Abstract
Introduction
Previous studies have shown that endothelial cell senescence is involved in cardiovascular diseases such as cardiac fibrosis, atherosclerosis and heart failure. Accumulating evidence indicates that apelin exerts protective effects on ageing-related endothelial dysfunction. In this study, we aim to investigate the role of the apelin/APJ axis in angiotensin II (AngII)-induced endothelium senescence and its associated mechanisms.
Material and methods
Senescence-related β-gal activity assay and western blot were used to evaluate human umbilical vein endothelial cell (HUVEC) senescence. In addition, DCFH-DA staining was carried out to detect the generation of reactive oxygen species (ROS). A validated, high-sensitivity real-time quantitative telomeric repeat amplification protocol (RQ-TRAP) was applied to determine telomerase activity in HUVECs, and a CCK-8 assay was employed to measure cellular viability.
Results
AngII induced an increase in SA-β-Gal-positive cells and upregulation on expression of P21 and PAI-1 compared to the control group (p < 0.05), while apelin against this process (p < 0.05). The protective effects were attenuated when APJ, AMPK and SIRT1 expression was knocked down (p < 0.05). Furthermore, apelin reduced AngII-induced ROS generation and enhanced telomerase activity in HUVECs (p < 0.05), which contributed to increased HUVEC viability as assessed by the CCK-8 assay (p < 0.05).
Conclusions
The apelin/APJ axis improved AngII-induced HUVEC senescence via the AMPK/SIRT1 signaling pathway, and the underlying mechanisms might be associated with reduced ROS production and enhanced telomerase activity.
Keywords: apelin, endothelium, senescence, mechanisms
Introduction
Ageing is an important and independent risk factor for cardiovascular diseases, which is considered at least partially due to ageing-related structural and functional alterations of the cardiovascular system [1]. Accumulating evidence has consistently demonstrated that ageing-related endothelial dysfunction plays a major role in cardiovascular disease development [2–4]. Apelin is a novel adipocyte-derived factor which has been identified as an endogenous ligand for the G protein-coupled receptor APJ [5]. In the cardiovascular system, apelin is predominantly expressed in endothelial cells, and the APJ receptor is expressed on endothelial cells, smooth muscle cells and cardiomyocytes [6]. Several active isoforms of apelin have been identified to date. Notably, a shorter peptide which consists of 13 amino acids exhibits the greatest activity [7]. Previous studies have revealed that apelin can promote angiogenesis and reduce ischemia-induced myocardial injury [8, 9]. Recently, some studies have also shown that the apelin/APJ pathway is beneficial for improving cardiac fibrosis [10]. In addition, in patients with heart failure, plasma apelin level is decreased while an increased plasma apelin level appears to improve left ventricular remodeling [11, 12]. Taken together, previous findings indicate that the apelin/APJ pathway is involved in the alterations of cardiovascular structure.
Prior studies have revealed that endothelium ageing is associated with endothelial dysfunction and atherogenesis [13–15], and activation of the apelin/APJ pathway appears to ameliorate and improve these pathological processes [9, 10]. However, the underlying mechanism is still elusive. There is a high homology between APJ receptor and angiotensin II receptor type I (AT1R), which shares identity of 54% in the transmembrane regions [16]. However, substantial evidence has shown that the apelin/APJ pathway has opposing actions to AngII/AT1R in regulation of the cardiovascular system. Meanwhile, a prior study indicated that AngII promoted the onset of senescence of endothelial cell [17]. In the present study, we therefore hypothesized that the apelin/APJ pathway might antagonize the AngII-induced endothelium senescence. Apelin is highly conserved among different species, and the C-terminal 13 amino acids (65−77) are completely conserved across all species studied [16], indicating that apelin-13 should be the best representative agent for experimental research. Therefore, in the current study, we used apelin-13 as a representative of apelin to investigate the above hypothesis.
Material and methods
Cells culture and treatment
Human umbilical vascular endothelial cells (HUVECs) were purchased from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. HUVECs were cultured in endothelial cell medium (ECM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin, 100 mg/ml streptomycin and 20 mg/ml vascular endothelial growth factor (VEGF). The cells were incubated at 37°C in a humidified environment with 5% CO2. HUVECs with the concentration of 105 per well were pre-treated with apelin-13 (10–8 M) (Sigma, USA) for 1 h and then treated with AngII (10–6 M) (Sigma, USA) for 24 h. The medium was not changed during culture and treatment.
