Abstract
Opiate tolerance and dependence is a worldwide public health problem and gives a significant burden to society. The aim of this study was to evaluate the effects of metformin (MET) on development and expression of morphine tolerance and dependence in rats. For induction of tolerance, morphine sulfate was injected (10 mg/kg, twice a day, s.c.) for 7 days. Animals received metformin (5 and 50 mg/kg, orally, daily) during the examination period for assessing the development of morphine tolerance and dependence. In order to evaluate the expression of morphine tolerance and dependence, single doses of MET (5 and 50 mg/kg, orally) were administered on day 7. Tail flick test was performed to assess the induction of morphine tolerance. For evaluation of morphine dependence, naloxone-induced jumping (5 mg/kg, s.c.) was monitored. Our results showed that 7 days coadministration of 50 mg/kg of MET significantly reduced the development of morphine analgesic tolerance versus morphine + saline treated rats (P < 0.001). Treatment with 50 mg/kg MET reduced the incidence and frequency of jumping in naloxone injected animals (P < 0.01). It is notable that single dose administration of MET, did not prevent the expression of analgesic tolerance and physical dependence to morphine. Based on these results, it can be concluded that MET attenuates the development of morphine analgesic tolerance and dependence in rats.
Keywords: Metformin, Morphine, Physical dependence, Tolerance
INTRODUCTION
For centuries, opioid drugs such as morphine have been widely used for management of acute and chronic pain(1). It has been shown that, chronic administration of morphine leads to development of analgesic tolerance and physical dependence in both humans and animals(2,3,4). Although morphine-induced effects have widely been investigated in previous works, precise mechanisms underlying morphine tolerance and dependence are still unclear(5,6). Opioid tolerance is characterized by an increased responsiveness to an opioid agonist such as morphine and is usually manifested by the need to use increasing doses to achieve the desired effect(7). Dependence to opioid drugs is a compulsive drug use and if the opiate consumption is ceased abruptly the withdrawal sings including back and leg pain, sleep disturbances, chronic fatigue, restlessness, and mood disorders will occur(8). Although our knowledge about opioid addiction and treatment are beginning to emerge but there is no desirable treatment for the morphine tolerance and dependency. These conditions can be controlled or attenuated by some drugs such as clonidine and methadone(9). Therefore, morphine-induced tolerance and dependence has become a real challenge that limits the clinical use of morphine(10,11). Opioid tolerance and dependence are complex phenomena that involve multifaceted mechanisms including free radicals generation(12), down-regulation of opioid receptors(11), inflammation of the central nervous system(9), activations of glutamate receptor N-methyl-D-aspartate (NMDA), and protein kinase C (PKC)(7).
Biguanide-class drugs such as metformin (MET) are commonly used to treat and prevent type 2 diabetes(13), obesity(14), and polycystic ovary syndrome(15). MET has pleiotropic effects that might be due to the anti-inflammatory(16), neuroprotective(17) and antioxidant properties(18) of the drug. MET inhibits the production of free radicals via decreasing the nicotinamide adenine dinucleotide phosphate oxidase expression(19). Moreover, several studies have shown the anti-inflammatory effects of MET in many experimental models of inflammation. These studies have suggested that anti-inflammatory effects of MET is mediated by inhibition of NF-κB signaling(20), reduction in levels of TNF-α and IL-6(16). Furthermore, the neuroprotective effects of MET has been shown in some studies(17,21,22). Pan et al. has reported that metformin can reduce morphine tolerance in mice. They suggested that metformin might inhibit microglial activation and further suppress central sensitization occurring in the spinal cord, which contributes to the attenuation of morphine tolerance(23). Thus, taken together, the present study was aimed to investigate the possible effect of MET on the development and expression of morphine-induced tolerance and dependence in rats.
