Abstract
Quinazoline is one of the most widespread scaffolds amongst natural and synthetic bioactive compounds. Recently the quinazoline derivatives and in particular the 4-anilinoquinazolines have attracted much attention for their anticancer properties due to their capability to stabilize the kinase activity of epidermal growth factor receptor (EGFR). A series of fifteen previously designed and synthesized 4-anilinoquinazoline analogs (4-18) were evaluated for cytotoxic activity on two breast cancer cell lines (MCF-7 and MDA-MB-468). Ligand efficiency and binding mode studies were also done and evaluated for their potentially EGFR inhibitory effects in comparison with imatinib and erlotinib as reference drugs. Among the tested 4-anilinoquinazolines, compound 11, which contains diethoxy at phenyl ring and morpholino pendants at positions 5 and 7 of the quinazoline ring, demonstrated the most potent biological activity on both cell lines. Our new quinazoline derivatives with different substituents such as cyclic or linear ethers and flour groups may be a promising cytotoxic lead compounds for further anti-breast cancer research.
Keywords: 4-Anilinoquinazoline, Cytotoxic activity, Docking, EGFR
INTRODUCTION
Overexpression of luminal (positive for estrogen or progesterone receptors) and epidermal growth factor receptors 2 (EGFR2 or HER2) play essential roles in breast cancer(1). There is an intractable and an aggressive subtype of breast cancer that is defined by the absence of estrogen, progesterone receptors, and HER2 which known as triple-negative breast cancer (TNBC). The therapeutic effect of EGFR inhibitors as potent suppressors of TNBC progression have been underwhelming(2,3). Recent improvements in the identification of EGFR inhibitors have created hope for the modulation of uncontrolled cell growth in cancer therapy especially for solid tumors. In recent years, many EGFR inhibitors with different molecular scaffolds have been discovered(4,5,6). Among them, ATP competitive inhibitors of 4-anilinoquinazolines analogs like erlotinib and gefitinib have been approved(7,8,9,10). There are also some reports showing the hopeful benefits from imatinib and combined treatment with a dual inhibitor of EGFR and imatinib(11,12). But unfortunately, these EGFR inhibitors have been disappointing in EGFR overexpressed TNBC because of signal pathways of Akt and HER3 in cells that could cause acquired resistance. Herein, in order to search for new promising anti-breast cancer compounds, a series of 4-anilinoquinazoline derivatives were designed and evaluated against imatinib using two cell lines MCF-7 and MDA-MB-468 (Fig. 1). For this purpose we attempted to set up predictive models in in silico analysis to design EGFR-inhibitors. Subsequently fifteen new anilinoquinazoline derivatives containing desirable substituents were considered to optimize the favorable scaffold. The main points for this optimization were ability of making polar interaction and inhibition of the tyrosine kinase activity.
Fig. 1.
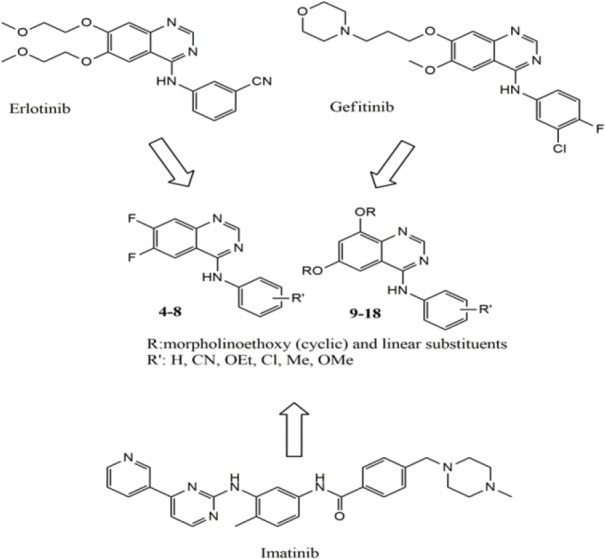
Structure based design of anti-breast cancer agents.
MATERIALS AND METHODS
Chemistry
All new synthesized compounds were chose from our previous study(13,14). A brief of general procedure for synthesis and their chemical structures are mentioned here. 4-aminoquinazoline derivatives were prepared from diflouro or dichloro anthranilic acid through 3 or 4 step reactions according to the procedure shown in Scheme 1. All compounds contained aniline pendant with various electron donating and withdrawing groups at position 4 of quinazoline ring. Chemical structures of all compounds are summerized in Table 1.
