Abstract
Insulin-like growth factor binding protein-3 (IGFBP-3) is a vital protein exist in circulation which interacts with high affinity to insulin-like growth factor (IGFs) altering their activities. Therefore, the interaction between IGFs and IGFBP-3 has a key role altering large spectrum of activities such as cell cycle progression, proliferation and apoptosis. Despite decades of research, the crystal structure of IGFBP-3 has not been identified possibly due to some technical challenge in its crystallizing. The three-dimensional (3D) structure of IGFBP-3 was predicted using homology modeling, Phyre2, and molecular dynamic. Its interaction with IGF-1 was also identified by HADDOCK software. IGFBP-3 has the most identity with other IGFBPs in N and C-domain; however, its linker domain has lower identity. Our data predicted that IGF-1 structurally interacts with N-domain and linker domain of IGFBP-3. Some conserved residues of IGFBP-3 such as Glu33, Arg36, Gly39, Arg60, Arg66, Asn109, and Ile146 interacts with Glu3, Asp12, Phe16, Gly19, Asp20, Arg21, and Glu58 of IGF-1. In addition, our data predict that the linker domain has a loop structure which covers post translational modification and interacts with IGF-1. The phosphorylation of Ser111 in linker domain, which previously has been shown to induce apoptosis make a repulsive force interrupting this interaction to IGF-1, which enables IGFBP-3 to induce apoptosis. The present study suggests that the linker domain has a key role in recognition of IGFBP-3 with IGF-1.
Keywords: Docking study, IGF-1, IGFBP-3, Linker domain, Molecular dynamic
INTRODUCTION
Insulin-like growth factors (IGFs) are members of the insulin super family of growth-promoting hormones participating growth and homeostasis of the whole organ in the human body(1,2,3). They interact to specific receptors such as type 1 IGF receptor (IGF-1R) and insulin receptor isoforms(4,5). The IGFs also interact with a member of super family of high-affinity IGF binding proteins (IGFBPs). Binding to IGFBPs regulates IGFs availability for receptor and increase their half-lives in circulation(6,7). IGFBPs contain six members (IGFBP-1 to IGFBP-6) which share both structural and functional similarities, affect the tissue distribution of IGFs enhancing access to their cell receptors(8). IGFBPs are involved in at least two categories. First of which is called “IGF-depended system” including IGF-1, IGF-2 and IGF-1R, acid-labile subunit (ALS), mannose 6-phosphate/IGF-2 receptor (M6P/IGF-2R), insulin receptor (IR), IGFBP-1 to IGFBP-6 and their proteases(9,10) while others are known as “IGF-independent” system(11,12). For instance, in the later system, IGFBPs were identified as an important regulator of cell fate decisions such as cell differentiation, cell cycle progression, and apoptosis(13,14,15,16). Several members of IGFBPs are present in human tissues, while IGFBP-3 is the most abundant IGF binding protein in human serum(17).
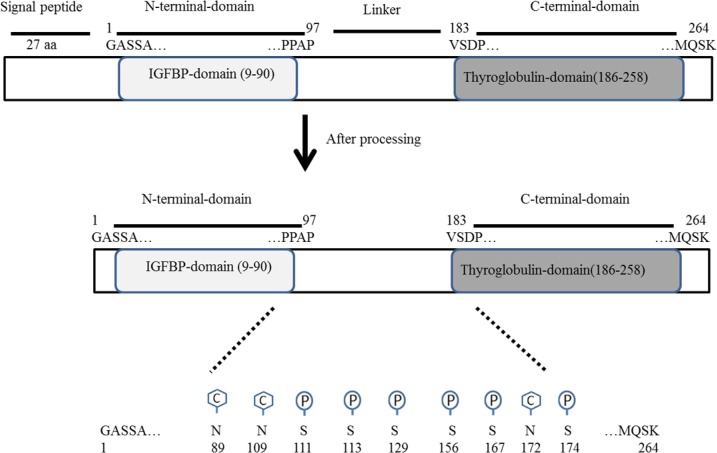
As indicated in Fig. 1, IGFBP-3 is a 291-amino acids protein comprising of a 27 amino acid signal peptide and a chain with 264 amino acids.
Fig. 1.
Schematic diagram of insulin-like growth factor binding protein 3 (IGFBP-3) and its domains which are processed to mature form. The C hexagonal stands and the P cycles represented respectively for carbohydrate and phosphorylation modifications.
The primary structure of IGFBP-3, similar to other IGFBPs, has three domain structures: a conserved N-terminal domain (residues 9-90), and a thyroglobulin type-1 domain in its C-terminal domain (residues 183-258) connected by a flexible “linker” domain. Post-translational modifications have been reported in the linker domain of IGFBP-3 that could alter its function(18). For instance, N-glycosylation sites which are located in the linker domain (Asn89, Asn109, and Asn172) have been shown to probably alter its affinity for glycosaminoglycan of the cell surface. In addition, IGFBP-3 might be phosphorylated by casein kinase-2 on Ser111, Ser113, and Ser156. Moreover, Ser129 and Ser174 might be phosphorylated by DNA dependent protein kinase. Further studies have shown that these phosphorylation of IGFBP-3 has no effect on IGF affinity but alter proteolysis properties and cell-surface binding(19,20,21).