RNA interference
In the following experiment, the APJ, AMPK and SIRT1 genes of HUVECs were knocked out using RNA interference. In brief, the HUVECs (with 70% confluence) were transfected with a plasmid containing siRNAs specific against the APJ, AMPK and SIRT1 genes and the scramble control for 48 h in accordance with the manufacturer’s instructions. The siRNAs were synthesized and purchased from RiboBio Company (China). The short hairpin RNA sequences were as follows: siAMPK-1: 5′-GAGGAGAGCTATTTGATTA-3′; siAMPK-2: 5′-GCAGAAGTATGTAGAGCAA-3′; siAMPK-3: 5′-ACACATGAATGCAAAGATA-3′. Meanwhile, three short hairpin RNA vectors were constructed to encode sequences targeting human SIRT1 mRNA (siSIRT1-001: 5′-GCT-AAGAATTTCAGGATTA-3′ and siSIRT1-002: 5′-GGAA-ATATATCCTGGACAA-3′ and siSIRT1-003: 5′-CCTTAA-AACTAGAGATCAA-3′). Thereafter, the HUVECs were harvested and used for the following experiments.
Senescence-associated β-gal activity assay
Senescence was detected using a senescence-associated β-galactosidase (SA-β-gal)-positive approach. The HUVECs were stained with 4′,6-diamino-phenylindole (DAPI) (0.2 µg/ml in 10 mmol/l Tris-HCl, pH 7.0, 10 mmol/l EDTA, 100 mmol/l NaCl) for 10 min and then the total number of HUVECs was counted. The cells were imaged under a bright-field microscope at a magnification of 200×. The senescent cells were observed in an optical microscope and counted from 5 random fields of vision.
Western blot
After treatments, the HUVECs were harvested and lysed with cell lysis solution at 4°C for 30 min. Loading buffer was added to cytosolic extracts, and after boiling for about 5 min, 30 µg of protein of each sample were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then the total gel was transferred into polyvinylidene fluoride (PVDF) membranes. The membranes were probed with anti-P21 (CST 2947), anti-PAI-1 (CST 11907), anti-APJ Receptor (AB 214369), anti-t-AMPK (CST 5832), anti-p-AMPK (CST 2535), anti-SIRT1 (CST 2493) and anti-GAPDH (GXP 199629) primary antibodies overnight at 4°C with 1 : 1000 dilution followed by horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG). The immunoreactive signals were visualized by enhanced chemiluminescence (ECL) detection. In order to quantify the protein expression, the X-ray films were scanned and analyzed with Image J 1.47i software.
Measurement of intracellular reactive oxygen species generation
Intracellular reactive oxygen species (ROS) generation was tested by the oxidative conversion of cell-permeable oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to fluorescent DCF. The HUVECs were cultured on 6-well plates with ECM. With different treatments, each well was washed 3 times with PBS, and 10 µM DCFH-DA solution in serum-free medium was added to the wells, and then the HUVECs were incubated at 37°C for a further 30 min in an incubator. After that, the cells were washed 5 times with PBS, and DCF fluorescence was measured over the entire field of vision using a fluorescence microscope connected to an imaging system (BX50-FLA, Olympus, Tokyo, Japan). The mean fluorescence intensity (MFI) of ROS from 5 random fields was measured using Image J 1.47i software and the MFI was used as an index of the amount of ROS. MFI in the AngII group was considered as 100% for comparison with other groups.
Real-time quantitative telomeric repeat amplification protocol (RQ-TRAP) assay
Telomerase activity in 1,000 HUVECs was assessed using a SYBR Green (Applied Biosystems, Foster City, CA) RQ-TRAP assay as described by Henning Wege [14]. The threshold cycle value (Ct) of each sample was compared with standard curves generated from serial dilutions of telomerase-positive 293T cell extracts (1000, 100, 10 and 1 cells). Standards and negative controls with heat-inactivated samples and lysis buffer were assayed on each plate. Each sample was analyzed and the telomerase activity was calculated relative to 293T cells. Telomerase activity in the control group was considered as 100% for comparison with other groups.
