Abstract
For adequate provision of preventive services, there is an interplay between activities at the healthcare practice, healthcare provider, and patient levels of the clinical encounter. Commonly used health promotion and behavior theoretical models address some of these three levels, but none fully account for all three. Building off of key components of many existing theoretical models, including the Health Belief Model, Theory of Planned Behavior/Theory of Reasoned Action, Social Cognitive Theory, Social Ecological Model, and the Systems Model of Clinical Preventive Care, we describe the development of the P3 (Practice-, Provider-, and Patient-level) Model for preventive care interventions. The P3 Model accounts for all three levels of the clinical encounter, and the factors that impact these levels, concurrently. This yields a model for preventive care that is applicable and adaptable to different settings, and that provides a framework for the development, implementation, and evaluation of preventive care promotion interventions. The applicability of the P3 Model is shown through two exemplar preventive care programs – immunization and colorectal cancer screening. The P3 Model allows interventions to be developed and evaluated in a modular approach, to allow more practical refinement and optimization of the intervention.
Keywords: Health promotion, Behavior change, Behavior change theory, Cancer prevention and control, Immunization
1. Introduction
Preventive care utilization is often suboptimal. For example, some safe and effective vaccines to prevent infectious diseases are underutilized. In the United States' (US) -2016 influenza season, only 59% of children 6 months to 17 years and 42% of adults 18 years and older received influenza vaccine (Centers for Disease Control and Prevention, 2016a). As of 2015, 42% of adolescent girls and 28% of adolescent boys were vaccinated against human papillomavirus (HPV) (Reagan-Steiner et al., 2016). These vaccine coverage levels stand in contrast to Healthy People 2020 goals (70% for influenza vaccine and 80% for human papillomavirus vaccine coverage) (US Department of Health and Human Services, 2017a).
Other preventive measures, including cancer screening, are also underused. For example, while colorectal cancer screening (a Healthy People 2020 leading health indicator) increased from 52% in 2008 (US Department of Health and Human Services, 2017b) to 65% in 2012 (Centers for Disease Control and Prevention, 2013), this is still below the Healthy People 2020 goal of screening of 70% of eligible patients (US Department of Health and Human Services, 2017b).
In the US, there are over 30,000 incident cancers attributed to HPV (Viens et al., 2016), between 3000 and 49,000 influenza-related deaths (Centers for Disease Control and Prevention, 2010), and 136,000 incident cases of colorectal cancer and 51,800 colorectal cancer deaths (Centers for Disease Control and Prevention, 2016b) annually. Worldwide, there were 610,000 incident cancers attributable to HPV in 2008 (Forman et al., 2012), and annually, there are 1.4 million incident cases of colorectal cancer and 693,900 colorectal cancer deaths (Favoriti et al., 2016). Given suboptimal prevention utilization, we are missing the opportunity to reduce morbidity and mortality from these preventable diseases.
Health promotion and behavioral science theories provide frameworks for understanding human behavior regarding preventive care. However, these theories have not always provided a comprehensive path to address the complex interaction of key players and settings involved in preventive care provision nor a clear path for practical adoption in the clinical setting.
2. Review of existing theoretical models
In our experience developing interventions for promotion of preventive health behaviors, we have identified four key limitations of commonly used theoretical models that may impact their practical application in an ever-evolving medical and preventive care landscape. A summary of these four conditions is presented here, with a detailed description of specific models and their potential limitations immediately following this summary.
First, some models (e.g. Health Belief Model [HBM], Theory of Reasoned Action [TRA], Theory of Planned Behavior [TPB]) focus on individual-level constructs at the expense of a comprehensive evaluation of the interaction between the individual patient and the broader components of the healthcare system (Crosby et al., 2013; Salazar et al., 2013). Second, some models (e.g. Shared Decision Making [SDM]) that address patient-physician communication focus on the actual communication processes and not on affecting the broader healthcare environment, and often focus on treatment modalities (e.g. cancer treatments) rather than prevention (Charles et al., 1999; Elwyn et al., 2012). Third, some models that address the interaction between the patient and other components of the healthcare system and the environment (e.g. Social Cognitive Theory [SCT], Bronfenbrenner's Model of Human Development) do not sufficiently account for the breadth of impacting factors on these components and their interactions (Bandura, 1986; Bronfenbrenner, 1979). Finally, some models that attempt to clarify the interactions between the patient and the healthcare environment (e.g. Systems Model of Clinical Preventive Care) do not specifically address non-clinician components (e.g. administrative staff; systems-level feedback and reminder/recall systems) of the practice-level healthcare environment (Walsh and McPhee, 1992).