Both experimental and clinical findings have demonstrated that IGFBP-3 has a tumor-suppressor function in some cancers including gastric, colorectal, and lung(22,23,24). However, other lines of studies have reported that IGFBP-3 shows oncogenic function in breast cancer(25). The IGFBP-3 effects are not restricted to cancer but are also observed in other disease states such as Alzheimer's and Parkinson's diseases as well as diabetes mellitus, retinopathy, cardiovascular disease, and brain ischemia(26,27,28,29,30,31). The precise mechanism delineating the role of IGFBP-3 in such disease is partially unknown.
According to multifaceted role of IGFBP-3 in the human body and lack of its experimentally established crystal structure(32), the aim of this study was theoretical investigation and prediction of its three-dimensional (3D) structure using homology modeling and molecular dynamic. Once the 3D structure of IGF-1/IGFBP-3 becomes available, it enhances rational drug design and development of small molecule targeting IGFBP-3 functions.
MATERIALS AND METHODS
Softwares
Modeller 9.10, Phyre2, Gromacs, HADDOCK softwares were used to identify the interaction of IGFBP-3 with IGFs. All molecular images were produced using VMD and PyMOL.
Target and template selection
The amino acids sequence of IGFBP-3 protein of Homo sapiens (P17936) was retrieved from the UniProt database (www.uniprot.org). All numbering of residues were based on excluding the signal peptide. Residue numbering is based on the mature sequence of IGFBP-3 (1-264 aa).
The IGFBP-3 basic local alignment search tool (BLAST) was performed to detect the most important residues of IGFBP-3 along mammals. Then protein data bank (PDB) structures of known proteins which published previously were selected as multiple templates(33,34,35,36).
Prediction of 3D model by protein homology/analogy recognition engine 2 and Modeller 9.10
Molecular modeling of IGFBP-3 proteins was performed using protein homology/analogy recognition engine 2 (Phyre2) and Modeller 9.10(37,38). Phyre2 is a free online server for homology modeling. It uses principles of homology modeling to generate reliable protein models resulting in extensive improvement of accuracy of 3D structure function and mutations prediction(38). Phyre2 also predicts and incorporates ligand binding sites and analyze the effects of amino acid variants in protein query. In addition Phyre2 also predicts some parts of proteins which have no detectable homology to known structures. The Phyre2 server is available at http://www.sbg.bio.ic.ac.uk/phyre2. A typical structure prediction will be returned after 24 h of submission. Furthermore, sequence alignment of template and target sequences were carried out by protein BLASTp against PDB database. Structure refinement and energy minimization were done and then 3D structure files were analyzed using Swiss PDB Viewer andModeller 9.10.
Ramachandran plots analysis
Ramachandran plots analysis by RAMPAGE assessment of the model was performed to determine the stereochemical quality and accuracy of the predicted model structure(37,39). The model with the minimum number of residues in the outlier region was selected for further studies.
Molecular dynamics simulations
Molecular dynamics (MD) simulation is an appropriate way of optimization and completion of protein folding. In MD simulation step, proteins can undergo conformational changes (folding/unfolding) causing changes in their substrate-binding site. All MD simulations were carried out by the GROMACS 4 package using the g431a1force field (Gromos87)(39). The Swiss PDB Viewer was employed in order to structural superimposition.
In our study, the chain A of IGF-1 protein (1PMX) was used as a template for simulation of water molecules represented using a simple point charge (SPC216) model. Chloride counter-ions were added by replacing water molecules to ensure the overall charge neutrality of the simulated system that comprised of IGFBP-3 or IGF-1. At first, an energy minimization process was carried out. After this step, position restraint procedure was performed in association with canonical (NVT) and isothermal–isobaric (NPT) ensembles. An NVT ensemble was adopted at the constant temperature of 323 K. After stabilization of temperature an NPT ensemble was performed. In this phase a constant pressure of 100 kPa was employed.
Docking protocol
Docking study is generally based on ab initio docking and data-driven docking. Ab initio docking considers just on the coordinates of the structures, despite any experimental information about the system; and experimental data is often used to improve the docking results. Whereas, data-driven docking directly process experimental or predictional information. In this study, docking was performed by HADDOCK program which is a popular docking program that follows a true data-driven strategy and can be applied for docking protein-protein, protein-nucleic acid, protein-oligosaccharide, and protein-small molecule complexes(40). At first, experimental data were entered in the form of active and passive residues of IGF-1 and IGFBP-3. Then the data were converted by HADDOCK into ambiguous interaction restraints (AIRs) used to drive the docking. The docking protocol consists of three stages: a rigid body energy minimization, a semi-flexible refinement in torsion angle space, and a final refinement in explicit solvent. After each of these stages, structures were scored and ranked, and the best structures were adopted for the next stage. The HADDOCK score is a sum of van der Waals, electrostatic, desolvation, and restraint violation energies together with buried surface area. After a successful docking run, clustered results were displayed and for every cluster, various energies that make up the HADDOCK score were presented.
