Abstract
Aging is a multifactorial process characterized by several features including low-grade inflammation, increased oxidative stress and reduced regenerative capacity, which ultimately lead to alteration in morpho-functional properties of skeletal muscle, thus promoting sarcopenia. This condition is characterized by a gradual loss of muscle mass due to an unbalance between protein synthesis and degradation, finally conveying in functional decline and disability. The development of specific therapeutic approaches able to block or reverse this condition may represent an invaluable tool for the promotion of a healthy aging among elderly people. It is well established that changes in the quantity and the quality of dietary proteins, as well as the intake of specific amino acids, are able to counteract some of the physiopathological processes related to the progression of the loss of muscle mass and may have beneficial effects in improving the anabolic response of muscle in the elderly. Taurine is a non-essential amino acid expressed in high concentration in several mammalian tissues and particularly in skeletal muscle where it is involved in the modulation of intracellular calcium concentration and ion channel regulation and where it also acts as an antioxidant and anti-inflammatory factor. The aim of this review is to summarize the pleiotropic effects of taurine on specific muscle targets and to discuss its role in regulating signaling pathways involved in the maintenance of muscle homeostasis. We also highlight the potential use of taurine as a therapeutic molecule for the amelioration of skeletal muscle function and performance severely compromised during aging.
Keywords: Taurine, sarcopenia, nutrition, amino acids, oxidative stress, inflammation, protein catabolism
1. INTRODUCTION
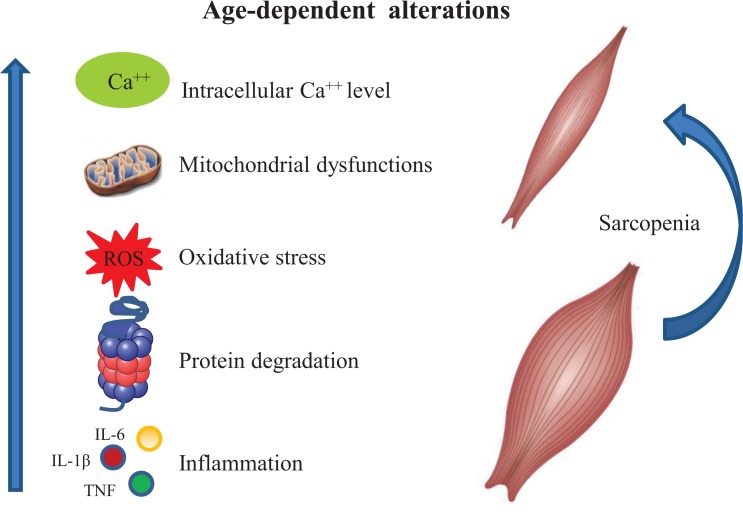
Aging is an inexorable and complex biological process characterized by a gradual decline in the physiological and biochemical functions of the principal systems, resulting in increasing risk of disability and loss of independence [1, 2]. During aging, several morpho-functional changes occur in skeletal muscle, such as the generalized loss of muscle mass, reduced myofiber size, and the progressive reduction in muscle strength, leading to a pathologic condition known as sarcopenia [3, 4] (Fig. 1).
Fig. (1).
Schematic representation of cellular processes involved in the onset of sarcopenia. During aging multifactorial events such as increased levels of intracellular Ca++ concentration, mitochondrial dysfunctions, protein catabolism, oxidative stress and inflammation lead to the onset of sarcopenia.
This progressive age-related muscle wasting process is associated with an increased prevalence of falls, a greater incidence of diseases and the loss of functional independence [4-6]. Sarcopenia might also involve intramuscular fat accumulation, fibrosis, chronic inflammation and a decreased ability of satellite cells to activate and proliferate following injury, thus leading to impaired muscle regeneration [7, 8]. While there are many possible causes for the age-related decline in skeletal muscle mass, it is generally accepted that changes in the regulation of skeletal muscle protein metabolism are responsible for the negative protein balance and are the result of an alteration between protein synthesis and breakdown rates [9, 10]. Protein balance is regulated by many factors that are each susceptible to change during the aging process, including hormone status, physical activity and nutrition [10-17]. Among the risk events for pathologic changes associated with aging, particular relevance should be directed to the diminished food intake, sedentary life style and reduced energy expenditure of older adults. Several interventions have been suggested to counteract sarcopenia. In particular, nutrition plays an important role in many age-associated diseases and is relevant to highlighting how chronic pathological conditions may influence the requirement for specific nutrients. In this context, amino acids are considered very important among nutrients because they not only form the structure of proteins but are also key signaling molecules involved in the regulation of protein metabolism [18, 19]. Moreover, it has been demonstrated that, in older individuals, chronic amino acid supplementation stimulates a muscle protein synthesis similar to that observed in young people [20] and increases muscle mass and function [21-23].
Therefore, nutritional strategies may be very useful in counteracting muscle loss during aging and stimulating muscle growth and strength in individuals currently affected by sarcopenia. The composition of meals and supplements and the administration modality appear to be critical factors to the successful use of nutrition as a treatment for sarcopenia.
