Abstract
During the recent years, more attentions have been focused on lipid base drug delivery system to overcome some limitations of conventional formulations. Among these delivery systems solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are promising delivery systems due to the ease of manufacturing processes, scale up capability, biocompatibility, and also biodegradability of formulation constituents and many other advantages which could be related to specific route of administration or nature of the materials are to be loaded to these delivery systems. The aim of this article is to review the advantages and limitations of these delivery systems based on the route of administration and to emphasis the effectiveness of such formulations.
Keywords: Drug delivery systems, Nanoparticles, Nanostructured lipid carriers (NLCs), Routes of administration, Solid lipid nanoparticles (SLNs)
1. INTRODUCTION
Lipid nanoparticles as drug delivery systems were considered from the beginning of the 19th century by professor R. H. Müller from Germany and Professor M. Gascon from Italy(1,2). These nanoparticles are manufactured from solid or mixture of solid and liquid lipids and stabilized by emulsifiers.
Lipids used in these nanoparticles are biocompatible and completely tolerated by the body like triglycerides, fatty acids, steroids, and waxes. In addition, using combination of emulsifiers could stabilize the formulations more efficiently. Lipid nanoparticles have many advantages in comparison to other particulate systems such as the ease of large-scale production(3), biocompatible and biodegradable nature of the materials(4), low toxicity potential(5), possibility of controlled and modified drug release(6), drug solubility enhancement and the possibility of both hydrophilic and lipophilic drug incorporation. Lipid nanoparticles are different from micro-emulsions, which are clear thermodynamically stable dispersion of oil and water that are stabilized by surfactants and cosurfactants(7,8). The most important parameters in lipid nanoparticles characterization are particle size and size distribution, zeta potential, polymorphism, degree of crystallinity, drug loading, entrapment efficiency, and drug release. There are three different types of lipid nanoparticles: homogenous drug-lipid matrix, drug enriched core and drug enriched shell. Drug release from lipid nanoparticles is mostly dependent on the matrix type and location of drug in matrix formulation; for example in the third type, drug release from the nanocarriers shows more sustained release profile. The composition of lipid matrix, surfactant concentration and manufacturing parameters, such as temperature and stirring rate, can also affect drug release profiles. Probably the most important reasons of using lipid nanoparticles, as a suitable alternative of previous polymeric nanoparticles, are the ease of large-scale production and their low toxicity potential(1).
2. TYPES OF LIPID NANOPARTICLES
Solid lipid nanoparticles (SLNs) are the first generation of lipid-based nanocarriers that are formulated from lipids, which are solid in the body temperature and stabilized by emulsifiers(1). SLNs have submicron (less than 1000 nm) sizes(9). They have numerous advantages such as drug protection against harsh environmental situations, ease of large scale production using high pressure homogenization technique, biocompatibility, and biodegradability(10). SLNs have also some disadvantages; because of their perfect crystalline structure, they have low drug loading efficiency(10) and the possibility of drug expulsion due to the crystallization process during the storage conditions. Another drawback is initial burst release(11) which usually occurs with these formulations. In SLNs drug molecules orients between the fatty acid chains or glycerides and during the storage periods and polymorphic changes in solid lipid structures there is a tendency to expulsion of previously dissolved drug in SLNs. Fig. 1 illustrates the actual place of drug orientation in SLNs and nanostructured lipid carriers (NLCs) schematically.
Fig. 1.
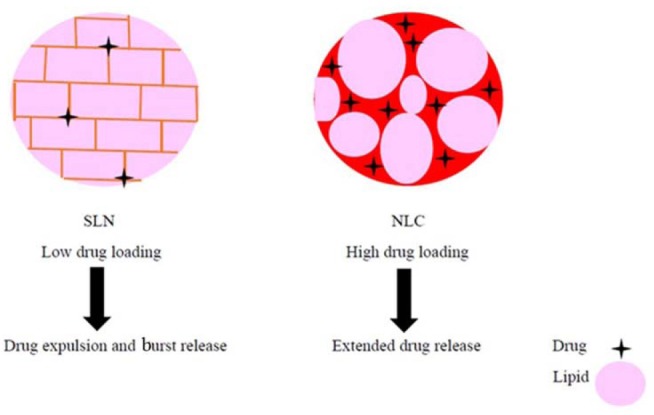
Schematic view of the solid lipid nanoparticle (SLN) and nanostructured lipid carriers (NLCs) showing the drug location within the lipid matrix.
NLCs are second generation of lipid-based nanocarriers formed from mixture of solid and liquid lipids and have unstructured-matrix due to the different moieties of the constituents of NLCs(2). NLCs were designed in order to overcome the SLNs limitations. NLCs have higher drug loading capacity because of imperfect crystal structure and could avoid drug expulsion by avoiding lipid crystallization during the manufacturing and storage periods. Due to the presence of liquid lipids in NLCs formulation expulsion of loaded drug after formulation and during the storage period is minimized. NLCs also can increase drug solubility in lipid matrix and they can show more controllable release profiles in comparison to SLNs(12). Although NLCs are solid in nature even in body temperature but they have low melting point than SLNs and due to their unstructured nature and imperfection in their crystalline behaviors provide more space for drug dissolution and payload in liquid part of the NLCs. In this regard, loading capacity in NLCs are more than SLNs. Previous researches also confirm on less susceptibility of NLCs than SLNs to gelation during the preparation and storage period, which is another advantage of NLCs, NLCs can facilitate separation of nanoparticle from the rest of the medium and dosage form preparation for parenteral administration(2,12).
3. METHODS OF LIPID NANO-PARTICLES PREPARATION
Lipid nanoparticles could be prepared by different methods such as hot and cold high pressure homogenization(13,14), solvent emulsification/evaporation(15), microemulsion formation technique(16), and ultrasonic solvent emulsification(3). Large-scale productions of lipid nanoparticles are mainly obtained by high pressure homogenization technique.
3.1. High pressure homogenization technique
3.1.1. Hot high pressure homogenization
In this method, lipid phase is heated up to 90 °C, then the hot lipid phase is dispersed in aqueous phase containing surfactants with same temperature. The pre-emulsion is homogenized at 90 °C under 3 cycles of high pressure homogenizer at 5 × 107 Pa. Finally, the obtained oil in water emulsion is cooled down to room temperature to solidify SLNs or NLCs(17).
3.1.2. Cold high pressure homogenization
In this method, the melted lipid phase is cooled to solidify and then ground to form lipid microparticles. Obtained lipid microparticles are dispersed in cool aqueous phase containing surfactants to form pre-suspension. Then the pre-suspension is homogenized under 5 cycles of high pressure homogenizer at room temperature and pressure of 1.5 × 108 Pa(18).