Scheme 1.
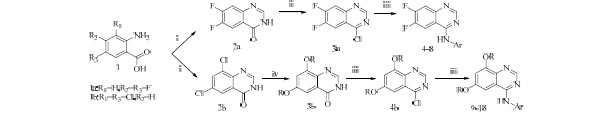
Synthesis pathway for the preparation of 4-aminoquinazoline derivatives. Reagents and conditions: (i) Formamide, micro wave; (ii) SOCl2, dimethylformamide (DMF), reflux, 20 h; (iii) Aniline derivatives, iPrOH/DMF, reflux, 20 h; (iv) NaH, ROH, DMF, reflux, 7 h.
Table 1.
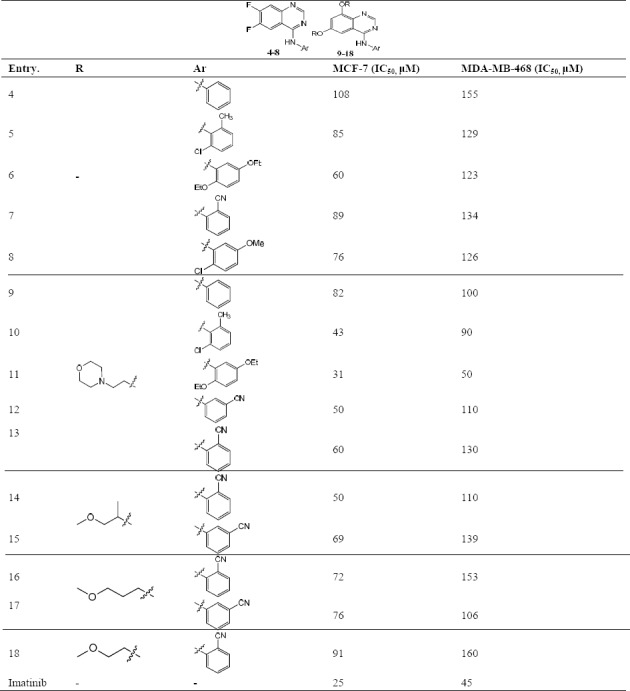
Inhibition of breast carcinoma proliferation (MCF-7 and MDA-MB-468) by compounds 4-18.
Assesments of in vitro anticancer activities
The effects of all the newly synthesized compounds on cell proliferation were evaluated by thiazolyl blue tetrazolium bromide (MTT) assay in two human breast carcinoma cell lines (MCF-7 and MDA-MB-468)(15). Cell lines were purchased from the national cell bank of Pasteur Institute of Iran (Tehran, I.R. Iran). In brief, cells from different cell lines were seeded in 96-well plates and incubated for 24 h to allow cell attachment. The cells were then incubated for another 24 h with various concentrations of the tested compounds and washed in phosphate buffered saline (PBS). Then 20 μL of MTT solution (5 mg/mL) were added to each well and an additional 4 h of incubation at 37 °C were done, and then the medium was discarded. Afterward dimethyl sulfoxide (DMSO) (60 μL) was added to each well, and the solution was vigorously mixed to dissolve the purple tetrazolium crystals. Finally the absorbance of each well was measured by plate reader at a test wavelength of 490 nm against a standard reference solution at 490 nm(16,17).
In silico studies
The chemical structures were drawn for geometry optimization using HyperChem software (Version 7, Hypercube Inc) under molecular mechanics MM+ method. PYRX molecular docking software has been employed for docking studies. The crystal structure of the EGFR-erlotinib complex was obtained from http://www.rcsb.org (PDB ID: 1M17). All bound waters and ligand were removed. The cubic grid box was created in 60 Å size (x, y, and z) and the center was placed on catalytic active site region where the erlotinib as native ligand was embedded. The properties (ligand efficiency (LE)) were calculated according to the presented method on http://www.molinspiration.com/cgi-bin/properties and binding mode analysis and images were generated with PYMOL.
