Abstract
Dengue is one of the most important arboviral infections worldwide, infecting up to 390 million people and causing 25,000 deaths annually. Although a licensed dengue vaccine is available, it is not efficacious against dengue serotypes that infect people living in South East Asia, where dengue is an endemic disease. Hence, there is an urgent need to develop an efficient dengue vaccine for this region. Data from different clinical trials indicate that a successful dengue vaccine must elicit both neutralizing antibodies and cell mediated immunity. This can be achieved by designing a multi-epitope peptide vaccine comprising B, CD8+ and CD4+ T cell epitopes. As recognition of T cell epitopes are restricted by human leukocyte antigens (HLA), T cell epitopes which are able to recognize several major HLAs will be preferentially included in the vaccine design. While peptide vaccines are safe, biocompatible and cost-effective, it is poorly immunogenic. Strategies to improve its immunogenicity by the use of long peptides, adjuvants and nanoparticle delivery mechanisms are discussed.
Keywords: Dengue, peptide vaccine, multi-epitope, synthetic peptides, HLA, cell mediated immunity
1. INTRODUCTION
Dengue is a mosquito-borne viral disease that occurs mainly in the tropical and subtropical regions [1]. It causes an annual estimated 390 million infections, of which 500,000 require hospitalization and 25,000 deaths are reported [2, 3]. Dengue is transmitted to humans by female Aedes aegypti or Aedes albopictus mosquitoes infected with the dengue virus. Most patients have asymptomatic or self-limiting Dengue Fever (DF), however others may suffer from life-threatening Dengue Haemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS) [4]. Severe dengue is a potentially deadly complication due to plasma leakage, fluid accumulation, respiratory distress, severe bleeding, and organ impairment.
Dengue virus belongs to the Flaviviridae family and is endemic in more than 100 countries worldwide [3]. Dengue infection can be caused by four distinct, but closely related serotypes, DENV-1, DENV-2, DENV-3 and DENV-4, sharing 65-70% sequence homology [5]. Dengue virus has a positive stranded ~11kb RNA genome, encoding for a polypeptide chain of approximately 3400 amino acids. This polypeptide is made up of three structural proteins (capsid, membrane and envelope), and seven non-structural (NS) proteins (NS1, 2a, 2b, 3, 4a, 4b and 5). The structural proteins form the external viral structure, while the NS proteins are involved in the viral replication machinery.
2. Host immunity against dengue virus
Immunity against viral infection can be divided into the innate and adaptive arms. Upon entry of viruses into the human host, it is first recognized by the innate immunity, which is the first line of defense against pathogens. Cells of the innate immunity recognize viral components as being ‘foreign’ by using different types of pattern recognition receptors such as Toll-like Receptors (TLRs), Nucleotide Oligomerization Domain-like receptors (NOD) and retinoic acid-inducible gene I-like receptors (RLRs). These receptors trigger downstream intracellular signaling cascades, eliciting the production of pro-inflammatory cytokines and chemokines [6, 7]. Of these, type I interferons (IFNs) are the primary cytokines that inhibit viral replication in both infected and uninfected cells [8]. In addition, type I IFNs promote the initiation of adaptive immune responses by mediating activation of Dendritic Cells (DCs), enhancing the effector functions of macrophages and lymphocyte, and stimulating the humoral response to neutralize viruses [9].
Adaptive immunity against viral infections are mediated by antibodies (humoral immunity) and cellular mediated immunity. Humoral immunity is mediated by B cells, which when stimulated by CD4+ T helper cells, secrete specific anti-viral antibodies. These antibodies “neutralize” the virus by binding to its envelope or capsid proteins, thereby preventing its attachment and entry into host cells. Antibodies can also function to opsonize viral particles and promote their destruction by phagocytes such as macrophages and neutrophils [9]. Viral neutralization and opsonization by antibodies are only effective when they are extracellular.