RESULTS
Prediction of 3D for IGFBP-3 structure by homology modeling Phyre2 and its validation by Ramachandran
A fast and accurate method for building and prediction 3D structure of a protein is homology modeling. Recently it has been accepted that the necessary condition for successful homology modeling is a sufficient similarity between the protein sequences. As indicated in Fig. 2 multiple sequence alignment as a reliable method for identifying highly conserved residues showed that the N-and C-domains of this protein contain cysteine-rich residues which are well conserved, while the mid-region (linker domain) is weakly conserved (Fig. 2). The N-terminal domain has twelve conserved cysteine residues and the C-terminal domain has six ones. Six intra domain disulfide bonds are found in the N-terminal domain (residues 13-40, 16-42, 24-43, 31-46, 54-61, and 67-87) and three in C-terminal domain (residues 186-213, 224-235, and 237-258). since no specific protein to use as a template is not present, this study used multiple-template for simulation of IGFBP-3 structure. The PDB ID of templates are as follows: 1BOE, 1H59, 2DSP and 1WQJ(33,34,35,36).
Fig. 2.

Multiple sequence alignments of mature forms of N and linker domains of IGFBP-3 with other IGFBP1-6 in human. There are numerous amino acids indicated in black color which strongly conserved almost in all sequences. Also amino acids indicated by stars, show conserved sites which are important in binding with IGF-1. Serine111 is indicated by a circle. Conserved in linker domain is low.
In this alignment, sequence of an unknown protein is aligned with sequences of known protein structures. Therefore, the 3D structure of human IGFBP-3 built through homology modeling using the 3D structure of templates should be suitable for modeling of IGFBP-3.From the 100 model produced by Modeller, the best model with the lowest value of the probability density function was selected as the initial model for further analysis. However, due to extra amount of loop in this predicted structure, it was not satisfying.
Phyre2 predicted structure for total sequence of IGFBP-3, similar with model predicted by Modeller software was not satisfying. In order to use Phyre2 its sequence was divided in three sections prior to 3D structure prediction. First part of this mature protein which includes residues of 1 to 97 called N-domain. The best homologous template was growth factor binding domain suggesting that this hydrophobic residues of the N-terminal domain participate in IGF binding properties which is confirmed by several studies(32,41).
Second parts of its structure which contains 98 to 183 residues called linker, has no satisfying structural homology with known proteins. Third domain called C-domain starts from 184 to 264 residues. Therefore, both the N-and C-domains of the IGFBP-3 act in a cooperative manner to bind IGF preventing IGF receptor binding which results in IGF activity reduction.
The structure prediction of IGFBP-3 has shown that 35.6 % of this protein contains 15.9% alpha helix (and 19.7% β-sheet (which can make flexibility to this protein for various interactions by other proteins like IGF-1 and IGF-2, transmembrane protein 219 (TMEM 219), ALS, retinoid X receptor (RXR) etc(42,43,44).
Comparison of six members of IGFBPs N-domain, that belong to IGF binding domain of IGFBP-3 with other N-domain of IGFBPs are shown in Table 1 and Fig. 3 illustrating that N-domain of IGFBP-5 have most identity in primary and secondary structure. The C-domain of IGFBP-3, which belongs to thyroglobulin type I domain, with C-domain of IGFBPs have shown most identity to IGFBP-5(43). Because of the lack of IGFBP-5 structure, next similar protein is IGBPP-6 suggesting that maybe there is a motif containing a helix and an anti-parallel β-sheet in the structure of IGFBP-3.
Table 1.
Comparison of insulin-like growth factor binding protein 3 (IGFBP-3) domains with other IGFBPs domains. Percentage of amino acid sequence identity BP3 and its domains in comparison with IGFBPs domain.
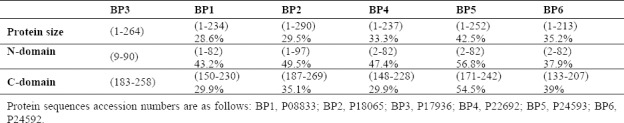
Fig. 3.
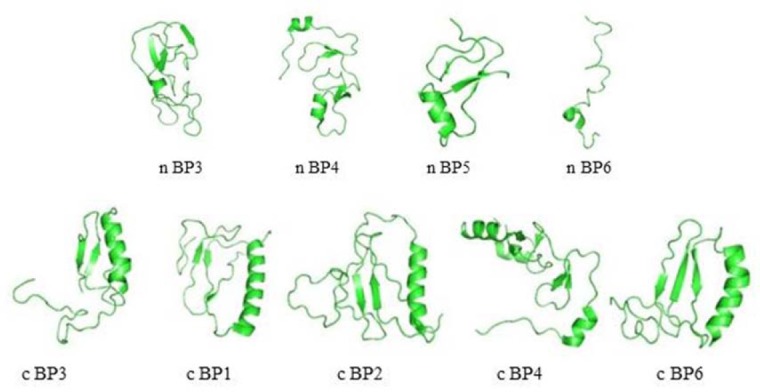
Overview of predicted secondary structure of insulin-like growth factor binding proteins (IGFBPs). Comparison of N-domain of insulin-like growth factor binding protein 3 (IGFBP-3) with IGFBP-4 (PDB code, 1WQJ), IGFBP-5 (PDB code, 1H59), and IGFBP-6 (PDB code, 2JM2). C-domain comparison of IGFBP-3 with IGFBP-1(PDB code, 1ZT5), IGFBP-2 (PDB code, 2H7T), IGFBP-4 (PDB code, 2DSP), and IGFBP-6 (PDB code, 1RMJ).