A detailed examination of the limitations of these models is presented below. Using key components from a variety of health behavior theoretical models, we have created the P3 (Practice-, Provider-, Patient-Level) Model for conceptualizing, developing, and testing interventions to improve preventive care utilization. A summary of these models, and their connection to constructs at the practice-, provider-, and patient-levels is shown in Table 1.
Table 1.
Practice-, Provider-, and/or Patient-specific levels in theoretical models assessed in developing the P3 Model.
| Practice-level | Provider-level | Patient-level | |
|---|---|---|---|
| Health Belief Model | X | ||
| Theory of Planned Behavior | X | ||
| Social Ecological Model | Xa | X | X |
| Social Cognitive Theory | X | X | |
| Shared Decision Making | X | X | |
| Systems Model of Clinical Preventive Care | X | X |
The concept of healthcare practice-specific factors is not directly addressed, rather this model considers ecological and environmental constructs.
The Health Belief Model (HBM) is one of the most widely recognized and used models to address patient-level prevention activities. The HBM focuses on an individual's perceptions of susceptibility to and severity of disease along with perceived barriers and self-efficacy related to utilizing the preventive service. The HBM includes modifying factors (e.g. sociodemographics, knowledge) that can impact these beliefs and perceptions, and cues to action (e.g. provider recommendation) that serve to advance acceptance of the preventive service (Dempsey et al., 2015; Mullen et al., 1995). However, the delivery of these cues to action may be equivocal rather than presenting straightforward recommendations, and these situations may be based on healthcare providers' perceptions of their patient's susceptibility to disease. With patients increasingly seeking collaborative healthcare decision making discussions (Lipstein et al., 2012), interactions between patient- and provider-level assessments of HBM constructs need to be explicitly included in health behavior theoretical models. For example, in the case of HPV vaccination, health care encounter recordings have documented providers not recommending HPV vaccination with the same directness and emphasis as for other routinely recommended adolescent vaccines (Clark and Kuter, 2014a; Dempsey et al., 2016). This may be due in part to some providers perceiving that 11- or 12-year-old patients are not at risk for a sexually transmitted virus, and therefore not in need of HPV vaccination (Perkins et al., 2014).
When compared to the HBM, the Theory of Planned Behavior (TPB) incorporates and attempts to address a wider variety of underlying beliefs that may affect attitudes, subjective norms, and perceived behavioral control. While this theory expands on the constructs in the Health Belief Model, it may have the same main limitation, namely that it is focused on individual patients' attitudes, beliefs, and perceptions, and do not fully incorporate external influences (Albert et al., 2012; Clark and Kuter, 2014b). The inclusion of social and subjective norms is a positive step in terms of addressing influencing factors, but is limited by focusing on the impact of those norms on the patient's intention to utilize the preventive behavior. The TPB does not account for the impact these external factors may also have on providers and practice staff (and the subsequent interactions they may have with patients). One notable addition to the TPB, relative to the HBM, is the concept of control over the decision to undertake the action. While individual-level control towards taking a preventive action is important, patients who choose to control the decision to not take the action (e.g. choose to exert decisional control to not receive a vaccine or undergo a screening) may need additional counseling from healthcare providers who strongly recommend the preventive action.
Communication between patients and healthcare providers is a key component of the clinical encounter. Over time, there has been a shift from a more paternalistic view of physician recommendations to the concept of shared decision making. While models have been posited to describe the methods for patient-physician communication under the shared-decision making (SDM) process (Charles et al., 1999; Elwyn et al., 2012), these models look at the point in time for the conversation, without addressing the broader environment, including other communication within the healthcare practice (e.g. intra-practice staff communication). Additionally, SDM models primarily focus on treatment modalities and the decision between two or more treatment options (e.g. different forms of chemotherapy for cancer treatment, each with their own benefits and risks). This type of discussion is not always amenable to preventive care, which is typically promoting a single action (e.g. a vaccination, a screening test) recommended for broad cross-sections of the population – if not the whole population. While treatment-based discussions may require a more collaborative SDM approach, SDM-based communication may not be as applicable for routine preventive services. Recent studies have shown that use of a presumptive communication style, where the activity is presented as the expected default, is effective in promoting vaccine uptake (Opel et al., 2013; Opel et al., 2015).
Social-ecological models, particularly Bronfenbrenner's Model of Human Development (Bronfenbrenner, 1979), highlight how individual health decisions exist within an ever-widening sphere of influence, from individual, interpersonal, organization, community, and policy levels. However, in its attempt to be comprehensive, Bronfenbrenner's model poses some issues in terms of clear adaptation for use, implementation, and analysis. For example, specific factors that may impact individual levels in the model or the interactions between levels are not clearly elucidated. Without an assessment of potential modifiable characteristics addressing these levels, it may be difficult to use this model for detailing specific intervention activities to improve preventive care. Additionally, the more distal components of Bronfenbrenner's model (e.g. political or policy related components) may not provide a clear means for intervention, even though their effects are often felt proximally. However, some of these distal policy issues may affect proximal recommendations for preventive care. For example, health plan-based incentives for meeting specific performance goals (Borenstein et al., 2004) can be tied into routine assessment and feedback processes at the level of the individual healthcare practice (Gilkey et al., 2014; Lebaron et al., 1999), and state-level policies regarding use of reporting systems (Centers for Disease Control and Prevention, 2015) can affect data quality and reporting for these feedback processes. This highlights the need for a model that accounts for all addressable components existing within the interaction of the multiple layers of a social-ecological model framework.
