Abstract
The role of angiogenesis in tumor progression and metastasis formation has been well recognized. Recent studies have reported that Trigonella foenum-graecum L. (fenugreek) seed extracts have potential anticancer properties. The current study was planned to investigate the anti-angiogenic activity of hydroalcoholic extract of fenugreek (HAEF) in vitro and in vivo. Effect of HAEF (50-3000 µg/mL) and thalidomide (200-3000 µmol/L), as a positive control, on the viability of human umbilical vein endothelial cells (HUVECs) and 3T3 fibroblast cells was assessed by thiazolyl blue tetrazolium bromide (MTT) assay. Effect of HAEF on vessel-like tube formation by HUVECs was examined in the matrigel-based assay. Furthermore, the chick chorioallantoic membrane (CAM) was used as in vivo model to study the anti-angiogenic effect of HAEF. HAEF, similar to thalidomide, significantly inhibited the viability of HUVECs and 3T3 cells dose-dependently after 24 h. Moreover, both HAEF and thalidomide significantly reduced tube formation by HUVECs in cell culture condition. In CAM model, HAEF and thalidomide caused a significant decline in the number of neovascular points and in the amount of grades 1 and 2 vessels. These findings revealed that fenugreek has cytotoxic and anti-angiogenic effects in vitro and in vivo. Therefore, this medicinal plant can be subjected to further investigations as antitumor agents.
Keywords: Angiogenesis, Cancer, Chorioallantoic membrane, Human umbilical vein endothelial cells, Thalidomide, Trigonella foenum-graecum
INTRODUCTION
Angiogenesis is one of the most important physiological processes that contribute in growth and progression of many natural and pathological events such as pregnancy and cancers, respectively. In tumor growth, angiogenesis has an essential role in supporting the tumor growth, survival, and metastases of cancerous cells(1).
Developing angiogenesis inhibitors with few side effects can be regarded as a desirable anticancer target(2,3). Angiogenesis inhibition by both new inhibitors and natural edible agent are interested in finding new ways to the effective therapeutic outcome with few side effects.
Several lines of studies have shown that certain medicinal plants have anticancer effects(4). Herbal extracts exert their anticancer effect through different mechanisms including inhibition of angiogenesis, cell proliferation, and induction of apoptosis(5,6). At present, a number of herbal agents such as vinca alkaloids (vincristine, vinblastine), taxol analogues, and podophyllotoxin derivatives are used for chemotherapy of cancer patients(7). There are many in vitro and in vivo studies on the anti-cancer and anti-angiogenic effects of plants(8,9,10).
Trigonella foenum-graecum is one of these important herbs; an annual herb belongs to the family of Leguminoseae, with the species name fenugreek(11). Recently, antineoplastic and chemopreventive effects of Trigonella foenum-graecum have been displayed in vitro and in vivo(12,13,14). The main effective compounds of Trigonella faenum-graecum are including saponins, mucilage, steroids, alkaloids, and unsaturated fatty acids. In addition, the main steroidal sapogenins in the seeds of Trigonella faenum-graecum have been considered to be Diosgenin and Yamogenin(11,15).
The seeds of Trigonella faenum-graecum L. have been considered an important part of the plant with different medicinal uses including anti-diabetic and anticancer effects(14,16). This plant also has therapeutic activity against hepatotoxicity(17), diabetes(18), and hyperlipidemia(19). Few of the studies have examined the anti-angiogenic effects of fenugreek as a possible mechanism for anticancer activity of this plant(20,21). Therefore, the current study was planned to investigate the anti-angiogenic activity of a hydroalcoholic extract of fenugreek (HAEF) in vitro and in vivo.
METHODS AND MATERIALS
Drugs and Chemicals
The human umbilical vein endothelial cells (HUVECs) and NIH/3T3 fibroblast cells were taken from Pasteur Institute of Iran (Tehran, I.R. Iran). Dulbecco's modified eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (USA). 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), penicillin/streptomycin and trypsin/EDTA solutions were obtained from Sigma (USA). Fertilized eggs were purchased from Morghdaran Toos Co. (Mashhad, I.R. Iran). Thalidomide was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Extract preparation
The seeds of fenugreek were purchased from Imam Reza pharmacy (Division of Medicinal Plants, Mashhad, I.R. Iran) and identified by Eng. Mohammadreza Joharchi (Ferdowsi University of Mashhad Herbarium, Mashhad, I.R. Iran). A voucher specimen (No. 30711) was deposited in the herbarium of Ferdowsi University of Mashhad (I.R. Iran).