3D Structure of IGF-1
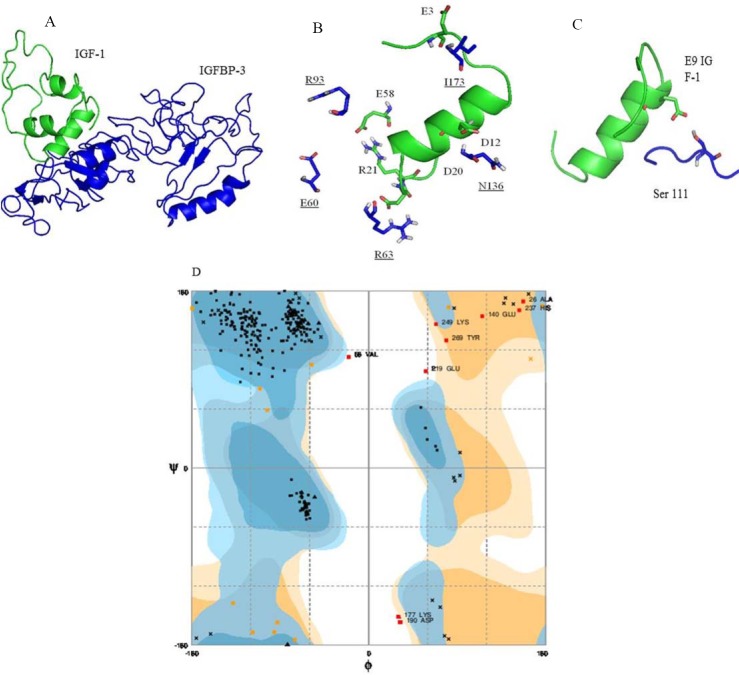
IGF-1 is a 70-amino acid protein showing many growth-promoting and metabolic activities(44). There are several crystallography 3D structures of IGFs by both nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography(44,45,46). Structure of IGFs consists of four parts and IGFs belongs to three helix bundle. The secondary structural principle of IGF-1 and IGF-2 is α-helix. The A part consists of helix 2 (Ile43-Cys47 of IGF-1 and Glu44-Phe48 of IGF-2) and helix 3 (Arg56-Tyr60 of IGF-1 and Ala54-Tyr59 of IGF-2), whereas the B part contains helix 1 (Gly7-Cys18 of IGF-1 and Gly10-Val20 of IGF-2)(46). The IGF-1 C and D parts with loop structure are highly flexible in solution (Fig. 4A). Mutagenesis studies have shown that three disulfide bonds including Cys6-Cys48, Cys18-Cys61, and Cys47-Cys52 stabilize its 3D fold(47).
Fig. 4.
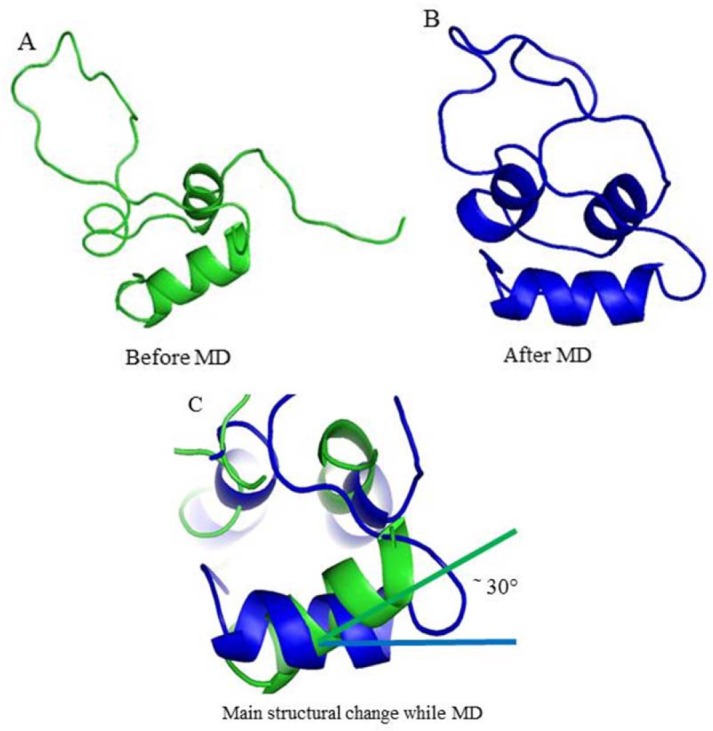
Three dimensional structure of insulin-like growth factor 1 (IGF-1) (PDB code, 1PMX). (A) Before molecular dynamics (MD), (B) after MD, and (C) structural superposition of IGF-1 before MD and after MD. IGF-1 structure change in helix-1 gives rotation angle of about 30°, which is main changing of IGF-1 through MD.
MD simulation of IGF-1 proceed in 20 ns, shown changing of the conformation. As shown changing of the conformation. As shown in Fig. 4B, MD influenced GF-1 folding as compacted structure. Superposition of IGF-1 before and after MD with Swiss PDB Viewer showed that helix-1 in IGF-1 rotated around 30° (Fig. 4C).
Docking prediction of IGFBP-3/IGF-1
Active and passive residues of IGF-1 should be entered to software
The selected template was then forwarded for the Modeller analysis and then final models predicted are shown in Fig. 5A. The active residues of IGF-1 involved in the interaction with IGFBP-3 are provided in Fig. 5B, 5C and Tables 2 and 3. All listed residues in Table 3 are located on the surface of IGF-1.The active residues Glu3, Glu9, Asp12, Phe16, Val17, Gly19, Asp20, Arg21, and Glu58 have central importance for the interaction(48,49). The distance side chain of these amino acids with corresponding amino acids in IGFBP-3 is less than 5 Å.