Once virus has infected the cells, its elimination is mediated by the killing of the infected cell by CD8+ cytotoxic T-lymphocytes (Tc). Viral proteins are degraded by the proteolytic enzymes in the endosome, and resulting peptides are loaded on either Major Histocompatibility Complex (MHC) class I or class II molecules. Recognition of viral peptides bound to MHC-I by T-cell Receptor (TCR), followed by co-stimulatory signals generated by CD4+ T-helper (Th) cells and DCs [10] is necessary to stimulate the activation of CD8+ T-cells. Activated CD8+ Tc release cytotoxic granules containing granzymes, perforins and granulysin that induce the apoptosis of viral-infected cells, and cytokine production of TNF-α and IFN-γ.
During the primary DENV infection, antibodies against the virus are able to neutralize the viral infection and confer lifelong protection against it. Upon heterologous infection with another DENV serotype, there is an initial phase of cross-protection. However, these antibodies are short-lived, and are superseded by the non-neutralizing cross-reactive antibodies [11]. They form antibody-virus complexes and bind to the FcγR receptors of circulating monocytes and macrophages, allowing the heterologous DENV to infect these cells more efficiently, leading to high viral replication and viral load, eventually causing a more severe form of dengue infection. This phenomenon is known as Antibody-Dependent Enhancement (ADE) [12, 13].
While the humoral response to secondary heterologous dengue infection leads to ADE, the equivalent aberrant response in the cellular immunity is termed as the “original antigenic sin” [14]. The original antigenic sin, or the Hoskins effect refers to the propensity of the immune system to favor the immunological memory of the first infecting antigen, when an altered version of the antigen is encountered later. In the case of dengue, during the secondary dengue infection (with a virus serotype different from the primary infection), the cellular immunity is dominated by cross-reactive memory T cells generated during the first infection. Although the cross-reactive T cells are activated more rapidly and are present in large numbers, they are inefficient in lysis of DENV-infected cells due to sub-optimal TCR triggering. This results in poor virus clearance from the host [14, 15].
The exact role of T cells, as being protective or pathologic during dengue infection is still being debated [16-18]. Initial research in this area mainly supports the pathological role of T cells in dengue. The delay in viral elimination leading to a more severe dengue immunopathology observed during secondary infections could be due to lower affinity CD8+ T cells that were dysfunctional [14]. Several studies highlighted the observation of DENV-specific T cells that produced high levels of TNF-α (versus IFN-γ) during secondary infection as a potential cause of immunopathology [19-21]. High amounts of TNF-α can lead to vascular leakage, a symptom of severe dengue. Accordingly, as IFN-γ is implicated in antiviral activity, the lower IFN-γ secretions observed during heterologous infection indicates reduced clearance of viral infected cells. Apart from the TNF-α / IFN-γ balance, the lack of cytotoxic activity of T cells is also a contributing factor to the negative role of T cells in secondary infections [22].
Of late however, data from studies on CD8+ T cells support its protective role in secondary dengue infections. A recent study by Rivino et al. (2015) reported that the DENV-specific CD8+ T cells in patients with acute secondary dengue infection had anti-viral effector phenotype, characterized by high IFN-γ, CD107a+ and low TNF-α cytokines [23]. It was previously suggested that high levels of TNF-α found in serum of severe dengue patients could be the causal factor of plasma leakage [24]. The TNF-α cytokine may not originate from dengue-specific T cells, as it has been reported that these cells were only detected in the blood of dengue infected patients post-viremia resolution, indicating that it was unlikely that T cells were linked to plasma leakage [25]. Furthermore, individual HLA alleles have been implicated in dengue susceptibility or protection. In a study performed on dengue seropositive individuals from Sri Lanka, the protective CD8+ T cells had higher response magnitude and were polyfunctional in terms of cytokine production [26].
3. Current prevention options for dengue
The burden and costs of dengue continue to grow, with an estimation of more than 96 million symptomatic dengue infections in the world annually. At present, the most successful method of controlling dengue is based primarily on mosquito vector control and dengue case management, but these strategies are only partially successful. Of late, there has been an increasing amount of research in the field of dengue vaccine development. Although one vaccine (Dengvaxia®) has been registered and several others are in late phases of clinical trials, so far, none has provided effective protection against all DENV serotypes. The challenge in dengue vaccine development arises from the presence of four serotypes of viruses that can cause the disease, and the previous observations that subsequent heterologous infection often results in a more severe form of dengue, implying that natural humoral or cell-mediated immune response against the primary dengue infection is insufficient to confer complete protection against the heterologous DENV.