RESULTS
In vitro anticancer activity
The quinazoline derivatives were screened in three different groups according to their chemical structures, including: derivatives of diflouro groups at positions 6 and 7 of the quinazoline ring (4-8), derivatives with cyclic diether substitutions at positions 5 and 7 of the quinazoline ring (9-13) and derivatives containing linear diether substitutions at positions 5 and 7 of the quinazoline core (14-18). As shown in Table 1, all compounds showed better anti-proliferation activity against MCF-7 (IC50 = 31- 106 μM) than MDA-MB-468 (IC50 = 50-160 μM) cell line. These data indicated compound 11 bearing diethoxy phenyl and morpholine ether pendants was the most potent cytotoxic agent with IC50 = 31 and 50 μM for MCF-7 and MDA-MB-468 cell lines, respectively. Among tested compounds in diflouro groups (4-8), compound 6 (Ar: 2,5- diethoxy phenyl) exhibited the minimum IC50 value for cytotoxic activity against the MCF-7 cell line. In the second group with morpholine moiety as cyclic ether at positions 6 and 7 of the quinazoline backbone (9-13) the order of cytotoxic activity was 11 (Ar: 2,5- diethoxy phenyl) > 10 (Ar: 2-chloro-6-methyl phenyl) > 12 (Ar: 3- benzonitrile) >13 (Ar: 2 benzonitrile) >9 (Ar: phenyl) with the range of IC50 values between 31 to 82 μM. In the third group of compounds with linear diether substitutions at positions 5 and 7 of the quinazoline core (14-18) the order of cytotoxic activity was 14 (Ar: 2 benzonitrile) > 15 (Ar: 3- benzonitrile) > 16 (Ar: 2 benzonitrile) > 17 (Ar: 3- benzonitrile > 18 (Ar: 2 benzonitrile) with the range of IC50 values between 50- 91 μM. The IC50 values for MDA-MB-468 cell line for all compounds were increased up to100 μM, except compounds 10 and 11 with IC50 values 90 and 50 μM, respectively.
In silico studies
Docking analysis
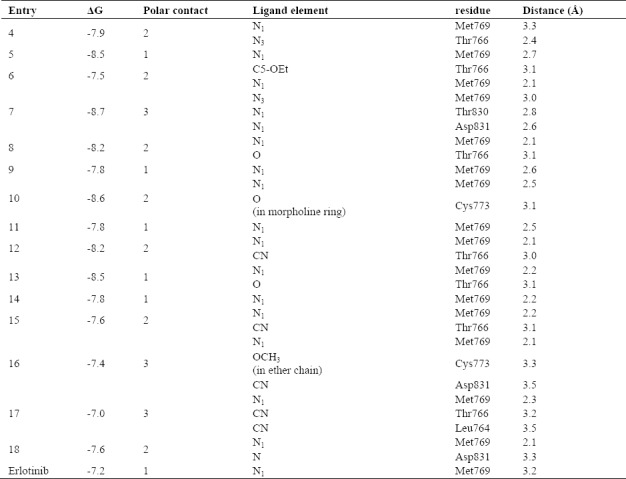
After docking the designed compounds into the active site of the EGFR complex structure, most of them showed better binding energy in comparison to erlotinib (-7.2 kcal/mol) as cognate ligand. Compound 7 with substitution of diflouro groups at positions 6 and 7 of the quinazoline ring and 2-benzonitrile ring seems to be a good lead molecule, which represented binding energy of -8.7 Kcal/mol. In the case of another cyano counterpart, compounds with cyclic and linear diether substituent, though having higher binding energy, but still was comparable with the standard drug (Fig. 2). Moreover the polar interactions with desirable residues in 8 Å distance may be improving the inhibitory activity of the ligands. As listed in Table 2, most of the compounds potentially are able to exhibit hydrogen bonds with Thr766 and Met769. Apart from N1 and N3, the O from ether substitutes on compounds 6 and 8 and also cyano group in compounds 12, 7, 15, 16, 17 and 18 could participate in a polar interaction. Binding of a morpholine ring in compounds 10 and ether chain in 16 with Cys773 may be improving inhibitory effects of the compounds. The non-contact residues in most of the binding site are Leu694, Lys721, Ala719, Gly772 and Pro770.
Fig. 2.