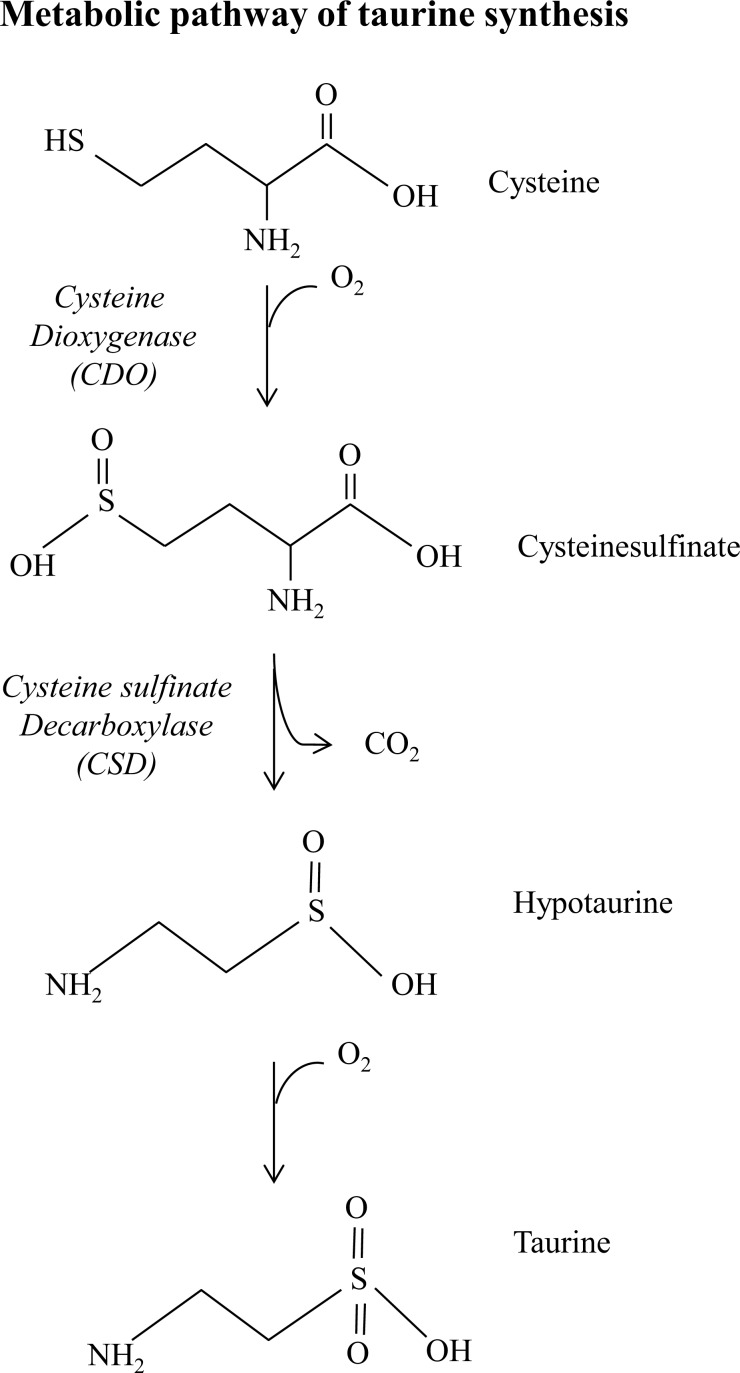
Taurine (2-aminoethane-sulfonic acid) is a sulfur-containing semi-essential amino acid not incorporated into proteins. In mammalian tissues, taurine is widely expressed and is the most abundant free amino acid in the heart, retina, skeletal muscle, brain, and leukocytes. Moreover, taurine plays an important role in several biological processes including cell development, cell signaling, membrane stability, Calcium-dependent excitation-contraction process and antioxidant defense [24]. In skeletal muscle, taurine is involved in the control of ion channel function, membrane stability and calcium homeostasis [25]. For instance, taurine treatment of dystrophic mdx mice improved grip strength, prevented exercise-induced muscle weakness and restored calcium homeostasis [26-28]. Taurine is synthesized from methionine and cysteine by the cysteine sulfinic acid decarboxylase (CSD) which is an enzyme present in the liver, kidney and brain [29] (Fig. 2).
Fig. (2).
Metabolic pathway of taurine synthesis. Endogenous taurine synthesis occurs in the liver via the cysteine sulfinic acid pathway. The metabolic reaction involves a first oxidation of the sulfhydryl group of cysteine to cysteine sulfinic acid by the enzyme cysteine dioxygenase. Cysteine sulfinic acid is then decarboxylated to hypotaurine by the cystyeine sulfinate decarboxylase. Taurine is synthesized through the hypotaurine dehydrogenase-dependent oxidation of hypotaurine.
Its intracellular concentration is guaranteed by the presence of a specific active transporter (TauT), ubiquitously expressed in many mammalian tissues, that concentrates taurine inside cells against its gradient [30]. Taurine is naturally found in numerous dietary components, especially in meat and seafood [31]. Some animal species, such as felines and foxes, are incapable of synthesizing taurine and, therefore, are highly dependent on its acquisition through the diet. The importance of such amino acid in the body is suggested by the fact that these animal species are also particularly susceptible to a deficient state, in which they develop severe pathological conditions, such as cardiomyopathy, retinal degeneration and reproductive defects [32-34]. This evidence supports the hypothesis that an alteration in the tissue content of taurine might play a role in tissue malfunction and that taurine supplementation may be used to pharmacologically control functions in which this amino acid plays a modulatory role.
The purpose of this review is to highlight the role of taurine in counteracting the impairment of skeletal muscle strength and function characteristic of senescent muscle. In particular, we discuss the potential effect of taurine on the pathogenic mediators of muscle aging and sarcopenia such as deregulated calcium levels and proteolytic systems, oxidative stress, inflammation, altered satellite cell activity and muscle regeneration.
2. PROTECTIVE MECHANISMS OF TAURINE AGAINST SARCOPENIA
2.1. Role of Taurine in Excitation-contraction Coupling and Muscle Performance
It is well established that levels of taurine gradually decrease during the aging process in several tissues [35]. Moreover, taurine-depleted skeletal muscle exhibits several abnormalities in its morphology and function resembling those that occur during aging [35]. Among these, alterations in the mechanisms of excitation-contraction coupling and muscle performance have been reported. Excitation-contraction coupling is a physiological process that converts the sarcolemmal action potential into muscle action and force generation. An important role in this process is played by the dihydropyridine receptors (DHPRs) which, in response to a depolarization of the muscle post-synaptic plasma membrane, activate the ryanodine receptors (RyRs) localized on the sarcoplasmic reticulum (SR), triggering calcium release. Calcium, in turn, promotes the interaction between actin and myosin, where it stimulates muscle contraction and is then pumped back into the SR or competitively bound. Disruption or uncoupling at any step of this process may affect muscle functionality [36]. Aging causes a reduction in the number of DHPRs, increases uncoupling between these receptors and RyRs, and consequently results in calcium release deficits [37, 38].
It has been demonstrated that taurine plays an essential role in the maintenance of muscle performance and excitation-contraction coupling even though the exact mechanism of these actions is still unknown. Interestingly, Huxtable and Bressler [39] showed that taurine enhances the rate of Ca++ and total Ca++ sequestering capacity of SR isolated from rat skeletal muscle, suggesting that a possible impairment of this process may occur in taurine-depleted muscle. This impairment could result in an increased cytosolic calcium level, ultimately leading to the observed alteration in the excitation-contraction coupling mechanism [40].
