Abstract
Natural plants have traditionally been used throughout the world for their anti-diabetic effects. The aim of the present study was to investigate the protective effect of hydroalcoholic extract of Trifolium pratens L. (T. pratense) on streptozotocin (STZ) cytotoxicity and insulin concentration from RIN-5F pancreatic β cell line. In this study, possible cytoprotective action of T. pratense extract (using pre-treatment, simultaneous, and post-treatment schedules) against STZ (30 mM) was evaluated using MTT assay. Apoptosis was quantified by fluorescent dye staining. Also, the effect of extract on insulin secretion in low and high glucose media was examined. Data were analyzed by one-way ANOVA test and P < 0.05 was considered significant. The viability of RIN-5F cells in 10, 20, 30, 40, and 60 μg/mL doses of T. pratense extract showed significant increases compared to control group (P < 0.001). STZ significantly reduced cell viability in a dose-dependent manner (P < 0.05). T. pratense extract in 20, 30, and 40 μg/mL doses had significant cytoprotective effect (P < 0.05) on cytotoxic action of STZ and this effect is greater in simultaneous treatment. STZ-mediated apoptotic death is reduced by extract. T. pratense extract treatment also, increased insulin concentration in cell culture medium. T. pratense had potent cytoprotective action, prevented apoptosis and increased insulin concentration in cell culture medium via the increase in pancreatic β cell number and/or insulin secretion. In addition, T. pratense enhanced viability of RIN-5F. Thus, T. pratense not only has anti-diabetic actions on β cells but also enhances their viability.
Keywords: Insulin secretion, Protective effect, Streptozotocin, Trifolium pratense L.
INTRODUCTION
Diabetes mellitus, commonly referred to as diabetes is one of the most common metabolic disorders characterized by high blood glucose. It is due to either the pancreas not producing enough insulin or the cells of the body not responding properly to the produced insulin. Chronic hyperglycemia in diabetic patients damages many of the body's systems, in particular eyes, kidneys, nerves, heart, and blood vessels(1).
The incidence of diabetes has increased rapidly over the past two decades, due to lack of physical activity, poor dietary habits, overweight or obesity, and psychological stress(2,3).
The World Health Organization has predicted that by 2030 the number of adults with diabetes would have almost doubled worldwide, from 177 million in 2000 to 370 million(4,5). This disease may become the strongest and deadliest leading cause of humans’ death in the future(2). Due to the limitation of currently available antidiabetic drugs especially in terms of efficacy and safety, development of new strategies are necessary for the prevention and management of this kind of disease.
Herbal remedies are among the best existing alternative therapies(6), which have been used since ancient times for the treatment of diabetes. In literature over 800 plants are reported for their antidiabetic properties and over 1200 plants are used in traditional medicine for reduction in blood glucose levels. To the date, more than 400 traditional plant treatments for diabetes have been reported, although only a small number of them have received clinical evaluation to assess their efficacy(7).
Numerous plant extracts containing isoflavones have been studied during the last decade for their protective effects against a variety of disorders, including cardiovascular disease, cancer, hyperlipidemia, and osteoporosis(8,9). In addition, the potential of dietary isoflavones in the prevention of diabetes has attracted increasing attention in recent years(9,10). Trifolium pratense L. (T. pratense), a member of Leguminosae or Fabaceae family, is a short-lived biennial plant which has been used as a health food for humans. It is probably native to Europe, western Asia, and northwest Africa, but today it has been naturalized in other continents(11). It has also been suggested in traditional medicine to treat of some human diseases such as whooping cough, asthma, eczema, and eye diseases(12). Our previous study showed the anticancer properties of T. pratens hydroalcoholic extract on glioma cell line (U87MG)(13).
An in vivo study suggested that the T. pratense extract had a significant effect on lowering the blood glucose levels and upregulated hepatic glucokinase of mice. So, it significantly improved the glucose homeostasis in mice(14). In the present study an in vitro effect of T. pratense extract on rat insulin secreted cell line (RIN-5F) was investigated.
