus GM-CSF alone in high-risk breast cancer patients to prevent recurrence. J. Clin. Oncol. 2012;31(Suppl.) Abstract 3097. [Google Scholar]
- 29.Lee TD. Distribution of HLA antigens in North American – Caucasians, North American blacks and orientals. In: Lee J, editor. The HLA System. Vol. 154. Springer-Verlag; NY, USA: 1990. [Google Scholar]
- 30.Patil R, Clifton GT, Holmes JP, et al. Clinical and immunologic responses of HLA-A3+ breast cancer patients vaccinated with the HER2/neu-derived peptide vaccine, E75, in a Phase I/II clinical trial. J. Am. Coll. Surg. 2010;210(2):140–147. doi: 10.1016/j.jamcollsurg.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Mimura K, Ando T, Poschke I, et al. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2 . Int. J. Cancer. 2011;128(2):390–401. doi: 10.1002/ijc.25613. [DOI] [PubMed] [Google Scholar]
- 32.Clifton GT, Peoples GE. Overcoming cancer immune tolerance and escape. Clin. Cancer Res. 2009;15(3):749–751. doi: 10.1158/1078-0432.CCR-08-2805. [DOI] [PubMed] [Google Scholar]
- 33.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Initial report demonstrating the safety of combining a HER2-targeting vaccine and trastuzumab.
- 34.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann. Surg. Oncol. 2006;13(8):1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]