The HAEF was prepared by macerating 150 g of the powder of fenugreek seeds in 2 L of 50% ethanol (v/v in water) at 37 °C. After 4 days, the extract was filtered and concentrated under reduced pressure using a rotary apparatus (Stuart, RE300, UK). The residual content was transferred into petri dish and was kept in an oven at 40 °C for drying up. The HAEF was kept at -20 °C until use in the in vitro and in vivo experiments.
Cell cultures and treatments
The HUVECs and 3T3 cells were cultured in high-glucose DMEM supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. The cells were incubated at 37 °C, in a humidified atmosphere (5% CO2 and 95% air). The culture medium was changed every 24 h. For cell viability assay, cells were harvested from culture flask (95% confluent) using trypsin/EDTA solution and seeded overnight in 96-well culture plate (2×104 cells/well). The culture medium on the 96 well-plate was removed and exchanged with the fresh one containing serial dilutions of HAEF (50-3000 µg/mL) or thalidomide (25-3000 μM) as reference drug, the cells were treated for 24 h and then the cell viability of HUVECs and 3T3 cells was determined using MTT test.
Five mg/mL of MTT in phosphate buffered saline (PBS) was added to each well with a final concentration of 0.05%, and the plates were incubated for 4 h at 37 °C, the reaction mixture was removed and 100 μL DMSO was added into each well. The optical density of formazan dye was read using microplate reader at a test wavelength of 570 nm and background wavelength of 630 nm. IC50 (50% inhibitory concentration) was expressed as the concentration of drug yielding 50% of dye reduction compared with untreated control.
Chicken chorioallantoic membrane angio-genesis model
Two hundred fertilized eggs were incubated at 37 °C and 70% relative humidity in a forced draught incubator. A small window was punctured on each egg of 8-day-old fertilized eggs and the HAEF (0, 250, 500, 1000, and 2000 µg/mL) were injected into the chorioallantoic sac. The negative and positive control eggs received sterile PBS and thalidomide (1000 μM), respectively. For each concentration 8 eggs were used for 5 times. The window in the shell was closed by wax and the eggs were maintained in the incubator. At day 12, the eggs were opened and the chorioallantoic membrane vasculatures were imaged using a stereo microscope equipped with a digital camera (Canon EOS 40D with Canon EF 100 mm f/2.8 USM macro lens Japan). Angiogenesis was quantified by counting the vessel grade types and neovascular point using Photoshop CS2 software (IMAGE J). To analyze the grades of angiogenesis, the type and the number of vessels were counted. On the basis of this fact, the grade 4 is determined by large vessels. Moreover, the branches were considered as subsequent grades (grades 1 to 3) according to decreasing of diameters of vessels. Furthermore, neovascularization points were counted and the results were expressed as a mean ± SEM (standard error of mean)(22).
Tube formation assay
Effect of fenugreek on in vitro angiogenesis was examined in HUVECs. First, a 96-well plate was coated with a thick layer of matrigel (80 μL/well) and stored for 30 min at 37 °C to allow solidify and polymerized. After culture HUVECs, the cells (1 × 105 cells) were seeded on the surface of the matrigel and treated with HAEF (0, 250, 500, 1000, and 2000 µg/mL). Thalidomide (1000 μM) was used as the positive control. After 8-18 h, the length of formed tubes was examined using a phase-contrast microscope equipped with a digital camera (Canon EOS 40D with Canon Japan).
Statistical analyses
All data in the different experimental groups were expressed as the mean ± SEM. The normality of data was performed by Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) and then Bonferroni post hoc test was performed for multiple-group comparisons. Statistical differences were considered to be significant if P < 0.05.
RESULTS
Effect of hydroalcoholic extract of fenugreek on the viability of 3T3 cells and HUVECs
Results of MTT assay demonstrated that HAEF significantly decreased the viability of HUVECs and 3T3 cells (Fig. 1). This effect of HAEF on both cells was concentration dependent and comparable to the effect of thalidomide (Fig. 2). The extract reduced the viability of 3T3 cells and HUVECs with IC50 values of 285.9 μg/mL and 478.8 μg/mL, respectively. The IC50 value of thalidomide was 917.2 μM for 3T3 cells and 1190 μM for HUVECs.