MATERIALS AND METHODS
Cell line and reagents
For this in vitro experimental study, rat RIN-5F cell line was obtained from the national cell bank of Iran (NCBI). Trypsin, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), streptozotocin (STZ), and glucose were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). RPMI 1640 and fetal bovine serum (FBS) were purchased from Gibco (Gibco Company, USA). All experiments were performed in triplicate and repeated independently at least three times. The study was approved by Ethical Committee of Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran (Code: kums.res.1395.46).
Extract preparation
Trifolium pratense seed was prepared from agricultural and engineering research institute (Karaj, I.R. Iran), cultured (spring 2017) in a farm and identified in terms of species by a botanist (Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran). Aerial parts of the herbs were dried and powdered, and 15 g of it was dissolved in 150 mL of 70% ethanol for 48 h in darkness. Then it was filtered through filter paper and dried to evaporate the alcohol at room temperature. Finally, the powder was dissolved in a serum-free cell culture medium, and passed through a 0.22 μm filter(13).
Cell culture conditions
Rat pancreatic β cell line (RIN-5F) was grown in polystyrene cell culture flasks containing RPMI 1640 (pH 7.4) supplemented with 10% FBS and without antibiotics. The cells were subcultured at regular intervals of 3 days till they attained confluence. Then, they were harvested by trypsin (0.25%)-ethylenediaminetetraacetic acid (EDTA) (0.02%) solution. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Dose and time optimization of extract
Rat pancreatic β cells (RIN-5F) were cultured in 96-well plates. The various concentrations of extract used were 1, 5, 10, 20, 30, 40, 60, 80, and 100 µg/mL and periods of incubation time were 1, 6, 12, and 24 h. Cell viability was determined by MTT assay(15).
Dose and time optimization of streptozotocin
Rat pancreatic β cells (RIN-5F) were cultured (5 × 104 cells/well) in 96-well plates. STZ was dissolved in cell culture medium. After 48 h of attachment period, cells were treated with different concentrations of STZ (1 mM-40 mM) and incubated 12, 24, and 48 h, after which the viability of cells was assayed using MTT assay as described previously. The IC50 value of STZ was obtained by nonlinear regression using GraphPad Prism 5 (GraphPad Software Inc, San Diego, USA).
Effect of extract on streptozotocin-induced cytotoxicity
For this study, cells were cultured in 96-well plates. Then cells were treated with optimal doses of extract and IC50 value of STZ and different periods of incubation. Three types of experiments were performed as described below.
-
(a)
Pre-treatment: In the pre-treatment study, RIN-5F cells were first incubated with different concentrations of extract for 5 h. Then, medium containing extract was removed and replaced with fresh medium containing STZ and incubated for 24 h. finally, cell viability was determined by MTT assay.
-
(b)
Simultaneous treatment: In simultaneous treatment study, RIN-5F cells were incubated with medium for 5 h and then extract and STZ were added simultaneously and incubated for 24 h. At the end of the incubation period, viability of cells was measured by MTT assay.
-
(c)
Post-treatment: In this study, RIN-5F cells were treated with STZ for 24 h. Then, medium was removed and replaced with fresh medium containing extract and incubated for an additional 5 h. MTT assay was performed.