Recently, it has been shown that taurine potentiates the rate of SR calcium uptake in both type I and type II human muscle fibers, probably acting within the lumen of the SR; moreover, low physiological taurine levels significantly reduce the degree of potentiation [41]. In a recent study, Goodman et al. [42] demonstrated that the skeletal muscle of taurine-supplemented rats exhibited increased force and greater resistance and recovery after tetanic stimulation. These effects are accompanied by an up-regulation of the calsequestrin 1 level, which is a calcium binding protein that plays a critical role in maintaining high amounts of calcium in SR tubules. These data suggest that in the presence of taurine skeletal muscle can store an increased amount of calcium leading to increased calcium availability for contraction.
2.2. Taurine and Calcium-dependent Signaling Pathways
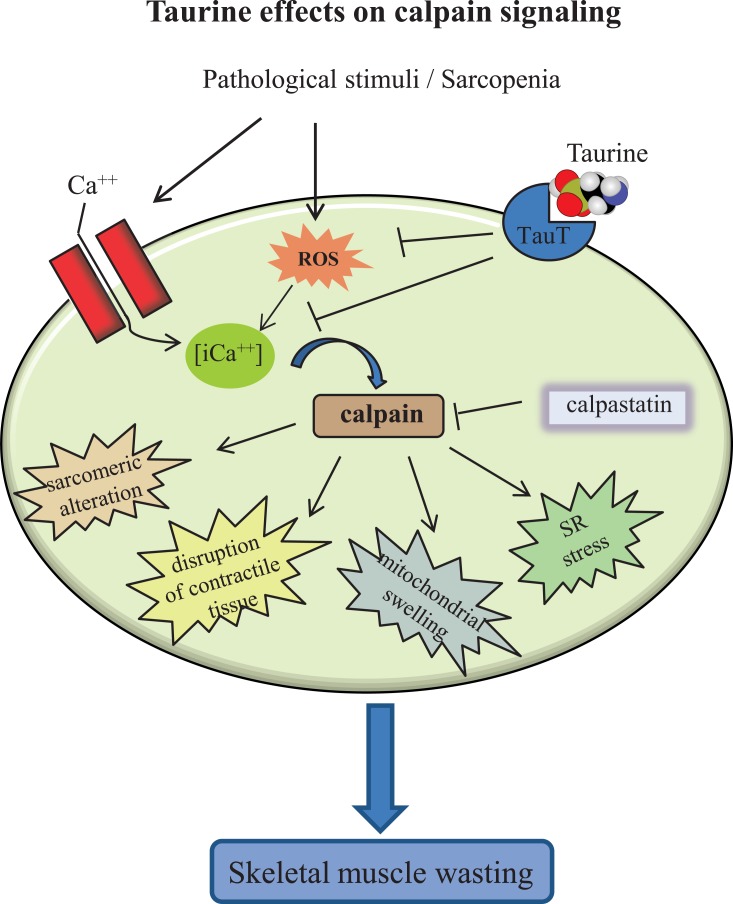
The effect of taurine in the modulation of SR calcium release suggests this amino acid is involved in the regulation of calcium-dependent signaling pathways. The need for an accurate modulation of intracellular calcium levels is, indeed, based on the observation that the dysregulation of calcium concentration leads to the activation of proteolytic systems, such as calpains and caspases, promoting muscle atrophy. Calpains are calcium-activated cysteine proteases, involved in a number of different biological processes including cytoskeletal remodeling [43], myofibril maintenance [44], and apoptosis [45]. They are also activated by several stimuli in which intracellular calcium homeostasis is affected, causing sarcomeric alteration [46], mitochondrial swelling, SR vacuolization [47, 48], and the disruption of contractile tissue [49].
The activity of calpains is regulated by the calpain inhibitor calpastatin, and their interaction is modulated by calcium concentrations. Interestingly, calpastatin over-expression attenuates muscle wasting and prevents the shift from slow to fast fiber type associated with disuse [50]. It has also been shown that acute taurine supplementation prevents calpain activation in norepinephrine-stimulated cardiomyocytes and that this effect is mediated via down-regulation of NADPH oxidase activation and the reduction of reactive oxygen species (ROS) [51]. These findings are in agreement with another study showing that taurine down-regulates calpain activity in a rat model of focal cerebral ischemia [52]. The discovery that the inhibition of NADPH oxidase-mediated calpain activation contributes to the anti-apoptotic effects of taurine offers new insights into the signaling mechanisms required for taurine protective effects, which may provide biological endpoints for the clinical evaluation of taurine as a potential therapeutic agent (Fig. 3).
Fig. (3).
Schematic representation of taurine effects on calpain pathway. Calpains are calcium-dependent proteases involved in many catabolic processes and are negatively regulated by calpastatin. In skeletal muscle, calpains contribute to sarcomeric alterations, mitochondrial dysfunction, sarcoplasmic reticulum (SR) stress, and contractile tissue decay. Taurine administration down-regulates intracellular [iCa++] and reactive oxygen species (ROS) generation, thus exerting a protective role against sarcopenia.
Another important pathway closely involved in calcium-dependent degradation of proteins in muscle fibers is the caspase pathway. Caspases represent a key component of the proteolytic system and the apoptotic machinery both of which become dysregulated during aging and neuromuscular diseases [53, 54]. Caspases are present in the cytoplasm as inactive proenzymes (procaspases). Under appropriate conditions, caspases can activate themselves through autocatalytic cleavage, and can also cleave and activate other caspases, thereby creating a self-amplifying cascade [55]. Indeed, caspases are classified as either initiator caspases (including caspase-2, 8, 9 and 10) or effector caspases (including caspases-3, 6 and 7). Initiator caspases cleave and activate downstream effector caspases which, in turn, cleave numerous cellular targets ultimately leading to cell death [55]. One of the proposed mechanisms involved in the activation of the caspase pathway during aging is endoplasmic reticulum (ER) stress, caused by calcium dysregulation or oxidative stress [56]. ER stress provokes an alteration in protein folding; thus, unfolded and misfolded proteins accumulate in the ER lumen, triggering the apoptotic pathway [57]. Interestingly, ER stress may be increased in tissue in which taurine is depleted, suggesting that endogenous taurine contributes to the stabilization of protein folding [58]. The efficacy of taurine in counteracting the actions of ER stress inducers is consistent with its role as an osmotically active substance and with the theory that organic osmolytes act as chemical chaperones [59]. Owing to this property, taurine may prevent intracellular volume changes resulting from alterations in plasma osmolality [34]. Indeed, it has been postulated that taurine could interact with membrane proteins leading to alterations in membrane functions and consequently to changes in the intracellular concentration of ions, in particular calcium [60]. Thus, the ability to modulate cellular ion concentrations and the regulation of cell volume together with its anti-apoptotic properties may be responsible for the protective effects of taurine against sarcopenia.
