Abstract
Polymorphism in the genes encoding CYP2C9 enzyme and VKORC1 reductase significantly influence warfarin dose requirement since patients with CYP2C9*2, CYP2C9*3 and VKORC1 mutant alleles require lower warfarin maintenance doses. Studies have reported the ethnic variations in the frequency of these genes within the various populations in Iran and other parts of the world. However, no such study has been done yet on Kurdish population in Kermanshah. From Kurdish population of Kermanshah province in Iran, a total of 110 patients who had heart surgery and taking warfarin, were genotyped for polymorphisms of VKORC1-1639 G>A, CYP2C9*2, and CYP2C9*3. Polymorphism genotyping was performed by sequencing as well as polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using restriction enzymes of MspI, AVAII and KpnI, respectively. The frequencies of VKORC1-1639 GG, GA, and AA genotypes were 42%, 36%, and 22%, respectively and for CYP2C9 1*/1*, 1*/2*, 2*/2*, 1*/3*, 3*/3*, 2*/3* were 71%, 17%, 5.4%, 1.8%, 4.5%, and 0%, respectively. The frequency of VKORC1-1639A allele was 42.3% and the frequencies of CYP2C9*2 and *3 alleles were 14% and 5.4%, respectively. It was indicated that low warfarin dose requirements are strongly associated with the presence of CYP2C9 and VKORC1-1639 variant alleles. Our results confirmed the supply to understand the distribution of genomic biomarkers related to the drugs metabolism for future planning health programs.
Keywords: Cytochrome P-450 CYP2C9, International normalized ratio, Polymorphism, Vitamin K1 epoxide reductase, Warfarin
INTRODUCTION
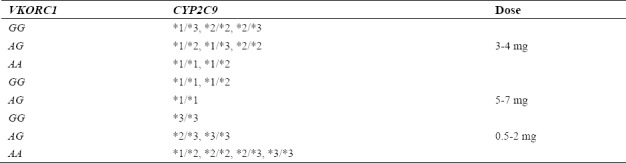
Warfarin is an anti-coagulation which is derived from coumarin and widely used in treating thrombosis by reducing the risk of blood clotting(1,2,3), (Fig. 1). The required warfarin dose among different individuals is influenced both by non-genetic factors including age, sex, weight, smoking, drug use, and genetic factors such as cytochrome P-450 family 2, subfamily C, polypeptide 9 (CYP2C9) and vitamin K epoxide reductase complex, and subunit 1 (VKORC1) genes(1,4). Clinical factors and variations in two genes considerably affect the already mentioned requirements for warfarin in various patients(5,6,7). The Food and Drug Administration (FDA) recommended an initial range of warfarin dose requirement for individuals based on their CYP2C9 and VKORC1 genotypes presented in Table 1.
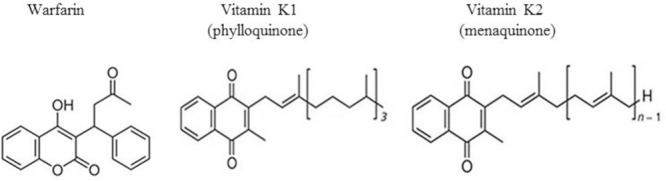
Fig. 1.
Chemical structure of warfarin, and two naturally forms of vitamin K1 (phylloquinone) and vitamin K2 (menaquinone)(32).
Table 1.
Food and Drug Administration (FDA) recommended initial doses (mg/day) requirements of warfarin for individuals based on their CYP2C9 and VKORC1 genotypes.
CYP2C9 is an essential enzyme expressed in the liver and transcribed by a gene located on the 10q22 chromosome. The gene is about 55 kb and contains 9 exons(8) having alleles among which CYP2C9*2 (single nucleotide polymorphisms (SNP) ID: rs1799853) (arginine- to -cysteine change at codon 144) and CYP2C9*3 (SNP ID: rs1057910) (isoleucine- to -leucine change at codon 359) can reduce the activity of CYP2C9 enzyme in comparison to CYP2C9*1, the wild type allele, due to the lower catalytic activity of the two allelic variants(9).