MATERIALS AND METHODS
Animals
A total of 56 male Wistar rats (weight 275 ± 25 g) were used in this study. Rats were obtained from the animal house of Rafsanjan University of Medical Sciences. Rats were housed in groups of four rats in a plastic cage maintained at a 12-h light/dark cycle (lights on 07:00 to 19:00) with free access to food and water and maintained at 23 ± 2.0 °C. All experimental procedures were carried out in accordance with the guidelines for the care and use of laboratory animals in the Rafsanjan University of Medical Sciences and the European Communities Council Directive 24 November 1986 (86/609/EEC) (IR.RUMS.REC.1396.134). Each rat was used only once in this study and each group consisted of 6-8 animals. All behavioral experiments were carried out at the same time of the day. The animals were divided into 8 experimental groups in this study:(1) saline, the animals received saline (as metformin vehicle) daily for 7 days; (2 and 3) metformin, the animals received metformin (5 and 50 mg/kg) daily for 7 days;(4) morphine + saline, the animals that received morphine (10 mg/kg twice a day, s.c.) and saline daily for 7 days (for assessing the development of morphine tolerance and dependence); (5 and 6) morphine + metformin, the animals received morphine (10 mg/kg twice a day, s.c.) and metformin (5 and 50 mg/kg, orally) daily for 7 days; (7 and 8) morphine + metformin, the animals received morphine (10 mg/kg twice a day, s.c.) daily for 7 days and metformin (5 and 50 mg/kg, orally) at day 7.
Drugs
The following drugs were used in this study: morphine sulphate (Temad, Iran), naloxone hydrochloride (Tolidaru, Iran), and MET (Merck, Germany). All drugs were dissolved in saline.
Morphine tolerance and dependence were induced in rats by a repeated injection of morphine (10 mg/kg; s.c.) twice daily (8:00 a.m and 8 p.m) for 7 consecutive days(23). Two doses of MET (5 and 50 mg/kg in volume of 5 mL, p.o.) were administered once a day for a 7-day period. Nociceptive response of the animals to noxious heat stimulus was measured by tail flick test every day. The control group received only vehicle (5 mL saline, p.o.).
Tail flick test
In order to assess the morphine analgesic effect, we used a tail flick analgesia meter apparatus (UGO BASILE, Italy). This apparatus was set to make a light beam focused on the ventral part of the animal's tail. Intensity of the heat was adjusted to elicit 2-4 s baseline responses. Tail-flick latency was considered as the time between tail exposure to radiant heat and tail withdrawal. The heat stimulus was discontinued after 10 s to avoid tissue damages (cut off point = 10 s). The animals were allowed to habituate to laboratory surroundings before tail flick test. Rats were lightly restrained in a poly(methyl methacrylate) (Plexiglas) rat restrainer box during the test. The rat's tail was exposed through gaps located in the inferior door of the restrainer. For 3 days before the first trial, rats were habituated to the test conditions to reduce their stress during the assessment. Antinociception was quantified by the percentage of maximum possible effect (MPE%), which was calculated according to the following equation:
MPE%=[(T1–T0)/(T2–T0)]×100 (1)
where, T0 is pre-treatment latency, T1 is post-treatment latency, and T2 is the cut off time(24).
Evaluation of morphine tolerance and dependence development
In order to examine the MET effects on the development of morphine tolerance and dependence, two doses of MET (5 and 50 mg/kg in volume of 5 mL; p.o.) or its vehicle (5 mL normal saline) were given during the period of examination. Every day of experiment, the animals were assessed for tolerance to morphine analgesic effects. Nociceptive response of the animals to noxious heat stimulus was measured by tail flick test and the decrease in response was used to assess the degree of tolerance. Moreover, dependence was evaluated by the incidence of jumping following administration of naloxone (5 mg/kg, i.p.) 2 h after the last dose of morphine on final (7th) day(25). Immediately, after the naloxone injection, each rat was placed in a Plexiglas box (35 cm long, 35 cm wide, 45 cm high) and frequency of jumps was recorded during 30 min. it is notable that the control group received only MET vehicle (5 mL saline, p.o.). The 5 and 50 mg/kg doses of MET or its vehicle were administered only on the seventh day, 45 min before morphine injection. Nociceptive response of the animals to noxious heat stimulus and physical dependence to morphine was evaluated by the methods that describe earlier.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed via GraphPad Prism version 6.01 for Windows (GraphPad Software, USA). Differences between the groups were tested with two-or one-way analysis of variance (ANOVA) followed by the Tukey post-hoc test. Differences between means were considered statistically significant if P < 0.05.