3.2. Solvent emulsification/evaporation technique
In this method, lipid phase is dissolved in an organic solvent such as acetone (organic phase). Then the organic phase is added to the aqueous phase (surfactant solution in water) under continuous stirring at 70-80 °C. The stirring will be continued until the organic phase is completely evaporated. Then obtained nanoemulsion is cooled (below 5 °C) to solidify lipid nanoparticles(15).
3.3. Microemulsion formation technique
In this method, lipids are melted at appropriate temperature and aqueous phase containing surfactants are heated up to same temperature. Then the hot aqueous phase will be added to the melted lipids under stirring at the same temperature. The hot oil in water microemulsion is dispersed in cold water at 1:50 ratio to solidify lipid nanoparticles(19).
3.4. Ultrasonic solvent emulsification technique
In this method, lipid phase is dissolved in an organic solvent such as dichloromethane and heated up to 50 °C. Then, aqueous phase containing surfactants and emulsifiers is heated up to the same temperature. After partial evaporation of dichloromethane, the aqueous phase is added to the organic phase under stirring at 50 °C. Obtained emulsion is sonicated for appropriate time and finally cooled in an ice bath to solidify lipid nanoparticles(3).
4. LIPID NANOPARTICLES APPLICATIONS AND DIFFERENT ROUTES OF ADMINISTRATION
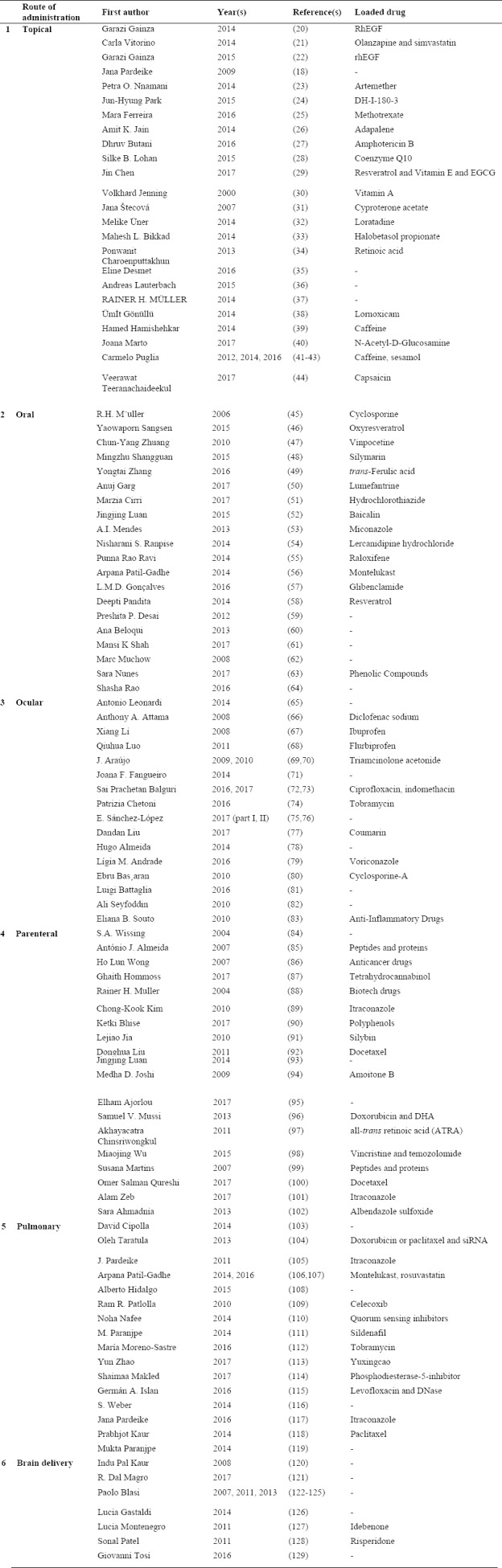
Numerous articles are reviewed and the results are categorized according to the routes of drug administration to six topics of topical, oral, parenteral, ocular, lung and brain delivery as shown in Table 1.
Table 1.
Different loaded active compound and routes of administration of lipid nanoparticles.
4.1. Topical route of administration
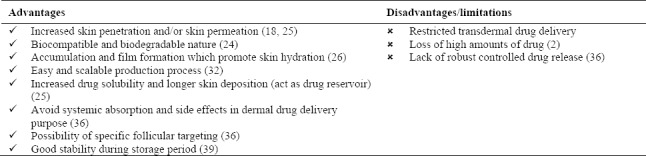
Skin related diseases are very common around the world. The major limitations for treatment of these diseases are low drug efficacy because of poor skin penetration or skin permeation of drugs from the most conventional formulations. Stratum corneum of epidermis is the major skin barrier and it should be bypassed through changing the penetration pathway from transcellular to paracellular or follicles. Lipid nanoparticles such as SLNs and NLCs have been developed to increase skin penetration or permeation. These particulate formulations are manufactured by mixing SLNs or NLCs with conventional formulations. They could be directly prepared in a one-step process which produce drug-loaded SLNs or NLCs. Lipid nanoparticles have so many advantages for topical drug delivery such as biocompatibility and biodegradability, controlled and extended drug release profile, close contact and strong skin adhesion, skin hydration and film formation in order to increase skin and dermal penetration (Table 2)(27,29,35,36,40).
Table 2.
Lipid nanoparticles advantages and disadvantages as topical drug delivery systems.
4.2. Oral route
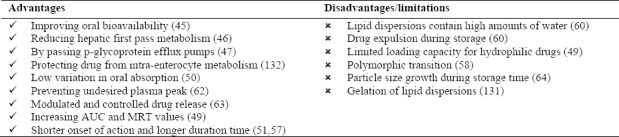
Oral drug administration is the most common route of drug delivery system because of the highest patient compliance. Low oral bioavailability due to limited drug solubility and/or high hepatic first pass effect are the most important limitations in oral drug delivery that should be overcome. Nanoparticle-based drug delivery systems were considered as suitable delivery system to increase oral bioavailability. Lipid nanoparticles such as SLNs and NLCs have the advantage of sustained drug release capability to maintain a constant plasma levels. In addition, nanoparticles with higher specific surface area and higher saturation solubility have more rapid dissolution rate that can accelerate the onset of drugs action. Other major barriers in oral drug delivery are p-glycoprotein efflux pumps and chemical or enzymatic degradation. Recent researches have shown that some specific lipids or surfactants, which are used in lipid nanoparticles, are capable of inhibiting p-glycoprotein efflux pumps. Drug-loaded lipid nanoparticles could reduce chemical or enzymatic degradation of the drugs which are embedded in a lipid matrix. Lipid nanoparticles could promote lymphatic transport and can bypass the liver and avoid hepatic first pass effect(50,51,52,130,131). Lipid nanoparticles advantages and disadvantages for oral route are listed in Table 3.