Evaluation of HUVEC viability
The HUVECs were seeded on 96-well plates at a density of 1 × 104 cells/well, and the CCK-8 assay was employed to measure cellular viability. After the indicated treatments, 10 µl of CCK-8 solution at a 1 : 10 dilution was added to every well and then the plate was incubated for 3 h in the incubator. Absorbance at 450 nm was measured with a microplate reader (Molecular Devices, Sunnyvale, CA, USA), and the means of optical density (OD) of 4 wells in the indicated groups were used to calculate the percentage of cell viability in relation to the control group, which was based on the following formula: cell viability (%) = (OD treatment group/OD control group) × 100%.
Statistical analysis
All data were presented as the mean ± SD. Differences between groups were analyzed by one-way analysis of variance (ANOVA) using the SPSS 19.0 software (SPSS, Chicago, IL, USA), followed by the LSD post hoc between-group comparison test. P < 0.05 was considered to be statistically significant.
Results
Apelin inhibited AngII-induced HUVEC senescence and intracellular ROS production
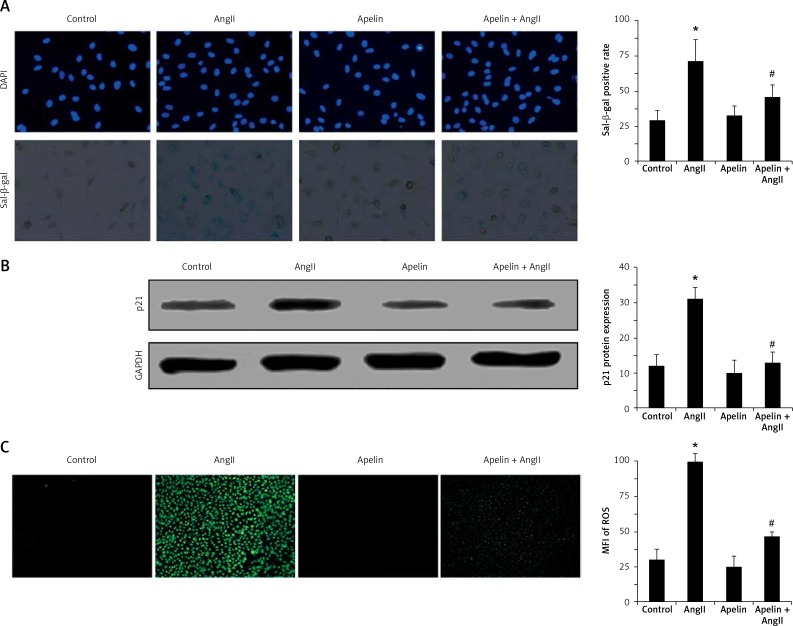
To assess the onset of senescence, senescence-associated β-galactosidase was detected. As shown in Figure 1 A, AngII induced an increase in SA-β-Gal-positive cells compared to the control group, demonstrating that HUVEC senescence was successfully induced by AngII. Of note, SA-β-Gal-positive cells were significantly reduced by apelin. We further tested the effects of apelin on the expression of p21 proteins in AngII-treated cells since P21 protein has been shown to increase during cell senescence. As shown in Figure 1 B, p21 expression in the apelin + AngII group was significantly lower compared to the AngII group.
Figure 1.
Apelin improved AngII-induced senescence and suppressed ROS production. A – Cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Cell senescence was evaluated by SA-β-gal activity. Representative photo micrographs showed SA-β-gal-positive cells (blue) in HUVECs. B – P21 expression was detected by western blot. The bar graphs show the quantification of the indicated proteins. C – Cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Cellular ROS was evaluated by DCFH-DA. Representative photo micrographs show ROS-positive cells (green) in HUVECs
The values are expressed as means ± SD of at least 3 independent experiments or 5 random fields of vision.*P < 0.05 vs. control; #P < 0.05 vs. AngII.
It has been well documented that cell senescence is closely associated with intracellular ROS production [18]. Therefore, we also tested the changes of ROS with and without apelin treatment using the H2DCF-DA labeling assay. As shown in Figure 1 C, AngII remarkably increased the H2DCF-DA oxidation level in HUVECs, while treatment with apelin significantly inhibited ROS production.