Social Cognitive Theory (Bandura, 1986) addresses the interaction between an individual and their environment, through a triadic reciprocal determinism (e.g. bidirectional effects between the individual, their environment, and the behavior under study) including factors related to the person, the behavior, and the environment. In addition to this reciprocity, Social Cognitive Theory is constructed upon individual-level concepts of outcome expectations, self-efficacy, incentive motivation, self-regulation, and moral disengagement, along with environment-level concepts of collective efficacy, observational learning, and facilitation (Bandura, 1986). Similar to the limitations described with regard to the HBM and TPB, there is not a clear role in Social Cognitive Theory for the physician encounter with the patient. These encounters may touch upon a number of Social Cognitive Theory concepts, while falling within the “environment” component of the reciprocal triad. This can be problematic as there would be no clearly delineated provider-level factors upon which to base intervention (e.g. provider assessment of susceptibility that may impact their decision to strongly recommend a preventive service).
The final model considered here is the Systems Model of Clinical Preventive Care (Walsh and McPhee, 1992). This model attempts to address numerous gaps identified across the preventive care continuum of patients and providers by identifying multiple factors (i.e. enabling, predisposing, reinforcing, situational, preventive activity, and healthcare delivery/organizational) that act on the patient and provider concomitantly. The Systems Model of Clinical Preventive Care provides a framework for accounting for this complex web of interactions and factors, and offers a clear set of considerations for developing, implementing, and evaluating preventive care interventions. However, this model does not fully incorporate healthcare practice-specific constructs that may impact recommendation or utilization of preventive care (e.g. systems-level reminders, appropriate use of electronic medical records and/or immunization information systems). These higher-level systems, which are not specific to a given provider within a clinic, need to be appropriately accounted for and utilized in the course of preventive care provision.
3. Methods for development of the P3 Model
As part of prior and ongoing vaccine acceptance and uptake studies, our team has developed the P3 Model, addressing the Practice-, Provider-, and Patient-level components involved in preventive care for vaccine promotion. This model takes important factors from the previously described models and incorporates additional factors to address the gaps and limitations of these models. Here, we describe the development of the P3 Model, showing how it addresses gaps left by other health promotion and behavior models, and how it can be adopted and adapted for other preventive care activities.
3.1. Provider- and Patient-level model components
The Systems Model of Clinical Preventive Care offers a clear path forward to concurrently address factors acting at the Provider- and Patient-levels of the clinical encounter. While the HBM and TPB are typically considered in the context of an individual patient's beliefs (Bronfenbrenner, 1979; Dempsey et al., 2015), interaction between providers and patients with regard to health belief constructs of the HBM and TPB is commonly used, if not always commonly recognized. For example, a clinician taking a family history from their 45-year-old patient may identify a history of colorectal cancer, which may indicate earlier initiation of screening (American Cancer Society, 2017) than generally recommended at 50 years of age (US Preventive Services Task Force, 2016). While this is considered standard clinical practice, it involves a provider basing their recommendation on their perception of the patient's susceptibility to disease and potential severity of disease.
On the other hand, for adolescent human papillomavirus (HPV) vaccination, providers' perceptions of disease susceptibility and severity may negatively impact their ability to provide a strong recommendation for vaccination (Clark and Kuter, 2014b). Typically, pediatricians do not see cervical or other anogenital cancer cases, and for providers who have cared for an adolescent patient since they were an infant, it may be difficult to envision their 11 or 12-year-old patient at risk for a sexually transmitted virus or the long-term cancers caused by this virus. This is compounded by parents having similar concerns envisioning their child being sexually active (Perkins et al., 2014). This interconnectedness between parents' and providers' attitudes around sexual risk may be a contributor to suboptimal provider recommendations for adolescent HPV vaccination (Perkins et al., 2014).
One of the major benefits of the Systems Model of Clinical Preventive Care is the assessment of factors acting independently as well as concomitantly on both the provider and the patient. From the standpoint of vaccination behaviors, the clearest use of the Systems Model of Clinical Preventive Care, as currently conceived, is to apply the constructs of the Health Belief Model to both the provider and the patient. It is not merely enough to rely on individual's perceived susceptibility or severity for a given infectious disease; providers now are basing their recommendation patterns on perceptions of disease susceptibility or severity for their patients and the broader patient population they serve.
