ABSTRACT
Kindlins play an important role in supporting integrin activation by cooperating with talin; however, the mechanistic details remain unclear. Here, we show that kindlins interacted directly with paxillin and that this interaction could support integrin αIIbβ3 activation. An exposed loop in the N-terminal F0 subdomain of kindlins was involved in mediating the interaction. Disruption of kindlin binding to paxillin by structure-based mutations significantly impaired the function of kindlins in supporting integrin αIIbβ3 activation. Both kindlin and talin were required for paxillin to enhance integrin activation. Interestingly, a direct interaction between paxillin and the talin head domain was also detectable. Mechanistically, paxillin, together with kindlin, was able to promote the binding of the talin head domain to integrin, suggesting that paxillin complexes with kindlin and talin to strengthen integrin activation. Specifically, we observed that crosstalk between kindlin-3 and the paxillin family in mouse platelets was involved in supporting integrin αIIbβ3 activation and in vivo platelet thrombus formation. Taken together, our findings uncover a novel mechanism by which kindlin supports integrin αIIbβ3 activation, which might be beneficial for developing safer anti-thrombotic therapies.
KEY WORDS: Integrin αIIbβ3, Kindlin, Paxillin, Talin, Platelets, Thrombosis
Summary: Kindlin supports integrin αIIbβ3 activation by forming a complex with paxillin and talin and thus enhancing talin binding to integrin.
INTRODUCTION
Integrins mediate cell adhesion and migration by interacting with their ligands in the surrounding microenvironment (Hynes, 2002). For many integrin members, a process of activation is required for them to be able to interact with soluble ligands, via conformational changes from a quiescent/bent state to an active/extended state, i.e. inside-out signaling (Dai et al., 2015; Ginsberg et al., 2005). The activation states of integrins are tightly controlled by interactions between α- and β-subunits at both the membrane-proximal cytoplasmic tails (CTs) and the transmembrane domains (Vinogradova et al., 2002; Yang et al., 2009; Zhu et al., 2009). Integrin conformational changes toward active states can be triggered by the binding of cytoplasmic integrin activators to the integrin β CTs (Liu et al., 2000; Ma et al., 2007; Moser et al., 2009b; Qin et al., 2004). Among many integrin β CT binding proteins, the talin head domain (TH) and the kindlin family members are essentially required to induce integrin activation (Moser et al., 2009b; Tadokoro et al., 2003). Both TH and kindlins are FERM domain-containing proteins, consisting of typical FERM subdomains (F1, F2 and F3) and an extra N-terminal F0 subdomain (Larjava et al., 2008). The F3 subdomains in TH and kindlins are responsible for binding to the integrin β CTs, but their binding sites in the β CTs are distinct: an NPLY/F motif in the middle of β CTs for TH and an NxxY/F motif at the C-termini of β CTs for kindlins (Harburger et al., 2009; Ma et al., 2008; Moser et al., 2008; Shi et al., 2007; Xu et al., 2015, 2014). Meanwhile, the F3 subdomain of TH also recognizes the membrane-proximal residues of the integrin β CTs and is thus capable of unclasping the integrin α/β CT complex and triggering integrin activation (Wegener et al., 2007); in addition, other subdomains in TH are functionally supportive by facilitating membrane attachment (Goult et al., 2010). Besides talin, multiple lines of evidence, from model cells to genetically modified mice to human patients, have clearly shown that kindlins are also essentially involved in supporting integrin activation (Larjava et al., 2008; Plow et al., 2009; Rognoni et al., 2016).
The kindlin family members, including kindlin-1, kindlin-2 and kindlin-3, share a high homology with each other but display distinct patterns of tissue-specific expression (Ussar et al., 2006). Kindlin-1 is rich in epithelial cells; a lack of kindlin-1 in humans causes Kindler syndrome, marked with serious skin fragility (Jobard et al., 2003; Siegel et al., 2003). Kindlin-2 has been found in multiple cell types; kindlin-2-deficient patients have not been identified so far, probably due to a lethal consequence, as evidenced in kindlin-2 knockout mice (Dowling et al., 2008; Montanez et al., 2008). Kindlin-3 is predominantly expressed in hematopoietic cells; human LAD-III patients with a kindlin-3 deficiency display severe bleeding disorders and recurrent infections as a result of dysfunctional integrins in both platelets and leukocytes (Kuijpers et al., 2008; Malinin et al., 2009; Svensson et al., 2009). Mice with deficiencies of kindlin-1 (Ussar et al., 2008) and kindlin-3 (Moser et al., 2009a, 2008) phenotypically recapitulate the Kindler syndrome and LAD-III patients, respectively. Despite their functional importance, the kindlin family members are incapable of unclasping the integrin α/β CT complex (Bledzka et al., 2012) and, consequently, insufficient to induce high integrin activation (Ma et al., 2008; Shi et al., 2007). Because significant interaction between kindlins and TH has not been observed (Bledzka et al., 2012; Yates et al., 2012a), it is mechanistically unclear how the kindlin family supports talin-induced integrin activation. Hypothetically, a bridging molecule might exist between kindlin and talin, which could promote integrin activation by facilitating the formation of a multiprotein complex of the key integrin activators.
In addition to β-integrins, multiple kindlin binding proteins (KBPs) have been identified, such as migfilin for kindlin-1 and kindlin-2 (Brahme et al., 2013; Tu et al., 2003), Src and F-actin for kindlin-2 (Bledzka et al., 2016; Liu et al., 2015; Qu et al., 2014), and RACK1 for kindlin-3 (Feng et al., 2012). It has been shown that these KBPs are involved in regulating integrin-mediated cell adhesion and spreading (outside-in signaling), but not integrin activation (inside-out signaling). ILK has even been identified as an integrin activator (Honda et al., 2009) and can also interact with kindlin-2 (Fukuda et al., 2014; Huet-Calderwood et al., 2014); however, it has no additional effect on integrin activation mediated by TH and kindlin-2 (Fukuda et al., 2014), indicating that ILK may not fit the category of bridging molecules. Interestingly, a recent study has disclosed that ADAP, a hematopoietic-specific adapter protein, can associate with both talin and kindlin-3 and promote integrin αIIbβ3 activation in platelets (Kasirer-Friede et al., 2014), which endorses the proposed bridging molecule model. However, the unexpectedly mild bleeding phenotype observed in ADAP−/− mice suggests that other candidates may also be required for supporting integrin αIIbβ3 activation in platelets (Kasirer-Friede et al., 2007).
In this study, we identify the paxillin (PXN) family members as KBPs and demonstrate that the PXN family members are able to promote platelet integrin αIIbβ3 activation by cooperating with kindlin and talin, thus highlighting a novel mechanism for kindlin-supported integrin activation.
RESULTS
PXN interacts with kindlin-1 and enhances integrin αIIbβ3 activation
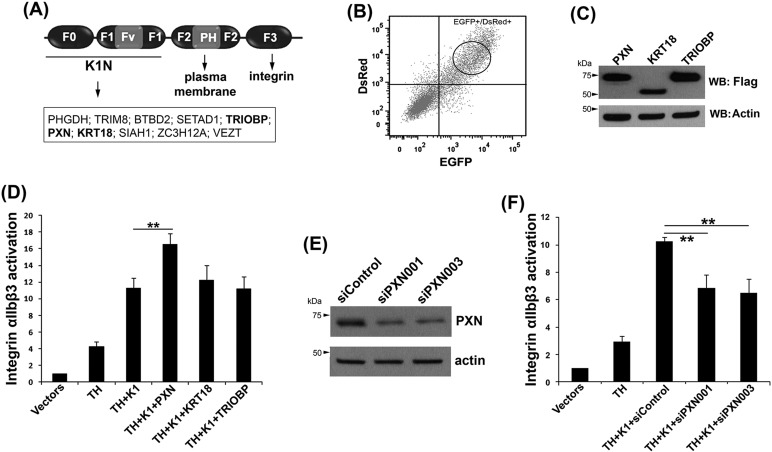
The kindlin family members are FERM domain-containing integrin activators (Fig. 1A). The F3 subdomain of kindlins directly interacts with the integrin β CT. The pleckstrin homology (PH) domain inserted in the F2 subdomian can associate with the plasma membrane and facilitate kindlin recruitment to integrin (Hart et al., 2013; Liu et al., 2011, 2012; Yates et al., 2012b). The N-termini of kindlins, including the F0 and F1 subdomains, are also involved in supporting integrin activation (Bouaouina et al., 2012; Goult et al., 2009; Ma et al., 2008). Although possible membrane-binding sites have been proposed in the F0+F1 subdomains of kindlins, the exact mechanism by which they support integrin activation remains unclear. To explore the underlying mechanism, the ProQuest™ yeast two-hybrid system was employed to screen and identify the binding partners for the F0+F1 subdomains of kindlin-1. After initial screening and subsequent verification, multiple kindlin-1 binding proteins were identified (Fig. 1A). Notably, KRT18, TRIOBP and paxillin (PXN) are cytoskeletal proteins, like talin and kindlin. To determine whether they play a role in regulating integrin activation, each of these kindlin-1 binding molecules was Flag-tagged and co-expressed with DsRed-fused TH and enhanced green fluorescent protein (EGFP)-fused kindlin-1 in CHO-αIIbβ3 cells. Integrin αIIbβ3 activation was analyzed by flow cytometry (FACS). PAC-1 and 2G12, two specific antibodies to integrin αIIbβ3, were used to stain activated and total integrins, respectively. The binding of PAC-1 to activated integrin αIIbβ3 in CHO cells induced by TH and kindlin-1 is specific (Fig. S1A). To ensure the similar expression of TH and kindlin-1 in different transfectants, DsRed and EGFP double-positive cells were gated (Fig. 1B; Fig. S1B). In addition, expression of the kindlin-1 binding proteins was also evaluated by western blotting (Fig. 1C). Interestingly, although PXN, KRT18 and TRIOBP could all be expressed in the transfected cells, we found that PXN, but not the other two, significantly enhanced integrin αIIbβ3 activation (Fig. 1D).
