Abstract
The function of δ opioid receptors (DOR) expressed by peripheral pain-sensing neurons (nociceptors) is regulated by both cyclooxygenase- and lipoxygenase (LOX)-dependent arachidonic acid (AA) metabolites. Whereas cyclooxygenase metabolites enhance responsiveness, LOX metabolites elicit a refractory, nonsignaling state of the DOR receptor system for antinociceptive signaling. In this study, using high-performance liquid chromatography-tandem mass spectrometry analyses, we have found that the 12-/15-LOX metabolites, 12-hydroxyeicosatetraenoic acid (HETE) and 15-HETE, were elevated after treatment of adult rat primary sensory neuron cultures with AA. Exogenously applied 12-HETE and 15-HETE, but not 5-HETE, completely prevented DOR and κ opioid receptor (KOR) agonist–mediated inhibition of prostaglandin E2 (PGE2)-stimulated cAMP accumulation, but not inhibition, by the 5-HT1 receptor agonist 5-carboxamidotryptamine in cultured peripheral sensory neurons and in Chinese hamster ovary (CHO) cells heterologously expressing DOR or KOR. Similarly, intraplantar injection of 12- or 15-HETE, either alone or in combination, prevented DOR agonist-mediated inhibition of PGE2-evoked thermal allodynia. Further, both AA- and carrageenan-mediated induction of the nonresponsive state of the DOR system was blocked by an intraplantar coinjection of the 12-/15-LOX inhibitors baicalein and luteolin. In contrast to the regulation of cAMP signaling, pretreatment with 12- and 15-HETE had no effect on either DOR or KOR agonist- mediated activation of extracellular signal-regulated kinase in peripheral sensory neurons or CHO cells. These results suggest that the analgesic efficacy of peripherally restricted opioids for treatment of inflammatory pain may be enhanced by adjunct inhibition of LOX activity.
Introduction
Treatment of pain disorders continues to be a major clinical challenge. Although drugs that target the μ opioid receptor (MOR; e.g., morphine and its analogs) are used most often for the treatment of moderate to severe pain, significant central nervous system (CNS)-mediated adverse effects limit their use. As an alternative to MOR, the δ opioid receptor (DOR) has attracted interest as a possible target for analgesic drug development (for review, see Gendron et al., 2015).
In addition to neurons in the CNS, opioid receptors [MOR, DOR, and κ opioid receptors (KOR)] are expressed on peripheral afferent pain-sensing neurons (nociceptors). Although peripheral MOR, DOR, and KOR are functionally inactive for analgesia under most basal conditions, they can produce profound analgesia when tissue is damaged or inflammation is present in the peripheral tissue (Stein and Lang, 2009; Stein, 2016). Similarly, in a rat behavioral model of nociception, peripheral opioid receptors are nonresponsive to agonist for antinociception, but after a brief pretreatment with an inflammatory mediator, such as bradykinin (BK) or arachidonic acid (AA), they become functionally competent to reduce thermal allodynia (Rowan et al., 2009; Berg et al., 2011; Sullivan et al., 2015). Moreover, in an ex vivo model system (i.e., primary cultures of peripheral sensory neurons), opioid receptor agonists do not inhibit adenylyl cyclase activity or neuropeptide release; however, brief pretreatment (“priming”) with BK or AA induces opioid receptor system functional competence for antinociceptive signaling (Patwardhan et al., 2005; Patwardhan et al., 2006; Berg et al., 2007a,b; Sullivan et al., 2015). This induction of responsiveness of peripheral opioid receptor antinociceptive systems is mediated by a cyclooxygenase-dependent metabolite of AA and is transient, returning to a nonresponsive state within 30–60 minutes (Berg et al., 2007a, 2011; Sullivan et al., 2015).
Recently, we discovered that DOR responsiveness is also regulated by a lipoxygenase (LOX)-sensitive metabolite of AA in peripheral sensory neurons (Sullivan et al., 2015). After induction of functional competence by administration of exogenous AA or carrageenan to the hind paw, the DOR system subsequently becomes unresponsive to agonist-stimulated antinociceptive signaling. This unresponsive state differs from that of the basal condition in that it is refractory to reinduction of functional competence by BK; however, treatment with LOX inhibitors permits reinduction of DOR functional competence by BK, indicating that the unresponsive state of the DOR system that follows AA or carrageenan is produced by LOX-dependent AA metabolites.
In this study, using high-performance liquid chromatography (HPLC) tandem mass spectrometry analyses (LC/MS/MS), we have identified LOX-dependent AA metabolites that are involved in producing the refractory, nonresponsive state of the DOR system for antinociceptive signaling in adult rat ex vivo and in vivo model systems. Results of this study suggest that the duration of peripheral opioid receptor responsiveness under inflammatory conditions may be increased by inhibition of 12-/15-LOX and further underscore the unique regulation of the function of opioid receptors expressed by peripheral sensory neurons.
Materials and Methods
Materials.
Bradykinin (BK), [D-Pen2,5]-enkephalin (DPDPE), U50488, 5-carboxamidotryptamine (5-CT), rolipram, and λ-carrageenan were purchased from Sigma-Aldrich (St Louis, MO); prostaglandin E2 (PGE2), 5-hydroxyeicosatetraenoic (HETE), 12-HETE, 15-HETE, luteolin (LUT), baicalein (BAIC), AA, and the LC-MS lipid standards (12-HETE, 15-HETE, and 5-HETE) were purchased from Cayman Chemicals (Ann Arbor, MI); The LC-MS lipid standards, eicosapentaenoic acid (EPA), leukotriene A4 (LTA4), linolenic acid (LA), and α-linolenic acid (ALA) were purchased from Avanti Polar Lipids (Alabaster, AL); [125I]-cAMP was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). For tissue cultures, collagenase was purchased from Worthington (Lakewood, NJ), and all other culture reagents were purchased from Invitrogen Corp (Carlsbad, CA). HPLC-grade acetonitrile and isopropanol were purchased from Fisher Scientific (Pittsburg, PA); MS-grade ammonium acetate was purchased from Fluka (St. Louis, MO).
Animals.
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 250–300 g were used for all behavioral and cell culture studies. Animals were housed for at least 1 week, with food and water available ad libitum before behavioral testing or harvesting of trigeminal ganglion cells. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to the International Association for the Study of Pain and federal guidelines.
Primary Culture of Rat Peripheral Sensory Neurons.