Given this, it is important to place existing frameworks of Provider-Patient interaction in the context of the reciprocal triadic causation of Social Cognitive Theory. The bidirectional nature of interactions between the provider and patient should be considered, particularly with regard to communication styles between these individuals. Understanding differences between paternalistic and SDM communication styles (Charles et al., 1999; Elwyn et al., 2012; Opel et al., 2013; Opel et al., 2015), and tailoring these styles to the communication needs of both parties is critical, and must be included as a separate component of any model addressing provider-patient interactions.
In a proof of concept study, our team tested the effectiveness of a practice-, provider-, and patient-intervention to improve antenatal influenza and Tdap vaccination in the obstetric setting (Chamberlain et al., 2015). Provider and patient-level components in this study included vaccine champions, posters, brochures, lapel buttons, provider talking points, an electronic tablet-based educational tutorial for patients, and peer-to-peer education for providers (Chamberlain et al., 2015). Despite non-significant differences in vaccine uptake, our results demonstrated enough promise to further develop and refine the P3 concept and intervention package. The majority of physical package components were positively associated with antenatal vaccine receipt, and from a patient awareness standpoint, there were signals that the educational messages were impactful. For example, intervention group women in their third trimester were more likely than similar non-intervention group women to request that their family members consider vaccination to benefit the infant (36% vs. 22%; risk ratio [RR] = 1.65, 95% confidence interval [CI]: 1.21, 2.26). Taking the results and feedback obtained from this pilot trial, we are further developing and fine turning this multi-component intervention approach for preventive care services.
3.2. Incorporating Practice-level components
Mullen et al. (1995) describe the concept of “setting” as an important component of health education for preventive care, citing the health care delivery system/organizational factors of Walsh and McPhee (1992). They acknowledge that the organizational and communication processes within healthcare offices and community settings are rarely addressed. We agree with this, and believe this needs to be extended not just to considering these factors, but incorporating “setting” as a separate intervention point. To this end, Practice has a distinct role in the P3 Model, with Practice-level components impacted by not just health care delivery system/organizational factors, but also reinforcing factors, situational factors, and cues to action.
Accounting for the Practice-level environment, and all of the factors that impact it, is supported by recommendations from the Community Services Task Force (The Community Guide, 2012; The Community Guide, 2013). For both cancer screening and vaccination (i.e. exemplar preventive services described here), there are a number of Community Guide recommendations related to the Practice-level. The Community Services Task Force recommends 13 of 18 general vaccination recommendations it considered; 6 of these 13 (Client Reminder and Recall Systems; Provider Assessment and Feedback; Provider Reminders; Standing Orders; Health Care System-Based Interventions Implemented in Combination; Immunization Information Systems) are based on Practice-level activities (The Community Guide, 2013). Additionally, 8 of 12 cancer screening recommendations considered by the Community Services Task Force are recommended for at least one type of cancer screening (i.e. breast, cervical, or colorectal); 4 of these 8 (Client Reminders; Reducing Structural Barriers; Provider Assessment and Feedback; Provider Reminder and Recall Systems) are based on Practice-level activities (The Community Guide, 2012) (Appendix Table 1).
Considering these Community Services Task Force recommendations, there are clear areas of focus for Practice-level activities across preventive services, including client and provider reminder systems and assessment and feedback activities. Additional practice-level components (e.g. establishment of a prevention champion, use of standing orders) have not been recommended across different modalities of prevention by the Community Services Task Force, but have been shown to be effective when incorporated into clinical practice (Baker et al., 2014). Adaptation of Practice-level activities for specific preventive services will need to be evaluated on a service-by-service basis to ensure applicability.
4. Results
4.1. The P3 Model
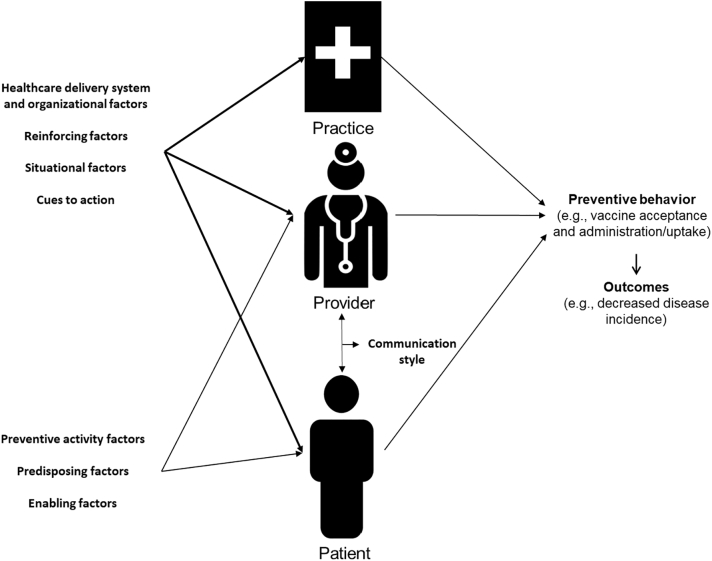
The P3 Model is presented in Fig. 1. The Practice-, Provider-, and Patient-level components are all presented independently, with some impacting factors (e.g. healthcare delivery system and organization; reinforcing; situational; cues to action) applicable to all three levels and some factors applicable to the Provider- and Patient-levels only (e.g. preventive activity attitudes and beliefs, and predisposing and enabling factors). Examples, and related implementation activities or considerations (in the case of predisposing factors), are presented in Table 2.