Fig. 1.
Paxillin interacts with the N-terminus of kindlin-1 and enhances integrin αIIbβ3 activation. (A) The subdomains of kindlins and their binding partners. (B) PXN, KRT18 and TRIOBP were constructed as Flag-tagged molecules and expressed in CHO-αIIbβ3 cells together with DsRed-fused talin head (TH) and EGFP-fused kindlin-1 (K1) by transient transfection. The transfected cells positive for both DsRed and EGFP were gated for further analysis. (C) The expression levels of Flag-tagged PXN, KRT18 and TRIOBP in the transfected cells were measured by western blotting (WB). (D) Integrin αIIbβ3 activation was expressed by normalized PAC-1 binding, as defined in Materials and Methods. (E) CHO-αIIbβ3 cells were transfected with the siRNA duplexes targeting PXN (siPXN001 and siPXN003) or a control siRNA duplex (siControl). Their efficiencies for knockdown of endogenous PXN were evaluated by western blotting. (F) Control and specific siRNA duplexes targeting for PXN were co-transfected in CHO-αIIbβ3 cells with TH and K1, as indicated, and their effects on integrin αIIbβ3 activation evaluated by the PAC-1 binding assay. Results in D and F represent the mean±s.d. of five experiments; **P<0.01.
Because EGFP–kindlin-1 was found to associate with endogenous PXN in CHO-αIIbβ3 cells (Fig. S2), we tested whether endogenous PXN participates in supporting integrin αIIbβ3 activation. Two different siRNA duplexes against PXN were selected, and each of them could efficiently knock down the expression of PXN (∼70% versus control siRNA) in CHO-αIIbβ3 cells (Fig. 1E). Knockdown of endogenous PXN by specific siRNAs significantly suppressed integrin αIIbβ3 activation induced by TH and kindlin-1 (Fig. 1F). Overexpression of exogenous PXN rescued integrin αIIbβ3 activation in these siRNA-treated cells (not shown), indicating that endogenous PXN is involved in supporting integrin activation. Together, these results suggest that PXN can interact with kindlin-1 and might play an important role in supporting integrin αIIbβ3 activation. As noted, integrin activation was only partially suppressed by siRNAs against PXN. This might be caused by incomplete knockdown of endogenous PXN (Fig. 1E) and/or the presence of another PXN family member, Hic-5 (hydrogen peroxide-inducible clone-5), in CHO-αIIbβ3 cells (Fig. S2).
PXN binds to the F0 subdomain of kindlin-1 and enhances integrin αIIbβ3 activation
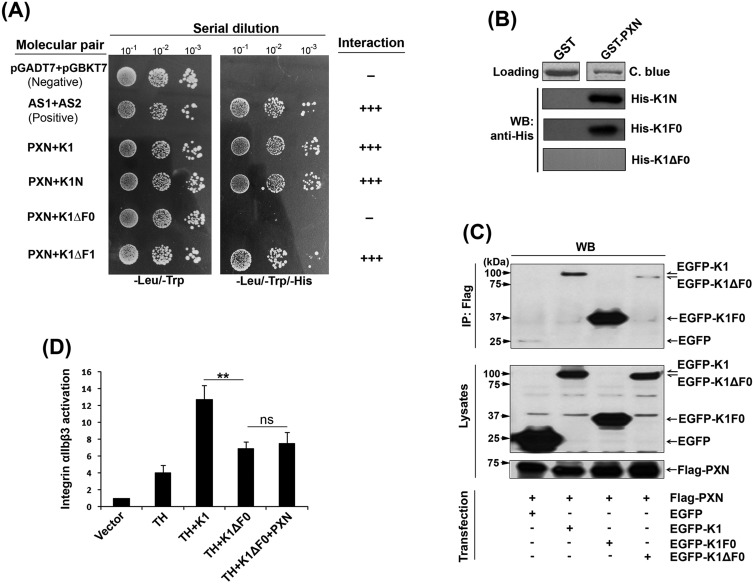
To validate the interaction between PXN and kindlin-1, another yeast two-hybrid system (Matchmaker Gold) was employed. Fig. 2A verifies that both the full-length kindlin-1 and the kindlin-1 (F0+F1) fragment interact with PXN. To further narrow down the PXN binding region in kindlin-1, deletion mutants of kindlin-1 were generated, with a lack of either the F0 or the F1 subdomain (kindlin-1-ΔF0 or kindlin-1-ΔF1). Specifically, deletion of the F0 subdomain, but not F1, disrupted the interaction of kindlin-1 with PXN (Fig. 2A), suggesting that the F0 subdomain in kindlin-1 is involved in mediating the interaction. To confirm this finding, these proteins were purified for pull-down assays (Fig. S3A). In contrast to GST alone, GST-fused PXN could bind to both kindlin-1 and the F0 subdomain of kindlin-1; however, it failed to interact with kindlin-1-ΔF0 (Fig. 2B). In addition, the full-length kindlin-1 and the F0 subdomain of kindlin-1 were able to interact with PXN in the lysates of transfected CHO cells; interaction of kindlin-1-ΔF0 with PXN was significantly compromised (Fig. 2C). These results demonstrate that PXN interacts with kindlin-1 via the N-terminal F0 subdomain. Furthermore, we observed that kindlin-1-ΔF0 had a significantly reduced ability to activate integrin αIIbβ3 (Fig. 2D), which might be a result of the disconnection between kindlin-1-ΔF0 and PXN in cells. Consistently, kindlin-1-ΔF0 failed to support PXN in enhancing integrin activation (Fig. 2D). Taken together, these results suggest that PXN supports integrin activation by directly interacting with kindlin-1 via the F0 subdomain.
Fig. 2.
The F0 subdomain of kindlin-1 interacts with PXN to enhance integrin αIIbβ3 activation. (A) The interaction of PXN with kindlin-1 (K1) and the indicated mutants were evaluated using the Matchmaker™ Gold yeast two-hybrid system as described in Materials and Methods. (B) GST and GST-tagged PXN (GST-PXN) were coupled to glutathione–Sepharose beads and incubated with the indicated His-tagged kindlin-1 fragments. The precipitated proteins on the beads were evaluated by either Coomassie Blue (C. blue) staining or western blotting (WB). (C) EGFP–kindlin-1 (EGFP–K1) and the indicated mutants were co-expressed with Flag–PXN in CHO cells, respectively. The interaction of Flag–PXN with the kindlin-1 variants was evaluated by CO-IP followed by western blotting (WB). (D) As indicated, different combinations of TH–DsRed (TH), EGFP–kindlin-1 (K1) or EGFP–K1ΔF0 (K1ΔF0), Flag–PXN (PXN), or their empty vectors were expressed in CHO-αIIbβ3 cells. The cells positive for both DsRed and EGFP were selected, and integrin αIIbβ3 activation in these cells was evaluated by the PAC-1 binding assay. Results represent the mean±s.d. of five experiments; **P<0.01; ns, not significant.
A conserved loop in the F0 subdomain of kindlin-1 is involved in mediating the interaction with PXN and supporting integrin αIIbβ3 activation
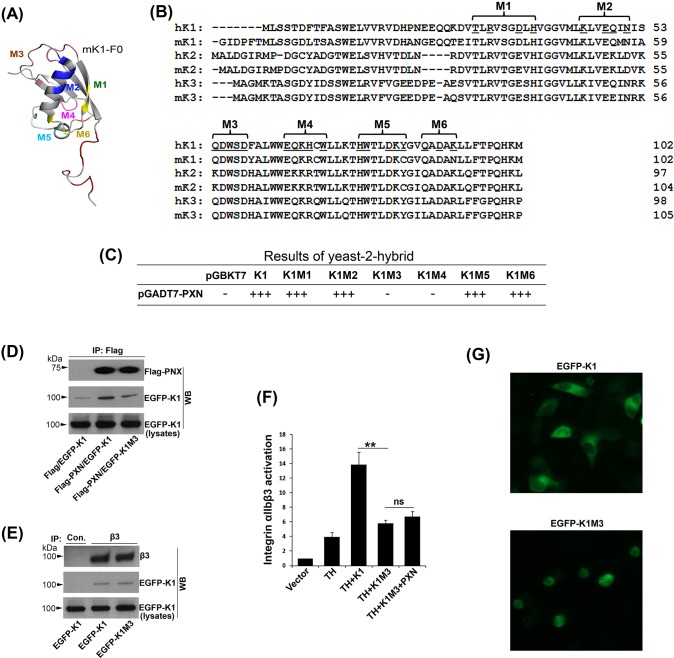
The F0 subdomains of kindlins adopt a ubiquitin-like fold (Goult et al., 2009; Li et al., 2017; Perera et al., 2011). To identify the PXN binding sites in kindlin-1 (Fig. 3A), the exposed surface residues in the F0 subdomain of kindlin-1 were selected on the basis of solvent accessibility (>30%) and grouped into six clusters (M1–M6). Accordingly, six mutants of kindlin-1 were generated by substitution of the exposed residues (underlined in Fig. 3B) in each of the clusters with alanine. Next, we evaluated the interactions of these kindlin-1 mutants with PXN using the yeast two-hybrid system, and found that the M3 and M4 mutants of kindlin-1 no longer interacted with PXN whereas the others still could (Fig. 3C). These results indicate that the exposed residues in the M3 and the M4 regions of kindlin-1 could constitute the binding sites for PXN. Because the M3 region is more conserved across the kindlin family members (Fig. 3C) and the M4 is involved in interfacing with the F1 subdomain in kindlins (Goult et al., 2009; Li et al., 2017), we focused on the M3 region for subsequent studies. When kindlin-1 and the M3 mutant were co-expressed with PXN in CHO-αIIbβ3 cells, we found that the M3 mutant of kindlin-1 exhibited a weaker interaction with PXN compared with its wild-type counterpart (∼65% reduction based on the results from three experiments), although they interacted equally with the β3-integrin (Fig. 3D,E). In addition, interaction of the M3 mutant of kindlin-1 with the integrin β3 CT was also confirmed in the yeast two-hybrid system (not shown). Together, these results indicate that the M3 region in kindlin-1 is involved in the interaction with PXN, but not with integrin. Consistently, the M3 mutant of kindlin-1 was less effective in supporting integrin activation, regardless of whether PXN was co-transfected (Fig. 3F). Furthermore, we found that when the M3 mutant of kindlin-1 was overexpressed in CHO-αIIbβ3 cells, these cells spread poorly on immobilized fibrinogen, whereas cells with overexpressed wild-type kindlin-1 spread well under the same conditions (Fig. 3G), indicating that the M3 mutant acts in a dominant negative manner to suppress integrin function.