Primary cultures of adult male rat trigeminal ganglion cells were prepared as previously described (Berg et al., 2007a, 2011, 2012). Briefly, fresh trigeminal ganglia were washed with Hanks’ balanced salt solution (HBSS; Ca++-, Mg++-free), digested with 3 mg/ml collagenase (30 minutes at 37°C), and centrifuged (1000 rpm, 1 minute). In the same solution, pellets were further digested with 0.1% trypsin (15 minutes) and 167 µg/ml DNase (10 minutes at 37°C). Next, cells were again centrifuged (2000 rpm, 2 minutes) and resuspended in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 100 ng/ml nerve growth factor, 10% fetal bovine serum, 1 × Pen/Strep; 1 × l-glutamine and mitotic inhibitors (7.5 µg/ml uridine and 17.5 mg/ml 5-fluoro-2′-deoxyuridine). After trituration to disrupt tissue, cells were seeded on polylysine-coated 48-well (for signaling experiments) or 10-cm plates (for LC-MS/MS experiments). Media was changed 24 hours and 48 hours after plating. On the 5th day of culture, cells were refed with serum-free DMEM without nerve growth factor. The cell-signaling assays and preparations of samples for mass spectrometry were performed on the 6th day of culture.
Chinese Hamster Ovary-K1 Cell Culture and Nucleofection.
Chinese hamster ovary (CHO-K1 cells were maintained in minimal essential medium α formulation (α-MEM) supplemented with 5% FBS; 24 hours before nucleofection, cells were seeded into T175 flasks (to provide ∼80% confluency). CHO cells were nucleofected with either mouse DOR or rat KOR cDNA cloned into pcDNA3 (2 × 106 cells, 100-µl volume, 2 µg of plasmid) using cell line kit T and nucleofector II according to the manufacturer’s directions (Lonza AG, Basel, Switzerland). Nuclefected cells were seeded into 48-well plates at a density of 105 cells per well in αMEM containing 10% FBS. Following a 24 hour plating period, cells were washed with HBSS) and placed into DMEM/F-12 [1:1] and cultured for an additional 24 hours before experiments.
Lipid Extraction.
Lipids were extracted from samples prepared from primary cultures using cold (−20°C) chloroform/methanol (2:1, v/v) in a 5:1 ratio over the sample volume (Gao et al., 2012). The mixtures were vortexed for 10 seconds and allowed to stand on ice for 30 minutes, followed by vortexing for an additional 10 seconds. After centrifugation at 13,800g for 10 minutes, 200 μl of the chloroform layer was removed and vacuum dried. Before LC-MS analysis, the lipid extracts were reconstituted in 200 μl of isopropanol containing 10 mM ammonium acetate.
LC-MS/MS.
Analysis of eicosanoids produced by treatment of primary cultures of peripheral sensory neurons was done as previously described (Gao et al., 2012). HPLC-ESI-MS/MS analyses were conducted on a Thermo Fisher Q Exactive fitted with a PicoChip nanospray source (New Objective, Woburn, MA) and a PicoChip column (Waters Atlantis dC18 column; 150 μm × 105 mm; 3-μm particles; Waters Corporation, Milford, MA). A 55-minute water-acetonitrile-isopropanol-ammonium acetate gradient was run at the flow rate of 1 μl/min. Mobile phase A was acetonitrile/water (40:60) containing 10 mM ammonium acetate, and mobile phase B was acetonitrile/isopropanol (10:90) containing 10 mM ammonium acetate. LC gradient was from 10% B to 99% B over 33 minutes and held at 99% B for 15 minutes. Data-dependent analyses were conducted using one full MS scan [m/z 200–2000; 70,000 resolution (m/z 300)] followed by six tandem MS scans with electrospray negative ion detection using normalized collision energy of 35 arbitrary units. Progenesis CoMet (Nonlinear Dynamics Limited, Durham, NC) was used to process the raw data files. Peak alignment and integration were performed, and the relative abundance was generated for each lipid among the different experimental groups. Fatty acids were identified by searching the following databases: METLIN (http://metlin.scripps.edu/index.php); lipid maps (http://www.lipidmaps.org/data/structure/); and HMDB (Human Metabolome Database; http://www.hmdb.ca/) using a 5-ppm mass tolerance. The putative lipid identifications were manually verified through examination of the MS/MS and in comparison with the retention times from commercially available standards (Cayman Chemicals and Avanti Polar Lipids).
Measurement of Cellular cAMP Levels.
Receptor-mediated inhibition of PGE2-stimulated adenylyl cyclase activity in cultured peripheral sensory neurons or CHO cells was measured as previously described (Sullivan et al., 2015). Briefly, cAMP accumulation was determined after a 15-minute incubation with the phosphodiesterase inhibitor rolipram (0.1 mM), with or without the adenylyl cyclase activator, PGE2 (1 μM), alone or in combination with the DOR agonist (DPDPE, 100 nM), KOR agonist (U50488, 100 nM), or the 5-HT1 receptor agonist (5-carboxamidotryptamine, 5-CT, 100 nM). Cells were washed twice with HBSS containing 20 mM HEPES, pH 7.4 (buffer) followed by pretreatment with BK (10 µM) or AA (50 µM) for 15 minutes at 37°C in buffer. All reactions were terminated by aspiration of the buffer and addition of 500 µl ice-cold absolute ethanol. Ethanol extracts were dried and reconstituted in buffer (100 µl 50 mM sodium acetate, pH 6.2), and the cAMP content was determined by radioimmunoassay.
To determine the effects of LOX metabolites on cAMP levels in primary cultures, cells were pretreated with either vehicle, 5-HETE (100 nM), 12-HETE (100 nM), 15-HETE (100 nM), or 12-HETE and 15-HETE combined, for 15–45 minutes followed by a 15-minute incubation with BK (10 µM) and subsequent measurement of basal and PGE2-stimuated cAMP levels, with and without receptor agonists. For experiments determining the effects of inhibition of 12- and 15-LOX for restoration of functional competence (Sullivan et al., 2015), we used a combination of the flavinoids, BAIC, and LUT, which have been shown to target selectively 12/15-LOX enzymes (Sadik et al., 2003; Deschamps et al., 2006; van Leyen et al., 2006; Lee and Kim, 2010). Briefly, cultures were washed and incubated with either vehicle or a combination of BAIC and LUT (each at 1-µM concentration) for 30 minutes before treatment with either AA or vehicle and an additional 15-minute incubation. Cells were washed and the responsiveness of DOR system was measured 15 minute or 60 minute later as described already.
To determine the effects 12- and 15-HETE on cAMP levels in CHO cells transiently expressing DOR or KOR, cells were pretreated with vehicle, 12-HETE (100 nM), 15-HETE (100 nM), or 12-HETE and 15-HETE combined for 15 minutes, followed by measurement of basal and PGE2-stimuated cAMP levels, with and without receptor agonists.
Measurement of Extracellular Signal-Regulated Kinase Activity.