Effect of extract on streptozotocin-induced apoptosis
Since T. pratense was able to prevent STZ-induced cytotoxic action in vitro, its ability to prevent apoptosis induced by this chemicals was investigated in the next step. RIN-5F cells were plated in 96-well plate. After attachment, cells were treated with extract (30 µg/mL) and/or STZ (30 mM) as described above. Apoptosis was evaluated by labeling the 3′-hydroxyl termini in DNA fragments using an In situ cell death detection kit, AP (Roche Diagnostics; Germany) according to the manufacturer's instructions. Briefly, after treatment, the cells were fixed with paraformaldehyde and permeabilized with Triton X-100 on ice. Then 50 μL of the TUNEL reaction mixture (label and enzyme solution) was added to each well, followed by incubation for 1 h at 37 °C. For differential staining of the cells, the propidium iodide (PI) staining solution was used. The plate was incubated for 5 min at room temperature. The cells were then rinsed three times with PBS and analyzed under a fluorescent microscope (Nikon Corporation, Tokyo, Japan). All the mentioned stages are performed in the dark. The apoptotic index of the cells was calculated as follows(16):
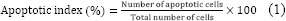
Insulin secretion assay
Rat pancreatic β cells (RIN-5F) were cultured in 24-well plates at 2 × 105 cells/well. After attachment the medium was removed and replaced with fresh medium containing low glucose (6.25 mM) or high glucose (12.5 mM) supplemented with various concentrations of extract. After 12 h, the aliquots in all wells were collected to determine the concentration of insulin in the media with the use of enzyme-linked immunosorbent assay (ELISA) kit (insulin ELISA kit, Ab100578, Abcam, Cambridge, UK) according to the manufacturer's instructions. The insulin secretion levels in cell culture medium were assessed by comparing them with the control insulin secretion level.
Statistical analysis
All data are presented as mean ± standard deviation of three independent experiments. Statistical evaluation was done using one-way analysis of variance with SPSS version 16.0 (SPSS Inc, Chicago, IL, USA) software, and differences were considered to be statistically significant when P < 0.05.
RESULTS
Extract effect on rat pancreatic β cells (RIN-5F) viability
The effects of various concentrations of extract (1, 5, 10, 20, 30, 40, 60, 80, and 100 µg/mL) on the viability of RIN-5F cells when incubated for different periods (1, 6, 12, and 24 h) were studied and these results are given in Fig. 1. It is evident that extract is not toxic to RIN-5F cells at the concentrations tested. In fact, some extract concentrations enhanced the viability of RIN-5F cells.
Fig. 1.
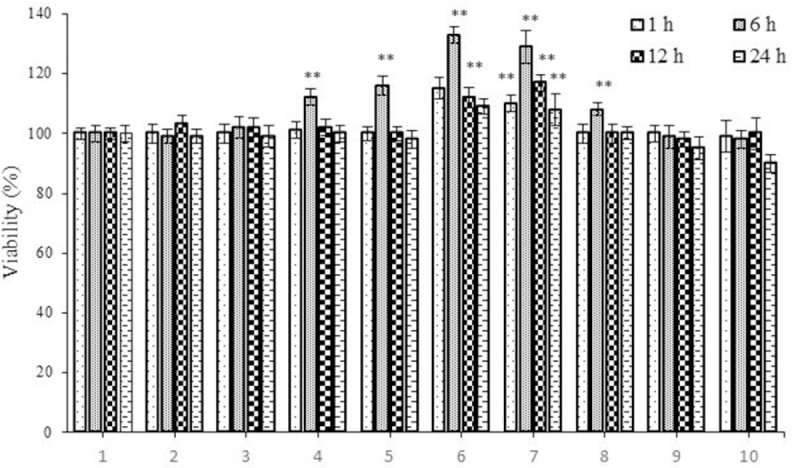
The effect of Trifolium pratense extract on viability of rat pancreatic β cells (RIN-5F). Cells were treated with extract for 1, 6, 12, and 24 h and viability was measured by MTT assay. Control wells were treated with equivalent amount of medium alone. The results show the mean ± SD from triplicated experiments. **P < 0.01 compared with control group.