Fig. 3.
Identification of PXN binding sites in the F0 subdomain of kindlin-1. (A) Structure of the F0 subdomain of kindlin-1 (PDB accession code 2KMC) was shown. The exposed surface residues were selected and highlighted (M1–M6). (B) Amino acid sequences of the F0 subdomains of kindlins of humans and mice. The exposed residues (underlined) were determined by solvent accessibility (>30%, Swiss PDB view) and mutated to alanine for functional analysis. (C) The interactions of kindlin-1 and the mutants (M1–M6) with PXN were evaluated using the Matchmaker™ Gold yeast two-hybrid system as described in Materials and Methods. (D) EGFP–kindlin-1 (EGFP-K1) and the M3 mutant (EGFP-K1M3) were expressed in CHO cells with Flag–PXN or the Flag vector, respectively. Their interactions were evaluated by CO-IP using an anti-Flag antibody. The co-precipitated EGFP–kindlin-1 and the M3 mutant were evaluated by western blotting (WB). (E) EGFP–kindlin-1 (EGFP-K1) and the M3 mutant (EGFP-K1M3) were expressed in CHO-αIIbβ3 cells. The interaction of β3-integrin with EGFP-K1 and EGFP-K1M3 was evaluated by CO-IP using an anti-β3 antibody followed by western blotting (WB). (F) Combinations of the indicated molecules were expressed in CHO-αIIbβ3 cells. Integrin αIIbβ3 activation in the positively transfected cells was evaluated by the PAC-1 binding assay. Results represent the mean±s.d. of five experiments; **P<0.01; ns, not significant. (G) CHO-αIIbβ3 cells expressing either EGFP-K1 or EGFP-K1M3 were allowed to spread on immobilized fibrinogen for 2 h at 37°C and the spreading of EGFP-positive cells was evaluated using fluorescence microscopy.
Structurally, the M3 residues in the F0 subdomain of kindlin-1 constitute a surface loop (Fig. 3A), which is distal from the known binding sites for other molecules such as F-actin and phospholipids (Bledzka et al., 2016; Perera et al., 2011), implying that the M3 region in kindlin-1 provides a unique docking site for PXN. In addition, when we mutated each of the residues in the M3 (Q54DWSD) of kindlin-1, we found that each of the conserved residues (DWSD) exhibited significant influence on integrin αIIbβ3 activation, except for the nonconserved Q54 (Fig. S4). Taken together, these findings suggest that the M3 loop in the F0 subdomain of kindlin-1 is responsible for interacting with PXN and supporting integrin αIIbβ3 activation.
Both kindlin-1 and TH are required for PXN to enhance integrin αIIbβ3 activation
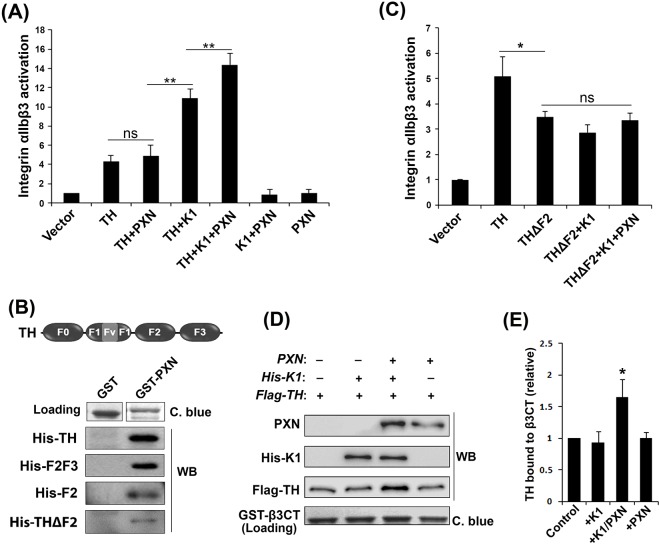
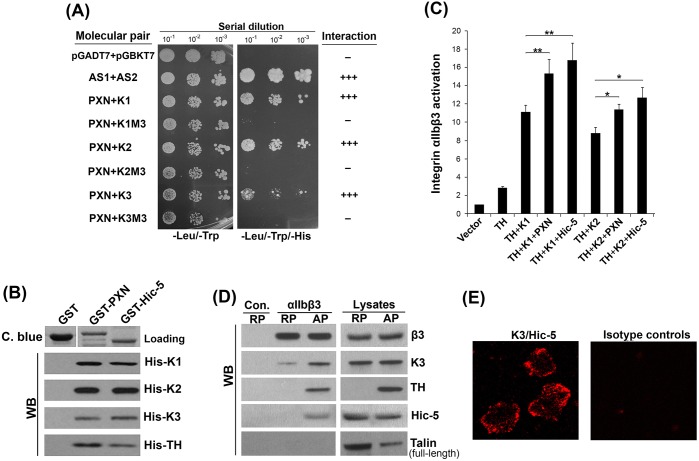
Next, we evaluated the involvement of TH and kindlin-1 in PXN-mediated enhancement of integrin αIIbβ3 activation. As shown in Fig. 4A, PXN alone failed to induce integrin activation. Combined with either TH or kindlin-1, PXN also failed to show further enhancement of integrin activation. The enhancement only occurred when both TH and kindlin-1 were present, suggesting that PXN functionally relies on both of them. Considering a previously identified association between PXN and talin (Salgia et al., 1995), our findings indicate that PXN may possibly complex with both TH and kindlin-1 in supporting integrin activation, through which binding of TH to integrin might be stabilized. To explore this idea, we performed pull-down assays using purified proteins and observed that PXN could directly interact with TH (Fig. 4B). We then dissected TH into the N-terminal TH-F0F1 and C-terminal TH-F2F3 fragments and found that PXN interacted with TH-F2F3 (Fig. 4B) but not with TH-F0F1 (not shown). Furthermore, we were able to detect a direct interaction between TH-F2 and PXN (Fig. 4B). Because of a high non-specific background, we failed to determine whether TH-F3 alone could interact with PXN; however, TH-ΔF2 exhibited the ability to bind to PXN (Fig. 4B), suggesting that TH-F2F3 contains more than one binding site for PXN. TH lacking the F2 subdomain exhibited a compromised effect on integrin activation, which may be ascribed to its functional disconnection with endogenous PXN in cells. In addition, TH-ΔF2 no longer cooperated with kindlin-1 to support integrin activation, even in the presence of PXN (Fig. 4C). Next, we tested whether PXN, together with kindlin-1, promotes the binding of TH to the integrin β3 CT. As shown in Fig. 4D,E, the binding of TH to the integrin β3 CT was significantly enhanced when both kindlin-1 and PXN were present; either kindlin-1 or PXN alone failed to enhance the binding. However, we failed to detect a significant enhancement of TH-ΔF2 binding to the integrin β3 CT in the presence of kindlin-1 and PXN (Fig. S3B). Furthermore, we found that, even in the absence of integrin, PXN could mediate formation of the complex between TH and kindlin-1 (Fig. S3C). Taken together, these results suggest that PXN enhances the binding of TH to integrin by complexing with kindlin-1 and TH, thus facilitating the ability of TH to support integrin activation.
Fig. 4.
PXN relies on both kindlin-1 and talin to enhance integrin activation. (A) Different combinations of Flag–PXN (PXN), DsRed-fused TH (TH), EGFP-fused kindlin-1 (K1) or their vectors were expressed in CHO-αIIbβ3 cells. Integrin αIIbβ3 activation in the positively transfected cells was evaluated by the PAC-1 binding assay. (B) Pull-down experiments were performed to evaluate the interaction of PXN with the subdomains of TH. The precipitates were determined by western blotting (WB). The loaded GST proteins were evaluated by Coomassie Blue (C. blue) staining. (C) DsRed-fused TH (TH) and the mutant lacking the F2 subdomain (THΔF2) were expressed in CHO-αIIbβ3 cells; THΔF2 was also co-expressed with EGFP-fused kindlin-1 (K1), with or without Flag–PXN (PXN). Integrin αIIbβ3 activation in the positively transfected cells was evaluated by the PAC-1 binding assay. (D,E) GST–β3CT protein was coupled to glutathione–Sepharose beads and used for incubation with purified Flag-tagged TH (Flag-TH) in the presence or absence of purified kindlin-1 (His-K1) and/or PXN. After washing, proteins precipitated with GST–β3CT were evaluated by western blotting (WB). The loaded GST–β3CT protein on the beads was evaluated by Coomassie Blue (C. blue) staining. The signals of TH bound to GST–β3CT were quantified using ImageJ software. The results represent mean±s.d. of four experiments; *P<0.05, **P<0.01; ns, not significant.