Receptor-mediated extracellular signal-regulated kinase (ERK) activation was determined by measuring phosphorylated ERK levels as described previously (Berg et al., 2011). Briefly, primary cultures or CHO cells transiently expressing either mouse DOR or rat KOR, washed twice with HBSS containing 20 mM HEPES, pH 7.4 (buffer), were preincubated for 15 minutes at 37°C (room air) with 12- and 15-HETE in combination (100 nM each) or vehicle. Cells were then further incubated with the DOR agonist DPDPE or the KOR agonist U50488 for 0–15 minutes at 37°C. When testing responses in primary sensory neuron cultures, BK (10 µM) was added during the 15-minute preincubation period. Reactions were terminated by aspiration of buffer and addition of 50 µl of lysis buffer supplied with the SureFire phospho-ERK (pERK) assay kit (PerkinElmer, Waltham, MA). Samples were processed according to the manufacturer’s directions. The fluorescence signal from pERK was measured in duplicate using a Fluostar microplate reader (BMG Labtech, Offenburg, Germany) with AlphaScreen settings.
Measurement of Thermal Allodynia in Rat Hind Paws.
Thermal allodynia was measured as previously described by observers blinded to treatment allocations (Rowan et al., 2009; Berg et al., 2011, 2012; Sullivan et al., 2015). Briefly, paw withdrawal latency (PWL) in response to a thermal (radiant heat) stimulus applied to the rat hind paw was measured before and after drug treatments (Hargreaves et al., 1988). The intensity of the radiant heat source was adjusted such that the average latency for paw withdrawal was 10 ± 2 seconds, with a cutoff of 25 seconds to avoid tissue damage. All drugs were injected intraplantarly locally into the plantar surface of the hind paw in a volume of 50 µl. Drug effects were measured as the change (seconds) from baseline PWL over time. The PWL measurements were taken in duplicate at least 30 seconds apart, and the average was used for statistical analysis. In all experiments, rats received pretreatments (described later) followed by an injection of DPDPE (20 µg) alone, PGE2 alone (0.3 µg), or a coinjection of PGE2 (0.3 µg) and DPDPE (20 µg). In experiments to test the effects of LOX metabolites, rats received intraplantarly injections of either vehicle, 12-HETE (0.1 µg) or 15-HETE (0.1 µg), alone or in combination, followed 45 minutes later with an injection of vehicle or BK (25 µg) to produce functional competence. After BK injection, rats received intraplantar injections of vehicle PGE2 or a coinjection of DPDPE and PGE2. In the experiments using carrageenan to induce inflammation, rats received an initial injection of either vehicle or carrageen (500 µg, 50 µl, intraplantar). Allodynia was measured at 15 minutes and 3 hours after the carrageenan injection. To test the effect of 12/15-LOX inhibition for restoration of DOR functional competence, rats received carrageenan injections followed 2.5 hours later with an injection of either vehicle or a coinjection (50 µl) of the 12/15-LOX inhibitors, BAIC (3 µg) and LUT (3 µg) (Sadik et al., 2003; Deschamps et al., 2006), collectively referred to as LOX inhibitor in the figure legends. DOR functional competence was assessed 30 minutes after administration of the LOX inhibitors by intraplantar injection of DPDPE (20 µg). In all experiments, PWLs were measured every 5 minutes in duplicate over a 20-minute period after the final injection.
Data Analysis.
Time-course data from behavioral and cellular experiments were analyzed with two-way analysis of variance followed by Tukey’s post-hoc test to compare the treatment effects over time. Area under the time course curve for behavioral experiments was calculated for individual rats using the trapezoidal method with Prism software (version 6.0, GraphPad Software, Inc., San Diego, CA) and is expressed as the mean ± S.E.M. of each group. Statistical analyses of the area under the time course curve data were done with one-way analysis of variance followed by Dunnett’s post hoc test to determine the significance from vehicle controls. For cellular experiments, statistical significance was assessed using one-way analysis of variance followed by Dunnett’s post hoc test to compare group means of test agents to the corresponding vehicle pretreatment. For all experiments, data are presented as mean ± S.E.M. and were analyzed using Prism software (version 6.0, GraphPad Software, Inc.). P < 0.05 was considered statistically significant.
Results
Identification of LOX Metabolites Produced by Exogenous Application of AA.
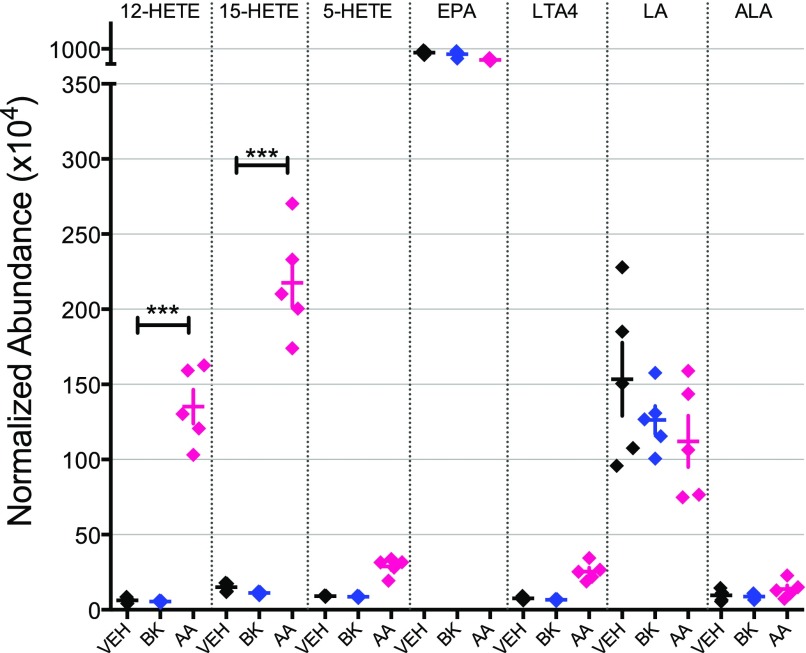
We have reported previously that treatment of peripheral sensory neurons in culture with AA for 15 minutes induces transient functional competence of the DOR system to inhibit adenylyl cyclase activity that is followed by a nonresponsive state that is resistant to reinduction of functional competence. This nonresponsive, refractory state does not occur after the induction of functional competence by treatment with BK and can be blocked by inhibitors of LOX (Sullivan et al., 2015). To identify the LOX-dependent metabolites of AA that lead to this refractory, nonresponsive state of the DOR system, we treated peripheral sensory neuron cultures for 15 minutes with vehicle, BK, or AA. As shown in Fig. 1, the eicosanoids 12-hydroxyeicosatetraenoic acid (12-HETE), 15-HETE, and 5-HETE, eicosapentaenoic acid, leukotriene A4 (LTA4), linolenic acid (LA), and α-linolenic acid (ALA) were identified by comparison with synthetic standards using LC-MS/MS. The levels of 12-HETE and 15-HETE were significantly greater in cultures treated with AA compared with vehicle (***P < 0.001, n = 5). By contrast, treatment with bradykinin did not increase the levels of either 12- or 15-HETE (Fig. 1).