Effect of streptozotocin on rat pancreatic β cells (RIN-5F) viability
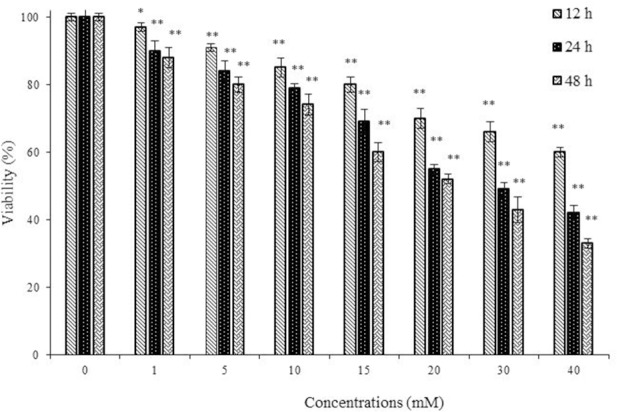
Studies with STZ showed that after exposure of RIN-5F cells to 1-40 mM for 12, 24, and 48 h, there was a significant decrease (P < 0.05) in their survival in a dose and time-dependent manner (Fig. 2). The IC50 values of STZ for 12, 24, and 48 h were 67.15, 35.21, and 23.39 mM, respectively. Based on these results, all subsequent studies were performed using 30 mM and an incubation time of 24 hr.
Fig. 2.
The effect of streptozotocin on viability of rat pancreatic β cells (RIN-5F). Cells were treated with extract for 12, 24, and 48 h and viability was measured by MTT assay. Control wells were treated with equivalent amount of medium alone. The results showed the mean ± SD from triplicated experiments. *P < 0.05 and **P < 0.01 compared with control group.
Effect of extract on streptozotocin-induced cytotoxicity
To evaluate the effect of the extract against the cytotoxic action of STZ on RIN-5F cells, 20, 30 and 40 µg/mL doses of the extract were tested individually against STZ (30 mM) using three treatment periods (Fig. 3). It is evident from the results shown in Fig. 3 that extract is most effective when used at 30 µg/mL even though other concentrations are also effective in protecting RIN-5F cells. These results showed that extract not only is non-toxic but, in fact, protected RIN-5F cells against the cytotoxic action of STZ. It was observed that of all the three treatment schedules employed in the present study, simultaneous treatment schedule with extract at a dose of 30 µg/mL is the most effective in protecting RIN-5F cells. (Simultaneous treatment > pre-treatment). Post treatment effect of the extract on STZ cytotoxicity was not significant when compared with STZ.
Fig. 3.
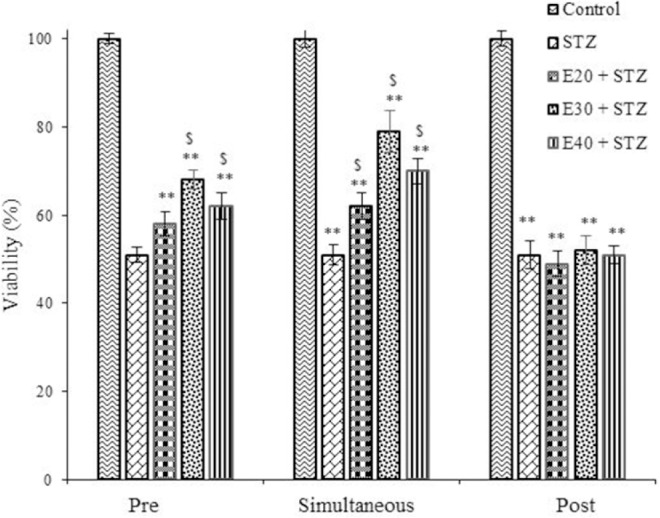
The effect of Trifolium pratense extract (20, 30, and 40 µg/mL) pre-, simultaneous, and post-treatment schedules on streptozotocin (STZ) induced cytotoxicity to rat pancreatic β cells (RIN-5F). The results showed the mean ± SD from triplicated experiments. **P < 0.01 compared with untreated control and $P < 0.01 compared with STZ-treated group. E, Trifolium pratense extract.