2.3. Effects of Taurine in Mitochondria-mediated Apoptosis and Oxidative Stress
It is generally accepted that mitochondrial dysfunction is a major factor contributing to the development of sarcopenia. Indeed, oxidative damage to mitochondrial components impairs cellular respiration and energy production and increases ROS generation, eventually leading to apoptotic events [61]. Notably, upregulation of myocyte apoptosis has been shown in animal models of premature aging as well as in aged rodents and humans [62-66]. The occurrence of apoptosis in skeletal muscle is evidenced, among others, by the expression of pro-apoptotic proteins such as Bax, caspase-3, Apoptosis Inducing Factor (AIF) and Apoptotic protease activating factor-1 (Apaf-1) [55, 67]. Interestingly, taurine has been found to ameliorate macroscopic and microscopic colitis through downregulating Bax expression and increasing the content of anti-apoptotic Bcl2 [68]. Moreover, taurine is able to suppress ischemia-induced apoptosis in cardiomyocytes by targeting the Apaf-1/caspase 9 complex which plays a crucial role in mitochondrion-mediated apoptosis [69]. These data highlight a cytoprotective role of taurine in several organs and tissues and reveal a novel molecular mechanism for the anti-apoptotic effect of taurine which may be harnessed to counteract sarcopenia.
Taurine has been found at particularly high concentrations in tissues exposed to elevated levels of oxidants, suggesting a role in the attenuation of oxidative stress [70-73]. Recently, it has been showed that taurine supplementation decreases the production of superoxide radicals in skeletal muscle, resulting in decreased lipid peroxidation and inflammation [74]. One possible mechanism by which taurine inhibits ROS generation has been proposed by Schaffer et al. and Jong et al. [73, 75] who proposed a novel antioxidant hypothesis, based on the presence of taurine-conjugated tRNAs in the mitochondria. Since tRNA conjugation is required for the normal translation of mitochondrial-encoded proteins, taurine deficiency reduces the expression of these respiratory chain components. As a result, a decrease in the electron flux through the electron transport chain occurs. Conversely, dysfunctional respiratory chain accumulates electron donors, which divert electrons from the respiratory chain to oxygen, forming superoxide anions. The restoration of taurine levels increases concentrations of conjugated tRNA, restores respiratory chain activity, and increases the synthesis of ATP at the expense of superoxide anion production [73, 75].
Recently, it was reported that transgenic mice knocked-out for the taurine transporter showed mitochondrial dysfunction in cardiomyocytes, characterized by reduced ATP production and elevated superoxide generation [76]. Such alterations were associated with activation of the ubiquitin-proteasome system (UPS) and autophagy [76]. Furthermore, Hansen et al. [77] demonstrated that taurine acts as a pH buffer in mammalian mitochondria, regulating the activity of specific enzymes such as pyruvate dehydrogenase, and that taurine depletion causes inadequate β-oxidation due to decreased pH buffering capacity, which consequently leads to metabolic dysfunction [77, 78].
All together, these observations suggest that the increased production of ROS during aging plays a key role in targeting a number of adaptive responses in skeletal muscle and strongly support a link between taurine activity, oxidative stress, mitochondrial dysfunction and apoptosis.
2.4. Taurine and Inflammation
Aging is accompanied by a chronic low-grade systemic inflammation state also known as inflamm-aging [79]. It is well established that tumor necrosis factor alpha (TNF-α) is increased in muscle wasting conditions [80, 81] and it is also known to be a potent activator of the nuclear factor kappa B (NF-κB) pathway in a variety of cell types, including muscle [82]. NF-κB is maintained in its inactive state by binding with a family of inhibitory proteins called inhibitors of kappa B (IkB). The increase in TNF-α levels induces the activation of an IκB kinase (IKK) complex that phosphorylates IkB, resulting in its ubiquitination and proteasomal degradation. This leads to nuclear translocation of NF-κB and transcription of NF-κB-mediated genes [83]. Activation of NF-κB in skeletal muscle leads to degradation of specific muscle proteins, induces inflammation and fibrosis, and blocks the regeneration of myofibers after injury/atrophy [84].
Taurine may provide a useful therapeutic approach to protect tissue damage from inflammation [85]. Indeed, the amino group of taurine can neutralize hypochlorous acid, one of the reactive species generated by neutrophils. In this reaction, taurine is converted to taurine chloramine, which is less toxic than hypochlorous acid and serves as a modulator of the immune system [86]. Thus, Tau-Cl, a stable oxidant, can be produced at the site of inflammation and down-regulate pro-inflammatory cytokine production leading to a significant reduction in the immune response. Moreover, recent reports have demonstrated that Tau-Cl exerts its effects upstream of IKK signaling pathway and inhibits the production of inflammatory mediators through a mechanism that, at least in part, involves the inhibition of NF-κB activation [87]. In this context, taurine might be involved in the down-regulation of inflammation and consequently favoring a functional activity of senescent muscle.