Apelin/APJ inhibited AngII-induced cell senescence via AMPK/SIRT1 pathway
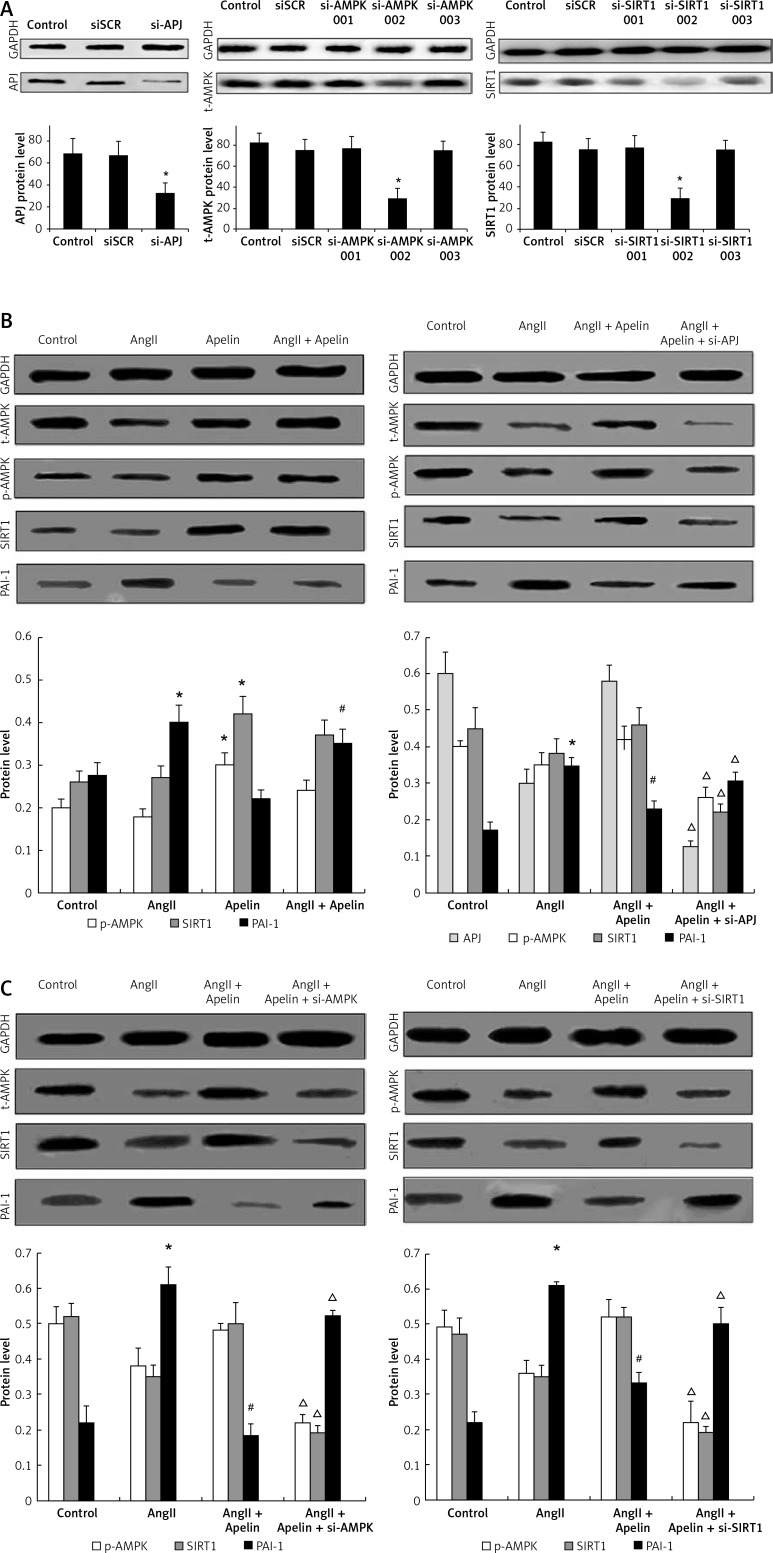
To determine whether the APJ/AMPK/SIRT1 pathway was involved in apelin-mediated HUVEC senescence improvement, a non-silencing scrambled control (SCR), siAPJ, siAMPK and siSIRT1 were used. After transfection of HUVECs with APJ siRNA, expression of APJ was determined using western blot and the results showed satisfactory transfection efficiency. As shown in Figure 2 A, siAMPK-2 significantly reduced t-AMPK expression. In contrast, siAMPK-1 and siAMPK-3 did not alter t-AMPK expression. In addition, we found that SIRT1 protein was also significantly decreased by siSIRT1-2.
Figure 2.