Fig. 1.
Relative abundance of fatty acid metabolites in cultures of peripheral sensory neurons treated for 15 minutes with either vehicle (Veh), BK (10 µM), or AA (50 µM). Eicosanoids (12-HETE, 15-HETE, and 5-HETE), EPA, LTA4, LA, and ALA were identified by comparison with synthetic standards using LC-MS/MS, and the average accumulation of metabolites was calculated for each treatment. Levels of 12-HETE and 15-HETE were significantly greater in cultures treated with AA compared with Veh (***P < 0.001). Data represent mean ± S.E.M. of five experiments.
Effect of 12-HETE and 15-HETE on DOR Function Ex Vivo.
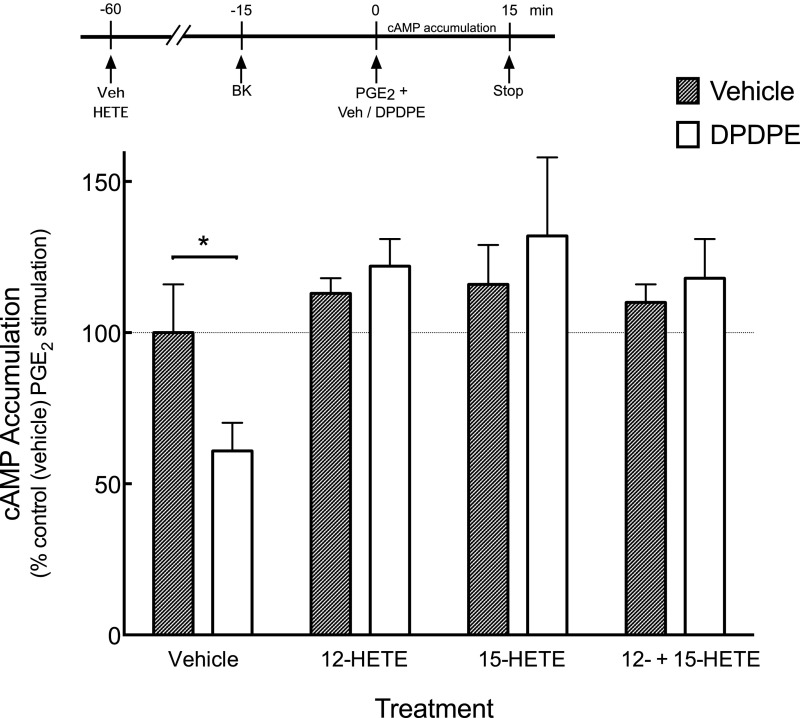
To determine whether 12- and 15-HETEs altered the responsiveness of the DOR system, we added them to cultures of peripheral sensory neurons where functional competence of the DOR system was induced with BK, as BK pretreatment does not increase 12- or 15-HETE levels (Fig. 1) or produce the refractory, nonresponsive state of the DOR system. As shown in Fig. 2, DPDPE reduced PGE2-stimulated cAMP levels by 40% ± 10%. In cells pretreated with 12-HETE or 15-HETE alone or in combination, DPDPE did not inhibit PGE2-stimulated cAMP accumulation (P < 0.05, n = 5 experiments). Pretreatment with either 12- or 15-HETE did not alter basal or PGE2-stimulated cAMP accumulation.
Fig. 2.
Effects of the LOX-dependent AA metabolites, 12-HETE, and 15-HETE, either alone or in combination, on DPDPE-mediated inhibition of PGE2-stimulated cAMP accumulation. Primary cultures of peripheral sensory neurons were pretreated with 12-HETE (100 nM), 15-HETE (100 nM), or 12-HETE and 15-HETE combined for 45 minutes before incubation with BK (10 µM) for an additional 15 minutes to induce DOR functional competence. The amount of cAMP accumulated (15 minutes, 37°C) in response to PGE2 (1 µM), with or without DPDPE (100 nM), was measured as described in Materials and Methods. Pretreatment with the HETEs had no effect on basal or PGE2 -stimulated cAMP accumulation levels, which were 1.2 ± 0.6 pmol/well and 187% ± 34% above basal, respectively. Data represent mean ± S.E.M. of five experiments. *P < 0.05 versus vehicle (no DPDPE).
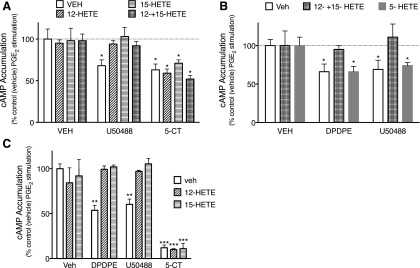
To determine whether 12- and 15-HETE reduced responsiveness of other receptor systems expressed by peripheral sensory neurons (Berg et al., 2011), we measured the inhibition of cAMP accumulation by the KOR agonist U50488 (using BK pretreatment to induce functional competence) and the serotonin (5-HT)1 receptor agonist 5-carboxamidotryptamine (5-CT), which does not require pretreatment with an inflammatory mediator to respond to agonist stimulation. As shown in Fig. 3A, treatment of cells with maximal KOR or 5-HT1 agonist concentrations inhibited PGE2-stimulated cAMP levels to a similar degree. Similar to the effects on the response of DOR to DPDPE, pretreatment with 12-HETE and/or 15-HETE also abolished the response to the KOR agonist U50488. By contrast, neither 12-HETE nor 15-HETE, either alone or in combination, altered the response to the 5-HT1 receptor agonist, 5-CT. We also tested the effects of the 5-LOX AA metabolite 5-HETE. As shown in Fig. 3B, exogenously applied 5-HETE did not alter the inhibition of adenylyl cyclase activity by either DPDPE or U50488 (Fig. 3B).
Fig. 3.
Effect of LOX metabolites on DOR-, KOR-, and 5-HT1-mediated inhibition of PGE2-stimulated cAMP accumulation in primary cultures of peripheral sensory neurons (A and B) or in CHO cells transfected with either DOR or KOR(C). (A) Pretreatment with the 12-/15-LOX-dependent AA metabolites, 12-HETE (100 nM), and 15-HETE (100 nM), alone or in combination, for 15 minutes, prevented inhibition of cAMP accumulation by the KOR agonist, U50488 (100 nM), but not the 5-HT1 receptor agonist, 5-CT (100 nM) in BK-pretreated cells. Data represent mean ± S.E.M. of five separate experiments. *P < 0.05. (B) In contrast to the effects of 12- and 15-HETE, pretreatment for 15 minutes with the 5-LOX–dependent AA metabolite 5-HETE (100 nM) had no effect on either DPDPE (DOR) or U50488 (KOR)-mediated inhibition of cAMP accumulation. (C) Pretreatment of transfected CHO cells for 15 minutes with 12-HETE (100 nM) or 15-HETE (100 nM) prevented inhibition of cAMP accumulation by the DOR agonist DPDPE or the KOR agonist U50488 (100 nM), but not the 5-HT1 receptor agonist, 5-CT (100 nM). Data represent mean ± S.E.M. of four separate experiments. *P < 0.05 versus vehicle pretreatment condition in the absence of opioid agonist.