Apoptosis assay
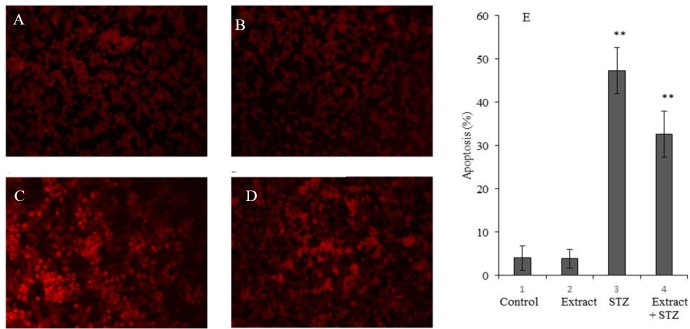
Rat pancreatic β cells (RIN-5F) were treated with extract and/or STZ. In these studies, extract was used at a dose of 30 µg/mL, the most effective concentration to protect RIN-5F cells against the cytotoxic action of STZ. The results of this study shown in Fig. 4 revealed that T. pratense preventes apoptosis induced by STZ significantly (P < 0.05).
Fig. 4.
The effect of Trifolium pratense extract (30 µg/mL) on streptozotocin (STZ)-induced apoptosis in rat pancreatic β cells (RIN-5F). (A) Untreated control cells, (B) cells treated with extract (30 µg/mL), (C) cells treated with STZ (30 mM), (D) cells treated with extract (30 µg/mL) + STZ (30 mM), and (E) columns mean percentage of apoptotic cells from three independent experiments. The data are expressed as the percentage of the control cells as the means ± SD. *P < 0.05; **P < 0.01 compared with control.
Insulin secretory assay
The concentration of insulin in cell culture medium was increased markedly after treatment with extract in all treated doses at glucose concentrations of 6.25 mm and 12.5 mm. The greatest effect in induction of insulin secretion was observed in the RIN-5F cells treated with 30 µg/mL of the extract (Fig. 5).
Fig. 5.
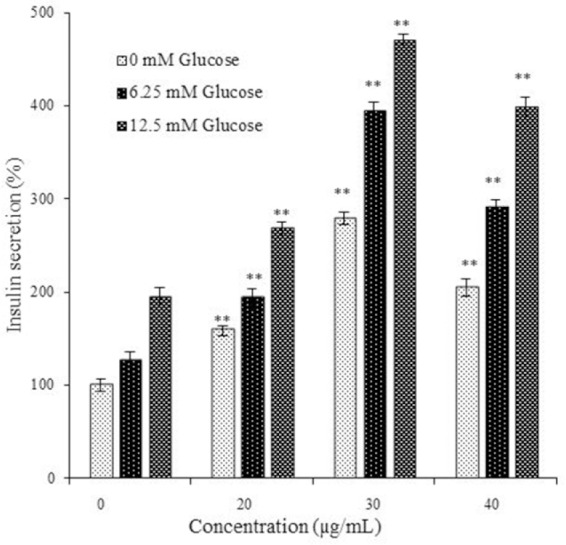
The effect of Trifolium pratense extract on glucose stimulated insulin release in rat pancreatic β cells (RIN-5F). The results showed the mean ± SD from triplicated experiments. **P < 0.01 compared with untreated control and $P < 0.01 compared with streptozotocin-treated group.
DISCUSSION
It is evident from the results of the present study that T. pratense extract prevented STZ-induced cytotoxicity in insulin-secreting pancreatic cells (RIN-5F) in vitro. STZ, a glucosamine derivative attached to cytotoxic methyl nitrosourea moiety, is toxic to β cells. STZ is taken up by pancreatic β cells via a glucose transporter (GLUT-2) and induces β cell death by alkylation of DNA. It causes DNA damage by activation of poly ADP-ribosylation resulting in depletion of cellular NAD+ and ATP, events that finally lead to the generation of superoxide, hydrogen peroxide and hydroxyl radicals(17).