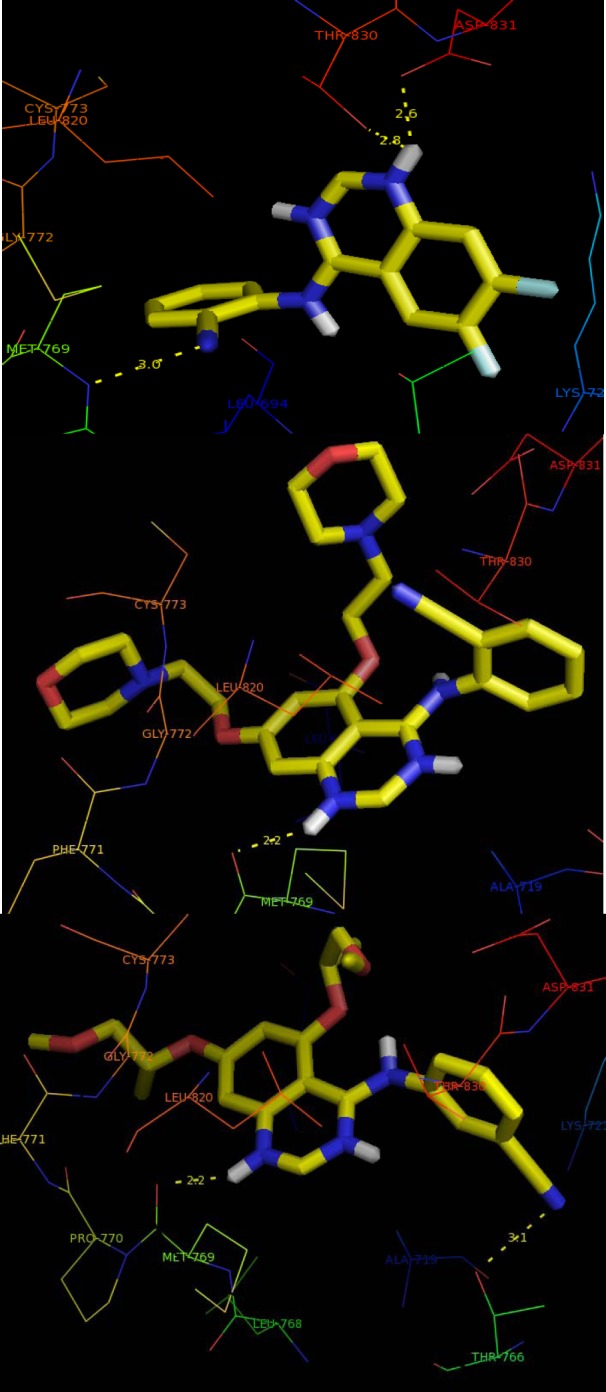
Binding mode of compounds 9, 13, and 17 (carbons, yellow; oxygens, red) with epidermal growth factor receptor (EGFR) enzyme (PDB ID: 1M17). The yellow dotted lines show the hydrogen bonds interaction.
Table 2.
Polar distance and binding site residues.
Efficiency indices
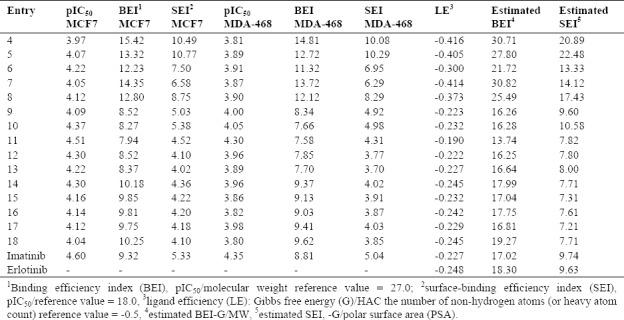
The concept of LE has been widely reported in drug discovery process of fragments, hits and lead selection and optimization. Different equations related to the potency and molecular properties used as LE definition in literatures. The ratio of potency to the molecular weight (MW) (define as pIC50/MW) and to the vanderwaals polar surface area (PSA) (define as pIC50/PSA) were utilized for two definitions of LE: binding efficiency index (BEI) and surface-binding efficiency index (SEI)(18,19,20). Most of the tested compounds had BEI more than imatinib as standard drug on both selected cell lines. As shown in Table 3, BEI of the cyclic ethers substituted compounds (9-13) were less than imatinib but their SEI were similar to this agent. Neither cyclic (9-13) nor linear (14-18) ethers substituted compounds but diflouro substitute's compounds (4-8) were active. Other normalizing parameters which used to estimate efficiencies are the ratio of ΔG and the number of non-hydrogen atoms, MW and PSA. Comparisons of the tested compounds with erlotinib indicated that compounds 9-13 had the closest values for these parameters to the reference drug.