Fig. 1.
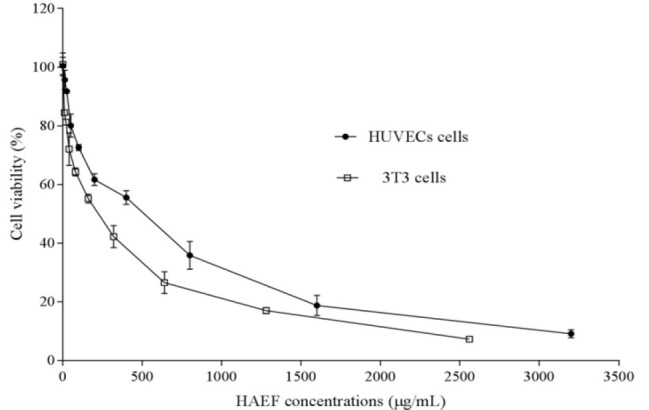
Effects of the hydroalcoholic extract of fenugreek (HAEF) on the viability of 3T3 fibroblast cells and human umbilical vein endothelial cells (HUVECs). The cells were treated for 24 h with various concentrations of HAEF and the percentage of viable cells was measured by MTT assay. Data are presented as mean ± SEM (n = 6).
Fig. 2.
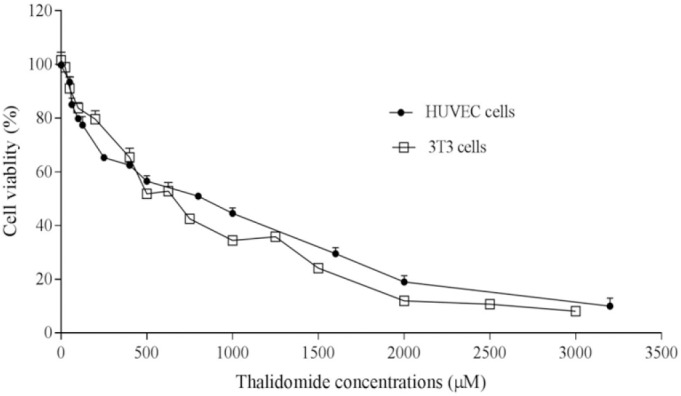
Effects of thalidomide on the viability of 3T3 fibroblast cells and human umbilical vein endothelial cells (HUVECs). The cells were treated for 24 h with various concentrations of thalidomide and the percentage of viable cells was measured by MTT assay. Data are presented as mean ± SEM (n = 6).
Effects of hydroalcoholic extract of fenugreek on angiogenesis in chorioallantoic membrane model
Figure 3 demonstrated the vasculature of chorioallantoic membrane (CAM) treated with HAEF. While control eggs showed dense vascularized CAM, this density was considerably decreased in HAEF treated eggs. As shown in Fig. 4, HAEF significantly reduced the formation of grades 1 and 2 of vessels types in the CAM at concentrations more than 500 μg/mL. On the other hand, similar to thalidomide, the effect of HAEF on the grades 3 and 4 vessels remained non-significant.
Fig. 3.
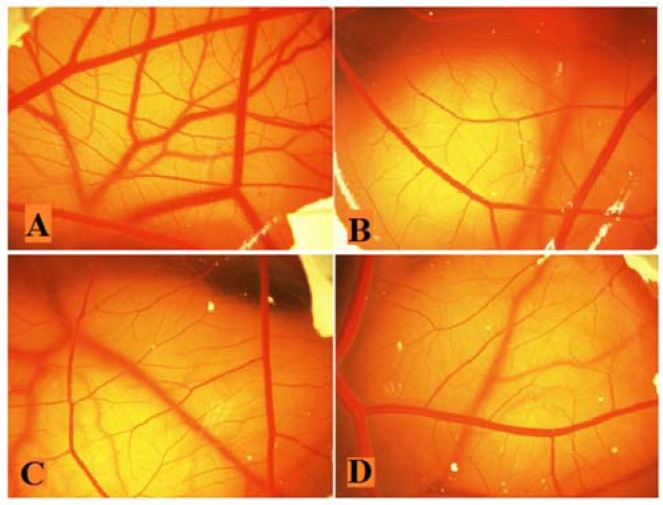
Effects of the hydroalcoholic extract of fenugreek (HAEF) and thalidomide on angiogenesis in the chicken chorioallantoic membrane (CAM) model. Representative photographs of the CAM treated with (A) vehicle (PBS), (B) 1000 μM of thalidomide, (C) 500 μg/mL of HAEF, and (D) 1000 μg/mL of HAEF on day 12. Magnification: × 20.