Fig. 5.
Prediction of protein-protein interaction of insulin-like growth factor binding protein 3 (IGFBP-3) with insulin-like growth factor 1 (IGF-1). (A) This predicted strucure showed that IGF-1 binds to N-domain and linker domain of IGFBP-3. (B) Underlined residues of IGFBP-3 are interacting with helix 1 of IGF-1. (C) This predicted structure showed that serine111 of IGFBP-3 is located near to Glu9 of IGF-1 whose phosphorylatin might interrupts this interaction through repulsive forces between phosphate group and glutamic acid. (D) Ramachandran plot of IGFBP-3. The dark, medium dark and white area represents respectively most favored, allowed, and disallowed regions.
Table 2.
The best cluster produced by HADDOCK for insulin-like growth factor binding protein (IGFBP-3) and insulin-like growth factor 1 (IGF-1).
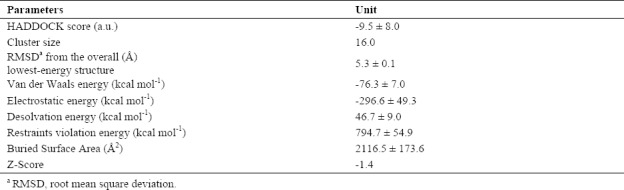
Table 3.
Residues of insulin-like growth factor 1 (IGF-1) interacting with insulin-like growth factor binding protein 3 (IGFBP-3) in the best predicted models with interaction distance between residues

Active and passive residues of IGFBP-3 should be entered to software
Computational docking is the prediction of the 3D structure of a bimolecular complex which uses the individual molecules. For IGFBP-3/IGF-1 interaction, HADDOCK clustered 159 structures in 16 clusters. Table 2 displays the best scoring structure with lower energy and Z-score that indicates standard deviations of a given cluster is separated from the mean of all clusters from the average. The best clusters of the predicted structure have some residues of IGFBP-3 and their distance which are interacting with the related structure of IGF-1 (Table 3). Z-score -1.4 and RMSD 5.3 Å for docking experiment suggested that the complex structure of IGFBP-3/IGF-1 is an acceptable model.
Fig. 5B and Fig. 5C summarize the active residues of IGFBP-3 correspond to interface residues of IGF-1 identified by docking experiment. The active residues Ile146, S111, Asn109, Arg60, Gly39, Arg36, and Glu33and Arg66 of IGFBP-3 are identified on the surface of protein.
Data obtained were rechecked by Ramachandran plot. This finding showed that 92.8% residues are in the most favored regions, 4.1% residues are in allowed regions while 3.1% residues are in disallowed region (Fig. 5D).
IGFBP-3 fragments with different lengths including the first 97 residues (1-97 IGFBP-3) are capable of binding IGFs, though with a lower affinity than intact IGFBP-3. The N-domains of IGFBP-3 like IGFBP-4 and -5 has rigid globular structures, whereas the C-domains of IGFBP-3 adopt a thyroglobulin type 1 fold contain some more flexible regions like that seem in IGFBP-1,-2 and -6 (Fig. 3). Abundant evidences show the importance of amino-terminal IGFBPs residues in interacting with the IGFs.(50,51).
DISCUSSION
IGFBP-3 is one the most abundant protein in serum which interacts with IGFs. Despite decades of unremitting research, the crystal structure of IGFBP-3 has not been elucidated; therefore, the present study was designed to predict 3D structure of IGFBP-3 in interaction with IGF-1. IGFBPs share the similar overall 3 domain structures, with N-, linker and C-domain. Linker connects N-and C-domains. Our results indicate that N-domain primary and secondary structure of IGFBP-3 is similar to N-domain of IGFBP-5 (Table 1 and Fig. 3). Forbes et al. indicated that N-domain of IGFBP-6 has different structure compared to IGFBP-4 and -5(31). The predicted secondary structure of N-domain seen in IGFBP-3 contains stranded β-sheet and α-helix that comparable to IGFBP-4 and 5(33). Comparative secondary structures of N-domain of IGFBPs are shown in Fig. 3 which might be involved in IGF binding. The 9th to 12th conserved cysteine residues of IGFBP-1 to IGFBP-5 located in N-domain. Site-directed mutagenesis in N-domain of IGFBPs is necessary for association with IGF binding affinity(48,49,50,51,52). The secondary structure of C-domain in IGFBPs adopt to thyroglobulin typ-1 structure. Fig. 3 also shows that C-domain of IGFBP-3 possess folding similar to thyroglobulin(41). The current study predicted that contains large amount of loop which make high flexibility for this protein (Fig. 5). Kalus, et al. describe the flexibility of IGFBP-5(32) which may enable it to bind to its partners such as IGFs, TMEM-219 and ALS(52,53). On the other hand, IGF-1 due to its flexible structure has a vital role in various stage of growth and metabolism(1,2,8). In this regard, our MD experiments showed that IGF-1 structure is flexible. The conformational rearrangement about 30° in helix-1 of IGF-1 enables high affinity interaction with IGFBP-3 (Fig. 4). Subsequently, this ability makes it a partner for various proteins such as IGF-1R and IGFBPs(12,45). Recently it has been shown that linking N-and C-domain of IGFBP-3 in collaboration with N-domain elevates affinity of IGF binding. Therefore isolated N-domain led to 10-fold reduction in IGF binding affinity than indicted IGFBP-3(2,51,54). In addition, mutational studies of IGFBP-3 have revealed that