The VKORC1 gene contains multiple polymorphisms associated with the variety of responses to warfarin. This gene is located in the promoter region and its polymorphism changes the expression of vitamin K epoxide reductase enzyme(10). The presence of a G nucleotide position 1639 instead of an A in VKORC1 gene (-1639 G>A) (SNP ID: rs9923231), increases the activity of the enzyme; therefore, promoter activity and then mRNA expression are reduced(11). VKORC1 is located on 16p11 chromosome and contains 3 exons and 5125 base pairs(8). The presence of CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A in individuals reduces the dose requirements for warfarin(12). Accordingly, it may be stated that CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A polymorphisms affect warfarin dose requirements to maintain a target international normalized ratio (INR = (prothrombin time (PT) measured ISI (international sensitivity index)/PT normal)) in warfarin users(13), (Table 1). The aim of the present study was to determine the prevalence of CYP2C9*2, CYP2C0*3, and VKORC1-1639 G>A polymorphisms and also their correlation to warfarin dose requirement in patients taking warfarin living in the city of Kermanshah, I.R. Iran.
MATERIALS AND METHODS
A total of 110 patients under warfarin therapy (59 males and 51 females) (warfarin tablet, 5 mg, Orion Corporation, Espoo, Finland) were enrolled in the present study. The patients had undergone heart surgery in the Imam Ali Hospital (Kermanshah, I.R. Iran). The research protocol was approved by Ethics Committee of the Kermanshah University of Medical Sciences in accordance with international agreements (ID number: 2260)(14). An informed consent form was voluntarily signed by all participants.
Blood samples were taken from patients to measure INR value and extract DNA. Genomic DNA was extracted from peripheral blood leukocytes by phenol-chloroform extraction method(15). The extracted DNA was stored at -20 °C until it was used. The CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A polymorphisms were genotyped using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The used primers were as follows: CYP2C9*2 forward: 5´-GTA TTT TGG CCT GAA ACC C-3´, CYP2C9*2 reverse: 5´ -GGC CTT GGT TTT TCT CAA CTC-3´(16); CYP2C9*3 forward: 5´-TGC ACG AGG TCC AGA GGT AC-3´, CYP2C9*3 reverse: 5´-ACA AAC TTA CCT TGG GAA TGA GA-3´(17); VKORC1-1639 G>A forward: 5´-GCC AGC AGG AGA GGG AAA TA-3´, VKORC1-1639 G>A reverse: 5´-AGT TTG GAC TAC GGT GCC T-3´(18). The PCR reaction consisted of buffer 10× (100 mM tris-HCl, 500 mM KCl, 50 mM MgCl2, pH 8.3), 0.2 mM dNTPs, 200 ng template DNA, and 1U AmpliTaq DNA polymerase. PCR thermal cycling conditions were as follows: for CYP2C9*2, one cycle of initial denaturation at 95 °C for 10 min, then 35 cycle of initial denaturation at 94 °C for 45 sec followed by annealing at 54 °C for 45 sec, and final extension at 72 °C for 10 min; for CYP2C9*3, one cycle of initial denaturation at 95 °C for 10 min, then 35 cycle of initial denaturation at 94 °C for 45 sec followed by annealing at 53 °C for 45 sec, and final extension at 72 °C for 10 min; for VKORC1-1639 G>A, one cycle of initial denaturation at 95 °C for 10 min, then 35 cycle of initial denaturation at 94 °C for 45 sec followed by annealing at 58 °C for 45 sec, and final extension at 72 °C for 10 min. PCR products were examined on 2% agarose gel with Gel Red DNA stain under ultraviolet light. The used restriction enzymes (Fermentas) were AVA II for CYP2C9*2, KpnI for CYP2C9*3, and MSP I for VKORC1(9,19). For restriction enzyme digestion of RCR products, 12 µL of PCR products was mixed with 1 µL restriction enzyme and 2 µL buffer, and incubated for 16 h at 37 °C. For genotypes sequencing, the samples were analyzed by DNA Sequencer apparatus (Macrogen, South Korea).
Data analysis
Deviation from Hardy-Weinberg Equilibrium (HWE) was tested using the calculator. The data was also analyzed by t-test using statistical package for social sciences (SPSS) version 18 and presented as the medians with 95% confidence intervals(20).
RESULTS
The results of the allele variants of CYP2C9*2, CYP2C9*3 and VKORC1-1639 G>A obtained from PCR-RFLP are shown in Figs. 2–4. The estimated genotype and allele frequencies of CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A and 0.054 (12 alleles), respectively and the VKORC1-1639 A allele frequency was 0.423.
Fig. 2.