Fig. 4.
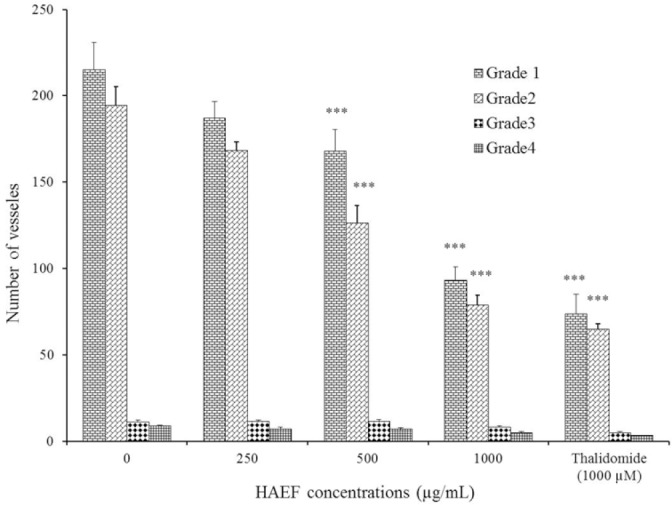
Effects of a hydroalcoholic extract of fenugreek (HAEF) and thalidomide on the number of vessels in the chicken chorioallantoic membrane model. Data are presented as mean ± SEM (n = 8). ***P < 0.001 versus related grade in control group (0 µM).
Figure 5 displayed the number of vascular points formed in CAM. In samples treated with low concentration (250 μg/mL) of HAEF there was no significant change in the number of neovascular points compared to control cells. However, in samples treated with high concentrations of HAEF (500, 1000, and 2000 μg/mL), a significant decrease was observed in the number of these points in comparison with control group (P < 0.001).
Fig. 5.
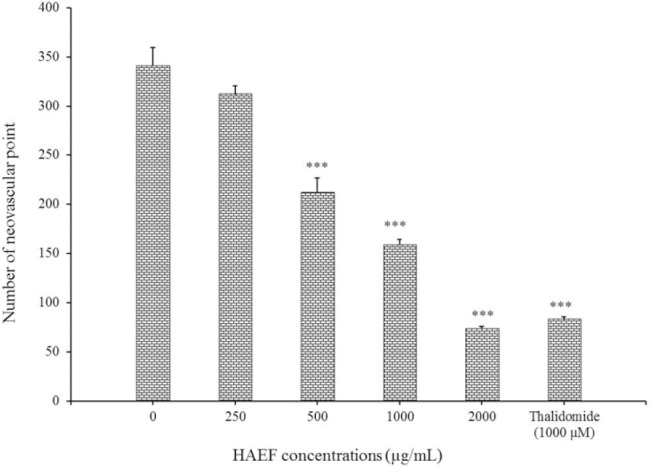
Effects of a hydroalcoholic extract of fenugreek (HAEF) and thalidomide on neovascular formation in the chicken chorioallantoic membrane model. Data are presented as mean ± SEM (n = 8). ***P < 0.001 versus control group (0 µM).
Effects of hydroalcoholic extract of fenugreek on tube formation by HUVECs
Induction of differentiation led to a change of HUVECs morphology into a tube-like shape. Similar to thalidomide, a significant reduction was seen in the length of the formed tube in HUVECs treated with HAEF compared to untreated cells (Fig. 6). This inhibitory effect of HAEF was concentration dependent and was statistically significant (P < 0.01) at all tested concentrations (Fig. 7).
Fig. 6.