Gly217Ser and Gln223Ala in C-domain disrupted IGF binding by 4 to 11 folds(55). Further studies have shown that mutation of residues 215-232 in IGFBP-3 interrupts interaction with ALS suggesting that its C-domain is more important in IGF-1 function(54). Taken together the N-and C-domains of IGFBP-3 were similar with corresponding domains in other IGFBPs. But, the linker is more likely to be different between IGFBPs (Fig. 2). Our data showed that most of post translational modifications of this protein are occurred in the linker (Fig. 1)(18,20). Mutagenesis studies by Song et al. on C-domain of IGFBP-5 revealed that the mutation of Gly217Ser and Gln223Ala disrupted the IGF binding affinity(53). Our docking studies have identified residues of IGFBP-3 that interact with IGF-1. This residues form the deep binding clef into IGF-1. The experimental results obtained from mutants of IGF-1, Glu3, Gly7, Leu10, Val17, and Phe25 have shown that these mutants are important residues for IGFBPs binding(9). Other important IGFBP-binding determinants of IGF-1 include Gln15 and Phe16 in the B-part and Phe49, Arg50, and Ser51 residues in A-part(48). Based on the model of the tertiary structure of IGF-1 proposed by Blundell, et al. it may be concluded that residues 3-4, 49-51, and 55-56 are on the surface of the IGF-1 molecule in locations suitable for interaction with IGFBP-3(49). The α-helical region of the B-domain (residues 8-18) also plays a critical role. Residues of Phe23, Tyr24, and Phe25 of IGF-1 are critical for IGF-1R binding; thus binding of IGF-1 to IGFBPs hinders its interaction with the IGF-1 receptor(49).
Clinical and practical studies have shown that phosphorylation of Ser111 located in the linker of IGFBP-3 significantly led to the induction of apoptosis(19,56). Therefore, our data suggested that this modification either interrupts hydrogen bond between Asp12 of IGF-1 and Gly108/Asn109 of IGFBP-3 or make repulsion force to repel Glu9 of IGF-1 (Fig. 5). Taken together, phosphorylation of Ser111 leads to separation of IGFBP-3 to induce apoptosis.
CONCLUSIONS
This study represents predicted structure of IGFBP-3 and defines contact residues that interact with IGF-1. These residues are located on surface of IGFBP-3 and affected the affinity of IGF-1. Our findings suggest that phosphorylation of IGFBP-3 (ser111) make a repulsive force to interrupt this interaction which enables IGFBP-3 to induce apoptosis. We propose employment of the information of this protein-protein interaction to develop new peptide drugs to treat various cancers. In addition, understanding of molecular interaction of IGFBP-3 to other ligands provides the basis of a specific tool or strategy for manipulation in IGF-depend and IGF-independ action.
ACKNOWLEDGEMENTS
This work was financially supported by the Bioinformatics Research Center (Grant No. 290319), Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab. 2016;27(6):375–391. doi: 10.1016/j.tem.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int. 2015. 2015 doi: 10.1155/2015/538019. Article ID 538019. DOI: 10.1155/2015/538019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nissley P, Lopaczynski W. Insulin-like growth factor receptors. Growth Factors. 1991;5(1):29–43. doi: 10.3109/08977199109000269. [DOI] [PubMed] [Google Scholar]
- 5.Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31(1):805–818. doi: 10.1007/s12032-013-0805-3. [DOI] [PubMed] [Google Scholar]
- 6.Rechler MM. Insulin-like growth factor binding proteins. In: McCormick D, editor. Vitamins and Hormones. Vol 47. San Deigo: Academic Press; 1993. pp. 8–14. [DOI] [PubMed] [Google Scholar]
- 7.Murekatete B, Shokoohmand A, McGovern J, Mohanty L, Meinert C, Hollier BG, et al. Targeting Insulin-Like Growth Factor-I and Extracellular Matrix Interactions in Melanoma Progression. Sci Rep. 2018;8(1):583–594. doi: 10.1038/s41598-017-19073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8(1):45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 9.Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16(4-5):421–439. doi: 10.1016/j.cytogfr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Khodadadi E, Panjepour M, Abbasian M, Broujeni ZK, Mofid MR. Cloning and expression of full-length human insulin-like growth factor binding protein 3 (IGFBP3) in the Escherichia coli. Adv Biomed Res. 2015;4:66–74. doi: 10.4103/2277-9175.153886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh Y, Rosenfeld RG. Contemporary Endocrinology. Totowa: Humana Press; 1999. IGF-independent actions of the IGF binding proteins; pp. 257–272. [Google Scholar]
- 12.Ansari A, Gheysarzadeh A, Mofid M.R. The interaction of insulin-like growth factor binding protein 3 (IGFBP-3) in insulin-like growth factor (IGF)-independent system. JIMS. 2017;35(451):1452–1461. [Google Scholar]