2.5. Taurine and Protein Imbalance
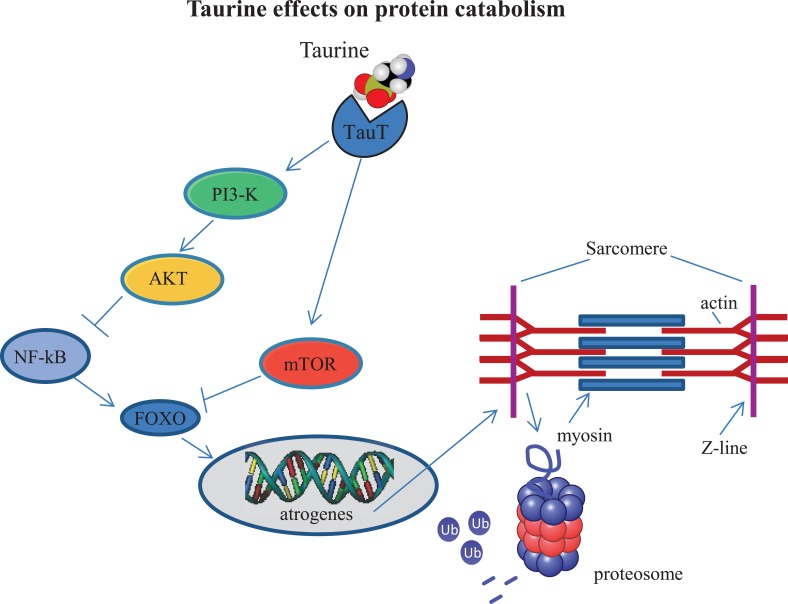
The UPS is a major proteolytic system in muscle. The muscle-specific ubiquitin-ligases, atrogin-1 (MAFbx) and Muscle RING Finger 1 (MuRF1) are up-regulated in old age and have therefore been involved in the pathogenesis [88]. In muscle, activation of the Phosphoinositide 3-kinase- Protein kinase B-Mammalian Target of Rapamycin (PI3K-AKT-mTOR) signaling promotes net protein accumulation by suppressing forkhead box protein O (FOXO) transcription factors [88], which control the expression of the atrogene program [89]. However, with aging, PI3K-AKT-mTOR signaling decreases, and consequently protein synthesis is reduced with a concomitant increase in proteolysis through the FOXO-dependent expression of the atrogene program [90]. Recently, it has been demonstrated that, in PC12 cells, taurine administration exerts neuroprotective effects against methamphetamine-induced damage, at least in part through mTOR-dependent pathway [91]. This finding suggests that taurine may influence protein catabolism in aged muscle through the stimulation of the mTOR-dependent pathway (Fig. 4).
Fig. (4).
Taurine may influence protein catabolism by down-regulating catabolic pathways. Taurine administration may inhibit atrogene expression through stimulating the Phosphoinositide 3-kinase- Protein kinase B-Mammalian Target of Rapamycin (AKT-PI3K-mTOR) pathway and subsequent blockade of Nuclear Factor κB (NF-κB) and Forkhead box O (FOXO) signaling.
In agreement with the role of taurine in counteracting protein synthesis decline, it has been shown that the up-regulation of MAFbx and MuRF1 observed in a mouse model of hind-limb unloading-induced atrophy is partially counteracted by taurine supplementation [30]. Although more detailed studies are needed to better clarify the molecular mechanisms underlying the taurine effects on the regulation of skeletal muscle mass, collectively these findings suggest that taurine administration might have a beneficial role in counteracting sarcopenia via the regulation of muscle protein turnover.
2.6. Taurine, Satellite Cells and Epigenetic Modifications
In addition to the mechanisms described above, deficiencies in muscle regeneration may also be involved in sarcopenia. Adult muscles are able to regenerate and respond to tissue damage by modifying their metabolic and contractile properties. However, a lower regenerative potential of aged muscle has been reported to be associated with a decline in satellite cell number and function [92, 93]. Conboy et al. [94] elegantly demonstrated that the regenerative potential of satellite cells can be dramatically improved in muscle of old mice after exposure to a young systemic environment. In addition, it has been shown that aged satellite cells, under standard culture conditions (i.e. Dulbecco’s Modified Eagle Medium supplemented with 5% horse serum), are able to differentiate without visible morphologic defects [7]. Interestingly, muscle differentiation was dramatically reduced when old satellite cells were cultured in autologous serum (isochronic culture conditions), whereas differentiation of aged satellite cells was rescued when cultured in serum obtained from young donors (heterologous/heterochronic conditions) [7]. These results emphasize the importance of the environment, which is created by circulating factors, but also by the local secretome of factors released by resident cells, including satellite cells as well as by the inflammatory milieu that characterizes the early steps of muscle regeneration [95].
As discussed above, aging is associated with increased ROS generation and heightened inflammation, leading to dysfunctions in a variety of cell signaling pathways and providing an unfavorable environment which can adversely affect satellite cell function and limit muscle repair in aging [96]. Considering the involvement of taurine in the modulation of calcium-dependent signaling pathways, attenuation of ROS generation and down-regulation of the pro-inflammatory cytokines secretion, it is possible to speculate that taurine administration may ameliorate the systemic environment. This, in turn, would influence the quantity and/or quality of satellite cells and their behavior during regeneration, slowing the aging process. Accordingly, a recent study by Gebara et al. [97] suggested a role for taurine in increasing hippocampal neurogenesis in old mice.
An additional mechanism of action of taurine involves the regulation of gene expression. MicroRNA (miRNAs) are small non-coding RNAs that regulate gene expression at post-transcriptional level. MiRNAs have been identified to be highly expressed in skeletal muscle, thereby indicating a possible regulatory role in muscle development and homeostasis [98]. MiRNAs also regulate the senescence program by targeting genes involved in cellular stress and tumor suppressor pathways. In addition, inflammatory mediators such as IL6 and IL8, which are up-regulated during aging, are targets of specific miRNAs that appear to be part of a compensatory response to protect against inflammation senescent primary fibroblasts [99]. Indeed, alteration and/or ablation of some miRNAs resulted in defects in satellite cell activation, proliferation and differentiation, altered muscle regeneration and promotion of muscle atrophy [100]. Emerging evidence also suggests that dietary intake, including administration of a number of different essential amino acids, may counteract muscle aging partly through miRNA modulation [101, 102].
It is still unknown whether increases in taurine intake may change in miRNA expression in muscle. However, through its effect on the maintenance of cellular integrity, redox homeostasis and inflammation, taurine administration may serve to overcome the anabolic resistance of aged muscle promoting muscle health in advanced age.