Dengvaxia® CYD-TDV by Sanofi Pasteur is currently the only vaccine approved for use in endemic populations. It is a tetravalent live-attenuated vaccine (LAV), composed of prM/E genes of the four DENV serotypes on the 17D yellow fever RNA backbone [27]. The yellow fever virus belongs to the same virus family as dengue, and has previously shown good immunogenicity and safety profile as a vaccine [28]. In general, Dengvaxia® showed higher efficacy in DENV seropositive recipients (having pre-existing anti-DENV antibodies) compared to seronegative recipients [29, 30]. The efficacy of Dengvaxia® varied according to serotype and age of the vaccinees. In individuals above 9 years old, higher overall protection was observed against DENV-4 (83.2%) and DENV-3 (73.6%), and was less efficient against DENV-1 (58.4%) and DENV-2 (47.1%) [31]. Protection against DENV-2 was particularly low in the Asian population at only 36.8% [31], although seroconversion was observed [32]. Efficacy of Dengvaxia® was higher against severe dengue and hospitalization in recipients above 9 years old (90.9% and 81.6%, respectively), compared to those below 9 years of age (44.5% and 56.1%, respectively) [31].
Reduced efficacies to all serotypes were observed in children less than 9 years old, in decreasing order from DENV-3 (62.1%), DENV-4 (51.7%), DENV-1 (46.6%) and DENV-2 (33.6%). In a large phase III study that involved 10,275 children from five Asian countries, Dengvaxia® CYD14 only produced an endpoint of 56.5% efficacy [29]. Asian children (2-5 years old) receiving Dengvaxia® had significantly higher hospitalization risk for virologically confirmed dengue cases, 15 cases in the vaccine group versus one case in the control group [30, 31, 33]. In addition, the number of serious adverse effects reported in the vaccinees (402 cases) was almost double of that in the control group (245 cases) [29], raising serious concerns against the use of Dengvaxia® as the preferred vaccine against dengue infection.
Although Dengvaxia® has several shortcomings, it is currently the only licensed vaccine against dengue infection. WHO has recommended the use of Dengvaxia® in countries where dengue is endemic, and for patients of 9 – 45 years old [34]. The lower efficacy of Dengvaxia® against DENV-1 and DENV-2, coupled with its low safety levels in children below 9 years old calls for the need of a more efficacious vaccine candidate, primarily in regions where DENV-1 and -2 are the prevailing serotypes, such as in Malaysia and Singapore [35-37]. The main mode of immune response of Dengvaxia® seems to be via the humoral route, by eliciting neutralizing antibodies. Despite the seroconversion against different serotypes, the lack of protection of Dengvaxia® (in particular against DENV-2) indicates that neutralizing antibodies alone are insufficient to confer protection, and there is a need for vaccine developers to pay attention on eliciting cellular immunity against dengue [26, 38].
Few other vaccine candidates are currently undergoing clinical trials, of which TV003/ TV005 and DENVax (TDV) are in the frontline [39]. TV003 and TV005 are tetravalent LAVs developed by the National Institute of Allergy and Infectious Diseases (NIAID) in the United States. The composition of TV003 and TV005 differs by its dose of the attenuated DENV-2, which was 10 fold higher in TV005 [40]. TV003/TV005 were attenuated by creating deletion of 30/31 base pairs in the 3’ untranslated for each serotype, and for DENV-2, the prM/E proteins were substituted on the rDENV-4∆30 backbone [41]. TV003 is currently in phase III clinical trial (ClinicalTrials.gov Identifier: NCT02406729) while TV005 is in phase II trial (NCT02678455). The overall tetravalent neutralizing antibody responses reported for TV003 and TV005 were 74.0% and 90.0%, respectively [39, 42]. Seroconversion rates for TV005 were more balanced between different serotypes, with 92% against DENV-1 and 97% against DENV 2-4, when compared to TV003 [42]. So far, results from clinical trials have been showing promising outcomes. In the phase II study, all 21 individuals vaccinated against TV003 and challenged with rDEN2∆30 did not develop viremia, neutropenia or rash and were protected from infection. All patients were also protected against the second challenge administered 6 months after the initial vaccination [43]. In contrast to Dengvaxia®, TV003 conferred higher degree of protection against DENV-2, and induced strong CD8+ T cell responses, directed against the highly conserved non-structural protein epitopes of all DENV serotypes [44].