Apelin/APJ inhibited AngII-induced senescence through AMPK/SIRT1 signal pathway. A – SIRT1, AMPK, APJ were respectively knocked down by siRNA transfection as described in the Material and methods section. APJ, AMPK and SIRT1 expression was detected by western blot. B – As shown in the left panel, cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Western blot analysis was performed. As shown in the right panel, APJ was knocked down. At 48 h after transfection, the cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Western blot analysis was performed C – As shown in the left panel, AMPK was knocked down. At 48 h after transfection, the cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Western blot analysis was performed. As shown in the right panel, SIRT1 was knocked down. At 48 h after transfection, the cells were pre-treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Western blot analysis was performed. The bar graphs show the quantification of the indicated protein
The values are expressed as means ± SD of at least 3 independent experiments.*P < 0.05 vs. control; #p < 0.05, vs. AngII; Δp < 0.05, vs. apelin + AngII
We further investigated whether APJ, AMPK and SIRT1 were associated with improvement of HUVEC senescence by apelin treatment. As shown in Figure 2 B, the expression levels of APJ, p-AMPK and SIRT1 were significantly increased by apelin treatment while PAI-1 protein was reduced. Furthermore, APJ knockdown led to an increase in PAI-1 expression and a decline in AMPK and SIRT1 expression, indicating that APJ might be an upstream regulator of the AMPK/SIRT1 pathway. As shown in Figure 2 C, PAI-1 reduction by apelin was also abrogated by siAMPK-2 and siSIRT1-2, indicating that the apelin/APJ axis improved AngII-induced cell senescence via the AMPK/SIRT1 pathway.
Apelin/APJ inhibited AngII-induced HUVEC senescence and intracellular ROS production via the AMPK/SIRT1 pathway
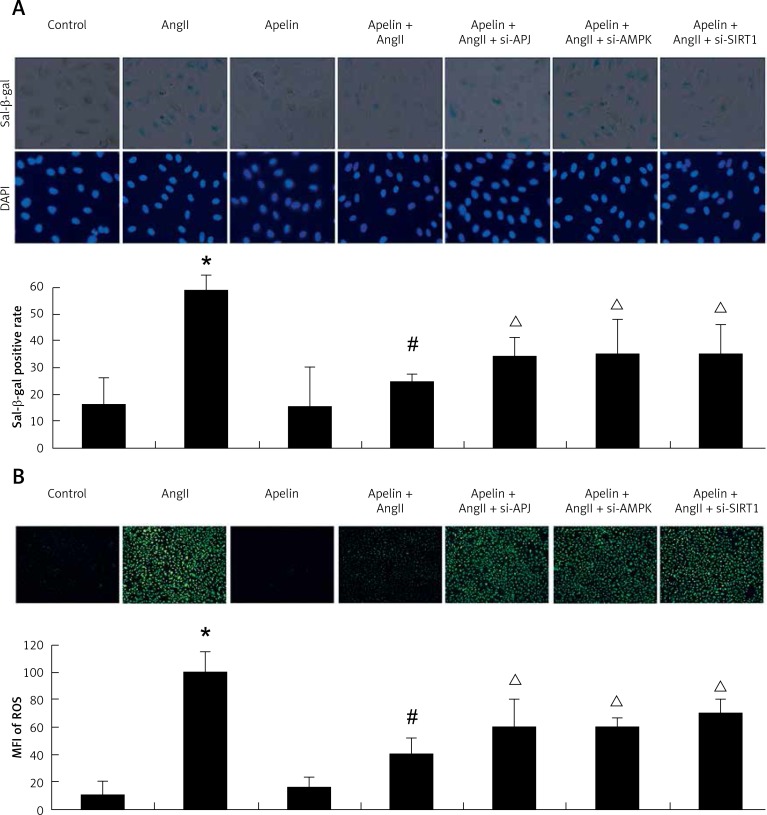
As shown in Figure 3 A, compared to the AngII group, addition of apelin reduced SA-β-Gal-positive cells. Of note, as shown in Figure 3 B APJ, AMPK and SIRT1 knockdown resulted in a significant increase in H2DCF-DA labeled cells, indicating that apelin’s reduction of AngII-induced ROS production was associated with the AMPK/SIRT1 pathway.
Figure 3.