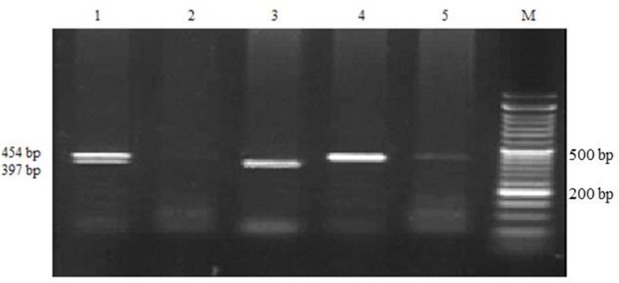
Agarose gel electrophoresis for CYP2C9*2 polymorphism detected by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and restriction enzyme AVA II. M, marker; lane 1, CT heterozygous; lane 2, negative control; lane 3, CC homozygous wild type; lanes 4 and 5, TT homozygous mutant type.
Fig. 4.
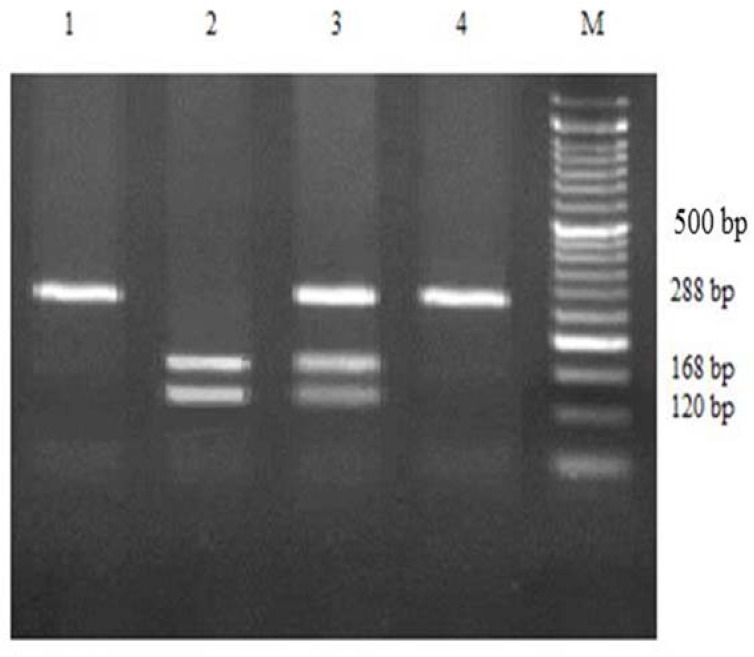
Agarose gel electrophoresis for VKORC1 G>A polymorphism detected by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and restriction enzyme MSP I. M, marker; lane 1 and 4, AA homozygous mutant type; lane 2, AC heterozygous; lane 3, GG homozygous wild type.
Fig. 3.
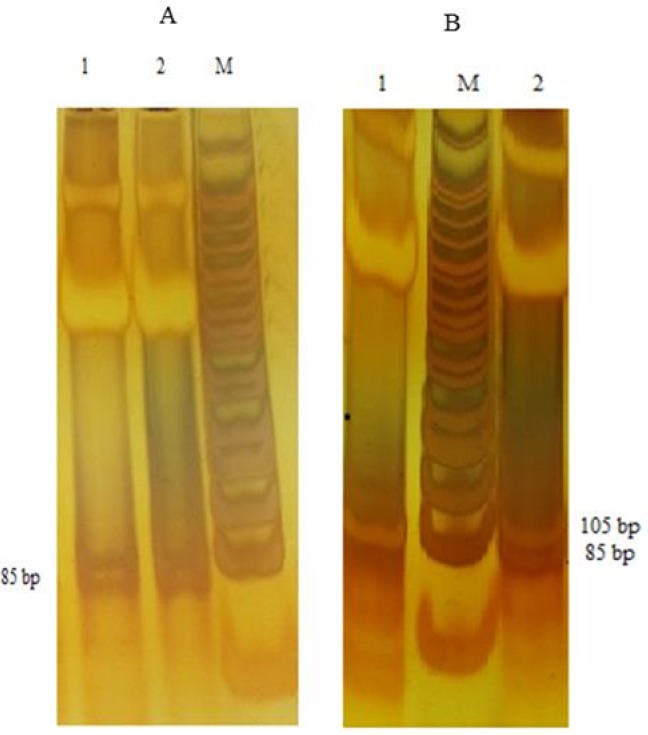
Agarose gel electrophoresis for CYP2C9*3 polymorphism detected by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and restriction enzyme KpnI. (A) M, marker; lane 2, CC homozygous mutant type. (B) M, marker; lane 1, AC heterozygous; lane 2, AA homozygous wild type.