PXN and Hic-5 interact with the kindlin family members and enhance integrin αIIbβ3 activation
We next sought to extend our finding to the kindlin family, which includes three members (kindlin-1, kindlin-2 and kindlin-3). As evaluated by the yeast two-hybrid system, PXN interacted with all kindlin members, but not with their M3 mutants (Fig. 5A); in addition, PXN interacted with the N-terminal fragments (F0+F1 or F0) of kindlins (not shown). Furthermore, we used pull-down assays to verify that PXN could directly interact with the N-terminal fragments (F0+F1) of all kindlins (Fig. S5). These results suggest that the interaction between PXN and kindlins is a common mechanism. Previously, it was reported that Hic-5, another key member of the PXN family, was able to play a supportive role in integrin αIIbβ3 activation in platelets (Kim-Kaneyama et al., 2012). Therefore, we tested whether Hic-5 can also interact with kindlins and TH. As shown in Fig. 5B, the results from pull-down experiments showed that Hic-5, similarly to PXN, interacted with all three kindlin members and TH. Functional analysis using CHO-αIIbβ3 cells also confirmed that Hic-5, like PXN, enhanced integrin αIIbβ3 activation mediated by TH and kindlin-1 or kindlin-2 (Fig. 5C). These results suggest that there may exist a general mechanism between the kindlin family members and PXN and/or Hic-5 to support integrin activation.
Fig. 5.
PXN family members interact with the kindlin family members and support integrin αIIbβ3 activation. (A) The interaction of PXN with different kindlins and their M3 mutants were evaluated using the Matchmaker™ Gold yeast two-hybrid system. (B) The interactions of PXN/Hic-5 with kindlins and TH were evaluated by pull-down assays. The precipitates were analyzed by western blotting (WB). Meanwhile, the loaded GST proteins on the beads was evaluated by Coomassie Blue (C. blue) staining. (C) As indicated, Flag–PXN (PXN) and Flag–Hic-5 (Hic-5) were each co-expressed with DsRed-fused TH (TH) and EGFP-fused kindlins (K1 and K2) in CHO-αIIbβ3 cells. Integrin αIIbβ3 activation in the positively transfected cells was measured by the PAC-1 binding assay. The results represent mean±s.d. *P<0.05, **P<0.01. (D) Washed human platelets were activated with TRAP-6 (7 μM, 15 min) or kept under resting conditions. Activated platelets (AP) and resting platelets (RP) were lysed and incubated with either control IgG or an antibody against αIIbβ3, in the presence of protein A/G agarose beads. After incubation for 6 h at 4°C, the beads were washed and the precipitated proteins on the beads analyzed by western blotting (WB). (E) Proximity ligation assay (PLA). Washed human platelets were spread on fibrinogen, PLA was performed and the proximity of Hic-5 and kindlin-3 was detected by confocal microscopy.
Kindlin-3 is primarily expressed in hematopoietic cells and plays an essential role in supporting integrin αIIbβ3 activation in platelets. However, the function of kindlin-3 is difficult to recapitulate in CHO-αIIbβ3 cells; instead, kindlin-1 and kindlin-2 have been widely used for exploring mechanistic details in this model system (Bialkowska et al., 2015; Harburger et al., 2009; Ma et al., 2008; Moser et al., 2008; Xu et al., 2013; Ye et al., 2013). To determine whether PXN and/or Hic-5 also support kindlin-mediated activation of integrin αIIbβ3, we sought to verify the association of kindlin-3, PXN/Hic-5 and talin in platelets. As is known, during platelet activation, talin can be cleaved to release the TH domain, which is responsible for binding to the integrin β CT and inducing integrin activation (Calderwood, 2004; Campbell and Ginsberg, 2004; Yan et al., 2001). As expected, Hic-5, the predominant PXN family member in human platelets (Rathore et al., 2007), was associated with integrin αIIbβ3, together with kindlin-3 and TH, in activated but not resting human platelets (Fig. 5D). These results indicate that the activation of integrin αIIbβ3 correlates with formation of a multiprotein complex in platelets. We failed to detect the association of full-length talin with integrin in platelets under either resting or activated conditions (Fig. 5D). In addition, when we used the proximity ligation assay (PLA) to evaluate the interaction of kindlin-3 and Hic-5 in human platelet spreading on immobilized fibrinogen, specific PLA signals of kindlin-3 and Hic-5 (reflected by the bright fluorescent spots) could be detected (Fig. 5E), demonstrating that there is a close proximity between kindlin-3 and Hic-5. Collectively, the above results suggest that the interactions between kindlins and the PXN family members might possibly constitute a new mechanism for support of integrin activation by facilitating the formation of a multiprotein complex.
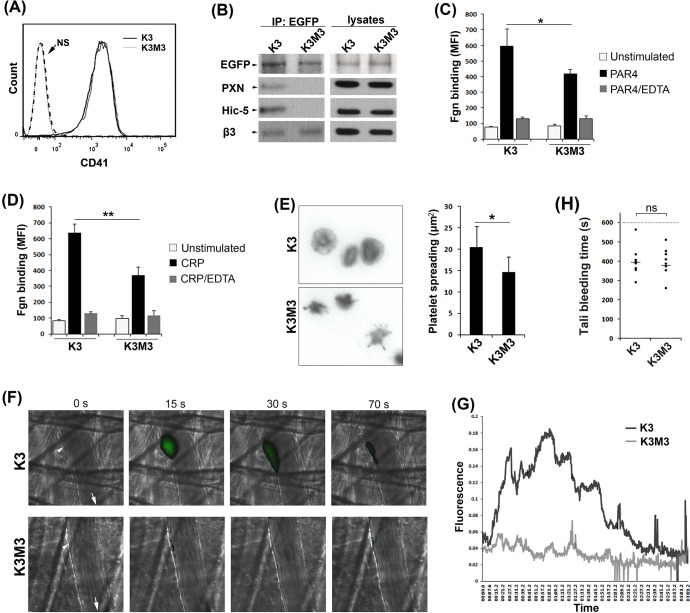
Disconnection of kindlin-3 with PXN/Hic-5 in mouse platelets compromises integrin αIIbβ3 activation and reduces platelet thrombus formation
Next, an in vivo kindlin-3 rescue strategy in mice was employed to functionally evaluate the crosstalk between kindlin-3 and PXN/Hic-5 in platelets. Kindlin-3-deficient bone marrow cells isolated from kindlin-3fl/flMx1-Cre mice were infected with lentiviral particles encoding either EGFP-fused wild-type kindlin-3 or the M3 mutant, and transplanted into lethally irradiated wild-type C57BL/6 recipient mice. At 6-8 weeks after bone marrow transplantation, platelets isolated from the recipients were analyzed by FACS to evaluate the EGFP-positive population. As shown in Fig. S6, platelets expressing either wild-type kindlin-3 or the M3 mutant showed a similarly heterogeneous pattern, probably caused by insufficient lentiviral transduction of the donor bone marrow cells. Nonetheless, we could perform functional analyses by gating the EGFP-positive platelets. Surface expression levels of integrin αIIbβ3 were comparable for platelets expressing EGFP-fused wild-type kindlin-3 and the M3 mutant (Fig. 6A). Co-immunoprecipitation (Co-IP) assays were performed using an anti-GFP antibody to immunoprecipitate EGFP-fused kindlin-3. As shown in Fig. 6B, wild-type kindlin-3 and the M3 mutant were precipitated from the respective platelet lysates. Although both PXN and Hic-5 were co-precipitated with wild-type kindlin-3, their association with the M3 mutant of kindlin-3 was less detectable. As expected, both wild-type kindlin-3 and the M3 mutant were able to interact with the integrin β3 subunit, validating the specificity of disconnection between the M3 mutant and PXN/Hic-5 in platelets. In addition, we evaluated the rescue effects of wild-type kindlin-3 and the M3 mutant on integrin αIIbβ3 activation. Platelets isolated from transplanted mice were stimulated with either PAR4 agonist peptides or collagen-related peptide (CRP) and incubated with Alexa Fluor 647-conjugated fibrinogen (a ligand of integrin αIIbβ3); the binding of fibrinogen to EGFP-positive platelets was analyzed by FACS. As shown in Fig. 6C,D, the binding levels of fibrinogen to platelets expressing wild-type kindlin-3 were significantly higher than for platelets expressing the M3 mutant. Importantly, the binding of fibrinogen to the two types of platelets could be reduced to similarly low levels when EDTA was present, showing the specificity of fibrinogen binding to integrin αIIbβ3. In addition, platelets expressing wild-type kindlin-3 spread well on fibrinogen, whereas platelets expressing the M3 mutant showed relatively less spreading (Fig. 6E), which is consistent with the observations in CHO cells (Fig. 3G). Together, these results indicate that interaction of kindlin-3 with PXN/Hic-5 in platelets plays a supportive role in integrin αIIbβ3 activation.
Fig. 6.