Natural medicinal plants have been used throughout the world for the therapy of diabetes. Our data showed that the hydro-alcoholic extract of T. pratense increased insulin concentration in RIN-5F cells culture medium. So, T. pratense potentially had a stimulatory effect on glucose-induced insulin secretion in vitro through inducing of insulin secretion from cells or increase the number of cells.
The chemical profile of T. pratense extract obtained using the high-performance liquid chromatography–ultraviolet (HPLC-UV) chromatogram showed that it was composed of isoflavones, flavonoids, pterocarpans, coumarins and tyramine(18). Its main isoflavones are biohanin A, formononetin, daizdein, genistein, pratensein, prunetin, pseudobaptigenin, calycosin, methylorobol, afrormosin, texasin, irilin B, and irilone(19). Numerous studies have demonstrated that, genistein, a naturally-occurring isoflavone, has antidiabetic effects, in particular, direct effects on β cell proliferation, glucose-stimulated insulin secretion and protection against apoptosis(20). We believe the effects of T. pratense extract obtained in this study may be associated with its genistein compound. These effects are structure-specific and not common to all flavonoids. At physiological concentrations (< 10 µM), a direct effect of genistein on β cells is through cAMP/PKA (cyclic AMP/ protein kinase A) signaling and epigenetic regulation of gene expression. The anti-diabetic effects of genistein in both in vitro and in vivo models and potential mechanisms underlying its direct effects on β cells were investigated by Gilberta et al.(20). Flavonoids, the other compound in T. pratense, are abundant in fruits and vegetables and increasing evidence demonstrates a positive relationship between consumption of flavonoid-rich foods and disease prevention. Studies supported the beneficial effects of dietary flavonoids on glucose and lipid homeostasis. It is stated that the beneficial effects of some flavonoids are at physiological concentrations and comparable to clinically-used antidiabetic drugs. Their antidiabetic effects are mediated through enhancing insulin secretion, reducing apoptosis and promoting proliferation of pancreatic β cells. They also decrease hyperglycemia through regulation of glucose metabolism, reducing insulin resistance, inflammation and oxidative stress in muscle and fat, and increasing glucose uptake in skeletal muscle and white adipose tissue(21). It was shown that pterocarpans from ethyl acetate extract of soy leaves improved plasma glucose and insulin levels in mice with type 2 diabetes by regulating β cell proliferation and insulin sensitivity(22). Also, plant extracts containing coumarins, secondary metabolites found widely in nature plants, exhibited antidiabetic activity. Both natural coumarins and coumarin derivatives have been reported to exhibit a promising therapeutic effect on diabetes and its complications due to repairing pancreatic β cells damage, improving insulin signaling and providing anti-inflammatory and antioxidative protection(23). The anti-diabetic activity of tyramine extracted from leaves of Cissus verticilllata was reported, in diabetic rats. The plasma glucose, triglycerides and total cholesterol of these rats showed significant reductions, after the treatment with the tyramine(24). It seems that T. pratense as a rich source of isoflavones, flavonoids, pterocarpans, coumarins and tyramine, can be a good medicinal herb for diabetes treatment.
CONCLUSION
The present study demonstrated that T. pratense extract protected RIN-5F cell line from STZ cytotoxicity and significantly potentiated the insulin secretion. These results could translate into beneficial effects of this herb in the management of diabetes. In future, clinical studies are suggested.