DENVax (TDV) is a tetravalent LAV developed by Takeda Pharmaceuticals, containing the prM and E genes of DEN-1, -3, and -4 on the DENV-2 PDK-53 genetic backbone [33]. The DENV2-PDK-53 has been attenuated by 53 serial passages in primary dog kidney cells, and further attenuated by gene mutation of NS3 [33, 39]. Phase I studies for DENVax were performed in participants aged 18-45 years old in the United States and Columbia. Different doses and routes of vaccine administration were evaluated. Neutralizing antibodies were produced against all serotypes regardless of dose and injection routes. Approximately 62% of the participants were able to seroconvert to all four serotypes 30 days after the second vaccination dose was administered [45]. Titers of neutralizing antibodies varied according to the serotype, with the highest against DENV-1 and -2, and lowest to DENV-4 [46]. In the multicentre, double-blind phase II studies conducted in Puerto Rico, Columbia, Singapore and Thailand, DENVax was well tolerated with no serious adverse events reported, irrespective of pre-vaccination dengue exposure profile and age of volunteers [46]. In addition to eliciting neutralizing antibodies, as the architecture of DENVax contained the genes that encode non-structural proteins of DENV-2, it was able to elicit multifunctional CD8+ T cell responses against NS1, NS3 and NS5 proteins of DENV-2, able to cross-react to the same proteins in other serotypes [33, 47, 48].
Other anti-dengue vaccine candidates that are currently in the pipeline include live attenuated vaccines, inactivated vaccines, recombinant vector vaccines, DNA vaccines and subunit vaccines [33, 49-53]. The peptide based vaccine strategy for dengue is currently intensely researched, via discovery of specific B, Tc and Th epitopes (Table 1) and represents an approach with great potential to prevent the disease.
Table 1.
Distribution of CD4+ and CD8+ epitopes based on dengue proteins.
| No. | Protein | No. of Epitopes | Subset | Refs. |
|---|---|---|---|---|
| 1 | C, NS2A, NS3, NS4B, NS5 | 8 | CD8 | [54] |
| 2 | C, NS4B, NS5 | 4 | CD8 | [55] |
| 3 | C | 2 | CD4 | [56] |
| 4 | NS3 | 2 | CD8 | [57] |
| 5 | NS3 | 1 | CD8 | [14] |
| 6 | NS3 | 3 | CD4 | [58] |
| 7 | NS3 | 2 | CD4 | [21] |
| 8 | NS3 | 1 | CD8 | [59] |
| 9 | NS3 | 1 | CD4 | [60] |
| 10 | NS3 | 1 | CD8 | [61] |
| 11 | NS3 | 1 | CD4 | [62] |
| 12 | NS3 | 2 | CD4 | [63] |
| 13 | NS3 | 1 | CD8 | [64] |
| 14 | NS3, NS4B, NS5 | 10 | CD8 | [65] |
| 15 | NS3 | 1 | CD8 | [22] |
| 16 | NS3 | 1 | CD8 | [66] |
| 17 | NS3 | 1 | CD8 | [20] |
| 18 | NS3, NS5 | 2 | CD8 | [67] |
| 19 | NS3 | 3 | CD8 | [68] |
| 20 | NS3, NS4B, NS5 | 5 | CD8 | [69] |
| 21 | NS5 | 14 | CD8 | [70] |
| 22 | E | 8 | CD8 | [71] |
| 23 | E | 3 | CD8 | [72] |