Disconnection of kindlin-3 with PXN/Hic-5 in mouse platelets disadvantages integrin αIIbβ3 activation and platelet thrombus formation. (A) Histograms showing the expression of integrin αIIb subunit (CD41) in platelets expressing either EGFP–K3 (K3) or EGFP–K3M3 (K3M3). Unstained (NS) platelets were used as negative controls. (B) Platelets expressing either EGFP–K3 or EGFP–K3M3 were lysed and used for Co-IP using an anti-GFP antibody. The precipitates were evaluated by western blotting (WB). (C,D) The binding of fibrinogen to platelets expressing either EGFP–K3 (K3) or EGFP–K3M3 (K3M3) was measured under stimulating conditions with either PAR4 agonist peptide (C) or CRP (D) in the presence or absence of EDTA (5 mM). The bound fibrinogen conjugated with Alexa Fluor 647 on platelets was measured by flow cytometry. (E) Platelets expressing either EGFP–K3 or EGFP–K3M3 were allowed to adhere and spread on immobilized fibrinogen for 45 min in the presence of PAR4 agonist peptide before fixation. The adherent platelets positive for EGFP were imaged by fluorescence microscopy and platelet spreading areas were measured. (F,G) Intravital microscopy was performed using a method described previously in transplanted mice (Kim et al., 2013). Platelet thrombus formation at the sites of laser-induced arteriolar wall injury was visualized and quantified. Data were collected from 15 sites in three of each type of mice. (H) A small segment of mouse tail (∼5 mm) was transected and the bleeding time monitored for 10 min. The time to first cessation in bleeding was recorded for each mouse. The results represent mean±s.d. ns, non-significant; *P<0.05, **P<0.01.
Furthermore, platelet thrombus formation in bone marrow transplanted mice was evaluated using fluorescent intravital microscopy. Mouse cremaster arteriolar walls underwent laser-induced injury, and platelet accumulation at the wound sites was visualized. As shown in Fig. 6F,G, after vascular injury, platelet thrombus formation quickly occurred in cremaster arterioles in mice expressing EGFP–kindlin-3 in platelets, and the formed thrombi were evident. However, platelet accumulation was only minimally detected in cremaster arterioles in mice expressing the M3 mutant of kindlin-3 in platelets under the same experimental conditions, indicating that these mice had a compromised ability to form platelet thrombus. Surprisingly, tail bleeding tests showed that the bleeding time was not significantly different between these two types of bone marrow transplanted mice, suggesting that the M3 mutant of kindlin-3 may have less effect on hemostasis (Fig. 6H). Taken together, the results from these in vivo experiments suggest that crosstalk of kindlin-3 with PXN and/or Hic-5 in mouse platelets is essential in supporting platelet thrombus formation while having minimal effects on normal hemostasis.
DISCUSSION
The important role of the kindlin family members in supporting integrin activation and functions has been highlighted in human genetic diseases and animal models. However, the mechanistic details remain largely unknown. In this study, the results of biochemical and functional analyses suggest a novel mechanism that could explain how kindlins support integrin αIIbβ3 activation. Our main findings are as follows: (1) Kindlins can interact with PXN and Hic-5 via their N-terminal F0 subdomain, where a conserved surface loop is involved in mediating the interaction. (2) Formation of a multiprotein complex of integrin/kindlin/PXN/talin can promote integrin activation, possibly by facilitating the binding of talin to integrin. (3) The binding of paxillin to kindlins might be crucial for supporting both integrin inside-out and outside-in signaling, as evidenced by soluble ligand binding and cell spreading, respectively. (4) Crosstalk between kindlin-3 and PXN/Hic-5 in platelets is required to support integrin αIIbβ3 activation. Importantly, such a mechanism can preferentially promote platelet thrombus formation in mice while having less effect on normal hemostasis, suggesting that it might be useful for developing specific anti-thrombotic strategies.
In a recently published paper, a distinguished research group also detected the interaction between PXN and kindlins (Theodosiou et al., 2016). The authors found that PXN interacted with the PH domain of kindlin-2, which is different from our current finding that the F0 subdomain in kindlins is responsible for binding to PXN. Using the yeast two-hybrid system, we did detect the interaction of PXN with the PH domain fragment of kindlin-1 (not shown), although we did not detect the binding signal between PXN and kindlin-1-ΔF0 (Fig. 2A), suggesting that the PXN binding site in the PH domain of kindlin-1 is buried by other subdomains. However, we failed to detect a significant binding signal between PXN and the PH domain fragment of kindlin-2, indicating that the binding might be relatively weak. In addition, the authors focused on β1-integrins and found that crosstalk between PXN and kindlin-2 could promote FAK-mediated cell spreading on fibronectin. In our current study, we disclose a novel mechanism by which kindlins can cooperate with PXN to enhance the binding of talin to integrin αIIbβ3, thus supporting platelet integrin αIIbβ3 function. Overexpression of the kindlin M3 mutants (with disabled ability to bind PXN) not only suppresses integrin αIIbβ3 activation (Fig. 3F) but also dramatically impairs integrin αIIbβ3-mediated cell spreading (Fig. 3G). These results demonstrate that the crosstalk between kindlins and PXN might be essential for supporting the bidirectional signaling of integrin αIIbβ3. However, we failed to detect significant influence of PXN on the binding of a β1-integrin ligand (fibronectin type-III repeats 9–11) to CHO cells under the same conditions (not shown), suggesting that the regulatory effect of PXN on integrin signaling may be integrin- and/or cell type-specific.
As a key scaffold protein, PXN has numerous binding partners (Brown and Turner, 2004). Importantly, many of the paxillin binding partners, such as ILK, vinculin, FAK, Src and even integrin subunits, are functionally associated with integrin signaling, suggesting that PXN might play multiple roles in regulating integrin activation. In addition, PXN may possibly induce integrin clustering by cooperating with kindlin (Ye et al., 2013). Interestingly, when comparing the surface distribution of integrin αIIbβ3 on CHO cells in suspension by confocal microscopy, significant differences were not detected between cells with and without exogenous expression of PXN (not shown). Nonetheless, possible regulation of integrin αIIbβ3 microclustering by PXN cannot be excluded. If avidity modulation does occur here, it probably relies on affinity modulation triggered by the talin head, because kindlin and PXN (individually or together) fail to induce integrin activation when the talin head is not present (Fig. 4A). In addition, disruption of the interaction between PXN and kindlin-3 significantly affects the binding of soluble fibrinogen to activated platelets (Fig. 6), indicating the involvement of affinity modulation. Obviously, more mechanistic details need to be explored in the future.
A previous study disclosed that Hic-5 plays a supportive role in integrin αIIbβ3 activation (Kim-Kaneyama et al., 2012), which is consistent with our current findings. However, another study claimed that Hic-5 is dispensable for integrin αIIbβ3 activation in mouse platelets (Popp et al., 2015). The mechanism for the discrepancy between these studies is unknown. Interestingly, one study has shown that knockdown of paxillin in mouse platelets by lentivirus-based shRNA could even elevate integrin αIIbβ3 activation (Sakata et al., 2014). These controversial observations indicate complications in understanding the complex roles of PXN and Hic-5 in regulating integrin αIIbβ3 activation in platelets. However, it is worth noting that these studies were based on deletion of either PXN or Hic-5 in platelets. Because PXN and Hic-5 interact with numerous important cytoskeletal and signaling molecules in cells (Brown and Turner, 2004), arbitrary deletion of PXN or Hic-5 might lead to significant signaling alternations in platelets. If so, the function of PXN and Hic-5 in supporting integrin activation might be buried in the integrated effects. In addition, PXN and Hic-5 may not function equally in supporting integrin activation in different cells. For example, we found that knockdown of endogenous Hic-5 in CHO-αIIbβ3 had a negligible effect on PAC-1 binding (not shown), indicating that endogenous PXN plays a major role in supporting integrin activation in these cells. In our study, we disconnected the crosstalk between kindlins and PXN/Hic-5 by introducing structure-based mutations in kindlins, which is more specific than knocking down kindlins. Our results from in vitro and in vivo analyses demonstrate that such crosstalk plays an important role in supporting integrin αIIbβ3 function. Nevertheless, functional specificity and possible compensation across different PXN family members need to be further explored. In addition, it is possible that more than one KBP in platelets is involved in the regulation of integrin αIIbβ3 activation. For example, ADAP, a hematopoietic cell-specific adaptor protein, was previously reported to be able to associate with talin and kindlin-3 and promote integrin αIIbβ3 activation (Kasirer-Friede et al., 2014).