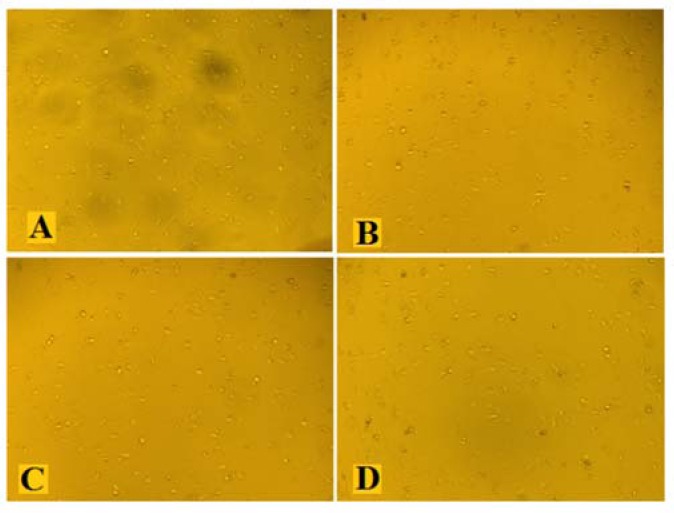
Effects of the hydroalcoholic extract of fenugreek (HAEF) and thalidomide on tube formation by human umbilical vein endothelial cells (HUVECs). Representative photographs of the HUVECs treated with (A) vehicle, (B) 1000 μM of thalidomide, (C) 500 μg/mL of HAEF, and (D) 1000 μg/mL of HAEF. Magnification × 40.
Fig. 7.
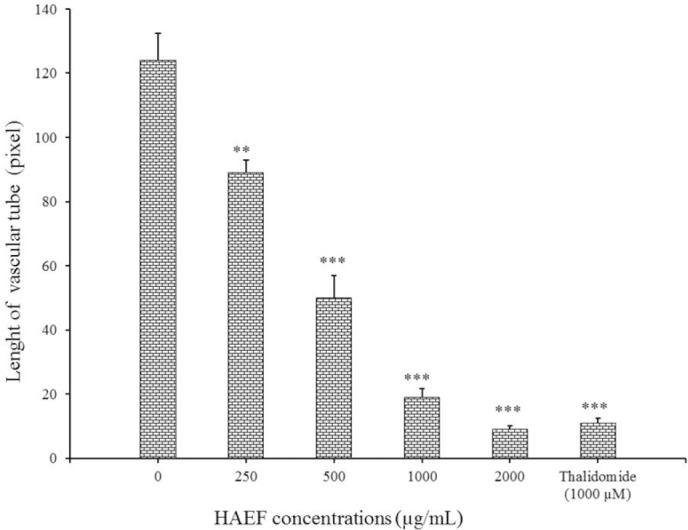
Effects of a hydro-alcoholic extract of fenugreek (HAEF) and thalidomide on the length of the vascular tubes formed by human umbilical vein endothelial cells (HUVECs) in collagen gels. Data are presented as mean ± SEM and calculated from three independent experiments. **P < 0.01 and ***P < 0.001 versus control group (0 µM).
DISCUSSION
Angiogenesis is a hallmark of tumor progression, invasion, and metastasis(23). Because of this crucial role of angiogenesis in the cancer progression, developing anti-angiogenic agents is a desirable target for cancer treatment and prevention(2). In the present study, survival of 3T3 cells and HUVECs that are used as laboratory model systems for the study of the function and pathology of endothelial cells were inhibited by an extract of fenugreek seed in a dose-dependent manner. Besides, it reduced tube formation of human umbilical vein endothelial cells and the neovascularization of CAM in vivo.
Numerous in vitro and in vivo methods are available assays for studying angiogenesis, each with its own advantages and limitations(24). Among them, tube formation assay is a promising method to determine the ability of compounds for increase or decrease of angiogenesis in endothelial cells. In addition, the CAM which exchanges gas and nutrient has a dense capillary network and is commonly used as an in vivo technique to study angiogenesis(25). Anti-angiogenic substances are of enormous interest, because of many common disorders, including obesity, atherosclerosis, arthritis, blindness, cancer, asthma, psoriasis, and infectious diseases associated with excess angiogenesis(26). On the other hand, anti-angiogenic agents may have some unwanted effects such as delayed wound healing(27).
The natural anti-angiogenic agents with greater effectiveness and lesser side effects is a great of interest(8,9). Fenugreek is usually used as a spice in food preparations because of the strong flavor and aroma and is used in folk medicines as leads to further drug development in modern medicine(28). We used maceration method for preparing fenugreek extract, which are preferred to thermal methods (e.g. Soxhlet technique) because it prevents destruction or degradation of compounds that may present inside the fenugreek parts.