In CHO cells heterologously expressing DOR or KOR and that do not require pretreatment with inflammatory mediators to elicit functional competence, pretreatment with 12- and 15-HETE also prevented DPDPE- and U50488-mediated inhibition of PGE2-stimulated cAMP accumulation (Fig. 3C). By contrast, pretreatment with 12- and 15-HETE had no effect on the ability of endogenously expressed 5-HT1B receptors to inhibit PGE2-stimulated cAMP accumulation.
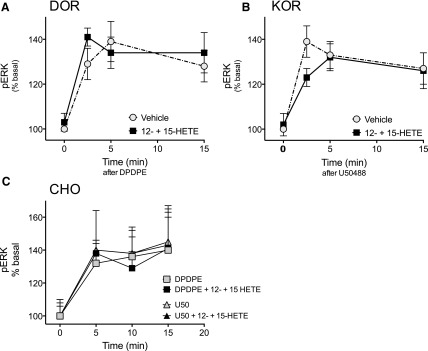
In addition to inhibition of cAMP signaling, activation of opioid receptors in peripheral nociceptors activates MAPKinase signaling cascades, such as ERK (Berg et al., 2011; Jamshidi et al., 2015). As shown in Fig. 4, the levels of pERK (an index of activation) increased in response to DPDPE (Fig. 4A) and U50488 (Fig. 4B). Administration of a combination of 12-HETE and 15-HETE did not alter the ability of either DPDPE or U50488 to activate ERK in peripheral sensory neurons or in CHO cells (Fig. 4). Taken together, these data suggest that the inhibitory effects of 12- and 15-HETE are both receptor system–dependent (alters opioid but not 5-HT1 receptor systems), as well as signaling pathway-selective (alters opioid receptor-mediated inhibition of cAMP signaling but not activation of ERK).
Fig. 4.
Effect of the 12-/15-LOX-dependent AA metabolites, 12- and 15-HETE, on DOR- and KOR-mediated activation of ERK in primary cultures of peripheral sensory neurons (A and B) or in CHO cells transfected with either DOR or KOR (C). Cells were pretreated for 15 minutes with 12-HETE (1 µM) and 15-HETE (1 µM) in combination, followed by 15 minutes’ incubation with BK (10 µM). Cells were then incubated with the DOR agonist DPDPE (100 nM) (A) or the KOR agonist U50488 (100 nM) (B) for the times indicated. (C) CHO cells transiently transfected with DOR or KOR were treated with 12-HETE (1 µM) and 15-HETE (1 µM) in combination for 15 minutes before incubation with DPDPE (100 nM) or U50488 (100 nM) for the times indicated. For all experiments, the levels of pERK were determined as described in Materials and Methods. Pretreatment with 12- and 15-HETE did not alter the magnitude or the time course for increases in ERK activation in response to either DPDPE or U50488. P > 0.05, two-way analysis of variance.
Effect of 12-HETE and 15-HETE on DOR Function In Vivo
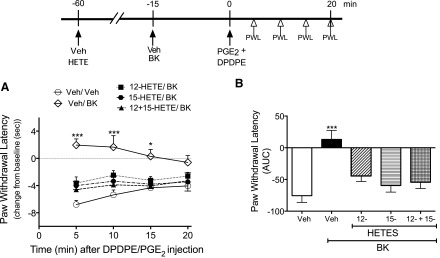
We next tested the effects of exogenous administration of 12- and 15-HETE on DOR responsiveness in a behavioral model of thermal nociception, where DOR functional competence was induced by administration of BK. As shown in Fig. 5, DPDPE reduced completely PGE2-evoked thermal allodynia after injection of BK into the rat hind paw. Administration of 12-HETE or 15-HETE, either alone or in combination, abolished DPDPE-mediated antiallodynia. Because HETEs have been shown to activate TRPV1 channels, albeit at micromolar concentrations (Hwang et al., 2000), we verified that injection of 12- and 15-HETE alone or in combination did not alter baseline PWL (Supplemental Fig. 1). Supplemental Fig. 2 shows that injection of 12- and 15-HETE did not alter the allodynia produced by intraplantar injection of BK.
Fig. 5.
Pretreatment with the 12-/15-LOX–dependent AA metabolites 12-HETE and 15-HETE attenuates DPDPE-mediated anti-allodynia. Rats received intraplantar injections of vehicle (Veh), 12-HETE (0.1 µg), 15-HETE (0.1 µg), or 12-HETE and 15-HETE combined. After 45 minutes, rats were injected with Veh or BK (25 µg), and then 15 minutes later received coinjections of PGE2 (0.3 µg) with DPDPE (20 µg). (A) PWL was measured before (baseline) and at 5-minute intervals after the PGE2/DPDPE injection for 20 minutes. Data were evaluated for statistical differences with two-way analysis of variance (ANOVA), followed by Tukey’s post hoc analysis. (B) Area under the individual time-course curves (AUC) for each pretreatment condition. Data were evaluated for statistical differences with one-way ANOVA, followed by Dunnett’s post hoc analysis. Data represent mean ± S.E.M. of 6 (Veh-Veh pretreatment), 9 (Veh-BK pretreatment), 12 (12-HETE/BK pretreatment), 9 (15-HETE/BK pretreatment), or 4 (12- + 15-HETE/BK pretreatment) animals per group. *P < 0.05, ***P < 0.001 compared with Veh/Veh/DPDPE + PGE2 (i.e., no pretreatment with BK). As shown in Supplemental Fig. 1 and 2, 12-HETE and 15-HETE did not alter either baseline PWL or PGE2 -evoked PWL.