In summary, we disclose that PXN and Hic-5, as a family of novel KBPs, are involved in supporting integrin αIIbβ3 activation by facilitating the formation of a multiprotein complex of integrin activators; this provides mechanistic insights into the regulation of integrin αIIbβ3 activation. Furthermore, we provide in vivo evidence to show that such a mechanism may be useful for developing specific anti-thrombotic therapeutics. Nonetheless, the heterogeneity of kindlin-3 expression in transplanted mice in this study means that further investigation using kindlin-3 knock-in mice are needed to specify the physiological and pathological significance of the identified mechanism.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal antibody (F1804) and rabbit polyclonal antibody (F7425) against Flag for Co-IP and western blotting were purchased from Sigma; mouse monoclonal antibody against GFP for CO-IP was kindly provided by Dr Boquan Jin (The Fourth Military Medical University, Xi'an, China); rabbit polyclonal antibody (A1544) against GFP for CO-IP and western blotting was purchased from Sigma; mouse monoclonal antibody (610052) against paxillin for western blotting was purchased from BD; mouse monoclonal antibody (611165) against Hic-5 for western blotting was purchased from BD; mouse monoclonal antibody (MAB1676) and rabbit polyclonal antibody (sc-15336) against talin for western blotting were purchased from EMD Millipore and Santa Cruz Biotechnology, respectively; mouse monoclonal antibody PAC-1 (340535) against activated integrin αIIbβ3 was purchased from BD; goat polyclonal antibody (sc-6627; sc-6626) against integrin β3 for western blotting was purchased from Santa Cruz Biotechnology; mouse monoclonal antibodies (sc-53073 and 631212) against 6×His tag for western blotting were purchased from Santa Cruz Biotechnology and Clontech, respectively; rabbit polyclonal antibodies against kindlin-3 peptides (GEVGEPAGTDPGLD; CERARGEELDEDLFLQ) were prepared at New England Peptide. A 1:1000 dilution of the primary antibodies was used for western blotting.
cDNA constructs, siRNA duplexes and transfection
The paxillin cDNA was kindly provided by Hisataka Sabe (Hokkaido University) and was subcloned into p3×Flag-CMV-10 vector (Sigma-Aldrich). Other cDNA constructs, including the KBPs, were obtained from either Addgene or ATCC. Different kindlin mutants were generated using a QuickChange site-directed mutagenesis kit (Agilent Technologies) and further verified by gene sequencing. The siRNA duplexes targeting paxillin in CHO cells [siRNA#001 5′-AAGAGAAGCCAAAGCGAAAUU-3′ (sense) and 5′-UUUCGCUUUGGCUUCUCUUUU-3′ (antisense); siRNA#003 5′-GCGCCAUCCUGGAGAACUAUU-5′ (sense) and 5′-UAGUUCUCCAGGAUGGCGCUU-3′ (antisense)] were ordered from Dharmacon. Transient transfection of CHO cells stably expressing integrin αIIbβ3 (CHO-αIIbβ3) was performed using either Lipofectamine 2000 or 3000 (Invitrogen).
Yeast two-hybrid systems
The ProQuest™ yeast two-hybrid system (Invitrogen, Carlsbad, CA) was employed for initial screening of KBPs. In brief, yeast MaV203 cells were sequentially transformed with a plasmid of pDBLeu-kindlin-1 encoding the N-terminal F0+F1 fragment of kindlin-1 and a human liver cDNA library in pPC86 vector. Primary positive clones were screened on selective media from at least 1×106 transformants and tested using three reporter assays. The positive clones were further verified by retransformation assays. Finally, the targeted cDNA inserts in positive clones were sequenced and analyzed by BLAST searches.
In addition, the Matchmaker™ Gold yeast two-hybrid system (Clontech, Mountain View, CA) was utilized to validate the interaction of known molecular pairs. Briefly, the two known molecules to be tested were fused with either the DNA binding domain or the activation domain of GAL4 in pGBKT7 or pGADT7 vector, and co-expressed in yeast strain Y2Hgold. Their interaction was evaluated by culturing the transformants on selective media by a serial dilution method. The growth of yeast cells on −Leu/−Trp medium indicates successful transformation; the growth of yeast cells on −Leu/−Trp/−His medium indicates a positive protein–protein interaction. The interactions were expressed as following: −, no growth with dilutions; +, growth only with a 10−1 dilution; ++, growth only with 10−1 and 10−2 dilutions; +++, growth with all three serial dilutions (Fig. 2A). A known molecular pair (AS1/AS2) (Liu et al., 2009) was used as a positive control; the empty vectors were transformed to serve as a negative control. In addition, auto-activation for each of the tested molecules was tested and excluded.
Integrin activation assays
Integrin αIIbβ3 activation was evaluated in CHO-K1 cells (ATCC) by the PAC-1 binding assay as described previously (Ma et al., 2008, 2006; Xu et al., 2013). Specifically, the talin head was cloned in pDsRed-Monomer-N1 vector; the kindlin family members were cloned in pEGFP-C2 vector; and KBPs, including paxillin and Hic-5, were cloned in p3×Flag-CMV-10 vector. Different combinations of TH–DsRed, EGFP–kindlin and Flag–KBP or their empty vectors (as indicated in each of the experiments) were transiently expressed in CHO cells stably expressing integrin αIIbβ3 (CHO-αIIbβ3 cells). At 24 h after transfection, cells were harvested and separated into two aliquots. One portion was incubated with PAC-1, an antibody specific for activated integrin αIIbβ3; the other portion was incubated with 2G12, an antibody for staining total integrin αIIbβ3. After incubation, the cells were further stained with Alexa Fluor 633-labeled secondary antibodies. Finally, flow cytometry analysis was performed. The cells positive for both DsRed and EGFP were gated and the binding of PAC-1 and 2G12 to these cells was analyzed. Dead cells were excluded by DAPI-positive staining. Integrin αIIbβ3 activation was expressed by the normalized median fluorescence intensity (MFI) of PAC-1 binding with total integrin αIIbβ3, as measured by 2G12 binding. We defined the integrin αIIbβ3 activation index of the sample transfected only with empty vectors as 1, and accordingly calculated integrin αIIbβ3 activation for other transfected samples.
Pulldown and co-immunoprecipitation assays
To evaluate the interactions of PXN and Hic-5 with kindlin and talin, purified GST and GST–PXN/Hic-5 proteins were coupled on glutathione–Sepharose beads and incubated with His-kindlin or His-TH proteins in PBS buffer containing 10% glycerol and 1% BSA overnight at 4°C. After incubation, the beads were washed and the precipitated proteins on the beads were analyzed by SDS-PAGE followed by Coomassie Blue staining or western blotting. To test whether PXN and kindlin can jointly promote the binding of TH to the integrin β CT, glutathione–Sepharose beads coated with GST–β3 CT were incubated with Flag–TH in the presence of either kindlin or PXN, or both; the amount of TH bound to the integrin β3 CT was evaluated by western blotting. For Co-IP assays to test the interaction of PXN and kindlin, Flag–PXN and EGFP–kindlin were transiently co-expressed in CHO cells. The transfected cells were lysed with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail) for 30 min on ice followed by high-speed centrifugation (14,500 g, 15 min). The supernatant was incubated with protein A/G agarose beads coupled with an anti-Flag antibody overnight at 4°C. To evaluate the association of PXN and Hic-5 with kindlin-3 or talin in platelets, washed human platelets, untreated or treated with TRAP-6 (7 μM, 15 min) under non-stirring conditions, were lysed with the lysis buffer (same as above) followed by high-speed centrifugation (14,500 g, 15 min) to remove the insoluble fraction; the supernatant was used for the Co-IP assays.
Proximity ligation assay
Freshly isolated human platelets were allowed to spread on immobilized fibrinogen for 45 min in the presence of TRAP-6 (10 μM), followed by fixation and permeabilization for PLA. In this assay, spread platelets were incubated with mouse anti-Hic-5 monoclonal and rabbit anti-kindlin-3 polyclonal primary antibodies and then stained with species-specific secondary antibodies, each attached to a unique short DNA strand, called PLUS and MINUS Duolink® PLA probes (Sigma-Aldrich), respectively. The proximity of these two probes was explored in three stages: (1) joining two other DNA oligonucleotides complementary for each of the probes into a circle by enzymatic ligation; (2) replicating the circle DNA strand via rolling circle PCR; and (3) highlighting the amplified DNA circles by complementary probes conjugated with a selected fluorescence reagent. Control mouse IgG and rabbit IgG antibodies were used to show the background. Finally, the signal was viewed by confocal microscopy.
Lentiviral transduction and bone marrow transplantation
Kindlin-3fl/flMx1-Cre mice were intraperitoneally injected with poly(I:C) at 6–8 weeks old to induce Mx1-cre-mediated kindlin-3 deletion in hematopoietic cells. Deficiency of kindlin-3 in platelets isolated from poly(I:C)-treated mice was verified by western blotting. Bone marrow cells were collected from these mice for lentiviral transduction to express EGFP–kindlin-3 or the M3 mutant. Finally, lentiviral-infected bone marrow cells were injected into lethally irradiated wild-type C57BL/6 male recipient mice. The transplanted recipients all survived but the non-transplanted control mice died about 2 weeks after irradiation, demonstrating that the transplanted bone marrow cells were functionally dominant in recipient mice. Functional analyses for platelets from the transplanted mice were performed at 6–8 weeks after the transplantation. Lentiviral particles used to express EGFP-fused kindlin-3 or the M3 mutant were prepared in the Viral Laboratory of Blood Center of Wisconsin.