Table 3.
Efficiency Indices: definitions and idealized reference values.
DISCUSSION
Previous researches had revealed that 4-anilinoquinazoline derivatives are EGFR inhibitors as their ATP-binding mimic and binding model might play a crucial role in its EGFR and antiproliferative activities(21). The design strategy of these compounds rose from the binding mode action of different substitutions on aniline moiety attached to the C4 position of the quinazoline ring. The presence of cyclic or linear ether chain and diflouro substitutions on the quinazoline nucleus are also effective. Structure-activity relationship (SAR) studies at cellular level for our 4-anilinoquinazolines demonstrated that compounds with cyclic ether (9-13) displayed more potent activities than those with linear ether (14-18) and diflouro analogues (4-8). It was reported that quinazoline nucleus interact with Met769 and Thr766 in the enzyme active site via two hydrogen bonds. In the binding site of EGFR there is also a lipophilic pocket, which occupied by the aniline ring of 4-anilinoquinazolines. In these type of quinazoline compounds substitutions at C-6 and C-7 of the quinazoline core act to improve the physical properties(22,23). Docking analysis and binding mode of designed compounds on EGFR made evident of polar interactions with two key residues: Met769 and Thr766 in most compounds. On the other hand binding indices calculation could correlate the experimental data with docking results. Similar trend exhibited in antiproliferative activities and LE against EGFR TK target. It demonstrated LE could predict the potency in parallel to the cytotoxic activity on the two tested cell lines.
CONCLUSION
Here we investigated some erlotinib analogues with 4-anilinoquinazoline scaffold which all have shown good cytotoxic activity. The docking studies simulated all tested compounds to the active site of EGFR TK target. The results revealed the polar interaction between quinazoline backbone and Met769 and Thr766 in catalytic active site. Additionally, the substitution on aniline moiety particularly ether and cyano played important roles in drug-receptor interactions. Furthermore, calculation of LE indices suggested a good prediction of binding parameters for target compounds and erlotinib as EGFR inhibitors for our future research. We hope the outcomes from the present study may be useful in future drug discovery and investigation of SAR of novel potent 4-anilinoquinazoline with cyclic ether substitution as potent EGFR inhibitors
ACKNOWLEDGMENT
This work was financially supported by a research Grant No. 89-01-36-2486 provided by the Vice Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, I.R. Iran. The authors also thank Osveh Pharmaceutical Company for supplying the Imatinib powder.
REFERENCES
- 1.Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121(10):3786–3788. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng LM, Chen YT, Huang CT, Chu PY, Wang WL, Liu CY, et al. Erlotinib derivative, devoid of EGFR kinase inhibiting effect, induced apoptosis of triple-negative breast cancer cells through modulating Elk-1/CIP2A signaling pathway. AACR. 2016;76(14) [Google Scholar]
- 3.Haines E, Schlienger S, Claing A. The small GTPase ADP-ribosylation factor 1 mediates the sensitivity of triple negative breast cancer cells to EGFR tyrosine kinase inhibitors. Cancer Biol Ther. 2015;16(10):1535–1547. doi: 10.1080/15384047.2015.1071737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noolvi MN, Patel HM. Synthesis, method optimization, anticancer activity of 2,3,7-trisubstituted quinazoline derivatives and targeting EGFR-tyrosine kinase by rational approach: 1st Cancer Update. Arabian J Chem. 2013;6(1):35–48. [Google Scholar]
- 5.Li RD, Zhang X, Li QY, Ge ZM, Li RT. Novel EGFR inhibitors prepared by combination of dithiocarbamic acid esters and 4-anilinoquinazolines. Bioorg Med Chem Lett. 2011;21(12):3637–3640. doi: 10.1016/j.bmcl.2011.04.096. [DOI] [PubMed] [Google Scholar]