Apelin improved AngII-induced ageing and reduced ROS production via AMPK/SIRT1 signaling pathway. A – SIRT1, AMPK, APJ were knocked down by siRNA transfection. At 24 h after transfection, the cells were treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Cell senescence was evaluated by SA-β-gal activity. Representative photomicrographs show SA-β-gal-positive cells (blue) in HUVECs. B – The level of intracellular ROS was assessed. Representative photomicrographs show ROS-positive cells (green) in HUVECs
The values are expressed as means ± SD of at least 3 independent experiments or 5 random fields of vision.*P < 0.05 vs. control; #p < 0.05 vs. AngII; Δp < 0.05 vs. apelin + AngII
Effects of apelin on telomerase activity in AngII-treated HUVEC
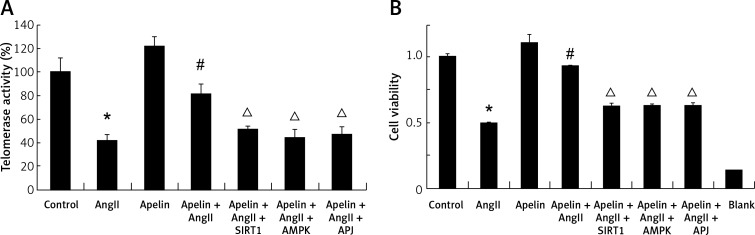
Initiation and development of endothelium senescence is known to be influenced by telomerase activity [19]. Therefore, we further tested whether treatment with apelin was capable of regulating telomerase activity in AngII-treated HUVECs. As presented in Figure 4 A, AngII significantly diminished telomerase activity. However, this effect was significantly reduced by treatment with apelin. Of note, treatment with siAPJ, siSIRT1 and siAMPK decreased telomerase activity. In addition, we also examined whether increased telomerase activity with apelin treatment was associated with improvement of cellular viability. As presented in Figure 4 B, apelin improved cell viability as indexed by CCK-8, and this protective effect was abolished by APJ, AMPK and SIRT1 knockdown.
Figure 4.
Apelin promoted telomerase activity in AngII-treated HUVEC. A – SIRT1, AMPK, APJ were knocked down by siRNA transfection. At 24 h after transfection, the cells were treated with apelin (10–8 M) for 1 h followed by exposure to AngII (10–6 M) for an additional 24 h. Telomerase activity was investigated by RQ-TRAP. Telomerase activity in the control group was considered as 100% compared to the other groups. B – Cell viability was detected by CCK-8 assay. The bar charts show the cell viability of the indicated group
The values are expressed as means ± SD of at least 3 independent experiments.*P < 0.05 vs. control; #p < 0.05, vs. AngII; Δp < 0.05, vs. apelin + AngII.
Discussion
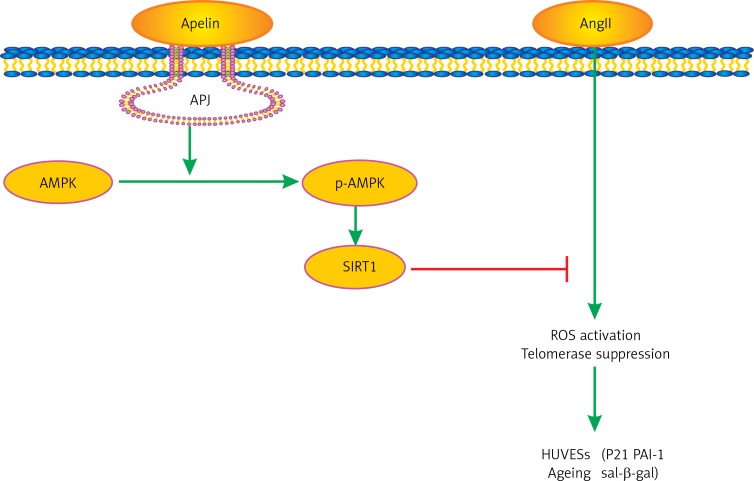
In the present study, we provide new evidence that apelin reduced AngII-induced HUVEC senescence, which in turn improved cellular viability. Importantly, we also demonstrated for the first time that these cellular protections were associated with the AMPK/SIRT1 signaling pathway (as presented in Figure 5). Moreover, our data suggested that ROS suppression and telomerase activation were also involved in these pathological alterations.
Figure 5.