This method is beneficial to avoiding any destruction or degradation of compounds that may present inside the fenugreek parts due to exposure to high temperature(29). In this study, the fenugreek seeds powder because most of the therapeutic effects was exposed to ethanol 50% to ensure that the majority of the polar and active ingredients have been extracted and isolated(18,30). Extract of fenugreek significantly attenuated cell viability in a dose-dependent manner in HUVECs and 3T3 fibroblasts. Moreover, our data showed that the extract was more toxic for 3T3 fibroblasts with lower IC50 comparing to the HUVEC. These findings suggested that the crude extract of fenugreek may induce unwanted effects associated with higher doses. The active compound responsible for the anti-angiogenic activity of the extract should be isolated for future works. In addition, in several studies thalidomide has been considered as a putative inhibitor of angiogenesis both in vivo and in vitro(31,32).
In the current study the potential effect of fenugreek extracts on the number of vessels formed in CAM was indicated that in the range up to 250 µg/mL had no significant effect on the number of these vessels, but at concentrations higher than 500 µg/mL, a significant inhibition was observed. Anti-angiogenic activity of fenugreek extract was associated with inhibition of endothelial cells migration. We also concluded that the number of neovascular points reduces at the range of 500-2000 µg/mL of fenugreek extract. This effect at a concentration of 2000 µg/mL was similar to 1000 μM of thalidomide used as a positive control (P > 0.05). Due to numerous researches on the anti-angiogenesis effect of fenugreek extract, we checked out the ability of endothelial cells to form three-dimensional structures. We assessed the effect of fenugreek extract on the reduced length of a tube made by endothelial cells. Evaluation of tube formation is one of the most specific methods for angiogenesis(33).
Compounds that can inhibit tube formation could be useful in many diseases, such as cancer because of receive nutrients through these tube vessels(34). The results revealed that the extract of fenugreek was able to reduce the length of HUVEC capillary tube at 250-2000 µg/mL. It is notable that length reduction of the tube created by the extract is the same to thalidomide. The mechanism of angiogenesis inhibition by fenugreek extract is currently not understood, but it is well documented that the main chemical components of fenugreek such as fibers, flavonoids, polysaccharides, saponins(13), and generally polyphenols tend to possess anti-angiogenic activity because of powerful antioxidant activity(35).
Antiangiogenic properties of flavonoids have been observed in the chick embryo chorioallantoic membrane since they can block the formation of new blood vessels(36). The vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) have been shown to be responsible for regulation of metastasis, angiogenesis, and tumor progression(1,37). Antiangiogenic effects of flavonoids mediated by regulating the expression of matrix metalloproteinases, VEGF, epidermal growth factor receptor (EGFR) and by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3-K)/Akt and extracellular signal-regulated kinases (ERK 1/2) signaling pathways(38). Scientists have demonstrated that certain flavonoids were powerful inhibitors of endothelial cell proliferation and VEGF/bFGF (basic fibroblast growth factor)-stimulated in vitro angiogenesis(39).
Furthermore, fenugreek has received considerable attention as a source of diosgenin. It is well documented that diosgenin inhibits angiogenesis dose-dependently by suppressing VEGF expression and therefore can affect angiogenesis(39,40). Several studies have shown that diosgenin induces apoptosis in different cancer cells including osteosarcoma, leukemia, and human colon carcinoma(41,42,43). Furthermore, cell proliferation is suppressed by diosgenin through disruption of Ca+2 homeostasis(44), activation of p53 and modulation of caspase-3 activity. Moreover, diosgenin inhibits NF-kB activity and NF-kB-regulated gene expression and subsequently decreasing proliferation(45). In addition, NF-kB upregulates the expression of several genes involved in tumor cell survival, including BCl-2, cIAP, BclXL, cFLIP, and Bfl-1(46).
In consistent with our findings, Al-Oqail et al. demonstrated that the seed oil of fenugreek although significantly decreased the viability of the cancerous cells (Hep-2, MCF-7, and WISH) dose-dependently but it has an antioxidant property and lower sensitivity towards the healthy cells more than cancerous cells(20). Furthermore, Al-Zubaidy et al. showed that fenugreek seeds induce anti-angiogenic activity in a concentration-dependent manner in ex vivo aortic ring model which was different to our study(21).