| 24 | C, PrM, M, E, NS1, NS2A, NS3, NS4A, NS4B, NS5 | 18 | CD4, CD8 | [73] |
| 25 | C, E, NS2B, NS3, NS4A, NS4B, NS5 | 13 | CD8 | [74] |
| 26 | C, M, E, NS2A, NS3, NS4B, NS5 | 25 | CD8 | [26] |
| 27 | E, NS2A, NS3, NS4B | 9 | CD8 | [75] |
| 28 | M, E, NS1, NS2A, NS2B, NS3, NS4A, NS4B | 16 | CD4, CD8 | [76] |
| 29 | C, NS1, NS3 | 5 | CD4, CD8 | [77] |
| 30 | E, NS2A, NS4A, NS4B | 7 | CD8 | [78] |
| 31 | NS2b, NS3, NS4a, NS4b, NS5 | 8 | CD8 | [79] |
| 32 | C, E, NS3 | 4 | CD4 | [80] |
| 33 | C, M, E, NS2A, NS4B, NS5 | 12 | CD8 | [81] |
| 34 | C, E, NS3, NS4B, NS5 | 5 | CD8 | [82] |
| 35 | E, NS4A, NS4B | 4 | CD8 | [15] |
| 36 | C, PrM, M, E, NS3, NS4A | 47 | CD4, CD8 | [83] |
| No. | Protein | No. of Epitopes | Subset | Refs. |
| 37 | C, M, E, NS1, NS2A, NS2B, NS3, NS4B NS5 | 30 | CD4, CD8 | [84] |
| 38 | E, NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5 | 19 | CD4, CD8 | [85] |
| 39 | E, NS1 | 2 | CD8 | [86] |
| 40 | NS1, NS3, NS5 | 16 | CD4 | [87] |
| 41 | NS3, NS5 | 5 | CD8 | [88] |
| 42 | E | 10 | CD4 | [89] |
| 43 | E | 3 | N/A | [90] |
| 44 | prM | 4 | N/A | [91] |
| 45 | E | 9 | CD4 | [92] |
| 46 | E, NS1, NS3, NS5 | 29 | N/A | [93] |
| 47 | E, NS2B, NS3, NS4A, NS4B, NS5 | 12 | CD4, CD8 | [94] |
| 48 | E | 7 | CD4 | [95] |
N/A – not available
4. Peptide vaccines compared to other types of dengue vaccinations
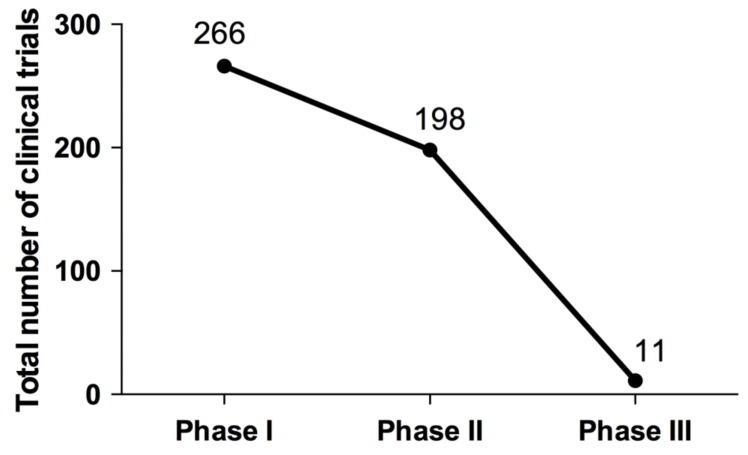
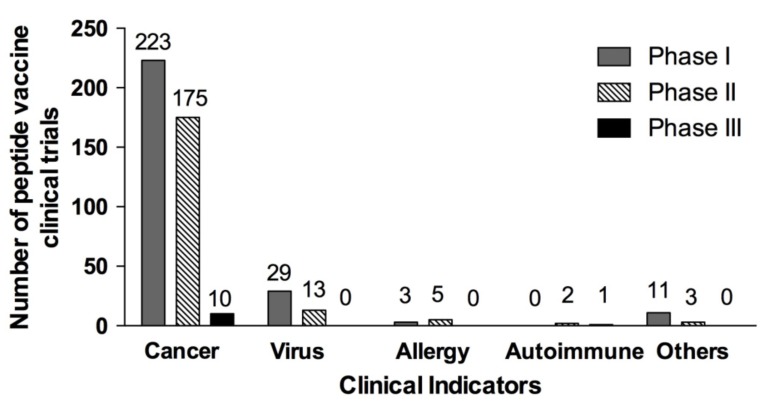
Peptide based vaccines are currently being developed to be used both as therapeutic or prophylactic treatment for a multitude of diseases, ranging from cancers and viral infections, to allergies and Alzheimer’s disease. A total of 475 clinical trials (266 in phase I, 198 in phase II, 11 in phase III) that used peptide vaccines for different diseases have been reported in ClinicalTrials.gov, a database of the US National Institute of Health (Fig. 1). So far, no peptide vaccine has reached phase IV trial. While the number of clinical trials reported from March 2014 (506 trials) [96] to date (15 June 2017, 475 trials) has not changed much, it still signifies a wide-spread interest of this vaccination strategy. Most of the peptide vaccines in clinical trials were against different cancers, followed by viral infections, allergy and auto-immune diseases (Fig. 2). The viral based peptide vaccines were in phase I (29 clinical trials) and phase II (13 clinical trials) against HIV, HBV, HCV and CMV (Fig. 2). No peptide vaccine against dengue is currently in clinical trial.
Fig. (1).
Total number of clinical trials based on study phase. Data is current as of 15 June 2017.
Fig. (2).
Number of clinical trials based on clinical indication and study phase. Data is current as of 15 June 2017. Others refer to studies on diabetes, malaria, Alzheimer’s disease and bacterial infections.