Effect of 12/15 LOX Inhibitors on DOR Function Ex Vivo and In Vivo
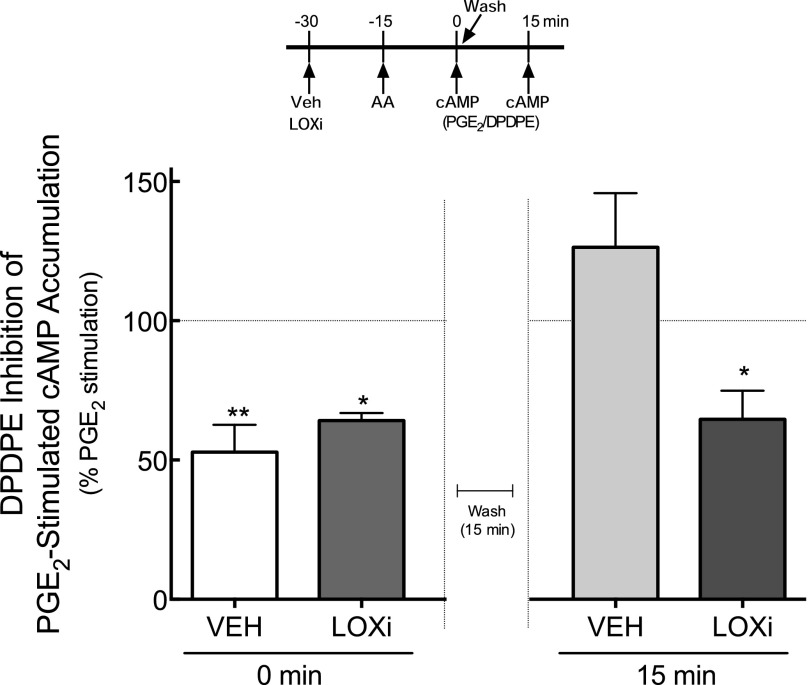
Previously, we reported that DOR system functional competence induced by AA treatment is transient, with DOR becoming nonresponsive (i.e., DPDPE becomes ineffective at inhibiting PGE2-stimulated cAMP accumulation) within 15 minutes of washout of AA (Sullivan et al., 2015). Thus, we next determined whether DOR responsiveness after AA treatment would be prolonged by inhibition of LOX. As shown in Fig. 6, pretreatment with a combination of the 12-/15-LOX inhibitors (i.e., LUT and BAIC) (Sadik et al., 2003; Deschamps et al., 2006) prolonged DOR functional competence induced by AA. Consistent with our previous results, the inhibition of cAMP accumulation in response to DPDPE was transient and returned to baseline PGE2-stimulated levels within 15 minutes of washout of AA. By contrast, in cells that were pretreated with the LOX inhibitors, DOR functional competence persisted when tested after 15 minutes of AA washout. Treatment with the LOX inhibitors did not alter either basal or PGE2-stimulated cAMP accumulation.
Fig. 6.
Inhibition of 12- and 15-LOX activity prolongs DOR functional competence for the inhibition of PGE2-stimulated cAMP accumulation in primary cultures of peripheral sensory neurons. Cells were incubated with or without the combination of 12-/15- LOX inhibitors (LOXi) luteolin (1 μM) and BAIC (1 μM) for 30 minutes before treatment with AA (50 µM) for 15 minutes. DPDPE (100 nM)-mediated inhibition of PGE2-stimulated cAMP was measured immediately, or cells were washed and DPDPE-mediated responses were determined 15 minutes later (see time line). LOX inhibitors did not alter basal or PGE2-stimulated cAMP accumulation, which were 0.6 ± 0.2 pmol/well and 205% ± 42% above basal, respectively. Data represent mean ± S.E.M. of four experiments * P < 0.05; **P < 0.01 compared with PGE2 in the absence of DPDPE.
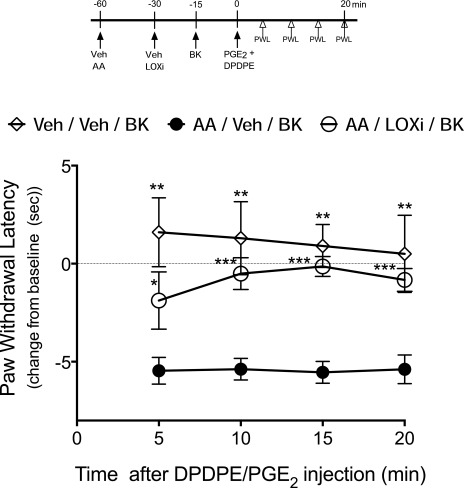
We have shown previously that after an initial induction of functional competence, intraplantar administration of AA also induces a subsequent nonresponsive state of the DOR system that is refractory to reinduction of functional competence by BK, similar to effects that occur in primary cultures of peripheral sensory neurons. Development of this refractory, nonresponsive state is blocked by a nonselective LOX inhibitor (Sullivan et al., 2015). Consistent with our previous data, preinjection of the 12-/15-LOX inhibitors LUT and BAIC restored DPDPE-mediated reduction in PGE2-evoked thermal allodynia following administration of AA (Fig. 7). As shown in Supplemental Fig. 2, injection of the LOX inhibitors did not alter PGE2-evoked thermal allodynia.
Fig. 7.
Inhibition of 12- and 15-LOX activity restores DOR system responsiveness for the reduction of PGE2-evoked thermal allodynia after AA treatment. Rats received injections of AA (3 μg, 50 μl, intraplantarly) 30 minutes before injections of Veh or the LOX inhibitors (LOXi) LUT (3 μg) and BAIC (3 μg) in combination (50 μl, intraplantarly). Rats were then injected with BK (25 μg, intraplantarly) 15 minutes later to induce functional competence; 15 minutes after BK administration, DOR responsiveness was assessed by injecting PGE2 (0.3 μg) along with DPDPE (20 μg) (50 µl, intraplantarly) and PWLs were measured before (baseline) and at 5-minute intervals after injection for 20 minutes. Data represent mean ± S.E.M. of the change from baseline PWL, six animals per group. *P < 0.05; **P < 0.01; ***P < 0.001 compared Veh-Veh.
DOR-Mediated Reduction of Thermal Nociception is Regulated by 12-LOX and 15-LOX Metabolites in the Carrageenan Model of Inflammatory Pain.
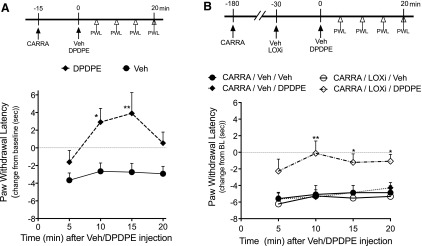
Intraplantar injection of carrageenan induces DOR functional competence for reduction of thermal allodynia when measured 15 minutes later, but DOR is nonresponsive when tested 3 hours after injection, even though the allodynia is still present (Sullivan et al., 2015) (Fig. 8, A and B); however, as shown in Fig. 8B, after administration of LOX inhibitors LUT and BAIC, DOR functional competence persisted through 3 hours. These data suggest that, similar to treatment with exogenous AA, carrageenan produces inflammatory mediators that induce functional competence and also produce 12-/15-LOX metabolites (12- and 15-HETE) that promote the nonresponsive state of the DOR system.