Fig. 1.
Graphical representation of the P3 (Practice-, Provider, and Patient-Level) model with identification of impacting factors (detailed in Table 2) and the levels they act on.
Table 2.
Factors impacting the Practice-, Provider-, and Patient-levels of the P3 Model, with related implementation considerations.
| Factor | Practice-level considerations | Provider-level considerations | Patient-level considerations |
|---|---|---|---|
| Healthcare delivery/organizational | Use of standing orders Vaccination promotion by all staff Prevention/Immunization champion Vaccine supply |
Access to care Availability of technology and personnel Organizational priorities Structure of office practice Reimbursement Coordination with community resources |
Access to care Coordination of resources |
| Reinforcing | Communication regarding practice vaccination policies “Culture of prevention” |
Patient satisfaction Support/approval of peers Case finding |
Social support/approval Inherent reinforcement value of the preventive activity |
| Situational | Flexibility to adapt to unscheduled/acute care visits for prevention promotion | Internal clues (e.g. symptom presentation) | Internal clues (e.g. symptom presentation) |
| Cues to action | Electronic medical record/IIS prompts Preventive service delivery rate feedback to practice |
Triggers to health behavior External clues (e.g. physician reminders) |
Triggers to health behavior External clues (e.g. physician reminders) |
| Preventive activity | N/A | Costs | Costs |
| Risks | Risks | ||
| Efficacy | Efficacy | ||
| Effectiveness | Effectiveness | ||
| Predisposing | N/A | Demographics (e.g. gender, race, ethnicity, language) Beliefs Attitudes Prior clinical experiences Personal health practices |
Demographics (e.g. gender, race, ethnicity, language) Health beliefs Attitudes Expectations Motivation Self-efficacy Health value orientation |
| Enabling | N/A | Training Technical expertise Knowledge Logistical factors Availability of materials |
Education Health knowledge Skills Income Logistical factors Physiologic factors |
| Communication style | N/A | Effective bidirectional provision and reception of information | |
The core factors in Table 2 are based on the Systems Model of Clinical Preventive Care, while the detailed list of considerations come from standard literature around considerations for clinical and preventive services research. The P3 Model is flexible and gives a systematic way to assess needs for the development, implementation, and evaluation of multi-level preventive care interventions. The various constructs and considerations can be operationalized in conjunction with one another, however, not all factors and constructs may be necessary in every approach.
Notably, the P3 Model allows for inclusion of factors at levels that may not be addressed fully through standard health promotion and behavioral models. For example, healthcare providers may hold beliefs and attitudes that a particular patient may not be at risk of acquiring HPV, either due to age or perceived risk of sexual activity (Perkins et al., 2014; Hughes et al., 2011). With the focus of models such as HBM and TPB on the attitudes and beliefs of the patient themselves, there is a missed opportunity for intervention on the part of healthcare provider's attitudes. Alternatively, some screening tests (e.g. colorectal cancer screening, mammography) may be conducted too frequently, relative to established recommendations, based on providers' standard of clinical practice (Bellizzi et al., 2011; Haas et al., 2016).
5. Discussion
5.1. Applying the P3 Model to preventive services
The P3 Model has its greatest utility in defining practical activities to undertake and evaluate as part of preventive care interventions. Based on the layout of the P3 Model (Fig. 1, Table 2), we have identified key activities applicable to immunizations and colorectal cancer screening (Table 3) interventions conducted under the P3 Model framework. While not an exhaustive list, these include: (a) best practices to identify patients needing the preventive service; (b) provider assessment and feedback; (c) using standing orders (which authorize nurses, pharmacists, and other trained healthcare personnel to administer vaccinations according to a protocol approved by an institution or physician); (d) establishing a prevention/immunization champion in the practice; (e) consistent preventive activity promotion and messaging across all points of patient contact during the clinical encounter; (f) relevant training for clinic staff; and (g) patient education programs tailored to the specific population (e.g. using print, audio-visual, or interactive tablet-based education, alone or in combination) (Table 3). We provide these examples as a guide for implementing interventions within the P3 Model, while highlighting the flexibility of this model to accommodate activities or materials most relevant to the population being served.