ACKNOWLEDGMENTS
This research project (No. 96277) conducted by Mozafar Khazaei, which was financially supported by the Kermanshah University of Medical Sciences, Kemanshah, I.R. Iran.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwin E, Sheeja E, Gupta V, Jain D. Fight diabetes the herbal way. Express Pharma Rev. 2006;1:41–42. [Google Scholar]
- 3.Jarald E, Joshi SB, Jain DC. Diabetes and herbal medicines. Iran J Pharmacol Ther. 2008;7(1):97–106. [Google Scholar]
- 4.Rowley WR, Bezold C. Creating public awareness: state 2025 diabetes forecasts. Popul Health Manag. 2012;15(4):194–200. doi: 10.1089/pop.2011.0053. [DOI] [PubMed] [Google Scholar]
- 5.Ahangarpour A, Heidari H, Salehizade Junghani M, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci. 2017;12(5):416–424. doi: 10.4103/1735-5362.213987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcury TA, Grzywacz JG, Bell RA, Neiberg RH, Lang W, Quandt SA. Herbal remedy use as health self-management among older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):S142–S149. doi: 10.1093/geronb/62.2.s142. [DOI] [PubMed] [Google Scholar]
- 7.Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S. Alternative therapies useful in the management of diabetes: A systematic review. J Pharm Bioallied Sci. 2011;3(4):504–512. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong MC, Emery PW, Preedy VR, Wiseman H. Health benefits of isoflavones in functional foods? Proteomic and meta-bonomic advances. Inflammo-pharmacology. 2008;16(5):235–239. doi: 10.1007/s10787-008-8023-x. [DOI] [PubMed] [Google Scholar]
- 9.Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53(1):7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 10.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 11.Rosso BS, Pagano EM. Evaluation of introduced and naturalised populations of red clover (Trifolium pratense L.) at pergamino EEA-INTA Argentina. Genet Resour Crop Evol. 2005;52(5):507–511. [Google Scholar]
- 12.Booth NL, Overk CR, Yao P, Totura S, Deng Y, Hedayat AS, et al. Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. J Agric Food Chem. 2006;54(4):1277–1282. doi: 10.1021/jf052927u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khazaei M, Pazhouhi M, Khazaei S. Evaluation of hydro-alcoholic extract of Trifolium Pratens L. for its anti-cancer potential on U87MG Cell line. Cell. 2018;20(3):412–421. doi: 10.22074/cellj.2018.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu L, Chen T, Zhong F, Hong Y, Chen L, Ye H. Red clover extract exerts antidiabetic and hypolipidemic effects in db/db mice. Exp Ther Med. 2012;4(4):699–704. doi: 10.3892/etm.2012.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khazaei M, Pazhouhi M. Temozolomide-mediated apoptotic death is improved by thymoquinone in U87MG cell line. Cancer Invest. 2017;35(4):225–236. doi: 10.1080/07357907.2017.1289383. [DOI] [PubMed] [Google Scholar]
- 16.Pazhouhi M, Sariri R, Rabzia A, Khazaei M. Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran J Basic Med Sci. 2016;19(8):890–898. [PMC free article] [PubMed] [Google Scholar]
- 17.Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12(1):60–66. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth NL, Overk CR, Yao P, Burdette JE, Nikolic D, Chen SN, et al. The Chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J Altern Complement Med. 2006;12(2):133–139. doi: 10.1089/acm.2006.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaurinovic B, Popovic M, Vlaisavljevic S, Schwartsova H, Vojinovic-Miloradov M. Antioxidant Profile of Trifolium pratense L. Molecules. 2012;17(9):11156–11172. doi: 10.3390/molecules170911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilberta ER, Liub D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4(2):200–212. doi: 10.1039/c2fo30199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777–1789. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim UH, Yoon JH, Li H, Kang JH, Ji HS, Park KH, et al. Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice. Molecules. 2014;19(11):18493–18510. doi: 10.3390/molecules191118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Yao Y, Li L. Coumarins as potential antidiabetic agents. J Pharm Pharmacol. 2017;69(10):1253–1264. doi: 10.1111/jphp.12774. [DOI] [PubMed] [Google Scholar]
- 24.Lino CS, Sales TP, Gomes PB, Amaral J, Alexandre FSA, Silveira ER, et al. Anti-diabetic activity of a fraction from Cissus verticillata and tyramine, its main bioactive constituent, in alloxan-induced diabetic rats. Am J Pharmacol Toxicol. 2007;2(4):178–188. [Google Scholar]