Table 3.
Lipid nanoparticles advantages and disadvantages as oral drug delivery systems.
4.3. Ocular administration
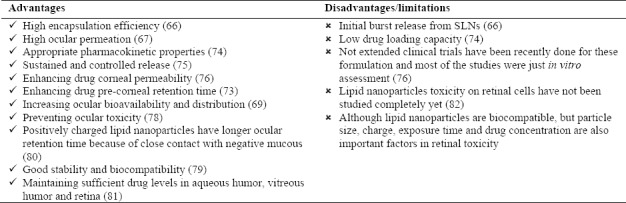
Ocular drug delivery has many limitations and remains challenging because of specific physiological and anatomical features of the eyes. Eyes are a very complex and sophisticated organ and have several barriers that should be overcome in order to reach specific ocular tissue. Novel drug delivery systems such as lipid nanoparticles were considered to overcome these barriers and improve ocular tissue bioavailability. Topical application is the most common route of drug delivery to the anterior segment of the eyes. This route of administration has many advantages and is the choice for superficial ocular diseases. Major barriers in this pathway are corneal epithelium, blood ocular barrier, conjunctival blood flow, and tear drainage. Lipid nanoparticles which are used as ocular drug delivery systems are capable of passing blood ocular barrier, obtain sustained and controlled drug release, protect drugs from lacrimal enzymes and prolong drug deposition and residence time in eyes. Treatment of ocular diseases, which involve posterior segment of the eyes, is very difficult. There are different ways to target posterior segment of the eyes.
Topical route is not a suitable way to target intraocular tissues; other routes that are used for this purpose are transscleral delivery (subconjunctival and retrobulbar injection), intravitreal route, subretinal injection, etc. Most of these ways are invasive, so novel drug delivery systems such as lipid nanoparticles could be an appropriate alternative. Gene therapy for the purpose of retinal targeting in retinal diseases was also considered using non-viral vectors gene delivery including SLNs and NLCs(73,74,75,76,81). A brief list of advantages and disadvantages of this route of administration are listed in Table 4.
Table 4.
Lipid nanoparticles advantages and disadvantages as ocular drug delivery systems.
4.4. Parenteral administration
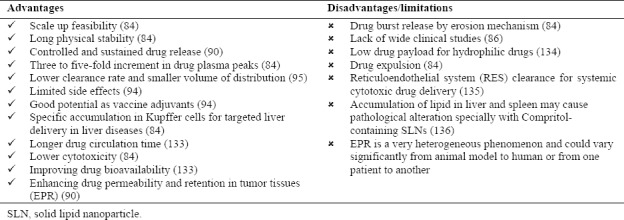
Nanomedicine and nanotechnology play an important role in improving the parenteral drug delivery. Lipid nanoparticles advantages and disadvantages as parenteral drug delivery systems are listed in Table 5. The most important advantages of lipid nanoparticles for this purpose are ease of scale up production, biocompatible and biodegradable nature of the formulation constituents, controlled and modified drug release pattern, preventing drug degradation and maintaining more constant serum levels of drugs. Drug-loaded lipid nanoparticles may be injected intravenously, subcutaneously, intramuscularly, and directlyto target organs. Drug release from lipid nanoparticles may occur via erosion (such as enzymatic degradation) or via diffusion which could support a sustained drug release. Recent researches have confirmed the capability of lipid nanoparticles in peptide and protein incorporation. In this context, SLNs are not suitable carrier due to limited drug loading capacity but NLCs are appropriate alternative. In this method peptides and proteins can be protected from harsh environmental conditions(92,93,97,100).
Table 5.
Lipid nanoparticles advantages and disadvantages as parenteral drug delivery systems.
4.5. Pulmonary delivery
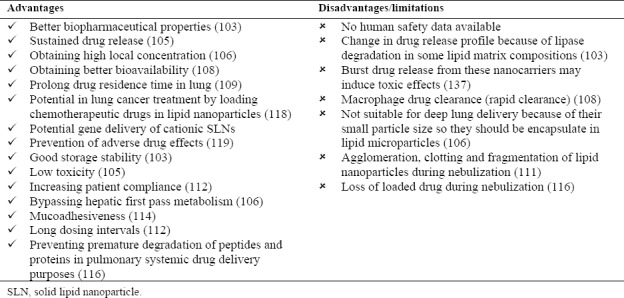
Pulmonary drug delivery is a relatively new approach, which has many advantages. It is a non-invasive route of drug delivery for both local and systemic administration. By this direct delivery system, drug dosage may be decreased and consequently drug adverse effects would be reduced. Direct drug inhalation can also accelerate onset of action. High drug accumulation in target site is another advantage of such administration route. Large surface area of pulmonary system and thin alveolar epithelium could guarantee high drug permeability. Lipid microparticles were used as delivery systems for lung targeting. These particulate systems showed good results such as drug bioavailability enhancement in comparison with conventional formulations. Lipid nanoparticles including SLNs and NLCs have been considered for pulmonary delivery. They have the advantage of sustaining drug release, biocompatibility and biodegradablity, lower toxicity and better stability in comparison with previously designed particulate systems. Pulmonary delivery of drug-loaded nanoparticles would result in high local concentration and can reduce systemic adverse effects. Also nanoparticles can achieve higher bioavailability for systemic delivery purposes. Lipid nanoparticles used in lung drug delivery, like other routes of administration, have the advantage of sustained drug delivery(103,114,117,118). Some of the most important advantages and limitations of this route of administration are listed in Table 6.
Table 6.
Lipid nanoparticles advantages and disadvantages as pulmonary drug delivery systems.