Currently, there are 103 clinical trial entries in ClinicalTrials.gov using the search terms “dengue” and “vaccine” (data accessed on 15 June 2017). This is a 72% increase of the number of trials, compared to the 60 studies reported in 2014 [41].
In dengue research, all four vaccines which are currently in the late clinical trial development stages (phase II and above) have been designed using the live attenuated virus (LAV) strategy. Although LAVs are very potent immunological stimulators, and are able to ideally generate appropriate immune responses to protect the host against subsequent viral infections, there are several risks associated with LAVs. As LAVs are living organisms, there is a risk of reversion to its original pathogenic form, which is capable of causing disease. This has been previously observed in the cases of vaccine-associated paralytic poliomyelitis and disease-causing vaccine-derived poliovirus associated with oral polio vaccine [97, 98]. LAVs could cause potential harm to immunocompromised patients (e.g. HIV infected) as they may not be able to respond adequately to the vaccine [99]. Other disadvantages include high storage and transportation costs, as LAVs usually require storage at low temperatures to remain active. In the case of dengue, as it can be caused by 4 serotypes, LAVs need to be prepared from all serotypes, and the resulting downstream growth and purification of these strains can be laborious and costly.
In an adaptive immunological response against foreign antigens, only short segments of the antigens (termed epitopes) are recognized by the B- and T-cell receptors [100]. Synthetic peptide vaccines (or “designer” vaccines) are composed of selected epitope(s) of the antigenic protein, thus enabling the induction of highly targeted immune responses. Using well defined antigen(s), the disadvantages commonly associated with whole pathogen vaccine, such as reversion to the wild type virus and contamination with other antigenic components can easily be avoided [101]. Compared to conventional vaccines, peptide synthesis is highly reproducible, fast and cost effective [96, 101]. However, despite its high safety profile compared to live attenuated vaccines, synthetic peptide vaccines are generally poorly immunogenic. They are susceptible to enzymatic degradation, making them unstable in vivo [101].
These shortcomings can be overcome by using several strategies, which will be explored in a greater detail in this review, such as increasing peptide length, addition of T-helper epitope sequences, addition of the third signal, adjuvants, delivery methods, or a combination of several approaches. The final goal is to develop a safe, reproducible and genetically stable vaccine, which is capable of inducing the appropriate immune response.