RESULTS
The effect of metformin on morphine analgesic effect
By this experiment, we can examine the effects of long term (7 days) MET treatment on nociceptive response in order to exclude the effect of MET on MPE%. As shown in Fig. 1, oral administration of MET (5 and 50 mg/kg), did not exhibit a significant change in nociception compared to normal rats (saline group) for the entire 7-day period of examination.
Fig. 1.
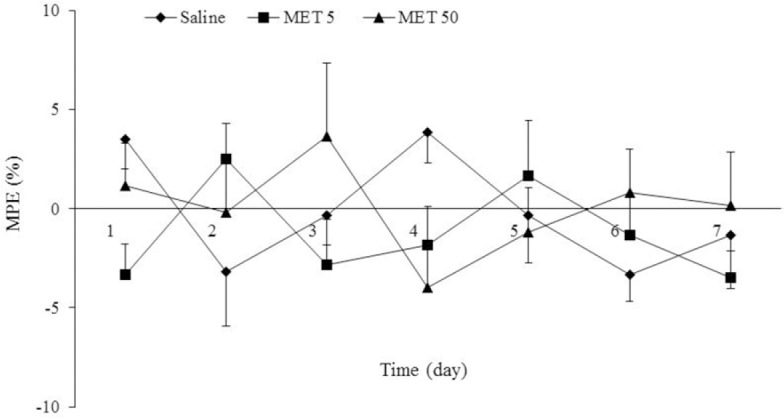
Antinociceptive activity of metformin (MET) at doses of 5 and 50 mg/kg in the tail flick test in rats. Antinociceptive effects are expressed as the percent of maximum possible effect (MPE%). There are no significant differences between various groups. Data are expressed as mean ± SEM of analgesic threshold of 6-8 rats.
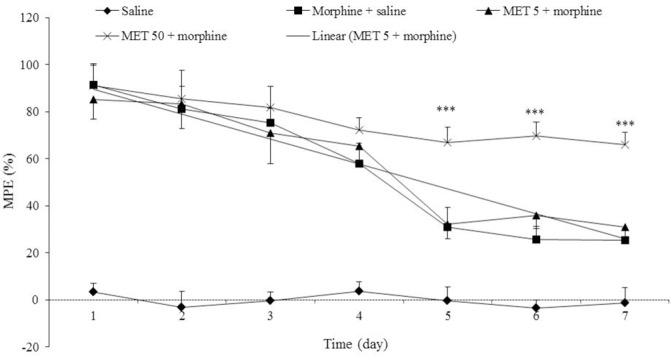
The effect of metformin administration on development of morphine analgesic tolerance
Figure 2 shows the effects of long-term administration of MET (5 and 50 mg/kg, p.o) on analgesic threshold in morphine treated animals during 7 days. MET at dose of 50 mg/kg, significantly increased the analgesic latency on days 5-7 with comparison to morphine-treated animals and prevented the induction of morphine antinociceptive tolerance (P < 0.001).
Fig. 2.
The effect of chronic treatment with metformin (MET) on morphine-induced tolerance. MET (5 and 50 mg/kg, p.o.) was administered 7 days with morphine (10 mg/kg twice a day, s.c). Antinociceptive response to morphine is shown as percent of maximum possible effect (MPE%). Data are expressed as mean ± SEM of 6-8 rats. ***P < 0.001 vs. morphine-treated group.
It is notable that, dose of 5 mg/kg metformin did not prevent the morphine tolerance in morphine-treated rats.
To clarify the role of acute MET administration on the expression of morphine tolerance, we administered this drug (5 and 50 mg/kg) only once 45 min before the dose of morphine on the day 7. Data showed that metformin did not prevent the expression of morphine tolerance in morphine-treated rats (Fig. 3).
Fig. 3.