4.6. Brain delivery
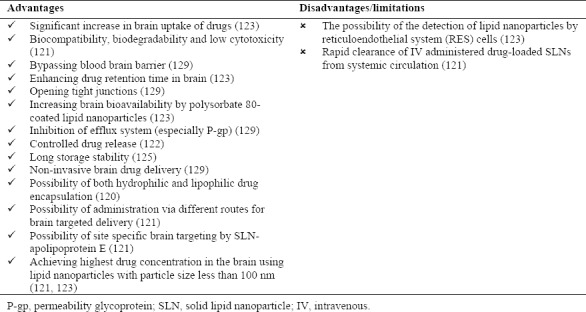
Drug delivery to the brain is one of the most important challenges in pharmaceutical sciences because of the presence of blood brain barrier (BBB). Nanoparticles with the advantage of small particle size and high drug encapsulation efficiency have been considered for specific targeting of brain tissues. Since nanoparticles can bypass reticuloendothelial system (RES), they are suitable as brain drug delivery systems. Two major obstacles in brain drug delivery are limited penetration of drugs across BBB and efflux of transported drugs from brain to blood circulation. Lipid nanoparticles such as SLNs and NLCs are one of the colloidal drug delivery systems that have been utilized to overcome these barriers. Lipid nanoparticles advantages and limitations as brain drug delivery systems are listed in Table 7. Lipid nanoparticles have the advantage of increasing drug retention time in blood of brain capillaries and inducing a drug gradient from blood to brain tissues, opening tight junctions to facilitate passage from BBB and transcytosis of drug-loaded lipid nanoparticles through the endothelium layer. Lipid nanoparticles are suitable for incorporating both lipophilic and hydrophilic drugs which could be administered via different routes(120,121,122,123,124,125,126,127,128,129). Previous researches emphasized on significant effect of surfactant suitability for brain drug delivery. Appropriate surfactants could be chosen according to their HLB and packing parameter. For site-specific brain drug delivery, polysorbates especially polysorbate 80, has shown best results. In addition, results showed that positively charged lipid nanoparticles induce better drug accumulation in the brain(123).
Table 7.
Lipid nanoparticles advantages and disadvantages as brain drug delivery systems
5. COMMERCIALLY AVAILABLE PRODUCTS FROM LIPID NANO-PARTICLES IN MARKET
Today, most of the commercially available products from lipid nanoparticles are cosmetic products such as Cutanova Cream Nano Repair Q10, Intensive Serum Nano Repair Q10, Cutanova Cream Nano Vital Q10, SURMER Crème Legère Nano-Protection, SURMER Crème Riche Nano-Restructurante, SURMER Elixir du Beauté Nano-Vitalisant, SURMER Masque Crème Nano-Hydratant, NanoLipid Restore CLR, NanoLipid Q10 CLR, NanoLipid Basic CLR, NanoLipid Repair CLR, IOPE SuperVital cream, serum, eye cream, extra moist softener and extra moist emulsion, NLC Deep Effect Eye Serum, NLC Deep Effect Repair Cream, NLC Deep Effect Reconstruction Cream, NLC Deep Effect Reconstruction Serum, Regenerations Creme Intensiv Scholl, Swiss Cellular White Illuminating Eye Essence, Swiss Cellular White Intensive Ampoules, SURMER Creme Contour Des Yeux Nano-Remodelante, Olivenöl Anti Falten Pflegekonzentrat, Olivenöl Augenpflegebalsam(18).
6. CONCLUSION
lipid nanoparticles are novel drug deivery systems which have many advantages over other colloidal and polymeric nanocarriers. The most important advantages of lipid carriers are their biocompatibility, biodegradability, ease of scalability, and controlled and modified release patterns. Among these two types of lipid nanoparticles (SLN and NLC), NLCs as a second generation of lipid nanoparticles, has shown better results for the purpose of targeted drug delivery and nowadays are more considered for different routes of administration. Lipid nanoparticles are suitable carriers for both hydrophilic and lipophilic drugs. They can be administered by different routes such as topical, oral, parenteral, ocular, pulmonary, brain drug delivery. These nanoparticles for each routes of administration have its own advantages and also limitations that should be considered. Lipid nanoaprticles are promising drug delivery systems for delivery of various pharmaceutically important active ingredients from small molecule to protein and gene in early future.
ACKNOWLEDGEMENT
The content of this paper is taken from the Pharm.D thesis (Grant No. 95010511769) submitted by Parisa Ghasemiyeh and was financially supported by the vice chancellery research of Shiraz University of Medical Sciences, Shiraz, I.R. Iran.
REFERENCES
- 1.MuÈller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 2.Beloqui A, Solinis MA, Rodriguez-Gascon A, Almeida AJ, Preat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, Chen D, Ren L, Zhao X, Qin J. Solid lipid nanoparticles for enhancing vinpocetine's oral bioavailability. J Control Release. 2006;114(1):53–59. doi: 10.1016/j.jconrel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Silva A, González-Mira E, García ML, Egea MA, Fonseca J, Silva R, et al. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf B Biointerfaces. 2011;86(1):158–165. doi: 10.1016/j.colsurfb.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz C, Mehnert W, Lucks JS, Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release. 1994;30(1):83–96. [Google Scholar]
- 6.zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm. 1998;45(2):149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Chen H, Luo Z, Fu X. Preparation of starch nanoparticles in water in oil microemulsion system and their drug delivery properties. Carbohydr Polym. 2016;138:192–200. doi: 10.1016/j.carbpol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12(11):1561–1572. doi: 10.1023/a:1016268311867. [DOI] [PubMed] [Google Scholar]
- 9.Doktorovova S, Kovacevic AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Yoon G, Park JW, Yoon IS. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharm Investig. 2013;43(5):353–362. [Google Scholar]
- 11.Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439–446. doi: 10.1016/j.ijpharm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Shidhaye SS, Vaidya R, Sutar S, Patwardhan A, Kadam VJ. Solid lipid nanoparticles and nanostructured lipid carriers-innovative generations of solid lipid carriers. Curr Drug Deliv. 2008;5(4):324–231. doi: 10.2174/156720108785915087. [DOI] [PubMed] [Google Scholar]
- 13.Kasongo KW, Muller RH, Walker RB. The use of hot and cold high pressure homogenization to enhance the loading capacity and encapsulation efficiency of nanostructured lipid carriers for the hydrophilic antiretroviral drug, didanosine for potential administration to paediatric patients. Pharm Dev Technol. 2012;17(3):353–362. doi: 10.3109/10837450.2010.542163. [DOI] [PubMed] [Google Scholar]
- 14.Souto EB, Müller RH. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J Microencapsul. 2006;23(4):377–388. doi: 10.1080/02652040500435295. [DOI] [PubMed] [Google Scholar]