There were 78 subjects with CYP2C9*1/*1 genotype (0.71), 19 subjects with CYP2C9*1/*2 genotype (0.17), 6 subjects with CYP2C9*2/2 genotype (0.054), 2 subjects with CYP2C9*1/*3 genotype (0.018), 5 subjects with CYP2C9*3/3 genotype (0.045), and none carried CYP2C9*2/*3 genotype. For VKORC1-1639 G>A polymorphism, there were 46 subjects with VKORC1-1639 GG genotype (0.42), 35 with VKORC1-1639 G>A genotype (0.32), and 29 with VKORC1-1639 AA genotype (0.26) (Table 2). The results of direct DNA sequencing from the patients with wild genotype and mutant genotype for each gene are shown in Fig. 5.
Table 2.
Genotype and allele frequencies of the CYP2C9*2, CYP2C9*3 alleles, and VKORC1-1639 G>A polymorphism in Kurdish population of Kermanshah.
Fig. 5.
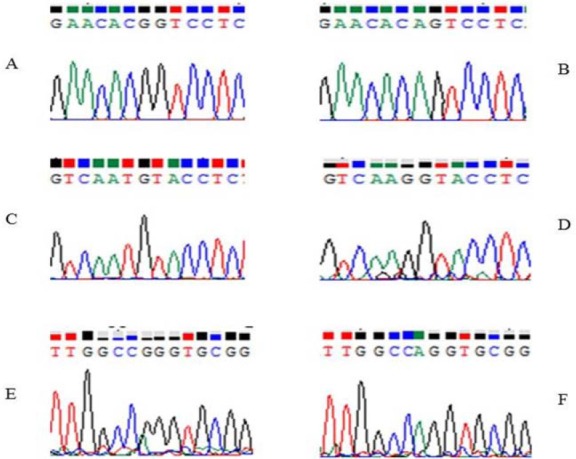
Sequence analysis of DNA samples. (A) Sequence of CYP2C9*1*1 genotype GG. (B) Sequence of CYP2C9*2*2 genotype AG. (C) Sequence of CYP2C9*1*1 genotype TG. (D) Sequence of CYP2C9*3*3 genotype GG. (E) Sequence of VKORC1-1639 GG. (F) Sequence of VKORC1-1639 AG.
Warfarin dosing is typically adjusted to maintain the INR at 2.5 ± 0.5 and at 3.0 ± 0.5 for higher risk patients, including those with certain mechanical heart valves(21). Considering the achieved data including the range of INR and calculating the genotype frequency, it was observed that warfarin dose requirements for reaching the target INR had a significant relationship with individuals´ genotype. Thereby, people with a mutation in CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A genes require less warfarin dose daily. Table 3 presents the relationship between warfarin dose requirements and individuals’ genotype. The calculated P values for each SNP are also shown in Table 4.
Table 3.
The warfarin dose requirement for the patients in our study to reach international normalized ratio (INR) goal of 2-3 according to the genotype (mg/day).
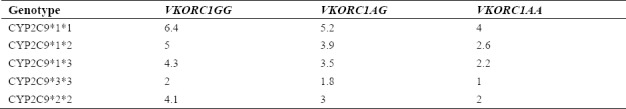
Table 4.
The calculated P values for each SNP.
![]()
DISCUSSION
Drug metabolism varies from one patient to another due to genetic differences. Accordingly, the required warfarin dose depends upon various factors including age, diet, sex, and last but not least, the genetics. Each society has its own specific gene polymorphism considered a significant determinant in drug metabolism. Patients with VKORC1, CYP2C9*2, and CYP2C9*3 mutant alleles require lower warfarin maintenance doses(1,4). Studies showed that the mutations on these genes may lead to an abnormal three-dimensional conformation of the molecule, which would have a remarkable impact on the protein structure. Furthermore, these mutations can alter the amino acid sequence of the proteins which result in altered enzymatic activity(22).