5. Viral T-cell epitopes are HLA restricted
Once the viral antigen is taken up by the Antigen Presenting Cells (APCs) such as DCs, it is processed by proteolytic degradation in the proteasomes, and the resulting peptides are transported into the endoplasmic reticulum. Here, CD8+ T cell epitopes of the virus binds to MHC class I molecule, and the complex is expressed on the cell surface of the APC [102]. MHC (or human leukocyte antigens, HLA in humans), are one of the most highly polymorphic genes determining specificity to antigen epitopes [103, 104]. MHC-I molecules are encoded by HLA-A, -B and –C (presenting CD8+ T cell epitopes), while MHC-II molecules are encoded by HLA-DP, -DQ and –DR genes (presenting CD4+ T cell epitopes). The polymorphic residues within the HLA molecule determine the peptide binding specificity, and this is termed HLA restriction. One of the main challenges in designing peptide vaccines is selecting the correct epitope, as T cell epitopes are restricted by HLA molecules. Furthermore, as there is a vast diversity of HLA types in a population, individual patients could recognize a different set of epitopes for the same antigen [101, 105]. However, several studies have demonstrated that some HLA supertypes can share high degree of peptide binding specificities. In approximately 90% of people, three peptide binding specificities are shared among HLA-A02, A03 and B07 [106, 107].
The HLA gene is located on chromosome 6p21 which is in close proximity to many other immune-related genes. Many studies have found that some HLA alleles were associated to disease susceptibility, while others were protective. Similarly, in dengue, studies investigating the association of HLA alleles to dengue susceptibility or protection have been carried out in many countries (Table 2). Most HLA associations have been investigated for class I HLA molecules, and very little is known regarding the role of HLA-II molecules. There seems to be a great diversity in HLA-alleles that confer susceptibility / protection across various populations (Table 2). This is perhaps due to two reasons. First, it has been shown that HLA alleles are specific to ethnicity and geographical location [108, 109]. Second, the four co-circulating dengue virus serotypes could further influence the HLA-specificity leading to the heterogeneous HLA-molecules associated with protection / susceptibility of dengue.
Table 2.
Susceptible and protective HLA to dengue based on population.
| Population | Susceptible HLA | Protective HLA | Reference |
|---|---|---|---|
| Malaysia (total) | HLA-B*53 | HLA-A*03 | [110] |
| HLA-B*18 | |||
| Malaysia (Malay population) | HLA-B*13 | [110] | |
| Philippines (children) | HLA-A*3301(severe dengue) | [111] | |
| Brazil (Latin American population) | HLA-B*44 | HLA-B*07, HLA-DR*13 | [112] |
| Brazil (White population) | HLA-DQ*01 | [113] | |
| Brazil | HLA-A*01 (DHF) | [114] | |
| Cuba | HLA-A*01 | HLA-B*14 | [115] |
| HLA-A*29 | |||
| HLA-A*31 (DHF) | HLA-DRB1*07 | [73] | |
| HLA-B*51 | HLA-DRB1*04 | ||
| Vietnam (children) | HLA-A*24 (DHF) | HLA-A*33 (DHF) | [88] |
| HLA-A*2402 (DHF/DSS) | HLA-DRB1*0901 (DSS) | [116] | |
| HLA-A*2403 (DHF/DSS) | |||
| HLA-A*2410 (DHF/DSS) | |||
| Mexico | HLA-DQB1*0302 (DHF, not DF) | HLA-B*35 (DF) | [117] |
| HLA-DQB1*0202 (DF only) | |||
| Sri Lanka | HLA-DRB1*08 (DHF/DSS) | [118] | |
| HLA-A*31 (DHF/DSS) | |||
| Thai | HLA-B*51 (DHF, secondary infection) | HLA-A*0203 | [119] |
| HLA-A*0207 (DHF) | HLA-B*44 | ||
| HLA-B*52 (DHF, primary infection) | HLA-B*62 | ||
| HLA-B*76 | |||
| HLA-B*77 | |||
| HLA-A*02 (secondary infection) | HLA-B44 (DHF, secondary infection) | [120] | |
| HLA-A*01 (secondary infection) | |||
| HLA-A*03 (secondary infection) | |||
| HLA-A*02 | HLA-B*13 | [121] | |
| Venezuela | HLA-B*57 (DF) | HLA-A*03 (DHF) | [122] |
| HLA-B*40 (DHF) | |||
| Jamaica | HLA-A*24 | HLA-A*23 | [123] |
| HLA-DRB5*01 | HLA-CW*04 | ||
| HLA-DRB5*02 | HLA-DQB1*02 | ||
| HLA-DQB1*03 |
6. T cell epitope mapping by experimental or predictive approaches
Epitopes can be broadly classified into linear or conformational epitopes, based on their interaction with the paratope. T cell epitopes (specific to either CD4+ or CD8+ cells) are linear, where its sequence is continuous, based on the primary amino acid sequence of the antigen. B cell epitopes can be linear or conformational. Conformational epitopes are based on the three-dimensional protein structure interaction with the B-cell receptor, therefore its amino acid sequence is discontinuous.