- 15.Chen DB, Yang TZ, Lu WL, Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull (Tokyo) 2001;49(11):1444–1447. doi: 10.1248/cpb.49.1444. [DOI] [PubMed] [Google Scholar]
- 16.Mojahedian MM, Daneshamouz S, Samani SM, Zargaran A. A novel method to produce solid lipid nanoparticles using n-butanol as an additional co-surfactant according to the o/w microemulsion quenching technique. Chem Phys Lipids. 2013;174:32–38. doi: 10.1016/j.chemphyslip.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Souto EB, Wissing SA, Barbosa CM, Müller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278(1):71–77. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Pardeike J, Hommoss A, Muller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1-2):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Shah RM, Malherbe F, Eldridge D, Palombo EA, Harding IH. Physicochemical characterization of solid lipid nanoparticles (SLNs) prepared by a novel microemulsion technique. J. Colloid Interface Sci. 2014;428:286–294. doi: 10.1016/j.jcis.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 20.Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, et al. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51–61. doi: 10.1016/j.jconrel.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Vitorino C, Almeida A, Sousa J, Lamarche I, Gobin P, Marchand S, et al. Passive and active strategies for transdermal delivery using co-encapsulating nanostructured lipid carriers: in vitro vs. in vivo studies. Eur J Pharm Biopharm. 2014;86(2):133–144. doi: 10.1016/j.ejpb.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Gainza G, Bonafonte DC, Moreno B, Aguirre JJ, Gutierrez FB, Villullas S, et al. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J Control Release. 2015;197:41–47. doi: 10.1016/j.jconrel.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Nnamani PO, Hansen S, Windbergs M, Lehr CM. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm. 2014;477(1-2):208–217. doi: 10.1016/j.ijpharm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Ban SJ, Ahmed T, Choi HS, Yoon HE, Yoon JH, et al. Development of DH-I-180-3 loaded lipid nanoparticle for photodynamic therapy. Int J Pharm. 2015;491(1-2):393–401. doi: 10.1016/j.ijpharm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira M, Silva E, Barreiros L, Segundo MA, Lima CSA, Reis S. Methotrexate loaded lipid nanoparticles for topical management of skin-related diseases: Design, characterization and skin permeation potential. Int J Pharm. 2016;512(1):14–21. doi: 10.1016/j.ijpharm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Jain AK, Jain A, Garg NK, Agarwal A, Jain A, Jain SA, et al. Adapalene loaded solid lipid nanoparticles gel: an effective approach for acne treatment. Colloids Surf B Biointerfaces. 2014;121:222–229. doi: 10.1016/j.colsurfb.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Butani D, Yewale C, Misra A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf B Biointerfaces. 2016;139:17–24. doi: 10.1016/j.colsurfb.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Lohan SB, Bauersachs S, Ahlberg S, Baisaeng N, Keck CM, Muller RH, et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur J Pharm Biopharm. 2015;89:201–207. doi: 10.1016/j.ejpb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Wei N, Lopez-Garcia M, Ambrose D, Lee J, Annelin C, et al. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur J Pharm Biopharm. 2017;117:286–291. doi: 10.1016/j.ejpb.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Jenning V, Gysler A, Schäfer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49(3):211–218. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 31.Stecova J, Mehnert W, Blaschke T, Kleuser B, Sivaramakrishnan R, Zouboulis CC, et al. Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: particle characterisation and skin uptake. Pharm Res. 2007;24(5):991–1000. doi: 10.1007/s11095-006-9225-9. [DOI] [PubMed] [Google Scholar]
- 32.Üner M, Karaman EF, Aydoğmuş Z. Solid lipid nanoparticles and nanostructured lipid carriers of loratadine for topical application: physicochemical stability and drug penetration through rat skin. Trop J Pharm Res. 2014;13(5):653–660. [Google Scholar]
- 33.Bikkad ML, Nathani AH, Mandlik SK, Shrotriya SN, Ranpise NS. Halobetasol propionate-loaded solid lipid nanoparticles (SLN) for skin targeting by topical delivery. J Liposome Res. 2014;24(2):113–123. doi: 10.3109/08982104.2013.843192. [DOI] [PubMed] [Google Scholar]
- 34.Charoenputtakhun P, Opanasopit P, Rojanarata T, Ngawhirunpat T. All-trans retinoic acid-loaded lipid nanoparticles as a transdermal drug delivery carrier. Pharm Dev Technol. 2014;19(2):164–172. doi: 10.3109/10837450.2013.763261. [DOI] [PubMed] [Google Scholar]
- 35.Desmet E, Van Gele M, Lambert J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin Drug Deliv. 2017;14(1):109–122. doi: 10.1080/17425247.2016.1206073. [DOI] [PubMed] [Google Scholar]
- 36.Lauterbach A, Muller-Goymann CC. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur J Pharm Biopharm. 2015;97(Pt A):152–163. doi: 10.1016/j.ejpb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Müller RH, Staufenbiel S, Keck CM. Lipid nanoparticles (SLN, NLC) for innovative consumer care and household products. H and PC Today. 2014;9(2):18–25. [Google Scholar]
- 38.Gonullu U, Uner M, Yener G, Karaman EF, Aydogmus Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015;65(1):1–13. doi: 10.1515/acph-2015-0009. [DOI] [PubMed] [Google Scholar]
- 39.Hamishehkar H, Shokri J, Fallahi S, Jahangiri A, Ghanbarzadeh S, Kouhsoltani M. Histopathological evaluation of caffeine-loaded solid lipid nanoparticles in efficient treatment of cellulite. Drug Dev Ind Pharm. 2015;41(10):1640–1646. doi: 10.3109/03639045.2014.980426. [DOI] [PubMed] [Google Scholar]
- 40.Marto J, Sangalli C, Capra P, Perugini P, Ascenso A, Goncalves L, et al. Development and characterization of new and scalable topical formulations containing N-acetyl-d-glucosamine-loaded solid lipid nanoparticles. Drug Dev Ind Pharm. 2017;43(11):1792–1800. doi: 10.1080/03639045.2017.1339083. [DOI] [PubMed] [Google Scholar]
- 41.Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin Drug Deliv. 2012;9(4):429–241. doi: 10.1517/17425247.2012.666967. [DOI] [PubMed] [Google Scholar]
- 42.Puglia C, Lauro MR, Offerta A, Crasci L, Micicche L, Panico AM, et al. Nanostructured lipid carriers (NLC) as vehicles for topical administration of sesamol: in vitro percutaneous absorption study and evaluation of antioxidant activity. Planta Med. 2017;83(5):398–404. doi: 10.1055/s-0042-105293. [DOI] [PubMed] [Google Scholar]