Pathway involved in the protective effect of apelin/APJ axis in AngII induced HUVECs ageing. Apelin/ APJ axis induces the phosphorylation of AMPK, causing SIRT1 activation. This process inhibits AngII-induced ROS generation and increases telomerase and finally improves AngII-induced HUVECs ageing
It is well known that endothelium senescence causes endothelial dysfunctional and ageing-related vascular disorders such as atherosclerosis [13]. A prior study revealed that AngII induced atherosclerosis by promoting vascular ageing [20]. It is also reported that AngII plays an important role in endothelium senescence [17]. It would be clinically relevant to improve AngII-induced endothelium senescence. It has been found that the apelin/APJ axis exerts the opposite physiological effects of AngII/AT1R. Lee et al. reported that apelin had hypotensive properties, which was in contrast to the vasopressor effects of angiotensin II [16]. Moreover, apelin reduced AngII-induced cardiovascular fibrosis and atherosclerosis [21, 22]. Interestingly and importantly, our current study showed that the apelin/APJ axis was involved in the ageing-related pathological alterations of HUVECs. This finding indicates that the apelin/APJ axis may play an important role in the regulation of endothelial dysfunction.
We have additionally investigated the underlying mechanisms of the endothelium-protective effects of the apelin/APJ axis. AMP-activated protein kinase (AMPK), a key regulator of metabolism, has been reported to be associated with ageing-related pathological changes in lower organisms such as yeast and worms [23, 24]. However, in higher organisms, the role of AMPK in the regulation of ageing-related changes is not clear yet. Some studies have reported that ageing increased AMPK activation [25], while others have reported decreased AMPK activity [26]. Previous studies have demonstrated that apelin can activate AMPK phosphorylation, which in turn inhibits the ageing-related cardiac fibrosis in animal models [10, 27], indicating a close connection between apelin and AMPK in ageing-related cardiovascular system changes. We observed that apelin-13 improved AMPK phosphorylation which in turn resulted in a reduction of AngII-induced HUVEC senescence. Notably, sirtuin 1 (SIRT1), an NAD+-dependent protein deacetylase regulated by AMPK, also has anti-ageing properties. Prior studies showed that SIRT1 improved ageing-related cardiovascular diseases by regulating a variety of cellular processes [28], and the AMPK/SIRT1 signaling pathway might play an important role in regulation of ageing. As expected, findings from our current study supported the notion that apelin attenuated AngII-induced HUVEC senescence through the AMPK/SIRT1 signaling pathway.
Improvement of ROS might be another mechanism. It has been reported that AngII-induced ROS generation in several pathophysiological processes such as vascular smooth muscle cell hypertrophy [29, 30]. Recent studies suggested that ROS generation accelerated the onset of cellular senescence [18, 31]. Moreover, Li et al. reported that AngII promoted cellular senescence and apoptosis via ROS [17]. We also found that apelin reduced ROS generation in AngII-treated HUVECs, which was abrogated by APJ knockout. In addition, AMPK and SIRT1 knockout also led to increased ROS generation, indicating that suppressing ROS generation might be associated with the endothelium-protective effects of the apelin/APJ axis.
It is generally believed that telomere length is a key factor in regulating cellular life span. Telomere shortening is like a molecular clock that triggers senescence. Telomerase, an RNA-directed DNA polymerase, is capable of maintaining the length of telomeres in endothelial cells and therefore regulates senescence [32]. A prior study showed that telomerase activation delayed endothelium senescence [33]. Therefore, it may be a new strategy for anti-ageing by regulating the telomerase activity. In this study, we investigated the role of telomerase activity in AngII-induced HUVEC senescence. Our data revealed that AngII diminished telomerase activity. However, apelin treatment enhanced telomerase activation and decreased HUVEC senescence. Telomerase activation induced by apelin was attenuated with APJ, AMPK and SIRT1 knockdown, indicating that apelin/APJ improved endothelium senescence through telomerase inactivation dependent on the AMPK/SIRT1.
In conclusion, our current study reveals that the apelin/APJ axis improved AngII-induced HUVEC senescence through the AMPK/SIRT1 signaling pathway, and the associated mechanisms might be related to decreased ROS production and increased telomerase activity. Our findings provide novel insights into the beneficial effects of apelin on ageing-related vascular diseases.
Acknowledgments
Rongfeng Yang and Wu Fang are co-first authors.