Table 3.
Practical applications of P3 Model to interventions targeted at improving immunization and colorectal cancer screening.
| Immunization | Colorectal cancer screening | |
|---|---|---|
| Practice-level |
|
|
| Provider-level |
|
|
| Patient-level |
|
|
With the flexibility of components, this model can be implemented across preventive care promotion and adapted to different clinical settings. One example of this flexibility is the increased provision of influenza vaccine in community-based settings (e.g. pharmacies and other retail locations) (Drozd et al., 2017). In these settings, there is still a need for the provider (e.g. the pharmacist) to recommend vaccination and be able to answer patient questions, while also including appropriate practice-level components (e.g. signage indicating that influenza vaccine is available, linkage of the pharmacy to immunization information systems).
5.2. Evaluation of interventions utilizing the P3 Model
Addressing various components of preventive care promotion through the Practice-, Provider-, and Patient-levels (Fig. 1) and accounting for key impacting factors (Table 2) provides a clear means to develop evaluation materials. For example, delineating specific activities as shown in Table 3 and comparing these activities to the impacting factors shown in Table 2, researchers have a framework for developing both process and outcome evaluation measures encompassing the depth and breadth of P3 Model-based interventions. Combining process evaluation with outcome evaluation in this way can identify specific intervention components critical to success, while noting components that can be removed or modified to create a more effective intervention. Using the P3 Model to design and evaluate interventions addresses the multifactorial nature of improving preventive care, and also allows interventions to be developed and evaluated in a modular approach to allow future refinement and optimization of the intervention.
6. Conclusions
We have described the development of the P3 (Practice, Provider, Patient) Model for use in promotion of preventive care, with examples presented for application to both vaccination and colorectal cancer screening. The P3 Model builds on the prior work related to individual-level health behavior models (i.e. HBM, TPB) (Glanz et al., 2008; DiClemente et al., 2013), ecological models (i.e. social ecological model, Social Cognitive Theory) (Glanz et al., 2008; DiClemente et al., 2013), communication strategies (Charles et al., 1999; Elwyn et al., 2012; Opel et al., 2013; Opel et al., 2015), and the Systems Model of Clinical Preventive Care (Walsh and McPhee, 1992), integrating key components into a comprehensive model for promotion of prevention activities. The P3 Model – including both the conceptual model and key activities or considerations for each component - provides a framework for the design, conduct, and evaluation of studies assessing the effectiveness of prevention promotion efforts. We have designed the P3 Model to focus on both practical application and agility with regard to different prevention modalities. The practical nature of this model, in terms of understanding key intervention points at each of the levels and developing and conducting evaluation of interventions, makes the P3 Model a flexible and adaptable framework for use across all types of preventive care promotion. The examples provided here are not meant to be an exhaustive list of applications of the P3 Model, but rather a framework for application of this model in a wide variety of settings.
Acknowledgments
Acknowledgments
This work was supported in part by the National Institutes of Health [grant number K01AI106961 (Bednarczyk); grant number R01AI110482 (Omer, Salmon, Chamberlain)]. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Financial disclosure
In the past, Dr. Salmon has served as a consultant for Merck, and conducted sponsored research for Crucell and Pfizer. Drs. Bednarczyk, Chamberlain, and Omer, and Ms. Mathewson have no conflicts of interest to declare.
Appendix A
Appendix Table 1.
Community Services Task Force recommendations for improving vaccination coverageb and cancer screening,c with linkage to relevant components in the P3 Model.
| Prevention type | Activity | Recommendation status | P3 Model level |
|---|---|---|---|
| Vaccination | Home Visits to Increase Vaccination Rates | Recommended | Patient |
| Requirements for Child Care, School, and College Attendance | Recommended | Patient | |
| Client Reminder and Recall Systems | Recommended | Practice | |
| Client or Family Incentive Rewards | Recommended | Practice and Patient | |
| Special Supplemental Nutrition Program for Women, Infants & Children (WIC) Settings | Recommended | Practice and Patient | |
| Provider Assessment and Feedback | Recommended | Practice | |
| Provider reminders | Recommended | Practice | |
| Standing orders | Recommended | Practice | |
| Health care system-based interventions implemented in combination | Recommended | Practice | |
| Community-based interventions implemented in combination | Recommended | Patient | |
| Reducing client out-of-pocket costs | Recommended | Patient | |
| Immunization information systems | Recommended | Practice | |
| Schools and organized child care centers | Recommended | Patient | |
| Client-held paper immunization records | Insufficient evidence | Patient | |
| Community-wide education when used alone | Insufficient evidence | Patient | |
| Monetary sanction policies | Insufficient evidence | Patient | |
| Provider education when used alone | Insufficient evidence | Provider | |
| Clinic-based client education when used alone | Insufficient evidence | Patient | |
| Cancer screening | Client reminders | Recommended | Practice |
| Small media | Recommended | Patient | |
| One-on-one education | Recommended | Provider and Patient | |
| Provider assessment and feedback | Recommended | Practice | |
| Provider reminder and recall systems | Recommended | Practice | |
| Group education | Recommendeda | Provider and Patient | |
| Reducing structural barriers | Recommendeda | Practice | |
| Reducing client out-of-pocket costs | Recommendeda | Patient | |
| Provider incentives | Insufficient evidence | Provider | |
| Client incentives | Insufficient evidence | Patient | |
| Mass media | Insufficient evidence | Patient | |
| Promoting informed decision making for cancer screening | Insufficient evidence | Provider and Patient |
Recommended for some forms of cancer screening
The Community Guide. Increasing Appropriate Vaccination - Evidence-Based Interventions for Your Community. 2013; https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed March 8, 2017.