Conclusion
Taurine may represent a promising nutritional agent to counteract the development and progression of sarcopenia through its actions on intracellular calcium homeostasis, protein metabolism, oxidative stress, inflammation, and muscle regeneration. Further research is needed to definitively establish the anti-sarcopenic properties of taurine, its potential synergistic activity with physical exercise and other nutritional aids, and the most effective supplementation regimen.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
The authors are grateful to Prof. Antonio Musarò for his insightful comments during the preparation of the manuscript. This work was supported by Progetto di ricerca d’interesse di Ateneo – Linea D.3.2 – Anno 2013, Università Cattolica del Sacro Cuore.
conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Brady A.O., Straight C.R., Evans E.M. Body composition, muscle capacity, and physical function in older adults: An integrated conceptual model. J. Aging Phys. Act. 2014;22(3):441–452. doi: 10.1123/japa.2013-0009. [DOI] [PubMed] [Google Scholar]
- 2.Buonocore D., Rucci S., Vandoni M., Negro M., Marzatico F. Oxidative system in aged skeletal muscle. Muscles Ligaments Tendons J. 2011;1(3):85–90. [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., Topinkova E., Vandewoude M., Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryall J.G., Schertzer J.D., Lynch G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9(4):213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 5.Carmeli E., Reznick A.Z., Coleman R., Carmeli V. Muscle strength and mass of lower extremities in relation to functional abilities in elderly adults. Gerontology. 2000;46(5):249–257. doi: 10.1159/000022168. [DOI] [PubMed] [Google Scholar]
- 6.Rolland Y., Czerwinski S. bellan Van, K.G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; Chumlea, W.M.; Vellas, B. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12(7):433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barberi L., Scicchitano B.M., De R.M., Bigot A., Duguez S., Wielgosik A., Stewart C., McPhee J., Conte M., Narici M., Franceschi C., Mouly V., Butler-Browne G., Musaro A. Age-dependent alteration in muscle regeneration: The critical role of tissue niche. Biogerontology. 2013;14(3):273–292. doi: 10.1007/s10522-013-9429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont N.A., Wang Y.X., Rudnicki M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005;82(5):1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 10.Morley J.E. Pharmacologic options for the treatment of sarcopenia. Calcif. Tissue Int. 2016;98(4):319–333. doi: 10.1007/s00223-015-0022-5. [DOI] [PubMed] [Google Scholar]
- 11.Basualto-Alarcon C., Varela D., Duran J., Maass R., Estrada M. Sarcopenia and androgens: A link between pathology and treatment. Front. Endocrinol. (Lausanne) 2014;5:217. doi: 10.3389/fendo.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon E.L., Durham W.J., Urban R.J., Sheffield-Moore M. Hormone treatment and muscle anabolism during aging: Androgens. Clin. Nutr. 2010;29(6):697–700. doi: 10.1016/j.clnu.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon E.L. Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids. 2013;45(3):431–441. doi: 10.1007/s00726-012-1438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horstman A.M., Dillon E.L., Urban R.J., Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67(11):1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore D.R. Keeping older muscle “young” through dietary protein and physical activity. Adv. Nutr. 2014;5(5):599S–607S. doi: 10.3945/an.113.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker D.K., Dickinson J.M., Timmerman K.L., Drummond M.J., Reidy P.T., Fry C.S., Gundermann D.M., Rasmussen B.B. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med. Sci. Sports Exerc. 2011;43(12):2249–2258. doi: 10.1249/MSS.0b013e318223b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch A.A. Nutritional influences on age-related skeletal muscle loss. Proc. Nutr. Soc. 2014;73(1):16–33. doi: 10.1017/S0029665113003698. [DOI] [PubMed] [Google Scholar]
- 18.Wu G., Wu Z., Dai Z., Yang Y., Wang W., Liu C., Wang B., Wang J., Yin Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44(4):1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- 19.Kimball S.R., Jefferson L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006;136(1) Suppl.:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 20.Paddon-Jones D., Sheffield-Moore M., Zhang X.J., Volpi E., Wolf S.E., Aarsland A., Ferrando A.A., Wolfe R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004;286(3):E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 21.Borsheim E., Bui Q.U., Tissier S., Kobayashi H., Ferrando A.A., Wolfe R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008;27(2):189–195. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillon E.L., Sheffield-Moore M., Paddon-Jones D., Gilkison C., Sanford A.P., Casperson S.L., Jiang J., Chinkes D.L., Urban R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 2009;94(5):1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tieland M. van de, R.O.; Dirks, M.L.; van der, Z.N.; Mensink, M.; van Loon, L.J.; de Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012;13(8):720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Huxtable R.J. Physiological actions of taurine. Physiol. Rev. 1992;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 25.Conte C.D., Tricarico D., Pierno S., Desaphy J.F., Liantonio A., Pusch M., Burdi R., Camerino C., Fraysse B., De L.A. Taurine and skeletal muscle disorders. Neurochem. Res. 2004;29(1):135–142. doi: 10.1023/b:nere.0000010442.89826.9c. [DOI] [PubMed] [Google Scholar]
- 26.Cozzoli A., Rolland J.F., Capogrosso R.F., Sblendorio V.T., Longo V., Simonetti S., Nico B., De L.A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of Duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011;37(3):243–256. doi: 10.1111/j.1365-2990.2010.01106.x. [DOI] [PubMed] [Google Scholar]
- 27.De Luca A., Pierno S., Liantonio A., Cetrone M., Camerino C., Fraysse B., Mirabella M., Servidei S., Ruegg U.T., Conte C.D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003;304(1):453–463. doi: 10.1124/jpet.102.041343. [DOI] [PubMed] [Google Scholar]
- 28.Terrill J.R., Grounds M.D., Arthur P.G. Taurine deficiency, synthesis and transport in the mdx mouse model for Duchenne Muscular Dystrophy. Int. J. Biochem. Cell Biol. 2015;66:141–148. doi: 10.1016/j.biocel.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Ripps H., Shen W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 30.De Luca A., Pierno S., Camerino D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojcik O.P., Koenig K.L., Zeleniuch-Jacquotte A., Costa M., Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208(1):19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faggiano A., Melis D., Alfieri R., De M.M., Filippella M., Milone F., Lombardi G., Colao A., Pivonello R. Sulfur amino acids in Cushing’s disease: Insight in homocysteine and taurine levels in patients with active and cured disease. J. Clin. Endocrinol. Metab. 2005;90(12):6616–6622. doi: 10.1210/jc.2005-0656. [DOI] [PubMed] [Google Scholar]