The study was supported by grants from the National Natural Science Foundation of China (NO. 81700263 and 81700323), the Natural Science Foundation of Guangdong Province of China (2016A030313248), the Science and Technology project of Guangzhou city of China (201604020129), and the Science and Technology project of Shenzhen city of China (JCYJ20170307161535847), and the study was supported by grants from the Research Project (Doctoral Innovation Program) of Health Planning system of Shenzhen city (SZBC2017007).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Barodka VM, Joshi BL, Berkowitz DE, Hogue CW, Jr, Nyhan D. Review article: implications of vascular aging. Anesthesia Analgesia. 2011;112:1048–60. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao J, Jin YF, Yang Z, et al. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–9. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- 3.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–7. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 4.Virdis A, Ghiadoni L, Giannarelli C, Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas. 2010;67:20–4. doi: 10.1016/j.maturitas.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Li H, Tang L, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through the RISK-GSK-3beta-mPTP pathway. Arch Med Sci. 2015;11:1065–73. doi: 10.5114/aoms.2015.54863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DK, Ferguson SS, George SR, O’Dowd BF. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem Biophys Res Commun. 2010;395:185–9. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- 8.Momiyama Y. Association between plasma apelin levels and coronary collateral development in patients with stable angina pectoris. Atherosclerosis. 2014;235:349–50. doi: 10.1016/j.atherosclerosis.2014.05.930. [DOI] [PubMed] [Google Scholar]
- 9.Novakova V, Sandhu GS, Dragomir-Daescu D, Klabusay M. Apelinergic system in endothelial cells and its role in angiogenesis in myocardial ischemia. Vascul Pharmacol. 2016;76:1–10. doi: 10.1016/j.vph.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Chen L, Lu L, Li L. The apelin-APJ axis: a novel potential therapeutic target for organ fibrosis. Clin Chim Acta. 2016;456:81–8. doi: 10.1016/j.cca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Chong KS, Gardner RS, Morton JJ, Ashley EA, McDonagh TA. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–60. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Francia P, Salvati A, Balla C, et al. Cardiac resynchronization therapy increases plasma levels of the endogenous inotrope apelin. Eur J Heart Fail. 2007;9:306–9. doi: 10.1016/j.ejheart.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I. Vascular cell senescence and vascular aging. J Mol Cell Cardiol. 2004;36:175–83. doi: 10.1016/j.yjmcc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Maizel J, Vasko R, Xavier S, Chen J, Cao J, Goligorsky MS. Vascular senescence and organ fibrosis. Proceedings of the Physiological Society. 2013 Proc 37th IUPS. [Google Scholar]
- 15.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–4. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Cheng R, T, Fan T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Guo X, Lei P, Shi S, Luo S, Cheng X. PI3K/Akt/uncoupling protein 2 signaling pathway may be involved in cell senescence and apoptosis induced by angiotensin II in human vascular endothelial cells. Mol Biol Rep. 2014;41:6931–7. doi: 10.1007/s11033-014-3580-0. [DOI] [PubMed] [Google Scholar]
- 18.Mikhed Y, Daiber A, Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int J Mol Sci. 2015;16:15918–53. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamino T, Komuro I. Role of telomere in endothelial dysfunction in atherosclerosis. Curr Opin Lipidol. 2002;13:537–43. doi: 10.1097/00041433-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–30. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Siddiquee K, Hampton J, Khan S, et al. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases PAI-1 production. J Hypertens. 2011;29:724–31. doi: 10.1097/HJH.0b013e32834347de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun HJ, Ali ZA, Kojima Y, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Investig. 2008;118:3343–54. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallottini V, Montanari L, Cavallini G, Bergamini E, Gori Z, Trentalance A. Mechanisms underlying the impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in aged rat liver. Mech Ageing Dev. 2004;125:633–9. doi: 10.1016/j.mad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Saupe KW. Effects of aging on cardiac and skeletal muscle AMPK activity: basal activity, allosteric activation, and response to in vivo hypoxemia in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1270. doi: 10.1152/ajpregu.00409.2004. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J Gerontol. 2005;60:21–7. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Reznick RM, Zong H, Li J, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metabol. 2007;5:151–6. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pchejetski D, Foussal C, Alfarano C, et al. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J. 2012;33:2360–9. doi: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- 28.Guarente L. Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–44. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SK. Role of oxygen-derived free radicals in acute angiotensin II: induced hypertensive vascular disease in the rat. Circ Res. 1990;66:722–34. doi: 10.1161/01.res.66.3.722. [DOI] [PubMed] [Google Scholar]
- 30.Ushiofukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox Is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–21. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 31.Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Renal Nutrition. 2012;22:86–9. doi: 10.1053/j.jrn.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Chang E, Cherry AM, et al. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–8. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 33.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000;87:540–2. doi: 10.1161/01.res.87.7.540. [DOI] [PubMed] [Google Scholar]