CONCLUSION
These findings revealed that fenugreek has cytotoxic and anti-angiogenic effects in vitro and in vivo. Therefore, this medicinal plant can be subjected to further investigations as anti-tumor agents. Moreover, further studies are needed in order to understand the mechanisms involved in the full anticancer potential of fenugreek.
ACKNOWLEDGMENTS
This project was financially supported via the Grant No. 951370 by the Iranian Ministry of Health and Medical Education, I.R. Iran.
REFERENCES
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health and Risk Manag. 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoeb M. Anti-cancer agents from medicinal plants. Bangladesh J Pharm. 2006;1(2):35–41. [Google Scholar]
- 5.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29(3):483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 6.Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/302426. Article ID: 302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug discovery Today. 2008;13:161–71. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Mirian M, Behrooeian M, Ghanadian M, Dana N, Sadeghi-Aliabadi H. Cytotoxicity and antiangiogenic effects of Rhus coriaria, Pistacia vera and Pistacia khinjuk oleoresin methanol extracts. Res Pharma Sci. 2015;10(3):233–240. [PMC free article] [PubMed] [Google Scholar]
- 9.Dana N, Javanmard ShH, Rafiee L. Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res pharma sci. 2015;10(2):117–124. [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh YJ, Huang HS, Leu YL, Peng KC, Chang CJ, Chang MY. Anticancer activity of Kalanchoe tubiflora extract against human lung cancer cells in vitro and in vivo. Environ Toxicol. 2016;31(11):1663–1673. doi: 10.1002/tox.22170. [DOI] [PubMed] [Google Scholar]
- 11.Petropoulos GA. Fenugreek: The Genus Trigonella. 1st ed. United States: CRC Press; 2002. pp. 1–7. [Google Scholar]
- 12.Sur P, Das M, Gomes A, Vedasiromoni J, Sahu NP, Banerjee S, et al. Trigonella foenum graecum (fenugreek) seed extract as an antineoplastic agent. Phytother Res. 2001;15(3):257–259. doi: 10.1002/ptr.718. [DOI] [PubMed] [Google Scholar]
- 13.Amin A, Alkaabi A, Al-Falasi S, Daoud SA. Chemopreventive activities of Trigonella foenum graecum (Fenugreek) against breast cancer. Cell biol int. 2005;29(8):687–694. doi: 10.1016/j.cellbi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Alsemari A, Alkhodairy F, Aldakan A, Al-Mohanna M, Bahoush E, Shinwari Z, et al. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum. BMC complement Altern Med. 2014;14:114–122. doi: 10.1186/1472-6882-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrafarin A, Qaderi A, Rezazadeh S, Naghdi Badi H, Noormohammadi G, Zand E. Bioengineering of important secondary metabolites and metabolic pathways in fenugreek (Trigonella foenum-graecum L.) J Med Plants. 2010;3(35):1–18. [Google Scholar]
- 16.Ribes G, Sauvaire Y, Costa CD, Baccou JC, Loubatieres-Mariani MM. Antidiabetic effects of subtractions from fenugreek seeds in diabetic dogs. Proc Soc Exp Biol Med. 1986;182(2):159–166. doi: 10.3181/00379727-182-42322. [DOI] [PubMed] [Google Scholar]
- 17.Das S. Hepatoprotective activity of methanol extract of fenugreek seeds on rats. Int J Pharm Sci Res. 2014;5(4):1506–1513. [Google Scholar]
- 18.Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Braz J Pharm Sci. 2013;49(3):413–422. [Google Scholar]
- 19.Sharma MS, Choudhary PR. Hypolipidemic effect of fenugreek seeds and its comparison with atorvastatin on experimentally induced hyperlipidemia. J Coll Physicians Surg Pak. 2014;24(8):539–542. [PubMed] [Google Scholar]
- 20.Al-Oqail MM, Farshori NN, Al-Sheddi ES, Musarrat J, Al-Khedhairy AA, Siddiqui MA. In vitro cytotoxic activity of seed oil of fenugreek against various cancer cell lines. Asian Pac J Cancer Prev. 2013;14(3):1829–1832. doi: 10.7314/apjcp.2013.14.3.1829. [DOI] [PubMed] [Google Scholar]
- 21.Al-Zubaidy AA, Sahib HB, Sadiq MH. Anti-angiogenig activity of fenugreek extracts: in vivo and ex vivo study. Int J Pharm Sci Rev Res. 2016;36(2):184–189. [Google Scholar]