The basic method used in this study was PCR-RFLP which is an appropriate method in SNP genotyping and mutation detection. Also, our sequencing results confirmed the results derived from RFLP-PCR. The frequencies of CYP2C9*2 and CYP2C9*3 alleles are different in various populations, as they are very common in some countries while being rare in others(9). Since individuals with CYP2C9*2 and CYP2C9*3 alleles have lower CYP2C9 enzyme activity, they are prone to bleeding following warfarin therapy. Individuals with CYP2C9*2 and CYP2C9*3 genotypes have 12% and 5% lower enzyme activity, respectively than the wild type. Therefore, studying the frequencies of CYP2C9*2 and CYP2C9*3 genes in populations with high frequency is extremely important prior to warfarin therapy(23). In this study, the frequency of VKORC1-1639 G>A SNP genotypes as well as the frequencies of CYP2C9*2 and CYP2C9*3 alleles were evaluated.
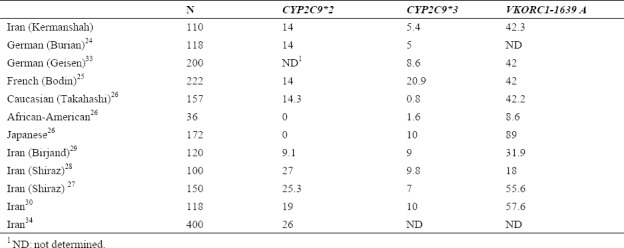
Based on our findings, allele frequency of CYP2C9*2 in Kermanshah population (14%) was similar to the results from German (14%)(24), French (14%)(25), and Caucasian (14.3%)(25) populations. In case of frequency of CYP2C9*3 allele, similar results were also obtained in our study (5.4%) compared to the report from German population (5%)(24) (Table 5). However, the estimated frequency of CYP2C9*2 and *2 alleles in our study compared with the reported corresponding results from Japanese, Caucasian, and African-American populations(26) showed a considerable difference (Table 5). Moreover, the frequency of VKORC1 in our study (42.3%) was similar to the results from the German, French, and Caucasian populations and different from the Japanese (89%) and African-American (8.6%) populations. In addition, our results were different from the findings obtained from the studies on other populations in Iran. For example, Azarpira, et al. in Shiraz determined the allele frequency of CYP2C9*2, CYP2C9*3 and VKORC1-1639 G>A with the frequencies of 25.3%, 9.8%, and 55.6%, respectively(27). In another study by Namazi, et al. in Shiraz(28), the allele frequency for CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A was obtained as 27%, 9%, and 18%, respectively (Table 5). In contrast to Kermanshah population, most of the subjects in Birjand showed more variant allele frequency of CYP2C9*2, CYP2C9*3, and VKORC1-1639 G>A as 9.1, 10, and 31.9%, respectively(29). Furthermore, Dalily and Ramazani in 2012(30) and Kameli, et al. in 2016(31) in two separate studies in Iran reported different genotype frequencies for CYP2C9*2, CYP2C9*3 and/or VKORC1 compared to the obtained results in our study (Table 5).
Table 5.
Comparative allele frequency (%) of three single nucleotide polymorphisms (SNPs) (CYP2C9*2, CYP2C9*3 and VKORC1-1639 A) in Iran and the different world populations.
Collectively, it can be concluded that the frequencies of CYP2C9*2, CYP2C9*3, and VKORC1 genes are different in various populations. Regarding the use of warfarin dose and individuals’ genotypes, it was observed that people having mutation in these genes require less warfarin doses to reach the target INR in comparison to people with no gene mutation(32). Therefore, it can be stated that warfarin dose requirement for reaching the target INR is influenced by individuals, genetics.
CONCLUSION
The role of VKORC1 and CYP2C9 polymorphisms has been long studied regarding warfarin therapy and to help clinicians with the new field of pharmacogenetics in order to determine the proper warfarin dose in patients treatment strategy. Our study confirmed the previous reports about the significance of pharmacogenetic testing in predicting a high risk of bleeding before initiation of anticoagulation with warfarin in the patients carrying either the VKORC1 variant (1639A) or the CYP2C92*, CYP2C9*3 alleles. According to the association between drug dosage and genotype, conducting regional studies based on races and geographical conditions is considered essential.
ACKNOWLEDGEMENT
This work was financially supported by the research grant No. 93221 from the Vice Chancellery of Research and Technology, Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran.