- 13.Rechler MM, Clemmons DR. Regulatory actions of insulin-like growth factor-binding proteins. Trends Endocrinol Metab. 1998;9(5):176–183. doi: 10.1016/s1043-2760(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 14.Alkharobi H, Alhodhodi A, Hawsawi Y, Alkafaji H, Devine D, El-Gendy R, et al. IGFBP-2 and-3 co-ordinately regulate IGF1 induced matrix mineralisation of differentiating human dental pulp cells. Stem Cell Res. 2016;17(3):517–522. doi: 10.1016/j.scr.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Liu X, Wang Y, Tian H, Xie Y, Li Q, et al. Insulin-like factor binding protein-3 promotes the G1 cell cycle arrest in several cancer cell lines. Gene. 2013;512(1):127–133. doi: 10.1016/j.gene.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 16.Perks C, McCaig C, Holly J. Differential insulin‐like growth factor (IGF)-independent interactions of IGF binding protein-3 and IGF binding protein-5 on apoptosis in human breast cancer cells. Involvement of the mitochondria. J Cell Biochem. 2001;80(2):248–258. doi: 10.1002/1097-4644(20010201)80:2<248::aid-jcb140>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Baxter RC. Insulin-like growth factor binding proteins in the human circulation: a review. Horm Res Paediatr. 1994;42(4-5):140–144. doi: 10.1159/000184186. [DOI] [PubMed] [Google Scholar]
- 18.Cobb LJ, Mehta H, Cohen P. Enhancing the apoptotic potential of insulin-like growth factor-binding protein-3 in prostate cancer by modulation of CK2 phosphorylation. Mol Endocrinol. 2009;23(10):1624–1633. doi: 10.1210/me.2008-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollowood A, Stewart C, Perks C, Pell J, Lai T, Alderson D, et al. Evidence implicating a mid‐region sequence of IGFBP-3 in its specific IGF-independent actions. J Cell Biochem. 2002;86(3):583–589. doi: 10.1002/jcb.10223. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Soderland D, Steinle JJ. TNFα Inhibits IGFBP-3 through Activation of p38α and Casein Kinase 2 in Human Retinal Endothelial Cells. PLoS One. 2014;9(7):e103578. doi: 10.1371/journal.pone.0103578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ST, Jang H-L, Lee J, Park SH, Park YS, Lim HY, et al. Clinical significance of IGFBP-3 methylation in patients with early stage gastric cancer. Transl Oncol. 2015;8(4):288–294. doi: 10.1016/j.tranon.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu T, Pappou EP, Guzzetta AA, de Freitas Calmon M, Sun L, Herrera A, et al. IGFBP-3 gene methylation in primary tumor predicts recurrence of stage II colorectal cancers. Ann Surg. 2016;263(2):337–344. doi: 10.1097/SLA.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho GY, Zheng SL, Cushman M, Perez-Soler R, Kim M, Xue X, et al. Associations of insulin and IGFBP-3 with lung cancer susceptibility in current smokers. J Natl Cancer Inst. 2016;108(7) doi: 10.1093/jnci/djw012. DOI: 10.1093/jnci/djw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scully T, Scott CD, de Silva HC, Firth SM, Twigg SM, Pintar JE, et al. Insulin-like growth factor binding protein-3 (IGFBP-3) enhances obesity-related breast tumorigenesis. Oncotarget. 2016;7(34):55491–55505. doi: 10.18632/oncotarget.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe K, Uemura K, Asada M, Maesako M, Akiyama H, Shimohama S, et al. The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer's disease. Mol Brain. 2015;8(1):82–95. doi: 10.1186/s13041-015-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhard FP, Heinzel S, Binder G, Weber K, Apel A, Roeben B, et al. Insulin-like growth factor 1 (IGF-1) in Parkinson's disease: potential as trait-, progression-and prediction marker and confounding factors. PLoS One. 2016;11(3):e0150552. doi: 10.1371/journal.pone.0150552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Retnakaran R. The Insulin-Like Growth Factor Axis: A New Player in Gestational Diabetes Mellitus. Diabetes? 2016;65(11):3246–3248. doi: 10.2337/dbi16-0048. [DOI] [PubMed] [Google Scholar]
- 28.Lofqvist C, Chen J, Connor KM, Smith AC, Aderman CM, Liu N, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A. 2007;104(25):10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen J, Stolk R, Pols H, Grobbee D, Lamberts S. Serum total IGF-I, free IGF-I, and IGFBP-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol. 1998;18(2):277–282. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- 30.Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28(9):1744–1748. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- 31.Forbes BE, McCarthy P, Norton R. Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol (Lausanne) 2012;3:38–50. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalus W, Zweckstetter M, Renner C, Sanchez Y, Georgescu J, Grol M, et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. EMBO J. 1998;17(22):6558–6572. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Żesławski W, Beisel HG, Kamionka M, Kalus W, Engh RA, Huber R, et al. The interaction of insulin‐like growth factor-I with the N-terminal domain of IGFBP-5. EMBO J. 2001;20(14):3638–3644. doi: 10.1093/emboj/20.14.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci U S A. 2006;103(35):13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siwanowicz I, Popowicz GM, Wisniewska M, Huber R, Kuenkele K-P, Lang K, et al. Structural basis for the regulation of insulin-like growth factors by IGF binding proteins. Structure. 2005;13(1):155–167. doi: 10.1016/j.str.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbari V, Moghim S, Mofid MR. Comparison of epothilone and taxol binding in yeast tubulin using molecular modeling. Avicenna J Med Biotechnol. 2011;3(4):167–175. [PMC free article] [PubMed] [Google Scholar]