Fig. 8.
The effect of LOX inhibitors on the duration of DOR system responsiveness to DPDPE on carrageenan-induced thermal allodynia. (A) Then, 15 minutes after intraplantar injection of carrageenan (CARRA, 500 µg), thermal allodynia was reduced completely by DPDPE (20 µg) Data represent mean ± S.E.M. of six rats/group. *P < 0.05; **P < 0.01 from CARRA-Veh. (B) Three hours after carrageenan injection, DPDPE no longer reduced thermal allodynia. After injection of the 12-/15-LOX inhibitors, reduction of carrageenan-induced thermal allodynia by DPDPE was restored. As shown in the time line, rats received intraplantar injections of Veh or a combination of the 12-LOX inhibitor, LUT (3 µg), and the 15-LOX inhibitor BAIC (3 µg) 2.5 hours after injection of carrageenan (500 µg). DPDPE (20 µg) was administered 30 minutes after LOX inhibitor pretreatment (i.e., responses to DPDPE were determined 3 hours after carrageenan administration). Data represent mean ± S.E.M. of six rats/group. *P < 0.05; **P < 0.01 from CARRA-Veh-DPDPE.
Discussion
Peripheral opioid receptors expressed on pain-sensing neurons are attractive potential targets for development of effective analgesics that would be devoid of severe CNS-mediated adverse effects, such as respiratory depression and addiction. As peripherally restricted MOR agonists can produce debilitating constipation (Grunkemeier et al., 2007), attention has shifted to peripheral DOR or KOR. If these receptors are to be valid targets for new drug development, it is important to understand their regulation.
Previous work has shown that peripheral opioid receptor systems expressed by pain-sensing neurons are regulated differently from those in the CNS or expressed heterologously. For example, acting at peripheral opioid receptors, opioid agonists do not promote antinociception or inhibit adenylyl cyclase activity unless cells are first exposed to inflammatory mediators that, acting via a COX-dependent AA metabolite, increase opioid agonist antinociceptive efficacy (Berg et al., 2007a,b, 2011; Sullivan et al., 2015). In addition to increasing opioid agonist efficacy, AA metabolites can also suppress peripheral opioid receptor antinociceptive signaling via the activity of lipoxygenase (LOX) enzymes (Sullivan et al., 2015). Here we identified that the 12/15-LOX-dependent AA metabolites, 12- and 15-HETE, are responsible for the nonresponsive state of the DOR system after administration of AA or carrageenan.
Lipoxygenases (5-, 12- and 15-LOX) are a family of enzymes involved in the oxidative metabolism of AA, and they catalyze the formation of corresponding hydroxyeicosatetraenoic acids (5-, 12- and 15-HETE). Using HPLC tandem mass spectrometry analyses (LC-MS/MS), we found that the 12/15-LOX-AA metabolites, 12-HETE and 15-HETE, but not 5-HETE, increased after treatment of primary cultures of peripheral sensory neurons with exogenously applied AA, but not with BK. Administration of 12-HETE and 15-HETE, either alone or in combination, blocked completely the inhibition of PGE2-stimulated cAMP accumulation in response to the DOR agonist DPDPE, whereas administration of 5-HETE had no effect. In vivo, intraplantar injection of either 12- or 15-HETE blocked completely DOR-mediated inhibition of PGE2-evoked thermal allodynia. Selective inhibitors of 12- and 15-LOX prevented the reduced responsiveness of the DOR system after AA treatment ex vivo and after AA and carrageenan treatment in vivo. These results suggest that 12- and 15-HETEs derived from the metabolism of AA by LOX are responsible for the refractory, nonresponsiveness state of the DOR system in peripheral sensory neurons.
The observation that BK administration was not effective at reinducing DOR responsiveness after exogenous administration of AA or after carrageenan injection suggests that perhaps 12- and 15-HETEs could interfere with BK signaling systems and the induction of opioid receptor system functional competence in peripheral sensory neurons; however, the allodynia produced by intraplantar injection of BK into the hind paw was not altered by exogenous AA (Sullivan) or by 12-/15-HETEs. Further, both 12- and 15-HETE blocked the inhibition of PGE2-stimulated cAMP accumulation in CHO cells heterologously expressing DOR or KOR, which do not require pretreatment with BK to induce responsiveness as do peripheral sensory neurons. These data suggest that the 12/15 LOX metabolites interfere with signaling by DOR and KOR.
When applied at micromolar concentrations, HETEs have been shown to activate transient receptor potential vanilloid-1 (TRPV1) channels and produce thermal hyperalgesia (Hwang et al., 2000; Sisignano et al., 2013; Kelly et al., 2015). Thus, it is possible that the reduced antinociceptive signaling by peripherally restricted DOR agonists is due to functional antagonism produced by 12- or 15-HETEs activating TRPV1; however, baseline PWL to thermal stimulation of the hind paw was not altered by intraplantar injection of 12- or 15-HETE at a dose of 0.1 µg (calculated to produce a concentration of ≈300 nM, assuming a volume of distribution of 1 ml in the paw), indicating that hyperalgesia produced by activation of TRPV1 channels by the HETEs was not responsible for the reduced antinociceptive effect of DOR agonists in vivo after AA or carrageenan.
Although it has been reported that 12-LOX–dependent AA metabolites mediate presynaptic inhibition of neurotransmitter release in neurons of the periaqueductal gray (Vaughan et al., 1997) and nucleus raphe magnus (Zhang and Pan, 2012) in response to opioid receptor activation, there are no reports of regulation of opioid receptor function by LOX metabolites. Since the 12- and 15-HETES reduced DOR and KOR receptor signaling, but not signaling of the 5-HT1 receptor, it is unlikely that the HETES target postreceptor mechanisms to reduce opioid receptor function in peripheral sensory neurons. 12-HETE has been shown to interact directly with G protein–coupled receptors, acting as an antagonist at thromboxane receptors (Siangjong et al., 2013) and as an agonist at GPR31 receptors (Powell and Rokach, 2015). It is unlikely that 12- or 15-HETE acts as an antagonist at DOR or KOR orthosteric binding sites because ERK activation in response to either DPDPE or U50488 was not altered in the presence of the HETEs. Our current working hypothesis is that 12- and 15-HETE may be allosteric regulators of DOR and KOR to reduce signaling through antinociceptive pathways (Gi), but not ERK.