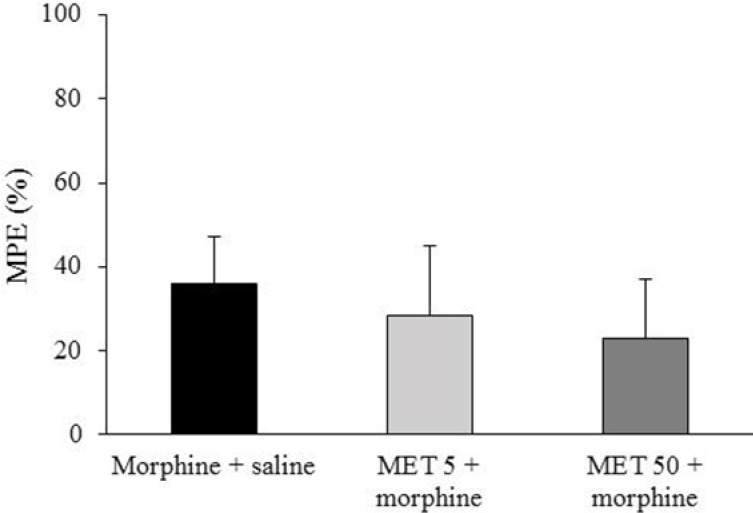
The effect of acute administration of metformin (MET) on the expression of morphine tolerance. MET (5 and 50 mg/kg) was administered 45 min before the last dose of morphine (10 mg/kg twice a day, s.c) on day 7. Antinociceptive response to morphine is shown as percent of maximum possible effect (MPE%). Data are expressed as mean ± SEM of 6-8 rats.
The effect of metformin administration on development of morphine dependence
As shown in Fig. 4, saline-treated animals exhibited no jumping behavior, whereas morphine-treated rats showed the jumping behavior, confirming development of morphine dependence (P < 0.001). Seven consecutive days’ administration of MET (50 mg/kg, p.o) before morphine injection significantly reduced the frequency of jumps (P < 0.01). Data showed that 7 days treatment with MET at dose of 5 mg/kg, did not prevent the frequency of jumps in morphine-treated rats (Fig. 4).
Fig. 4.
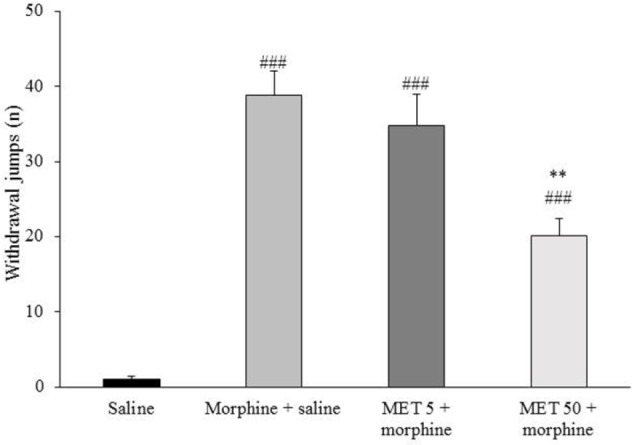
Effects of chronic administration of metformin (MET) on naloxone-precipitated morphine withdrawal jump. Data are expressed as mean ± SEM of 6-8 rats. ### P < 0.001 vs. saline-treated group and ** P < 0.01 vs. morphine-treated group.
Data showed that metformin in both doses (5 and 50 mg/kg) did not prevent the expression of behavioral withdrawal sign (jumping) associated with morphine dependence (Fig. 5).
Fig. 5.
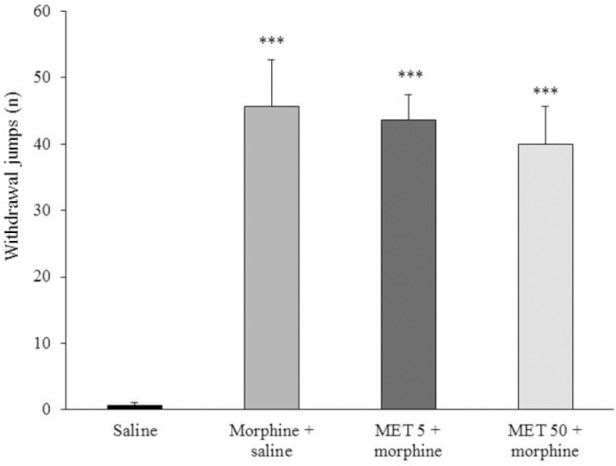
The effect of acute treatment with metformin (MET) on naloxone-precipitated morphine withdrawal sign (jumping). MET (5 and 50 mg/kg) was administered 45 min before the last dose of morphine (10 mg/kg twice a day, s.c) on day 7. Data are expressed as mean ± SEM of 6-8 rats. *** P < 0.001 vs. saline-treated group.