- 43.Puglia C, Offerta A, Tirendi GG, Tarico MS, Curreri S, Bonina F, et al. Design of solid lipid nanoparticles for caffeine topical administration. Drug Deliv. 2016;23(1):36–40. doi: 10.3109/10717544.2014.903011. [DOI] [PubMed] [Google Scholar]
- 44.Teeranachaideekul V, Chantaburanan T, Junyaprasert VB. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J Drug Deliv Sci Technol. 2017;39:300–307. [Google Scholar]
- 45.Muller RH, Runge S, Ravelli V, Mehnert W, Thunemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–89. doi: 10.1016/j.ijpharm.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 46.Sangsen Y, Wiwattanawongsa K, Likhitwitayawuid K, Sritularak B, Wiwattanapatapee R. Modification of oral absorption of oxyresveratrol using lipid based nanoparticles. Colloids Surf B Biointerfaces. 2015;131:182–190. doi: 10.1016/j.colsurfb.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 47.Zhuang CY, Li N, Wang M, Zhang XN, Pan WS, Peng JJ, et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1-2):179–185. doi: 10.1016/j.ijpharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Shangguan M, Qi J, Lu Y, Wu W. Comparison of the oral bioavailability of silymarin-loaded lipid nanoparticles with their artificial lipolysate counterparts: implications on the contribution of integral structure. Int J Pharm. 2015;489(1-2):195–202. doi: 10.1016/j.ijpharm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Li Z, Zhang K, Yang G, Wang Z, Zhao J, et al. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans -ferulic acid ascompared with conventional solid lipid nanoparticles. Int J Pharm. 2016;511(1):57–64. doi: 10.1016/j.ijpharm.2016.06.131. [DOI] [PubMed] [Google Scholar]
- 50.Garg A, Bhalala K, Tomar DS, Wahajuddin In-situ single pass intestinal permeability and pharmacokinetic study of developed Lumefantrine loaded solid lipid nanoparticles. Int J Pharm. 2017;516(1-2):120–30. doi: 10.1016/j.ijpharm.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 51.Cirri M, Mennini N, Maestrelli F, Mura P, Ghelardini C, Mannelli DCL. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int J Pharm. 2017;521(1-2):73–83. doi: 10.1016/j.ijpharm.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Luan J, Zheng F, Yang X, Yu A, Zhai G. Nanostructured lipid carriers for oral delivery of baicalin: In vitro and in vivo evaluation. Colloids Surf. A. 2015;466:154–159. [Google Scholar]
- 53.Mendes AI, Silva AC, Catita JA, Cerqueira F, Gabriel C, Lopes CM. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf B Biointerfaces. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 54.Ranpise NS, Korabu SS, Ghodake VN. Second generation lipid nanoparticles (NLC) as an oral drug carrier for delivery of lercanidipine hydrochloride. Colloids Surf B Biointerfaces. 2014;116:81–87. doi: 10.1016/j.colsurfb.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Ravi PR, Aditya N, Kathuria H, Malekar S, Vats R. Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur J Pharm Biopharm. 2014;87(1):114–124. doi: 10.1016/j.ejpb.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Patil-Gadhe A, Pokharkar V. Montelukast-loaded nanostructured lipid carriers: part I oral bioavailability improvement. Eur J Pharm Biopharm. 2014;88(1):160–168. doi: 10.1016/j.ejpb.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Goncalves LMd, Maestrelli F, Mannelli DCL, Ghelardini C, Almeida AJ, Mura P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm. 2016;102:41–50. doi: 10.1016/j.ejpb.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Pandita D, Kumar S, Poonia N, Lather V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int. 2014;62:1165–1174. [Google Scholar]
- 59.Desai PP, Date AA, Patravale VB. Overcoming poor oral bioavailability using nanoparticle formulations - opportunities and limitations. Drug Discov Today Technol. 2012;9(2):e71–e174. doi: 10.1016/j.ddtec.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Beloqui A, Solinis MA, Delgado A, Evora C, Isla A, Rodriguez-Gascon A. Fate of nanostructured lipid carriers (NLCs) following the oral route: design, pharmacokinetics and biodistribution. J Microencapsul. 2014;31(1):1–8. doi: 10.3109/02652048.2013.788090. [DOI] [PubMed] [Google Scholar]
- 61.Shah MK. Solid lipid nanoparticles (SLN) for oral drug delivery: an overview. J Nanomed Nanosci. 2017 [Google Scholar]
- 62.Muchow M, Maincent P, Müller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34(12):1394–1405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 63.Nunes S, Madureira AR, Campos D, Sarmento B, Gomes AM, Pintado M, et al. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit Rev Food Sci Nutr. 2017;57(9):1863–1873. doi: 10.1080/10408398.2015.1031337. [DOI] [PubMed] [Google Scholar]
- 64.Rao S, Prestidge CA. Polymer-lipid hybrid systems: merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin Drug Deliv. 2016;13(5):691–707. doi: 10.1517/17425247.2016.1151872. [DOI] [PubMed] [Google Scholar]
- 65.Leonardi A, Bucolo C, Romano GL, Platania CB, Drago F, Puglisi G, et al. Influence of different surfactants on the technological properties and in vivo ocular tolerability of lipid nanoparticles. Int J Pharm. 2014;470(1-2):133–140. doi: 10.1016/j.ijpharm.2014.04.061. [DOI] [PubMed] [Google Scholar]
- 66.Attama AA, Reichl S, Müller-Goymann CC. Diclofenac sodium delivery to the eye: in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. International journal of pharmaceutics. 2008;355(1-2):307–313. doi: 10.1016/j.ijpharm.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Nie Sf, Kong J, Li N, Ju CY, Pan WS. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int J Pharm. 2008;363(1-2):177–182. doi: 10.1016/j.ijpharm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Luo Q, Zhao J, Zhang X, Pan W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int J Pharm. 2011;403(1-2):185–191. doi: 10.1016/j.ijpharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: safety on drug delivery. Nanomedicine. 2009;5(4):394–401. doi: 10.1016/j.nano.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Araújo J, Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1-2):167–75. doi: 10.1016/j.ijpharm.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 71.Fangueiro JF, Andreani T, Egea MA, Garcia ML, Souto SB, Silva AM, et al. Design of cationic lipid nanoparticles for ocular delivery: development, characterization and cytotoxicity. Int J Pharm. 2014;461(1-2):64–73. doi: 10.1016/j.ijpharm.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 72.Balguri SP, Adelli GR, Janga KY, Bhagav P, Majumdar S. Ocular disposition of ciprofloxacin from topical, PEGylated nanostructured lipid carriers: Effect of molecular weight and density of poly (ethylene) glycol. Int J Pharm. 2017;529(1-2):32–43. doi: 10.1016/j.ijpharm.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur J Pharm Biopharm. 2016;109:224–235. doi: 10.1016/j.ejpb.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM, et al. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur J Pharm Biopharm. 2016;109:214–223. doi: 10.1016/j.ejpb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye - Part I - Barriers and determining factors in ocular delivery. Eur J Pharm Biopharm. 2017;110:70–75. doi: 10.1016/j.ejpb.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye - Part II - Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58–69. doi: 10.1016/j.ejpb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Liu D, Li J, Cheng B, Wu Q, Pan H. Ex Vivo and in Vivo evaluation of the effect of coating a coumarin-6-labeled nanostructured lipid carrier with chitosan-N-acetylcysteine on rabbit ocular distribution. Mol Pharm. 2017;14(8):2639–2648. doi: 10.1021/acs.molpharmaceut.7b00069. [DOI] [PubMed] [Google Scholar]