Functional analysis of transplanted mouse platelets
Platelets were isolated from the peripheral blood of bone marrow transplanted mice, then washed and diluted in Tyrode's buffer supplemented with 1 mM Ca2+ and 1 mM Mg2+. To test integrin αIIbβ3 activation, platelets were incubated with Alexa Fluor 647-conjugated fibrinogen for 25 min at room temperature in the absence or presence of either PAR4 agonist peptides (AYPGKF, 150 µM) or CRP (2 μg/ml). The expression of integrin αIIbβ3 was evaluated by a phycoerythrin-conjugated anti-CD41 antibody. After incubation, platelets were fixed with 2% paraformaldehyde for flow cytometry analysis. Platelet spreading was tested on immobilized fibrinogen. To evaluate the interaction of EGFP–kindlin-3 and PXN/Hic-5 in mouse platelets, protein A/G agarose beads pre-coupled with an anti-EGFP antibody were incubated with platelet lysates. Co-precipitation of EGFP–kindlin-3 and PXN/Hic-5 on the beads was quantified by western blotting. Intravital imaging of platelet thrombus formation in mouse cremaster arterioles was performed in the Thrombosis Core at Blood Research Institute of Blood Center of Wisconsin. Mice were anesthetized and the body temperature was maintained at 37°C using a thermoregulated heating pad. The cremaster muscle was exposed and superfused with warm saline (37°C) during the experiment. Arteriolar wall injury was induced with a micropoint laser ablation system (Intelligent Imaging Innovations). Fluorescence images were captured using a high-speed camera (Orca Flash4.0, Hamamatsu). Data were collected for at least 2 min following vessel injury. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Statistics
P values were calculated with a two-tailed Student’s t-test.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: G.C.W.; Methodology: M.L.S.; Validation: J.G.; Formal analysis: J.W., G.C.W., Z.X., Y.-Q.M.; Investigation: J.G., M.H., J.L., K.M., P.S., Z.C., Y.H., Y.Z., M.L.S., C.J., J.W., Z.X., Y.-Q.M.; Writing - original draft: Z.X.; Writing - review & editing: Y.-Q.M.; Supervision: Y.-Q.M.; Project administration: Z.X., Y.-Q.M.; Funding acquisition: Y.-Q.M.
Funding
This work was supported by grants from the National Institutes of Health (HL131654) and the National Natural Science Foundation of China (31370748, 31571177 and 31500618).
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.205641.supplemental
References
- Bialkowska K., Byzova T. V. and Plow E. F. (2015). Site-specific phosphorylation of kindlin-3 protein regulates its capacity to control cellular responses mediated by integrin alphaIIbbeta3. J. Biol. Chem. 290, 6226-6242. 10.1074/jbc.M114.634436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledzka K., Liu J., Xu Z., Perera H. D., Yadav S. P., Bialkowska K., Qin J., Ma Y.-Q. and Plow E. F. (2012). Spatial coordination of kindlin-2 with talin head domain in interaction with integrin beta cytoplasmic tails. J. Biol. Chem. 287, 24585-24594. 10.1074/jbc.M111.336743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledzka K., Bialkowska K., Sossey-Alaoui K., Vaynberg J., Pluskota E., Qin J. and Plow E. F. (2016). Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J. Cell Biol. 213, 97-108. 10.1083/jcb.201501006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaouina M., Goult B. T., Huet-Calderwood C., Bate N., Brahme N. N., Barsukov I. L., Critchley D. R. and Calderwood D. A. (2012). A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated alphaIIbbeta3 integrin coactivation. J. Biol. Chem. 287, 6979-6990. 10.1074/jbc.M111.330845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahme N. N., Harburger D. S., Kemp-O'Brien K., Stewart R., Raghavan S., Parsons M. and Calderwood D. A. (2013). Kindlin binds migfilin tandem LIM domains and regulates migfilin focal adhesion localization and recruitment dynamics. J. Biol. Chem. 288, 35604-35616. 10.1074/jbc.M113.483016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C. and Turner C. E. (2004). Paxillin: adapting to change. Physiol. Rev. 84, 1315-1339. 10.1152/physrev.00002.2004 [DOI] [PubMed] [Google Scholar]
- Calderwood D. A. (2004). Talin controls integrin activation. Biochem. Soc. Trans. 32, 434-437. 10.1042/bst0320434 [DOI] [PubMed] [Google Scholar]
- Campbell I. D. and Ginsberg M. H. (2004). The talin-tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 29, 429-435. 10.1016/j.tibs.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Dai A., Ye F., Taylor D. W., Hu G., Ginsberg M. H. and Taylor K. A. (2015). The structure of a full-length membrane-embedded integrin bound to a physiological ligand. J. Biol. Chem. 290, 27168-27175. 10.1074/jbc.M115.682377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. J., Gibbs E., Russell M., Goldman D., Minarcik J., Golden J. A. and Feldman E. L. (2008). Kindlin-2 Is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ. Res. 102, 423-431. 10.1161/CIRCRESAHA.107.161489 [DOI] [PubMed] [Google Scholar]
- Feng C., Li Y.-F., Yau Y.-H., Lee H.-S., Tang X.-Y., Xue Z.-H., Zhou Y.-C., Lim W.-M., Cornvik T. C., Ruedl C. et al. (2012). Kindlin-3 mediates integrin alphaLbeta2 outside-in signaling, and it interacts with scaffold protein receptor for activated-C kinase 1 (RACK1). J. Biol. Chem. 287, 10714-10726. 10.1074/jbc.M111.299594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Bledzka K., Yang J., Perera H. D., Plow E. F. and Qin J. (2014). Molecular basis of kindlin-2 binding to integrin-linked kinase pseudokinase for regulating cell adhesion. J. Biol. Chem. 289, 28363-28375. 10.1074/jbc.M114.596692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Partridge A. and Shattil S. J. (2005). Integrin regulation. Curr. Opin. Cell Biol. 17, 509-516. 10.1016/j.ceb.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Goult B. T., Bouaouina M., Harburger D. S., Bate N., Patel B., Anthis N. J., Campbell I. D., Calderwood D. A., Barsukov I. L., Roberts G. C. et al. (2009). The structure of the N-terminus of kindlin-1: a domain important for alphaiibbeta3 integrin activation. J. Mol. Biol. 394, 944-956. 10.1016/j.jmb.2009.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goult B. T., Bouaouina M., Elliott P. R., Bate N., Patel B., Gingras A. R., Grossmann J. G., Roberts G. C. K., Calderwood D. A., Critchley D. R. et al. (2010). Structure of a double ubiquitin-like domain in the talin head: a role in integrin activation. EMBO J. 29, 1069-1080. 10.1038/emboj.2010.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger D. S., Bouaouina M. and Calderwood D. A. (2009). Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284, 11485-11497. 10.1074/jbc.M809233200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R., Stanley P., Chakravarty P. and Hogg N. (2013). The kindlin 3 pleckstrin homology domain has an essential role in lymphocyte function-associated antigen 1 (LFA-1) integrin-mediated B cell adhesion and migration. J. Biol. Chem. 288, 14852-14862. 10.1074/jbc.M112.434621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Shirotani-Ikejima H., Tadokoro S., Maeda Y., Kinoshita T., Tomiyama Y. and Miyata T. (2009). Integrin-linked kinase associated with integrin activation. Blood 113, 5304-5313. 10.1182/blood-2008-07-169136 [DOI] [PubMed] [Google Scholar]
- Huet-Calderwood C., Brahme N. N., Kumar N., Stiegler A. L., Raghavan S., Boggon T. J. and Calderwood D. A. (2014). Differences in binding to the ILK complex determines kindlin isoform adhesion localization and integrin activation. J. Cell Sci. 127, 4308-4321. 10.1242/jcs.155879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Jobard F., Bouadjar B., Caux F., Hadj-Rabia S., Has C., Matsuda F., Weissenbach J., Lathrop M., Prud'homme J. F. and Fischer J. (2003). Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum. Mol. Genet. 12, 925-935. 10.1093/hmg/ddg097 [DOI] [PubMed] [Google Scholar]
- Kasirer-Friede A., Moran B., Nagrampa-Orje J., Swanson K., Ruggeri Z. M., Schraven B., Neel B. G., Koretzky G. and Shattil S. J. (2007). ADAP is required for normal alphaIIbbeta3 activation by VWF/GP Ib-IX-V and other agonists. Blood 109, 1018-1025. 10.1182/blood-2006-05-022301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasirer-Friede A., Kang J., Kahner B., Ye F., Ginsberg M. H. and Shattil S. J. (2014). ADAP interactions with talin and kindlin promote platelet integrin alphaIIbbeta3 activation and stable fibrinogen binding. Blood 123, 3156-3165. 10.1182/blood-2013-08-520627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Barazia A. and Cho J. (2013). Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus Formation in live mice. J. Vis. Exp. 74, e50329 10.3791/50329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Kaneyama J.-R., Miyauchi A., Lei X.-F., Arita S., Mino T., Takeda N., Kou K., Eto K., Yoshida T., Miyazaki T. et al. (2012). Identification of Hic-5 as a novel regulatory factor for integrin alphaIIbbeta3 activation and platelet aggregation in mice. J. Thromb. Haemost. 10, 1867-1874. 10.1111/j.1538-7836.2012.04856.x [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., van de Vijver E., Weterman M. A., de Boer M., Tool A. T. J., van den Berg T. K., Moser M., Jakobs M. E., Seeger K., Sanal O. et al. (2008). LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113, 4740-4746. 10.1182/blood-2008-10-182154 [DOI] [PubMed] [Google Scholar]
- Larjava H., Plow E. F. and Wu C. (2008). Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203-1208. 10.1038/embor.2008.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Deng Y., Sun K., Yang H., Liu J., Wang M., Zhang Z., Lin J., Wu C., Wei Z. et al. (2017). Structural basis of kindlin-mediated integrin recognition and activation. Proc. Natl. Acad. Sci. USA 114, 9349-9354. 10.1073/pnas.1703064114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Calderwood D. A. and Ginsberg M. H. (2000). Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563-3571. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhu Y., Gao J., Yu F., Dong A. and Shen W.-H. (2009). Molecular and reverse genetic characterization of NUCLEOSOME ASSEMBLY PROTEIN1 (NAP1) genes unravels their function in transcription and nucleotide excision repair in Arabidopsis thaliana. Plant J. 59, 27-38. 10.1111/j.1365-313X.2009.03844.x [DOI] [PubMed] [Google Scholar]