DISCUSSION
In the present study, we investigated the MET effect on development and expression of morphine tolerance and dependence in rats. Our results suggest that long term oral treatment with MET (50 mg/kg) significantly attenuated development of morphine tolerance and dependence in rats. We also showed that acute oral treatment with MET (5 and 50 mg/kg) does not inhibit expression of morphine tolerance and dependence in morphine dependent rats. Moreover, we found that oral administration of MET (5 and 50 mg/kg) has no significant effect on thermal nociception in non-morphine dependent rats. It is well established that, chronic morphine administration, alters the synaptic plasticity and structural changes in the brain(26). Moreover, it was shown that, opioid administration has neurotoxic effects and cause neuron degeneration in the rat brain(27). On the other hand, in different studies neuroprotective effects of MET have been reported by inhibiting apoptosis(21,22). Accordingly, MET may possibly prevent the pathological conditions associated with long term morphine-induced antinociceptive tolerance and dependence through the neuroprotective effects. Emerging studies have shown that inflammatory mediators such as IL-1β, IL-6, TNF-α, and NF-kB have critical roles in the development of morphine tolerance and dependence(28,29). Previous studies have reported the anti-inflammatory effects of MET. MET exhibited anti-inflammatory effects on human monocytes/ macrophages by reducing the levels of TNF-α and attenuating the mitogen-activated protein kinases (MAPKs) as well as NF-κB signaling(20). Moreover, long term administration of MET regulated pancreatic inflammation via reduction in inflammatory cytokines such as TNF-α and IL-6(16). Hence, it seems that these effects could be possible mechanisms in the effects of MET on induction of morphine tolerance and dependence. It is well established that activation of NMDA receptors has important role in the development of morphine tolerance and dependence(30). It has been shown that NMDA receptor antagonist such as MK-801 could prevent morphine-induced tolerance and dependence(31). In this regard, a recent investigation by Zhou et al. has demonstrated that MET directly inhibits glutamate-induced neurotoxicity via inhibition of NMDA receptor(17). Lee et al. showed that phenformin, another drug from the biguanide-class having a similar effect like MET, exhibits neuroprotective effects through modulating the NMDA receptors in a manner, which decreases glutamate-induced calcium influx(32). So, there is the possibility that MET treatment could prevent developing morphine tolerance and dependence via inhibition of glutamate neurotransmitter and NMDA receptors. The role of nitric oxide (NO) in morphine tolerance and dependence has been well characterized(33). It has been reported that repeated exposure to morphine elevated the level of NO that contributes to neuronal adaptations(33). Therefore, inhibition of NO synthase (NOS) and NO production ameliorates morphine withdrawal signs in animal models(34,35). Previous works demonstrated the inhibitory effects of MET on NO production. Chung et al. showed that MET protects against diabetic neuronal impairment which induced by glycosylation end product in human neural stem cells via decreasing the expression of inducible nitric oxide synthase(36). Moreover, it has been reported that MET has anti-aging effect by reducing NO levels(37). Accordingly, MET may possibly show these effects through the suppression of NO. It was demonstrated that morphine administration increased oxidative stress in brain and spinal cord(38,39). Importantly, several investigations reveal that pharmacologic inhibition of superoxide and peroxynitrite with antioxidant compounds can prevent or inverse the characteristic pathologies associated with morphine-induced tolerance and dependence(40,41,42). The antioxidant properties of MET have been demonstrated in previous studies. In a study, it has shown that metformin decreases the intracellular reactive oxygen species (ROS) levels by increasing the expression of the antioxidant thioredoxin(43). Additionally, metformin has been shown to attenuate intracellular ROS amount by augmentation the antioxidative glutathione system activity(44). Furthermore, it has been reported that MET could reduce malondialdehyde, protein carbonyl and ROS in brain of naturally aged and accelerated senescence model of rat(37) implying that the effect of MET might be possibly mediated by antioxidative mechanism.