- 33.Huxtable R.J. Expanding the circle 1975-1999: Sulfur biochemistry and insights on the biological functions of taurine. Adv. Exp. Med. Biol. 2000;483:1–25. [PubMed] [Google Scholar]
- 34.Schaffer S.W., Jong C.J., Ramila K.C., Azuma J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010;17(Suppl. 1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierno S., De L.A., Camerino C., Huxtable R.J., Camerino D.C. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. J. Pharmacol. Exp. Ther. 1998;286(3):1183–1190. [PubMed] [Google Scholar]
- 36.Manini T.M., Clark B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J. Nutr. Health Aging. 2000;4(3):162–164. [PubMed] [Google Scholar]
- 38.Renganathan M., Messi M.L., Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 1997;157(3):247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- 39.Huxtable R.J., Bressler R. Effect of taurine on a muscle intracellular membrane. Biochim. Biophys. Acta. 1973;323(4):573–583. doi: 10.1016/0005-2736(73)90165-x. [DOI] [PubMed] [Google Scholar]
- 40.De Luca A., Pierno S., Camerino D.C. Effect of taurine depletion on excitation-contraction coupling and Cl- conductance of rat skeletal muscle. Eur. J. Pharmacol. 1996;296(2):215–222. doi: 10.1016/0014-2999(95)00702-4. [DOI] [PubMed] [Google Scholar]
- 41.Dutka T.L., Lamboley C.R., Murphy R.M., Lamb G.D. Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2014;117(7):797–805. doi: 10.1152/japplphysiol.00494.2014. [DOI] [PubMed] [Google Scholar]
- 42.Goodman C.A., Horvath D., Stathis C., Mori T., Croft K., Murphy R.M., Hayes A. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J. Appl. Physiol. 2009;107(1):144–154. doi: 10.1152/japplphysiol.00040.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebart M.C., Benyamin Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006;273(15):3415–3426. doi: 10.1111/j.1742-4658.2006.05350.x. [DOI] [PubMed] [Google Scholar]
- 44.Goll D.E., Neti G., Mares S.W., Thompson V.F. Myofibrillar protein turnover: The proteasome and the calpains. J. Anim. Sci. 2008;86(14) Suppl.:E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 45.Johnson J.D., Han Z., Otani K., Ye H., Zhang Y., Wu H., Horikawa Y., Misler S., Bell G.I., Polonsky K.S. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J. Biol. Chem. 2004;279(23):24794–24802. doi: 10.1074/jbc.M401216200. [DOI] [PubMed] [Google Scholar]
- 46.Belcastro A.N., Shewchuk L.D., Raj D.A. Exercise-induced muscle injury: A calpain hypothesis. Mol. Cell. Biochem. 1998;179(1-2):135–145. doi: 10.1023/a:1006816123601. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong R.B., Warren G.L., Warren J.A. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 48.Friden J., Sfakianos P.N., Hargens A.R. Blood indices of muscle injury associated with eccentric muscle contractions. J. Orthop. Res. 1989;7(1):142–145. doi: 10.1002/jor.1100070120. [DOI] [PubMed] [Google Scholar]
- 49.Cannon J.G., Meydani S.N., Fielding R.A., Fiatarone M.A., Meydani M., Farhangmehr M., Orencole S.F., Blumberg J.B., Evans W.J. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am. J. Physiol. 1991;260(6 Pt 2):R1235–R1240. doi: 10.1152/ajpregu.1991.260.6.R1235. [DOI] [PubMed] [Google Scholar]
- 50.Tidball J.G., Spencer M.J. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J. Physiol. 2002;545(Pt 3):819–828. doi: 10.1113/jphysiol.2002.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y., Arnold J.M., Pampillo M., Babwah A.V., Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic. Biol. Med. 2009;46(1):51–61. doi: 10.1016/j.freeradbiomed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Sun M., Zhao Y., Xu C. Cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion. Cell. Mol. Neurobiol. 2008;28(1):71–85. doi: 10.1007/s10571-007-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupont-Versteegden E.E. Apoptosis in muscle atrophy: Relevance to sarcopenia. Exp. Gerontol. 2005;40(6):473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Scicchitano B.M., Faraldi M., Musaro A. The proteolytic systems of muscle wasting. Recent Adv. DNA Gene Seq. 2015;9(1):26–35. doi: 10.2174/2352092209999150911121502. [DOI] [PubMed] [Google Scholar]
- 55.Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 56.Puzianowska-Kuznicka M., Kuznicki J. The ER and ageing II: Calcium homeostasis. Ageing Res. Rev. 2009;8(3):160–172. doi: 10.1016/j.arr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Rutkowski D.T., Kaufman R.J. That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem. Sci. 2007;32(10):469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Ito T., Yoshikawa N., Inui T., Miyazaki N., Schaffer S.W., Azuma J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS One. 2014;9(9):e107409. doi: 10.1371/journal.pone.0107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bandyopadhyay A., Saxena K., Kasturia N., Dalal V., Bhatt N., Rajkumar A., Maity S., Sengupta S., Chakraborty K. Chemical chaperones assist intracellular folding to buffer mutational variations. Nat. Chem. Biol. 2012;8(3):238–245. doi: 10.1038/nchembio.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timbrell J.A., Seabra V., Waterfield C.J. The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. 1995;26(3):453–462. doi: 10.1016/0306-3623(94)00203-y. [DOI] [PubMed] [Google Scholar]
- 61.Wohlgemuth S.E., Seo A.Y., Marzetti E., Lees H.A., Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp. Gerontol. 2010;45(2):138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirks A.J., Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic. Biol. Med. 2004;36(1):27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Jang Y.C., Lustgarten M.S., Liu Y., Muller F.L., Bhattacharya A., Liang H., Salmon A.B., Brooks S.V., Larkin L., Hayworth C.R., Richardson A., Van R.H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24(5):1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marzetti E., Hwang J.C., Lees H.A., Wohlgemuth S.E., Dupont-Versteegden E.E., Carter C.S., Bernabei R., Leeuwenburgh C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim. Biophys. Acta. 2010;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marzetti E., Lees H.A., Manini T.M., Buford T.W., Aranda J.M., Jr, Calvani R., Capuani G., Marsiske M., Lott D.J., Vandenborne K., Bernabei R., Pahor M., Leeuwenburgh C., Wohlgemuth S.E. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: An exploratory study. PLoS One. 2012;7(2):e32829. doi: 10.1371/journal.pone.0032829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walston J., Fedarko N., Yang H., Leng S., Beamer B., Espinoza S., Lipton A., Zheng H., Becker K. The physical and biological characterization of a frail mouse model. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(4):391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marzetti E., Calvani R., Cesari M., Buford T.W., Lorenzi M., Behnke B.J., Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013;45(10):2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giris M., Depboylu B., Dogru-Abbasoglu S., Erbil Y., Olgac V., Alis H., ykac-Toker G., Uysal M. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin. Exp. Immunol. 2008;152(1):102–110. doi: 10.1111/j.1365-2249.2008.03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takatani T., Takahashi K., Uozumi Y., Shikata E., Yamamoto Y., Ito T., Matsuda T., Schaffer S.W., Fujio Y., Azuma J. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am. J. Physiol. Cell Physiol. 2004;287(4):C949–C953. doi: 10.1152/ajpcell.00042.2004. [DOI] [PubMed] [Google Scholar]