- 78.Almeida H, Amaral MH, Lobão P, Silva AC, Loboa JM. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: present and future considerations. J Pharm Pharm Sci. 2014;17(3):278–293. doi: 10.18433/j3dp43. [DOI] [PubMed] [Google Scholar]
- 79.Andrade LM, Rocha KA, De Sá FA, Marreto RN, Lima EM, Gratieri T, et al. Voriconazole-loaded nanostructured lipid carriers for ocular drug delivery. Cornea. 2016;35(6):866–871. doi: 10.1097/ICO.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 80.Basaran E, Demirel M, Sirmagül B, Yazan Y. Cyclosporine-A incorporated cationic solid lipid nanoparticles for ocular delivery. J Microencapsul. 2010;27(1):37–47. doi: 10.3109/02652040902846883. [DOI] [PubMed] [Google Scholar]
- 81.Battaglia L, Serpe L, Foglietta F, Muntoni E, Gallarate M, Rodriguez DPA, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv. 2016;13(12):1743–1757. doi: 10.1080/17425247.2016.1201059. [DOI] [PubMed] [Google Scholar]
- 82.Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17(7):467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 83.Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res. 2010;35(7):537–552. doi: 10.3109/02713681003760168. [DOI] [PubMed] [Google Scholar]
- 84.Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56(9):1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Hommoss G, Pyo SM, Müller RH. Mucoadhesive tetrahydrocannabinol-loaded NLC - Formulation optimization and long-term physicochemical stability. Eur J Pharm Biopharm. 2017;117:408–417. doi: 10.1016/j.ejpb.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs--a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1-3):151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Kim JK, Park JS, Kim CK. Development of a binary lipid nanoparticles formulation of itraconazole for parenteral administration and controlled release. Int J Pharm. 2010;383(1-2):209–215. doi: 10.1016/j.ijpharm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Bhise K, Kashaw SK, Sau S, Iyer AK. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int J Pharm. 2017;526(1-2):506–515. doi: 10.1016/j.ijpharm.2017.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia L, Zhang D, Li Z, Duan C, Wang Y, Feng F, et al. Nanostructured lipid carriers for parenteral delivery of silybin: Biodistribution and pharmacokinetic studies. Colloids Surf B Biointerfaces. 2010;80(2):213–218. doi: 10.1016/j.colsurfb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Liu D, Liu Z, Wang L, Zhang C, Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf B Biointerfaces. 2011;85(2):262–269. doi: 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 93.Luan J, Zhang D, Hao L, Qi L, Liu X, Guo H, et al. Preparation, characterization and pharmacokinetics of Amoitone B-loaded long circulating nanostructured lipid carriers. Colloids and surfaces B, Biointerfaces. 2014;114:255–60. doi: 10.1016/j.colsurfb.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 94.Joshi MD, Müller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 2009;71(2):161–172. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Ajorlou E, Khosroushahi AY. Trends on polymer- and lipid-based nanostructures for parenteral drug delivery to tumors. Cancer Chemother Pharmacol. 2017;79(2):251–265. doi: 10.1007/s00280-016-3168-6. [DOI] [PubMed] [Google Scholar]
- 96.Mussi SV, Sawant R, Perche F, Oliveira MC, Azevedo RB, Ferreira LA, et al. Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm Res. 2014;31(8):1882–1892. doi: 10.1007/s11095-013-1290-2. [DOI] [PubMed] [Google Scholar]
- 97.Chinsriwongkul A, Chareanputtakhun P, Ngawhirunpat T, Rojanarata T, Sila-on W, Ruktanonchai U, et al. Nanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drug. AAPS PharmSciTech. 2012;13(1):150–158. doi: 10.1208/s12249-011-9733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu M, Fan Y, Lv S, Xiao B, Ye M, Zhu X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: a comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016;23(8):2720–2725. doi: 10.3109/10717544.2015.1058434. [DOI] [PubMed] [Google Scholar]
- 99.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. Int J Nanomedicine. 2007;2(4):595–607. [PMC free article] [PubMed] [Google Scholar]
- 100.Qureshi OS, Kim HS, Zeb A, Choi JS, Kim HS, Kwon JE, et al. Sustained release docetaxel-incorporated lipid nanoparticles with improved pharmacokinetics for oral and parenteral administration. J Microencapsul. 2017;34(3):250–261. doi: 10.1080/02652048.2017.1337247. [DOI] [PubMed] [Google Scholar]
- 101.Zeb A, Qureshi OS, Kim HS, Kim MS, Kang JH, Park JS, et al. High payload itraconazole-incorporated lipid nanoparticles with modulated release property for oral and parenteral administration. J Pharm Pharmacol. 2017;69(8):955–966. doi: 10.1111/jphp.12727. [DOI] [PubMed] [Google Scholar]
- 102.Ahmadnia S, Moazeni M, Mohammadi-Samani S, Oryan A. In vivo evaluation of the efficacy of albendazole sulfoxide and albendazole sulfoxide loaded solid lipid nanoparticles against hydatid cyst. Exp Parasitol. 2013;135(2):314–319. doi: 10.1016/j.exppara.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Cipolla D, Shekunov B, Blanchard J, Hickey A. Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev. 2014;75:53–80. doi: 10.1016/j.addr.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pardeike J, Weber S, Haber T, Wagner J, Zarfl HP, Plank H, et al. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int J Pharm. 2011;419(1-2):329–338. doi: 10.1016/j.ijpharm.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 106.Patil-Gadhe A, Kyadarkunte A, Patole M, Pokharkar V. Montelukast-loaded nanostructured lipid carriers: part II pulmonary drug delivery and in vitro-in vivo aerosol performance. Eur J Pharm Biopharm. 2014;88(1):169–177. doi: 10.1016/j.ejpb.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 107.Patil-Gadhe A, Pokharkar V. Pulmonary targeting potential of rosuvastatin loaded nanostructured lipid carrier: Optimization by factorial design. Int J Pharm. 2016;501(1-2):199–210. doi: 10.1016/j.ijpharm.2016.01.080. [DOI] [PubMed] [Google Scholar]