CONCLUSION
In conclusion, we found that acute administration of MET had no effect on expression of morphine tolerance and dependence. In the other hands, long term MET administration could attenuate the development of morphine tolerance and dependence. This phenomenon may reveal that MET needs more time to change/regulate the cellular and molecular mechanisms involved in morphine tolerance and dependence attenuation. We also found, for the first time, that oral administration of MET alone has no analgesic effect. Our study supports the idea that co-administration of metformin and morphine might be a helpful approach for increasing the clinical use of morphine and other opioids in treating chronic pain and attenuating the tolerance following repetitive morphine treatment. Also, one of the most important strategies for the treatment of drug addiction is reducing the withdrawal syndrome. Results of our study suggest that MET could attenuate withdrawal symptoms. However, further investigations are required to unveil the precise underlying mechanisms.
ACKNOWLEDGEMENTS
This project was financially supported via the Grant No. 20/1216 by the Rafsanjan University of Medical Sciences, Rafsanjan, I.R. Iran
REFERENCES
- 1.Somogyi AA, Barratt DT, Coller JK. Pharmacogenetics of opioids. Clin Pharmacol Ther. 2007;81(3):429–444. doi: 10.1038/sj.clpt.6100095. [DOI] [PubMed] [Google Scholar]
- 2.Hajhashemi V, Dehdashti Kh. Antinociceptive effect of clavulanic acid and its preventive activity against development of morphine tolerance and dependence in animal models. Res Pharm Sci. 2014;9(5):315–321. [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannadi A, Hajhashemi V, Abrishami R. Effects of the Persian Carum copticum fruit extracts on morphine withdrawal syndrome in mice. Res Pharm Sci. 2012;7(3):127–131. [PMC free article] [PubMed] [Google Scholar]
- 4.Rezazadeh H, Hosseini Kahnouei M, Hassanshahi G, Allahtavakoli M, Shamsizadeh A, Roohbakhsh A, et al. Regulatory effects of chronic low-dose morphine on nitric oxide level along with baroreflex sensitivity in two-kidney one-clip hypertensive rats. Iran J Kidney Dis. 2014;8(3):194–200. [PubMed] [Google Scholar]
- 5.DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs. 2007;8(3):113–121. doi: 10.1016/j.pmn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164(4):1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin W, Chun W, Ling L, Wei W. Role of melatonin in the prevention of morphine-induced hyperalgesia and spinal glial activation in rats: protein kinase C pathway involved. Int J Neurosci. 2012;122(3):154–163. doi: 10.3109/00207454.2011.635828. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 9.Joseph H, Stancliff S, Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt Sinai J Med. 2000;67(5-6):347–364. [PubMed] [Google Scholar]
- 10.Song L, Wu C, Zuo Y. Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol. 2015;15:12–20. doi: 10.1186/1471-2253-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261(2):669–677. [PubMed] [Google Scholar]
- 12.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 14.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323–329. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 15.Gundelach T, Rodewald M, Bekes I, Janni W, Hancke K. Metformin for the treatment of polycystic ovary syndrome. Med Monatsschr Pharm. 2016;39(2):75–78. [PubMed] [Google Scholar]
- 16.Liu SN, Liu Q, Sun SJ, Hou SC, Wang Y, Shen ZF. Metformin ameliorates β-cell dysfunction by regulating inflammation production, ion and hormone homeostasis of pancreas in diabetic KKAy mice. Yao Xue Xue Bao. 2014;49(11):1554–1562. [PubMed] [Google Scholar]
- 17.Zhou C, Sun R, Zhuang S, Sun C, Jiang Y, Cui Y, et al. Metformin prevents cerebellar granule neurons against glutamate-induced neurotoxicity. Brain Res Bull. 2016;121:241–245. doi: 10.1016/j.brainresbull.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi I, Khaluoi A, Kaeidi A, Shamsizadeh A, Heydari S, Allahtavakoli M. Protective effect of metformin on D-galactose-induced aging model in mice. Iran J Basic Med Sci. 2017;21(1):19–25. doi: 10.22038/IJBMS.2017.24331.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefont-Rousselot D, Raji B, Walrand S, Gardes-Albert M, Jore D, Legrand A, et al. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 2003;52(5):586–589. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 20.Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K, Bołdys A, Okopień B. Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFκB signaling. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(10):1103–1115. doi: 10.1007/s00210-016-1277-8. [DOI] [PubMed] [Google Scholar]