- 22.Mortazavian SM, Parsaee H, Mousavi SH, Tayarani-Najaran Z, Ghorbani A, Sadeghnia HR. Acetylcholinesterase inhibitors promote angiogenesis in chick chorioallantoic membrane and inhibit apoptosis of endothelial cells. Int J Alzheimer Dis. 2013;2013 doi: 10.1155/2013/121068. DOI: 10.1155/2013/121068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 24.Cockerill GW, Gamble JR, Vadas MA. Angiogenesis: models and modulators. Int Rev Cytol. 1995;159:113–160. doi: 10.1016/s0074-7696(08)62106-3. [DOI] [PubMed] [Google Scholar]
- 25.Ribatti D. Chicken chorioallantoic membrane angiogenesis model. Methods Mol Biol. 2012;843:47–57. doi: 10.1007/978-1-61779-523-7_5. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 27.Bodnar RJ. Anti-angiogenic drugs: involvement in cutaneous side effects and wound-healing complication. Adv Wound Care. 2014;3(10):635–346. doi: 10.1089/wound.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thirunavukkarasu V, Anuradha CV, Viswanathan P. Protective effect of fenugreek (Trigonella foenum graecum) seeds in experimental ethanol toxicity. Phytother Res. 2003;17(7):737–743. doi: 10.1002/ptr.1198. [DOI] [PubMed] [Google Scholar]
- 29.Widsten P, Laine JE, Qvintus-Leino P, Tuominen S. Effect of high-temperature defibration on the chemical structure of hardwood. Holzforschung. 2002;56:51–59. [Google Scholar]
- 30.Zhao XL, Zhao YF, Guo SC, Song HS, Wang D, Gong P. Synthesis and anti-tumor activities of novel [1,2,4] triazolo [1,5-a] pyrimidines. Molecules. 2007;12(5):1136–1146. doi: 10.3390/12051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7(7):1888–1893. [PubMed] [Google Scholar]
- 33.Francescone RA, 3rd, Faibish M, Shao R. A matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp. 2011 doi: 10.3791/3040. DOI: 10.3791/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isaji M, Miyata H, Ajisawa Y, Takehana Y, Yoshimura N. Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J pharmacol. 1997;122(6):1061–1066. doi: 10.1038/sj.bjp.0701493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem U, Ahmad B, Ahmad M, Hussain K, Bukhari NI, Ashraf M. Evaluation of anti-angiogenic activity of latex and extracts of Euphorbia helioscopia using chorioallontoic membrane (CAM) assay. Int J Agric Biol. 2015;17:339–344. [Google Scholar]
- 36.Oak MH, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nut Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. 1992;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 38.Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008;57(4):259–265. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Tang YM, Yu SL, Han YW, Kou JP, Liu BL, et al. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015;13(8):578–587. doi: 10.1016/S1875-5364(15)30053-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen PS, Shih YW, Huang HC, Cheng HW. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One. 2011;6(5):e20164. doi: 10.1371/journal.pone.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moalic S, Liagre B, Corbière C, Bianchi A, Dauça M, Bordji K, et al. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001;506(3):225–230. doi: 10.1016/s0014-5793(01)02924-6. [DOI] [PubMed] [Google Scholar]
- 42.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1392–1398. [PubMed] [Google Scholar]
- 43.Hibasami H, Moteki H, Ishikawa K, Katsuzaki H, Imai K, Yoshioka K, et al. Protodioscin isolated from fenugreek (Trigonella foenumgraecum L.) induces cell death and morphological change indicative of apoptosis in leukemic cell line H-60, but not in gastric cancer cell line KATO III. Int J Mol Med. 2003;11:23–26. [PubMed] [Google Scholar]
- 44.Liu MJ, Wang Z, Ju Y, Wong RN, Wu QY. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer chemother pharmacol. 2005;55:79–90. doi: 10.1007/s00280-004-0849-3. [DOI] [PubMed] [Google Scholar]
- 45.Corbiere C, Liagre B, Bianchi A, Bordji K, Dauca M, Netter P, et al. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol. 2003;22(4):899–905. [PubMed] [Google Scholar]
- 46.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]