The Community Guide. Cancer Prevention and Control: Cancer Screening - Evidence-Based Interventions for Your Community. 2012; https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed March 8, 2017.
References
- Albert S.M., Nowalk M.P., Yonas M.A., Zimmerman R.K., Ahmed F. Standing orders for influenza and pneumococcal polysaccharide vaccination: correlates identified in a national survey of U.S. primary care physicians. BMC Fam. Pract. 2012;13:22. doi: 10.1186/1471-2296-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society American Cancer Society Recommendations for Colorectal Cancer Early Detection. 2017. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html
- Baker D.W., Brown T., Buchanan D.R. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern. Med. 2014;174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- Bandura A. Prentice-Hall; Englewood Cliffs NJ: 1986. Social Foundations of Thought and Action: A Social Cognitive Theory. [Google Scholar]
- Bellizzi K.M., Breslau E.S., Burness A., Waldron W. Prevalence of cancer screening in older, racially diverse adults: still screening after all these years. Arch. Intern. Med. 2011;171(22):2031–2037. doi: 10.1001/archinternmed.2011.570. [DOI] [PubMed] [Google Scholar]
- Borenstein J., Badamgarav E., Henning J.M., Gano A.D., Jr., Weingarten S.R. The association between quality improvement activities performed by managed care organizations and quality of care. Am. J. Med. 2004;117(5):297–304. doi: 10.1016/j.amjmed.2004.02.046. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Harvard University Press; Cambridge MA: 1979. The Ecology of Human Development. [Google Scholar]
- Centers for Disease Control and Prevention Estimates of deaths associated with seasonal influenza — United States, 1976-2007. MMWR Morb. Mortal. Wkly Rep. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb. Mortal. Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Survey of state immunization information system legislation. 2015. https://www2a.cdc.gov/vaccines/iis/iissurvey/legislation-survey.asp
- Centers for Disease Control and Prevention Flu Vaccination Coverage, United States, 2015–16 Influenza Season. 2016. https://www.cdc.gov/flu/fluvaxview/coverage-1516estimates.htm
- Centers for Disease Control and Prevention Colorectal cancer statistics. 2016. https://www.cdc.gov/cancer/colorectal/statistics/
- Chamberlain A.T., Seib K., Ault K.A. Improving influenza and Tdap vaccination during pregnancy: a cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine. 2015;33(30):3571–3579. doi: 10.1016/j.vaccine.2015.05.048. [DOI] [PubMed] [Google Scholar]
- Charles C., Gafni A., Whelan T. Decision-making in the physician–patient encounter: revisiting the shared treatment decision-making model. Soc. Sci. Med. 1999;49(5):651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- Clark L., Kuter B. 2014. An Investigation of the Recommendation Styles and Same-Day Vaccination Rates for Pediatricians Discussing HPV Vaccine with Adolescent Patients and Their Caregivers; Abstract #652. IDWeek. (Philadelphia, PA) [Google Scholar]
- Clark L., Kuter B. An Investigation of the Recommendation Styles and Same-Day Vaccination Rates for Pediatricians Discussing Hpv Vaccine with Adolescent Patients & their Caregivers. 2014. https://idsa.confex.com/idsa/2014/webprogram/Paper47037.html
- Crosby R., Salazar L., diClemente R. Value-expectancy theories. In: DiClemente R., Salazar L., Crosby R., editors. Health Behavior Theory for Public Health: Principles, Foundations, and Applications. Jones & Bartlett; Burlington MA: 2013. [Google Scholar]
- Dempsey A.F., Pyrzanowski J., Brewer S., Barnard J., Sevick C., O'Leary S.T. Acceptability of using standing orders to deliver human papillomavirus vaccines in the outpatient obstetrician/gynecologist setting. Vaccine. 2015;33(15):1773–1779. doi: 10.1016/j.vaccine.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Dempsey A.F., Lockhart S., Campagna E.J., Pyrzanowski J., Barnard J., ST O.L. Providers' time spent and tools used when discussing the HPV vaccine with parents of adolescents. Vaccine. 2016;34(50):6217–6222. doi: 10.1016/j.vaccine.2016.10.083. [DOI] [PubMed] [Google Scholar]
- DiClemente R., Salazar L., Crosby R., editors. Health Behavior Theory for Public Health: Principles, Foundations, and Applications. Jones & Bartlett; Burlington MA: 2013. [Google Scholar]