- 6.Waiker DK, Karthikeyan C, Poongavanam V, Kongsted J, Lozach O, Meijer L, et al. Synthesis, biological evaluation and molecular modelling studies of 4-anilinoquinazoline derivatives as protein kinase inhibitors. Bioorg Med Chem. 2014;22(6):1909–1915. doi: 10.1016/j.bmc.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Mowafy S, Farag NA, Abouzid KA. Design, synthesis and in vitro anti-proliferative activity of 4,6-quinazolinediamines as potent EGFR-TK inhibitors. Eur J Med Chem. 2013;61:132–145. doi: 10.1016/j.ejmech.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Lü S, Zheng W, Ji L, Luo Q, Hao X, Li X, et al. Synthesis, characterization, screening and docking analysis of 4-anilinoquinazoline derivatives as tyrosine kinase inhibitors. Eur J Med Chem. 2013;61:84–94. doi: 10.1016/j.ejmech.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Nasab RR, Hassanzadeh F, Khodarahmi GA, Mirzaei M, Rostami M, Abadi AJ. Synthesis, characterization, cytotoxic screening, and density functional theory studies of new derivatives of quinazolin-4 (3H)-one Schiff bases. Res Pharm Sci. 2017;12(6):444–455. doi: 10.4103/1735-5362.217425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafari E, Khajouei MR, Hassanzadeh F, Hakimelahi GH, Khodarahmi GA. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci. 2016;11(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Wang Y, Guan B, Rao Q, Wang J, Ma H, et al. C-kit and PDGFRA gene mutations in triple negative breast cancer. Int J Clin Exp Pathol. 2014;7(7):4280–4285. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YL, Overstreet AM, Chen MS, Wang J, Zhao HJ, Ho PC, et al. Combined inhibition of EGFR and c-ABL suppresses the growth of triple-negative breast cancer growth through inhibition of HOTAIR. Oncotarget. 2015;6(13):11150–11161. doi: 10.18632/oncotarget.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haghighijoo Z, Rezaei Z, Taheri S, Jani M, Khabnadideh S. A rapid and convenient method for synthesis of anilinoquinazoline: an improved synthesis of erlotinib derivatives. Trends Pharmacol Sci. 2015;1(3):173–178. [Google Scholar]
- 14.Haghighijoo Z, Eskandari M, Khabnadideh S. Method optimization for synthesis of trisubstitued quinazoline derivatives. Med Res Arch. 2017;5(5) [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi-Farani A, Foroumadi A, Rezvani Kashani M, Aliabadi A. N-Phenyl-2-p-tolylthiazole-4-carboxamide derivatives: Synthesis and cytotoxicity evaluation as anticancer agents. Iran J Basic Med Sci. 2014;17(7):502–508. [PMC free article] [PubMed] [Google Scholar]
- 17.Aliabadi A, Hasanvand Z, Kiani A, Mirabdali SS. Synthesis and in-vitro cytotoxicity assessment of N-(5-(Benzylthio)-1, 3, 4-thiadiazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide with potential anticancer activity. Iran J Pharm Res. 2013;12(4):687–693. [PMC free article] [PubMed] [Google Scholar]
- 18.Abad-Zapatero C, Metz JT. Ligand efficiency indices as guideposts for drug discovery. Drug Discov Today. 2005;10(7):464–469. doi: 10.1016/S1359-6446(05)03386-6. [DOI] [PubMed] [Google Scholar]
- 19.Abad-Zapatero C, Perišić O, Wass J, Bento AP, Overington J, Al-Lazikani B, et al. Ligand efficiency indices for an effective mapping of chemico-biological space: the concept of an atlas-like representation. Drug Discov Today. 2010;15(19-20):804–811. doi: 10.1016/j.drudis.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Abad-Zapatero C. Ligand efficiency indices for effective drug discovery. Expert Opin Drug Discov. 2007;2(4):469–488. doi: 10.1517/17460441.2.4.469. [DOI] [PubMed] [Google Scholar]
- 21.Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. 2002;277(48):46265–46272. doi: 10.1074/jbc.M207135200. [DOI] [PubMed] [Google Scholar]
- 22.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Liu Y, Lemmon MA, Radhakrishnan R. Erlotinib binds both inactive and active conformations of the EGFR tyrosine kinase domain. Biochem J. 2012;448(3):417–423. doi: 10.1042/BJ20121513. [DOI] [PMC free article] [PubMed] [Google Scholar]