REFERENCES
- 1.Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost. 2008;34(8):709–733. doi: 10.1055/s-0029-1145254. [DOI] [PubMed] [Google Scholar]
- 2.Saminathan R, Bai J, Sadrolodabaee L, Karthik GM, Singh O, Subramaniyan K, et al. Vkorc1 pharmacogenetics and pharmacoproteomics in patients on warfarin anticoagulant therapy: transthyretin precursor as a potential biomarker. PloS One. 2010;5(12):e15064. doi: 10.1371/journal.pone.0015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer TP, O’Kane DJ, Baudhuin LM, Wiley CL, Fortini A, Fisher PK, et al. Warfarin sensitivity genotyping: a review of the literature and summary of patient experience. Mayo Clin Proc. 2009;84(12):1079–1094. doi: 10.4065/mcp.2009.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahin MH, Khalifa SI, Gong Y, Hammad LN, Sallam MT, El Shafey M, et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics. 2011;21(3):130–135. doi: 10.1097/FPC.0b013e3283436b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5(3):e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G, et al. Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb Haemost. 2010;104(4):750–754. doi: 10.1160/TH09-11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. PLoS One. 2012;7(8):e44064. doi: 10.1371/journal.pone.0044064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung E, Patsopoulos NA, Belknap SM, O’Rourke DJ, Robb JF, Anderson JL, et al. Effect of genetic variants, especially CYP2C9 and VKORC1, on the pharmacology of warfarin. Semin Thromb Hemost. 2012;38(8):893–904. doi: 10.1055/s-0032-1328891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seng KC, Gin GG, Sangkar JV, Phipps E. Frequency of cytochrome P450 2C9 (CYP2C9) alleles in three ethnic groups in Malaysia. Asia Pac J Mol Biol Biothechnol. 2003;11(2):83–91. [Google Scholar]
- 10.Cosan DT, Yazıcı HU, Colak E, Soyocak A, Degirmenci I, Kurt H, et al. Susceptiveness of vitamin K epoxide reductase complex subunit 1 gene polymorphism in essential hypertension. Genet Test Mol Biomarkers. 2017;21(5):292–297. doi: 10.1089/gtmb.2016.0311. [DOI] [PubMed] [Google Scholar]
- 11.Madhan S, Kumar DK, Kumar DT, Balachander J, Adithan C. Effect of cyp2c9 and vkorc1 genetic polymorphisms on warfarin dose requirement in south indian population. Indian J Physiol Pharmacol. 2013;57(3):308–317. [Google Scholar]
- 12.Kaur A, Khan F, Agrawal SS, Kapoor A, Agarwal SK, Phadke SR. Cytochrome P450 (CYP2C9*2,* 3) & vitamin-k epoxide reductase complex (VKORC1-1639g<A) gene polymorphisms & their effect on acenocoumarol dose in patients with mechanical heart valve replacement. Indian J Med Res. 2013;137:203–209. [PMC free article] [PubMed] [Google Scholar]
- 13.Nahar R, Deb R, Saxena R, Puri RD, Verma IC. Variability in CYP2C9 allele frequency: a pilot study of its predicted impact on warfarin response among healthy south and north indians. Pharmacol Rep. 2013;65:187–194. doi: 10.1016/s1734-1140(13)70977-0. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association. World medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic acids Res. 1976;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak-Göttl U, Dietrich K, Schaffranek D, Eldin NS, Yasui Y, Geisen C, et al. In pediatric patients, age has more impact on dosing of vitamin k antagonists than VKORC1 or CYP2C9 genotypes. Blood. 2010;116(26):6101–6105. doi: 10.1182/blood-2010-05-283861. [DOI] [PubMed] [Google Scholar]
- 17.Aomori T, Yamamoto K, Oguchi-Katayama A, Kawai Y, Ishidao T, Mitani Y, et al. Rapid single-nucleotide polymorphism detection of cytochrome P450 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genes for the warfarin dose adjustment by the SMart-amplification process version 2. Clin Chem. 2009;55(4):804–812. doi: 10.1373/clinchem.2008.115295. [DOI] [PubMed] [Google Scholar]