- 108.Hidalgo A, Cruz A, Perez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm. 2015;95(Pt A):117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144(2):233–241. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nafee N, Husari A, Maurer CK, Lu C, de Rossi C, Steinbach A, et al. Antibiotic-free nanotherapeutics: ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J Control Release. 2014;192:131–140. doi: 10.1016/j.jconrel.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 111.Paranjpe M, Finke JH, Richter C, Gothsch T, Kwade A, Buttgenbach S, et al. Physicochemical characterization of sildenafil-loaded solid lipid nanoparticle dispersions (SLN) for pulmonary application. Int J Pharm. 2014;476(1-2):41–49. doi: 10.1016/j.ijpharm.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 112.Moreno-Sastre M, Pastor M, Esquisabel A, Sans E, Vinas M, Fleischer A, et al. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016;498(1-2):263–273. doi: 10.1016/j.ijpharm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y, Chang YX, Hu X, Liu CY, Quan LH, Liao YH. Solid lipid nanoparticles for sustained pulmonary delivery of Yuxingcao essential oil: Preparation, characterization and in vivo evaluation. Int J Pharm. 2017;516(1-2):364–371. doi: 10.1016/j.ijpharm.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 114.Makled S, Nafee N, Boraie N. Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor. Int J Pharm. 2017;517(1-2):312–321. doi: 10.1016/j.ijpharm.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 115.Islan GA, Tornello PC, Abraham GA, Duran N, Castro GR. Smart lipid nanoparticles containing levofloxacin and DNase for lung delivery. Design and characterization. Colloids Surf B Biointerfaces. 2016;143:168–176. doi: 10.1016/j.colsurfb.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 116.Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 117.Pardeike J, Weber S, Zarfl HP, Pagitz M, Zimmer A. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur J Pharm Biopharm. 2016;108:269–276. doi: 10.1016/j.ejpb.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 118.Kaur P, Garg T, Rath G, Murthy RS, Goyal AK. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016;23(6):1912–125. doi: 10.3109/10717544.2014.993486. [DOI] [PubMed] [Google Scholar]
- 119.Paranjpe M, Muller-Goymann CC. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 2014;15(4):5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 121.Dal Magro R, Ornaghi F, Cambianica I, Beretta S, Re F, Musicanti C, et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J Control Release. 2017;249:103–110. doi: 10.1016/j.jconrel.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 122.Blasi P, Giovagnoli S, Schoubben A, Puglia C, Bonina F, Rossi C, et al. Lipid nanoparticles for brain targeting I. Formulation optimization. Int J Pharm. 2011;419(1-2):287–295. doi: 10.1016/j.ijpharm.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 123.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Blasi P, Schoubben A, Romano GV, Giovagnoli S, Michele DA, Ricci M. Lipid nanoparticles for brain targeting II. Technological characterization. Colloids Surf B Biointerfaces. 2013;110:130–137. doi: 10.1016/j.colsurfb.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 125.Blasi P, Schoubben A, Traina G, Manfroni G, Barberini L, Alberti PF, et al. Lipid nanoparticles for brain targeting III. Long-term stability and in vivo toxicity. Int J Pharm. 2013;454(1):316–323. doi: 10.1016/j.ijpharm.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 126.Gastaldi L, Battaglia L, Peira E, Chirio D, Muntoni E, Solazzi I, et al. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur J Pharm Biopharm. 2014;87(3):433–444. doi: 10.1016/j.ejpb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Montenegro L, Campisi A, Sarpietro MG, Carbone C, Acquaviva R, Raciti G, et al. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev Ind Pharm. 2011;37(6):737–746. doi: 10.3109/03639045.2010.539231. [DOI] [PubMed] [Google Scholar]
- 128.Patel S, Chavhan S, Soni H, Babbar AK, Mathur R, Mishra AK, et al. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target. 2011;19(6):468–474. doi: 10.3109/1061186X.2010.523787. [DOI] [PubMed] [Google Scholar]
- 129.Tosi G, Musumeci T, Ruozi B, Carbone C, Belletti D, Pignatello R, et al. The “fate” of polymeric and lipid nanoparticles for brain delivery and targeting: Strategies and mechanism of blood-brain barrier crossing and trafficking into the central nervous system. J Drug Deliv Sci Technol. 2016;32:66–76. [Google Scholar]
- 130.Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MH, Silva AM, et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv. 2012;2012:1–10. doi: 10.1155/2012/750891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tran TH, Ramasamy T, Truong DH, Choi HG, Yong CS, Kim JO. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 2014;15(6):1509–1515. doi: 10.1208/s12249-014-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan S, Baboota S, Ali J, Khan S, Narang RS, Narang JK. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int J Pharm Investig. 2015;5(4):182–191. doi: 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, Majidi J, Yousefi M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif Cells Nanomed Biotechnol. 2016;44(4):1051–1061. doi: 10.3109/21691401.2014.998830. [DOI] [PubMed] [Google Scholar]
- 134.Attama AA. SLN, NLC, LDC: state of the art in drug and active delivery. Recent Pat Drug Deliv Formul. 2011;5(3):178–187. doi: 10.2174/187221111797200524. [DOI] [PubMed] [Google Scholar]
- 135.Selvamuthukumar S, Velmurugan R. Nanostructured lipid carriers: a potential drug carrier for cancer chemotherapy. Lipids Health Dis. 2012;11(1):159–166. doi: 10.1186/1476-511X-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prasad D, Chauhan H. Nanotoxicity of Polymeric and Solid Lipid Nanoparticles. In: Sutariya VB, Pathak Y, editors. Biointeractions of Nanomaterials. Florida: CRC Press; 2014. p. 151. [Google Scholar]
- 137.Xiang QY, Wang MT, Chen F, Gong T, Jian YL, Zhang ZR, et al. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res. 2007;30(4):519–525. doi: 10.1007/BF02980228. [DOI] [PubMed] [Google Scholar]