- Drozd E.M., Miller L., Johnsrud M. Impact of pharmacist immunization authority on seasonal influenza immunization rates across states. Clin. Ther. 2017;39(8):1563–1580.e1517. doi: 10.1016/j.clinthera.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Elwyn G., Frosch D., Thomson R. Shared decision making: a model for clinical practice. J. Gen. Intern. Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoriti P., Carbone G., Greco M., Pirozzi F., Pirozzi R.E.M., Corcione F. Worldwide burden of colorectal cancer: a review. Updat. Surg. 2016;68(1):7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- Forman D., de Martel C., Lacey C.J. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Gilkey M.B., Moss J.L., Roberts A.J., Dayton A.M., Grimshaw A.H., Brewer N.T. Comparing in-person and webinar delivery of an immunization quality improvement program: a process evaluation of the adolescent AFIX trial. Implement. Sci. 2014;9:21. doi: 10.1186/1748-5908-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K., Rimer B., Viswanath K., editors. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; San Francisco CA: 2008. [Google Scholar]
- Haas J.S., Sprague B.L., Klabunde C.N. Provider attitudes and screening practices following changes in breast and cervical Cancer screening guidelines. J. Gen. Intern. Med. 2016;31(1):52–59. doi: 10.1007/s11606-015-3449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.C., Jones A.L., Feemster K.A., Fiks A.G. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr. 2011;11:74. doi: 10.1186/1471-2431-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaron C.W., Mercer J.T., Massoudi M.S. Changes in clinic vaccination coverage after institution of measurement and feedback in 4 states and 2 cities. Arch. Pediatr. Adolesc. Med. 1999;153(8):879–886. doi: 10.1001/archpedi.153.8.879. [DOI] [PubMed] [Google Scholar]
- Lipstein E.A., Brinkman W.B., Britto M.T. What is known about parents' treatment decisions? A narrative review of pediatric decision making. Med. Decis. Mak. 2012;32(2):246–258. doi: 10.1177/0272989X11421528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen P.D., Evans D., Forster J. Settings as an important dimension in health education/promotion policy, programs, and research. Health Educ. Q. 1995;22(3):329–345. doi: 10.1177/109019819402200306. [DOI] [PubMed] [Google Scholar]
- Opel D.J., Heritage J., Taylor J.A. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037–1046. doi: 10.1542/peds.2013-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel D.J., Mangione-Smith R., Robinson J.D. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am. J. Public Health. 2015;105(10):1998–2004. doi: 10.2105/AJPH.2014.302425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins R.B., Clark J.A., Apte G. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics. 2014;134(3):e666–e674. doi: 10.1542/peds.2014-0442. [DOI] [PubMed] [Google Scholar]
- Reagan-Steiner S., Yankey D., Jeyarajah J. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- Salazar L., Crosby R., Noar S., Walker J., diClemente R. Models based on perceived threat and fear appeals. In: DiClemente R., Salazar L., Crosby R., editors. Health Behavior Theory for Public Health: Principles, Foundations, and Applications. Jones & Bartlett; Burlington MA: 2013. [Google Scholar]
- The Community Guide Cancer Prevention and Control: Cancer Screening - Evidence-Based Interventions for Your Community. 2012. https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf
- The Community Guide Increasing Appropriate Vaccination - Evidence-Based Interventions for Your Community. 2013. https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf
- US Department of Health and Human Services Immunization and infectious diseases | healthy people 2020. 2017. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives
- US Department of Health and Human Services Cancer | healthy people 2020. 2017. https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives
- US Preventive Services Task Force Screening for colorectal cancer: us preventive services task force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- Viens L.J., Henley S.J., Watson M. Human papillomavirus-associated cancers - United States, 2008–2012. MMWR Morb. Mortal. Wkly Rep. 2016;65(26):661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- Walsh J.M., McPhee S.J. A systems model of clinical preventive care: an analysis of factors influencing patient and physician. Health Educ. Q. 1992;19(2):157–175. doi: 10.1177/109019819201900202. [DOI] [PubMed] [Google Scholar]