- 18.Rathore SS, Agarwal SK, Pande S, Mittal T, Mittal B. The impact of VKORC1-1639 G> A polymorphism on the maintenance dose of oral anticoagulants for thromboembolic prophylaxis in north india: A pilot study. Indian J Hum Genet. 2011;17(Suppl 1):S54–s57. doi: 10.4103/0971-6866.80360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen MS, Lee M, Chen JJ, Chuang HP, Lu LS, Chen CH, et al. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther. 2008;84:83–89. doi: 10.1038/sj.clpt.6100453. [DOI] [PubMed] [Google Scholar]
- 20.Weir BS. Genetic data analysis II. Trends Genet. 1997;13(9):379. [Google Scholar]
- 21.Flockhart DA, O’kane D, Williams MS, Watson MS, Gage B, Gandolfi R, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med. 2008;10(2):139–150. doi: 10.1097/GIM.0b013e318163c35f. [DOI] [PubMed] [Google Scholar]
- 22.D’ambrosio RL, D’andrea G, Cafolla A, Faillace F, Margaglione M. A new vitamin K epoxide reductase complex subunit‐1 (VKORC1) mutation in a patient with decreased stability of CYP2C9 enzyme. J Thromb Haemost. 2007;5:191–193. doi: 10.1111/j.1538-7836.2006.02261.x. [DOI] [PubMed] [Google Scholar]
- 23.Sosa-Macías M, Lazalde-Ramos BP, Galaviz-Hernández C, Rangel-Villalobos H, Salazar-Flores J, Martínez-Sevilla V, et al. Influence of admixture components on CYP2C9*2 allele frequency in eight indigenous populations from Northwest Mexico. Pharmacogenomics J. 2013;13(6):567–572. doi: 10.1038/tpj.2012.52. [DOI] [PubMed] [Google Scholar]
- 24.Burian M, Grösch S, Tegeder I, Geisslinger G. Validation of a new fluorogenic real‐time pcr assay for detection of CYP2C9 allelic variants and CYP2C9 allelic distribution in a german population. Br J Clin Pharmacol. 2002;54(4):518–521. doi: 10.1046/j.1365-2125.2002.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, Funck-Brentano C, et al. Cytochrome P450 2c9 (CYP2C9) and vitamin K epoxide reductase (.(VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood, 2005;106:135–140. doi: 10.1182/blood-2005-01-0341. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra-and inter-population differences in maintenance dose of warfarin in japanese, caucasians and african-americans. Pharmacogenet Genomics. 2006;16(2):101–110. doi: 10.1097/01.fpc.0000184955.08453.a8. [DOI] [PubMed] [Google Scholar]
- 27.Azarpira N, Namazi S, Hendijani F, Banan M, Darai M. Investigation of allele and genotype frequencies of CYP2C9, CYP2C19 and VKORC1 in Iran. Pharmacol Rep. 2010;62(4):740–746. doi: 10.1016/s1734-1140(10)70332-7. [DOI] [PubMed] [Google Scholar]
- 28.Namazi S, Azarpira N, Hendijani F, Khorshid MB, Vessal G, Mehdipour AR. The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran. Clin Ther. 2010;32(6):1050–1060. doi: 10.1016/j.clinthera.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Razavi FE, Zarban A, Hajipoor F, Naseri M. The allele frequency of CYP2C9 and VKORC1 in the Southern Khorasan population. Res Pharm Sci. 2017;12:211–221. doi: 10.4103/1735-5362.207202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kameli R, Hasanzad M, Tahmasebi Fard Z, Babanejad M, Imeni M, Feizi Barnaji L, et al. Association between cytochrome P450 2 C9 and vitamin K epoxide reductase complex subunit 1 polymorphisms with warfarin dose among Iranian patients. Res Mol Med. 2016;4(4):38–44. [Google Scholar]
- 31.Dean L. Warfarin Therapy and the Genotypes CYP2C9 and VKORC1. In: Rubinstein W, Pratt V, McLeod H, Dean L, Malheiro A, editors. Medical Genetics Summaries. Bethesda: 2016. pp. 405–414. [Google Scholar]
- 32.Valente E, Lingafelter EC, Porter WR, Trager WF. Structure of warfarin in solution. J Med Chem. 1977;20(11):1489–1493. doi: 10.1021/jm00221a025. [DOI] [PubMed] [Google Scholar]
- 33.Geisen C, Watzka M, Sittinger K, Steffens M, Daugela L, Seifried E, et al. Vkorc 1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost. 2005;94(4):773–779. doi: 10.1160/TH05-04-0290. [DOI] [PubMed] [Google Scholar]
- 34.Dalily F, Ramazani A. Frequency of single nucleotide polymorphisms of cytochrome P450 CYP2C9 in an Iranian population using TaqMan genotyping assay. Res Pharm Sci. 2012;7(5):S451. [Google Scholar]