In contrast to the inhibitory effects on DOR function in response to exogenous administration of AA and the inflammatory conditions induced by carrageenan, it is curious that administration of the inflammatory mediator BK did not promote a refractory nonresponsive state of the DOR system (Sullivan et al., 2015). BK treatment leads to endogenous production of AA that is metabolized by COX to induce functional competence of opioid receptors in peripheral sensory neurons (Patwardhan et al., 2005; Berg et al., 2007a). It is possible that BK activation of bradykinin B2 receptors and Gq-protein-mediated phospholipid signaling elicits compartmentalized release of AA that is available for metabolism by COX, but not by 12-/15-LOX enzymes in nociceptors. Preferential metabolism of AA by certain enzymes in cells has been reported. For example, activation of cytosolic phospholipase A2 signaling by 5-HT2A and 5-HT2C receptors results in differential AA metabolism by cytochrome P450 versus COX enzymes, respectively (Berg et al., 1998). Further, activation of protease-activated receptors 1 and 4, which stimulate cytosolic phospholipase A2 signaling in platelets, differentially elicits AA metabolism by COX-1 versus 12-LOX, respectively (Holinstat et al., 2011).
After initial induction of DOR system responsiveness by intraplantar injection of carrageenan, the DOR system became unresponsive to agonist activation when tested 3 hours after carrageenan. Responsiveness was restored by inhibitors of 12-/15-LOX. Carrageenan elicits an endogenous inflammatory response that mimics that which occurs naturally, suggesting that the nonresponsive state of the peripheral DOR system could also occur in inflammatory conditions where 12-/15-LOX metabolites of AA are generated, such as rheumatoid arthritis (Deleuran et al., 1994; Liagre et al., 1997) and osteoarthritis (Kelly et al., 2015). It is possible that a LOX-dependent nonresponsive state of peripheral opioid receptor systems may underlie the cases where some clinical studies have failed to demonstrate effectiveness of peripherally restricted opioids for the treatment of pain (for reviews, see Vadivelu et al., 2011; Nielsen et al., 2015).
In summary, we have identified 12- and 15-HETE as the LOX-dependent AA metabolites that produce a refractory, nonresponsiveness state of the DOR system for antinociceptive signaling in peripheral pain-sensing neurons that occurs after exogenous administration of AA or carrageenan. Inhibition of 12/15 LOX extended the duration of responsiveness of the DOR system to at least 3 hours. Since 12/15 LOX metabolites are produced in a variety of inflammatory states, inhibition of 12/15 LOX activities may be an important adjunct for sustained peripheral opioid receptor function that will allow peripherally restricted opioid medications to block pain neurotransmission to the CNS without the dose-limiting and life-threatening CNS-mediated adverse effects.
Acknowledgments
The authors thank Dr. Bill Clarke for helpful discussions and comments.
Abbreviations
- AA
arachidonic acid
- ALA
α-linolenic acid
- BAIC
baicalein
- BK
bradykinin
- CHO
Chinese hamster ovary
- CNS
central nervous system
- DOR
δ opioid receptor
- DPDPE
[D-Pen2,5]-enkephalin
- ERK
extracellular signal-regulated kinase
- 5-HETE
5- hydroxyeicosatetraenoic
- 12-HETE
12- hydroxyeicosatetraenoic
- 15-HETE
15- hydroxyeicosatetraenoic
- HBSS
Hanks’ balanced salt solution
- HPLC
high-performance liquid chromatography
- LA
linolenic acid
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygensase
- LTA4
leukotriene A4
- LUT
luteolin
- pERK
phosphorylated ERK
- PGE2
prostaglandin E2
- PWL
paw withdrawal latency
Authorship Contributions
Participated in research design: Berg, Sullivan.
Conducted experiments: Chavera, Pando, Gao, Sullivan.
Performed data analysis: Berg, Gao, Sullivan.
Wrote or contributed to the writing of the manuscript: Berg, Sullivan.
Footnotes
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Berg KA, Maayani S, Clarke WP. (1998) Interactions between effectors linked to serotonin receptors. Ann N Y Acad Sci 861:111–120. [DOI] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007a) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA, et al. (2012) Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol 81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Zardeneta G, Hargreaves KM, Clarke WP, Milam SB. (2007b) Integrins regulate opioid receptor signaling in trigeminal ganglion neurons. Neuroscience 144:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuran B, Iversen L, Kristensen M, Field M, Kragballe K, Thestrup-Pedersen K, Stengaard-Pedersen K. (1994) Interleukin-8 secretion and 15-lipoxygenase activity in rheumatoid arthritis: in vitro anti-inflammatory effects by interleukin-4 and interleukin-10, but not by interleukin-1 receptor antagonist protein. Br J Rheumatol 33:520–525. [DOI] [PubMed] [Google Scholar]
- Deschamps JD, Kenyon VA, Holman TR. (2006) Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem 14:4295–4301. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Q, Meng D, Isaac G, Zhao R, Fillmore TL, Chu RK, Zhou J, Tang K, Hu Z, et al. (2012) A reversed-phase capillary ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) method for comprehensive top-down/bottom-up lipid profiling. Anal Bioanal Chem 402:2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Mittal N, Beaudry H, Walwyn W. (2015) Recent advances on the δ opioid receptor: from trafficking to function. Br J Pharmacol 172:403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. (2007) The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 5:1126–1139, quiz 1121–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA, Hamm HE. (2011) Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2α differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol 31:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, et al. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97:6155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA. (2015) Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, et al. (2015) Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis 74:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim GH. (2010) Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J Food Sci 75:H212–H217. [DOI] [PubMed] [Google Scholar]
- Liagre B, Vergne P, Rigaud M, Beneytout JL. (1997) Expression of arachidonate platelet-type 12-lipoxygenase in human rheumatoid arthritis type B synoviocytes. FEBS Lett 414:159–164. [DOI] [PubMed] [Google Scholar]
- Nielsen BN, Henneberg SW, Schmiegelow K, Friis SM, Rømsing J. (2015) Peripherally applied opioids for postoperative pain: evidence of an analgesic effect? A systematic review and meta-analysis. Acta Anaesthesiol Scand 59:830–845. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. (2006) PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain 125:114–124. [DOI] [PubMed] [Google Scholar]
- Powell WS, Rokach J. (2015) Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta 1851:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. (2009) Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol 602:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik CD, Sies H, Schewe T. (2003) Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem Pharmacol 65:773–781. [DOI] [PubMed] [Google Scholar]
- Siangjong L, Gauthier KM, Pfister SL, Smyth EM, Campbell WB. (2013) Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 304:H382–H392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisignano M, Angioni C, Ferreiros N, Schuh CD, Suo J, Schreiber Y, Dawes JM, Antunes-Martins A, Bennett DL, McMahon SB, et al. (2013) Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS One 8:e81228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. (2016) Opioid receptors. Annu Rev Med 67:433–451. [DOI] [PubMed] [Google Scholar]
- Stein C, Lang LJ. (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP. (2015) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. (2011) Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag 7:55–68. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. (2006) Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37:3014–3018. [DOI] [PubMed] [Google Scholar]