- 70.Green T.R., Fellman J.H., Eicher A.L., Pratt K.L. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim. Biophys. Acta. 1991;1073(1):91–97. doi: 10.1016/0304-4165(91)90187-l. [DOI] [PubMed] [Google Scholar]
- 71.Jeon S.H., Lee M.Y., Rahman M.M., Kim S.J., Kim G.B., Park S.Y., Hong C.U., Kim S.Z., Kim J.S., Kang H.S. The antioxidant, taurine reduced lipopolysaccharide (LPS)-induced generation of ROS, and activation of MAPKs and Bax in cultured pneumocytes. Pulm. Pharmacol. Ther. 2009;22(6):562–566. doi: 10.1016/j.pupt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira M.W., Minotto J.B., de Oliveira M.R., Zanotto-Filho A., Behr G.A., Rocha R.F., Moreira J.C., Klamt F. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 2010;62(1):185–193. doi: 10.1016/s1734-1140(10)70256-5. [DOI] [PubMed] [Google Scholar]
- 73.Schaffer S.W., Azuma J., Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can. J. Physiol. Pharmacol. 2009;87(2):91–99. doi: 10.1139/Y08-110. [DOI] [PubMed] [Google Scholar]
- 74.Silva L.A., Silveira P.C., Ronsani M.M., Souza P.S., Scheffer D., Vieira L.C., Benetti M., De Souza C.T., Pinho R.A. Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise. Cell Biochem. Funct. 2011;29(1):43–49. doi: 10.1002/cbf.1716. [DOI] [PubMed] [Google Scholar]
- 75.Jong C.J., Azuma J., Schaffer S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids. 2012;42(6):2223–2232. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 76.Jong C.J., Ito T., Schaffer S.W. The ubiquitin-proteasome system and autophagy are defective in the taurine-deficient heart. Amino Acids. 2015;47(12):2609–2622. doi: 10.1007/s00726-015-2053-7. [DOI] [PubMed] [Google Scholar]
- 77.Hansen S.H., Birkedal H., Wibrand F., Grunnet N. Taurine and regulation of mitochondrial metabolism. Adv. Exp. Med. Biol. 2015;803:397–405. doi: 10.1007/978-3-319-15126-7_30. [DOI] [PubMed] [Google Scholar]
- 78.Terman A., Brunk U.T. Myocyte aging and mitochondrial turnover. Exp. Gerontol. 2004;39(5):701–705. doi: 10.1016/j.exger.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Franceschi C. Inflammaging as a major characteristic of old people: Can it be prevented or cured? Nutr. Rev. 2007;65(12 Pt 2):S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 80.Guttridge D.C., Mayo M.W., Madrid L.V., Wang C.Y., Baldwin A.S., Jr NF-kappaB-induced loss of MyoD messenger RNA: Possible role in muscle decay and cachexia. Science. 2000;28(5488):2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 81.Reid M.B., Li Y.P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2001;2(5):269–272. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 83.Peterson J.M., Bakkar N., Guttridge D.C. NF-kappaB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 2011;96:85–119. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 84.Li H., Malhotra S., Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. (Berl.) 2008;86(10):1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schuller-Levis G.B., Park E. Taurine: New implications for an old amino acid. FEMS Microbiol. Lett. 2003;226(2):195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 86.Kim C., Jang J.S., Cho M.R., Agarawal S.R., Cha Y.N. Taurine chloramine induces heme oxygenase-1 expression via Nrf2 activation in murine macrophages. Int. Immunopharmacol. 2010;10(4):440–446. doi: 10.1016/j.intimp.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 87.Barua M., Liu Y., Quinn M.R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: Decreased NF-kappaB activation and IkappaB kinase activity. J. Immunol. 2001;167(4):2275–2281. doi: 10.4049/jimmunol.167.4.2275. [DOI] [PubMed] [Google Scholar]
- 88.Glass D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010;346:267–278. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 89.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Latres E., Amini A.R., Amini A.A., Griffiths J., Martin F.J., Wei Y., Lin H.C., Yancopoulos G.D., Glass D.J. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 2005;280(4):2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- 91.Li Y., Hu Z., Chen B., Bu Q., Lu W., Deng Y., Zhu R., Shao X., Hou J., Zhao J., Li H., Zhang B., Huang Y., Lv L., Zhao Y., Cen X. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol. Lett. 2012;215(1):1–7. doi: 10.1016/j.toxlet.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 92.Charge S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 93.Musarò A. The basis of muscle regeneration. 2014.
- 94.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 95.Le Bihan M.C., Bigot A., Jensen S.S., Dennis J.L., Rogowska-Wrzesinska A., Laine J., Gache V., Furling D., Jensen O.N., Voit T., Mouly V., Coulton G.R., Butler-Browne G. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J. Proteomics. 2012;77:344–356. doi: 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Fulle S., Di D.S., Puglielli C., Pietrangelo T., Beccafico S., Bellomo R., Protasi F., Fano G. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp. Gerontol. 2005;40(3):189–197. doi: 10.1016/j.exger.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Gebara E., Udry F., Sultan S., Toni N. Taurine increases hippocampal neurogenesis in aging mice. Stem Cell Res. (Amst.) 2015;14(3):369–379. doi: 10.1016/j.scr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Rippo M.R., Olivieri F., Monsurro V., Prattichizzo F., Albertini M.C., Procopio A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014;56:154–163. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Bhaumik D., Scott G.K., Schokrpur S., Patil C.K., Orjalo A.V., Rodier F., Lithgow G.J., Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany N.Y.) 2009;1(4):402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown D.M., Goljanek-Whysall K. 2015.
- 101.Drummond M.J., Glynn E.L., Fry C.S., Dhanani S., Volpi E., Rasmussen B.B. Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J. Nutr. 2009;139(12):2279–2284. doi: 10.3945/jn.109.112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rivas D.A., Lessard S.J., Rice N.P., Lustgarten M.S., So K., Goodyear L.J., Parnell L.D., Fielding R.A. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. 2014;28(9):4133–4147. doi: 10.1096/fj.14-254490. [DOI] [PMC free article] [PubMed] [Google Scholar]