- 38.Lovell SC, Davis IW, Arendall WB, De Bakker PI, Word JM, Prisant MG, et al. Structure validation by Cα geometry:phi,psi and Cbeta deviation. Proteins. 2003;50(3):437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 39.Hess B, Kutzner C, Van Der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4(3):435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein− protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125(7):1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- 41.Payet LD, Wang X-H, Baxter RC, Firth SM. Amino-and carboxyl-terminal fragments of insulin-like growth factor (IGF) binding protein-3 cooperate to bind IGFs with high affinity and inhibit IGF receptor interactions. Endocrinology. 2003;144(7):2797–2806. doi: 10.1210/en.2003-0102. [DOI] [PubMed] [Google Scholar]
- 42.Moreno-Santos I, Castellano-Castillo D, Lara MF, Fernandez-Garcia JC, Tinahones FJ, Macias-Gonzalez M. IGFBP-3 Interacts with the Vitamin D Receptor in Insulin Signaling Associated with Obesity in Visceral Adipose Tissue. Int J Mol Sci. 2017;18(11):2349–2364. doi: 10.3390/ijms18112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimasaki S, Shimonaka M, Zhang H-P, Ling N. Identification of five different insulin-like growth factor binding proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human. J Biol Chem. 1991;266(16):10646–10653. [PubMed] [Google Scholar]
- 44.Jafari S, Babaeipour V, Seyedi HE, Rahaie M, Mofid M, Haddad L, et al. Recombinant production of mecasermin in E. coli expression system. Res Pharm Sci. 2014;9(6):453–461. [PMC free article] [PubMed] [Google Scholar]
- 45.Brown J, Delaine C, Zaccheo OJ, Sieb C, Gilbert RJ, Van Boxel G, et al. Structure and functional analysis of the IGF-II/IGF2R interaction. EMBO J. 2008;27(1):265–276. doi: 10.1038/sj.emboj.7601938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci U S A. 2006;103(35):13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato A, Koyama S, Yamada H, Suzuki S, Tamura K, Kobayashi M, et al. Three-dimensional solution structure of a disulfide bond isomer of the human insulin-like growth factor-I. Chem Biol Drug Des. 2000;56(4):218–230. doi: 10.1034/j.1399-3011.2000.00769.x. [DOI] [PubMed] [Google Scholar]
- 48.Dubaquié Y, Lowman HB. Total alanine-scanning mutagenesis of insulin-like growth factor I (IGF-I) identifies differential binding epitopes for IGFBP-1 and IGFBP-3. Biochemistry. 1999;38(20):6386–6396. doi: 10.1021/bi990089p. [DOI] [PubMed] [Google Scholar]
- 49.Blundell T, Bedarkar S, Rinderknecht E, Humbel R. Insulin-like growth factor: a model for tertiary structure accounting for immunoreactivity and receptor binding. Proc Natl Acad Sci U S A. 1978;75(1):180–184. doi: 10.1073/pnas.75.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. J Biol Chem. 2002;277(12):10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- 51.Imai Y, Moralez A, Andag U, Clarke JB, Busby WH, Clemmons DR. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and-5 markedly reduce IGF-I binding and alter their biologic actions. J Biol Chem. 2000;275(24):18188–18194. doi: 10.1074/jbc.M000070200. [DOI] [PubMed] [Google Scholar]
- 52.Carrick FE, Forbes BE, Wallace JC. BIAcore analysis of bovine insulin-like growth factor (IGF)-binding protein-2 identifies major IGF binding site determinants in both the amino-and carboxyl-terminal domains. J Biol Chem. 2001;276(29):27120–27128. doi: 10.1074/jbc.M101317200. [DOI] [PubMed] [Google Scholar]
- 53.Fowlkes JL, Thrailkill KM, George-Nascimento C, Rosenberg CK, Serra DM. Heparin-binding, highly basic regions within the thyroglobulin type-1 repeat of insulin-like growth factor (IGF)-binding proteins (IGFBPs) -3, -5, and -6 inhibit IGFBP-4 degradation. Endocrinology. 1997;138(6):2280–2285. doi: 10.1210/endo.138.6.5182. [DOI] [PubMed] [Google Scholar]
- 54.Yan X, Forbes BE, McNeil KA, Baxter RC, Firth SM. Role of N-and C-terminal residues of insulin-like growth factor (IGF)-binding protein-3 in regulating IGF complex formation and receptor activation. J Biol Chem. 2004;279(51):53232–53240. doi: 10.1074/jbc.M409345200. [DOI] [PubMed] [Google Scholar]
- 55.Shand JH, Beattie J, Song H, Phillips K, Kelly SM, Flint DJ, et al. Specific amino acid substitutions determine the differential contribution of the N-and C-terminal domains of insulin-like growth factor (IGF)-binding protein-5 in binding IGF-I. J Biol Chem. 2003;278(20):17859–17866. doi: 10.1074/jbc.M300526200. [DOI] [PubMed] [Google Scholar]
- 56.Hoeck WG, Mukku VR. Identification of the major sites of phosphorylation in IGF binding protein‐3. J Cell Biochem. 1994;56(2):262–273. doi: 10.1002/jcb.240560220. [DOI] [PubMed] [Google Scholar]