- Liu J., Fukuda K., Xu Z., Ma Y.-Q., Hirbawi J., Mao X., Wu C., Plow E. F. and Qin J. (2011). Structural basis of phosphoinositide binding to Kindlin-2 pleckstrin homology domain in regulating integrin activation. J. Biol. Chem. 286, 43334-43342. 10.1074/jbc.M111.295352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhu Y., Ye S. and Zhang R. (2012). Crystal structure of kindlin-2 PH domain reveals a conformational transition for its membrane anchoring and regulation of integrin activation. Protein Cell 3, 434-440. 10.1007/s13238-012-2046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Lu D., Wang X., Wan J., Liu C. and Zhang H. (2015). Kindlin-2 phosphorylation by Src at Y193 enhances Src activity and is involved in Migfilin recruitment to the focal adhesions. FEBS 589, 2001-2010. 10.1016/j.febslet.2015.05.038 [DOI] [PubMed] [Google Scholar]
- Ma Y.-Q., Yang J., Pesho M. M., Vinogradova O., Qin J. and Plow E. F. (2006). Regulation of integrin alpha(IIb)beta(3) activation by distinct regions of its cytoplasmic tails. Biochemistry 45, 6656-6662. 10.1021/bi060279h [DOI] [PubMed] [Google Scholar]
- Ma Y.-Q., Qin J. and Plow E. F. (2007). Platelet integrin alpha(IIb)beta(3): activation mechanisms. J. Thromb. Haemost 5, 1345-1352. 10.1111/j.1538-7836.2007.02537.x [DOI] [PubMed] [Google Scholar]
- Ma Y.-Q., Qin J., Wu C. and Plow E. F. (2008). Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J. Cell Biol. 181, 439-446. 10.1083/jcb.200710196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin N. L., Zhang L., Choi J., Ciocea A., Razorenova O., Ma Y.-Q., Podrez E. A., Tosi M., Lennon D. P., Caplin A. I. et al. (2009). A point mutation in kindlin-3 ablates activation of three integrin subfamilies in humans. Nat. Med. 15, 313-318. 10.1038/nm.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E., Ussar S., Schifferer M., Bosl M., Zent R., Moser M. and Fassler R. (2008). Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325-1330. 10.1101/gad.469408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M. and Fässler R. (2008). Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325-330. 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- Moser M., Bauer M., Schmid S., Ruppert R., Schmidt S., Sixt M., Wang H.-V., Sperandio M. and Fässler R. (2009a). Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15, 300-305. 10.1038/nm.1921 [DOI] [PubMed] [Google Scholar]
- Moser M., Legate K. R., Zent R. and Fassler R. (2009b). The tail of integrins, talin, and kindlins. Science 324, 895-899. 10.1126/science.1163865 [DOI] [PubMed] [Google Scholar]
- Perera H. D., Ma Y.-Q., Yang J., Hirbawi J., Plow E. F. and Qin J. (2011). Membrane binding of the N-terminal ubiquitin-like domain of kindlin-2 is crucial for its regulation of integrin activation. Structure 19, 1664-1671. 10.1016/j.str.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Qin J. and Byzova T. (2009). Kindling the flame of integrin activation and function with kindlins. Curr. Opin Hematol. 16, 323-328. 10.1097/MOH.0b013e32832ea389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M., Thielmann I., Nieswandt B. and Stegner D. (2015). Normal platelet integrin function in mice lacking hydrogen peroxide-induced clone-5 (Hic-5). PLoS ONE 10, e0133429 10.1371/journal.pone.0133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Vinogradova O. and Plow E. F. (2004). Integrin bidirectional signaling: a molecular view. PLoS. Biol. 2, e169 10.1371/journal.pbio.0020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Tu Y., Guan J.-L., Xiao G. and Wu C. (2014). Kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch in the integrin outside-in signaling circuit. J. Biol. Chem. 289, 31001-31013. 10.1074/jbc.M114.580811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore V. B., Okada M., Newman P. J. and Newman D. K. (2007). Paxillin family members function as Csk-binding proteins that regulate Lyn activity in human and murine platelets. Biochem. J. 403, 275-281. 10.1042/BJ20061618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E., Ruppert R. and Fässler R. (2016). The kindlin family: functions, signaling properties and implications for human disease. J. Cell Sci. 129, 17-27. 10.1242/jcs.161190 [DOI] [PubMed] [Google Scholar]
- Sakata A., Ohmori T., Nishimura S., Suzuki H., Madoiwa S., Mimuro J., Kario K. and Sakata Y. (2014). Paxillin is an intrinsic negative regulator of platelet activation in mice. Thromb. J. 12, 1 10.1186/1477-9560-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R., Brunkhorst B., Pisick E., Li J. L., Lo S. H., Chen L. B. and Griffin J. D. (1995). Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL. Oncogene 11, 1149-1155. [PubMed] [Google Scholar]
- Shi X., Ma Y.-Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F. and Wu C. (2007). The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455-20466. 10.1074/jbc.M611680200 [DOI] [PubMed] [Google Scholar]
- Siegel D. H., Ashton G. H. S., Penagos H. G., Lee J. V., Feiler H. S., Wilhelmsen K. C., South A. P., Smith F. J. D., Prescott A. R., Wessagowit V. et al. (2003). Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am. J. Hum. Genet. 73, 174-187. 10.1086/376609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Howarth K., McDowall A., Patzak I., Evans R., Ussar S., Moser M., Metin A., Fried M., Tomlinson I. et al. (2009). Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat. Med. 15, 306-312. 10.1038/nm.1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H. and Calderwood D. A. (2003). Talin binding to integrin tails: a final common step in integrin activation. Science 302, 103-106. 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- Theodosiou M., Widmaier M., Böttcher R. T., Rognoni E., Veelders M., Bharadwaj M., Lambacher A., Austen K., Müller D. J., Zent R. et al. (2016). Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife 5, e10130 10.7554/eLife.10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Wu S., Shi X., Chen K. and Wu C. (2003). Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37-47. 10.1016/S0092-8674(03)00163-6 [DOI] [PubMed] [Google Scholar]
- Ussar S., Wang H.-V., Linder S., Fässler R. and Moser M. (2006). The Kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142-3151. 10.1016/j.yexcr.2006.06.030 [DOI] [PubMed] [Google Scholar]
- Ussar S., Moser M., Widmaier M., Rognoni E., Harrer C., Genzel-Boroviczeny O. and Fässler R. (2008). Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS. Genet 4, e1000289 10.1371/journal.pgen.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova O., Velyvis A., Velyviene A., Hu B., Haas T. A., Plow E. F. and Qin J. (2002). A structural mechanism of integrin àIIbá3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110, 587-597. 10.1016/S0092-8674(02)00906-6 [DOI] [PubMed] [Google Scholar]
- Wegener K. L., Partridge A. W., Han J., Pickford A. R., Liddington R. C., Ginsberg M. H. and Campbell I. D. (2007). Structural basis of integrin activation by talin. Cell 128, 171-182. 10.1016/j.cell.2006.10.048 [DOI] [PubMed] [Google Scholar]
- Xu Z., Gao J., Hong J. and Ma Y.-Q. (2013). Integrity of kindlin-2 FERM subdomains is required for supporting integrin activation. Biochem. Biophys. Res. Commun. 434, 382-387. 10.1016/j.bbrc.2013.03.086 [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen X., Zhi H., Gao J., Bialkowska K., Byzova T. V., Pluskota E., White G. C., Liu J., Plow E. F. et al. (2014). Direct interaction of kindlin-3 with integrin alphaIIbbeta3 in platelets is required for supporting arterial thrombosis in mice. Arterioscler. Thromb. Vasc. Biol. 34, 1961-1967. 10.1161/ATVBAHA.114.303851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Cai J., Gao J., White G. C., Chen F. and Ma Y.-Q. (2015). Interaction of kindlin-3 and beta2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood 126, 373-377. 10.1182/blood-2015-03-636720 [DOI] [PubMed] [Google Scholar]
- Yan B., Calderwood D. A., Yaspan B. and Ginsberg M. H. (2001). Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 276, 28164-28170. 10.1074/jbc.M104161200 [DOI] [PubMed] [Google Scholar]
- Yang J., Ma Y.-Q., Page R. C., Misra S., Plow E. F. and Qin J. (2009). Structure of an integrin alphaIIb beta3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc. Natl. Acad. Sci. USA 106, 17729-17734. 10.1073/pnas.0909589106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. A., Füzéry A. K., Bonet R., Campbell I. D. and Gilbert R. J. C. (2012a). Biophysical analysis of Kindlin-3 reveals an elongated conformation and maps integrin binding to the membrane-distal beta-subunit NPXY motif. J. Biol. Chem. 287, 37715-37731. 10.1074/jbc.M112.415208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. A., Lumb C. N., Brahme N. N., Zalyte R., Bird L. E., De C. L., Owens R. J., Calderwood D. A., Sansom M. S. P. and Gilbert R. J. C. (2012b). Structural and functional characterisation of the kindlin-1 pleckstrin homology domain. J. Biol. Chem. 287, 43246-43261. 10.1074/jbc.M112.422089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Petrich B. G., Anekal P., Lefort C. T., Kasirer-Friede A., Shattil S. J., Ruppert R., Moser M., Fassler R. and Ginsberg M. H. (2013). The mechanism of kindlin-mediated activation of integrin alphaIIbbeta3. Curr. Biol. 23, 2288-2295. 10.1016/j.cub.2013.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Luo B.-H., Barth P., Schonbrun J., Baker D. and Springer T. A. (2009). The structure of a receptor with two associating transmembrane domains on the cell surface: integrin alphaIIbbeta3. Mol. Cell 34, 234-249. 10.1016/j.molcel.2009.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.