- 21.Deng T, Zheng YR, Hou WW, Yuan Y, Shen Z, Wu XL, et al. Pre-stroke metformin treatment is neuroprotective involving AMPK reduction. Neurochem Res. 2016;41(10):2719–2727. doi: 10.1007/s11064-016-1988-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, et al. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016;477(4):534–540. doi: 10.1016/j.bbrc.2016.05.148. [DOI] [PubMed] [Google Scholar]
- 23.Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, et al. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation. 2016;13(1):294–301. doi: 10.1186/s12974-016-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajializadeh Z, Nasri S, Kaeidi A, Sheibani V, Rasoulian B, Esmaeili-Mahani S. Inhibitory effect of Thymus caramanicus Jalas on hyperglycemia-induced apoptosis in in vitro and in vivo models of diabetic neuropathic pain. J Ethnopharmacol. 2014;153(3):596–603. doi: 10.1016/j.jep.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Nunez C, Foldes A, Laorden ML, Milanes MV, Kovacs KJ. Activation of stress-related hypothalamic neuropeptide gene expression during morphine withdrawal. J Neurochem. 2007;101(4):1060–1071. doi: 10.1111/j.1471-4159.2006.04421.x. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, et al. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004;114(8):1001–1011. doi: 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- 28.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 29.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, et al. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117(11):3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Xin X, Xie GX, Palmer PP, Huang YG. Molecular and cellular mechanisms of the age-dependency of opioid analgesia and tolerance. Mol Pain. 2012;8:38–43. doi: 10.1186/1744-8069-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bujalska-Zadrozny M, Duda K. Additive effect of combined application of magnesium and MK-801 on analgesic action of morphine. Pharmacology. 2014;93(3-4):113–119. doi: 10.1159/000358255. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Chan SL, Lu C, Lane MA, Mattson MP. Phenformin suppresses calcium responses to glutamate and protects hippocampal neurons against excitotoxicity. Exp Neurol. 2002;175(1):161–167. doi: 10.1006/exnr.2002.7864. [DOI] [PubMed] [Google Scholar]
- 33.Dambisya YM, Lee TL. Role of nitric oxide in the induction and expression of morphine tolerance and dependence in mice. Br J Pharmacol. 1996;117(5):914–918. doi: 10.1111/j.1476-5381.1996.tb15280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao JL, Ding HL, He JH, Zhang LC, Duan SM, Zeng YM. The spinal nitric oxide involved in the inhibitory effect of midazolam on morphine-induced analgesia tolerance. Pharmacol Biochem Behav. 2005;80(3):493–503. doi: 10.1016/j.pbb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Machelska H, Ziólkowska B, Mika J, Przewlocka B, Przewlocki R. Chronic morphine increases biosynthesis of nitric oxide synthase in the rat spinal cord. Neuroreport. 1997;8(12):2743–2747. doi: 10.1097/00001756-199708180-00020. [DOI] [PubMed] [Google Scholar]
- 36.Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen YL, Pei D, et al. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352(1):75–83. doi: 10.1016/j.yexcr.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Garg G, Singh S, Singh AK, Rizvi SI. Antiaging effect of metformin on brain in naturally aged and accelerated senescence model of rat. Rejuvenation Res. 2017;20(3):173–182. doi: 10.1089/rej.2016.1883. [DOI] [PubMed] [Google Scholar]
- 38.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, et al. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J Pharmacol Exp Ther. 2009;329(1):64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 2011;51(5):951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori T, Ito S, Matsubayashi K, Sawaguchi T. Comparison of nitric oxide synthase inhibitors, phospholipase A2 inhibitor and free radical scavengers as attenuators of opioid withdrawal syndrome. Behav Pharmacol. 2007;18(8):725–729. doi: 10.1097/FBP.0b013e3282f18da6. [DOI] [PubMed] [Google Scholar]
- 41.Ozmen I, Naziroglu M, Alici HA, Sahin F, Cengiz M, Eren I. Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem Res. 2007;32(1):19–25. doi: 10.1007/s11064-006-9217-5. [DOI] [PubMed] [Google Scholar]
- 42.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, et al. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117(11):3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396(2):199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48(2):353–357. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]