Current vaccination formulations against dengue target the humoral immunity, and have been shown to be insufficient to effectively protect against the infection. Cytotoxic T cells play an important role in killing infected cells and control viremia of dengue virus. Hence, we focus the scope of this review on the use of T cell epitopes in peptide vaccine formulations, and the different strategies that could be employed to improve immunogenicity of the vaccine formulation.
The identification of T cell epitopes of antigens is the first step in designing peptide-based vaccines. This can be achieved either experimentally, using functional assays, or by in silico predictive approaches, with each method having its own advantages and limitations. An experimental method that is commonly used to identify T cell epitopes in dengue is by synthesizing a peptide library consisting of short overlapping synthetic peptides, spanning either the whole virus protein, or focusing on a specific protein of interest. These peptides are then tested using a functional assay such as ELISPOT to measure the release IFN-γ from PBMCs isolated from patients [83, 84, 124], intracellular cytokine staining of IFN-γ and TNF-α from patients CD4+ and CD8+ T cells of patients [19], or in vitro lymphocyte proliferation response of peptide-primed lymphocytes [89]. Alternatively, similar functional assays may also be performed using purified recombinant protein fragments [89]. MHC-microarray is another technique performed by spotting MHC-I molecules on microarray slides, and interacting it with peptides
that can be recognized by T-cells. By adding anti-cytokine antibodies to this assay, the amount of cytokine secreted by the target T cell can be measured, similar to that in an ELISPOT. This method has been used to map T cell epitopes in HIV [125], but has not gained popularity due to high cost and complexity of sample preparation and instrumentation. In cell ELISAs, the solid multi-well surface is first coated with an anti-cytokine antibody, followed by the addition of peptides and T cells. If the T cells recognize the peptide, it would secrete cytokines which can be detected by capture of the solid-phase antibody. T cell epitope mapping of a multiple sclerosis related intracellular pathogen, Mycobacterium avium, has been delineated using this method [126].
The binding of peptide epitope to HLA molecules is the pre-requisite of peptide-TCR recognition and eventually T cell activation and differentiation. Identification of the antigenic peptides that bind to HLA molecules in vivo can now be performed using immunoproteomics approaches. Compared to other experimental approaches, this method identifies clinically-relevant epitopes in a straightforward and direct manner, not subjected to any biases such as selection of dominant epitopes based on its level of IFN-γ secretion, or predictive scores of HLA-binding based on certain algorithms. This approach has been used to identify the MHC-I binding epitopes of dengue virus [54, 55]. The MHC-I epitopes identified originated from both structural and non-structural DENV proteins, indicating that Tc epitopes of DENV is more wide-spread than previously thought [26]. More importantly, this approach enabled the identification of two promiscuous Tc epitopes, binding to HLA-A2 and HLA-A24 [54], which allows for broader vaccine coverage and efficacy.
While experimental methods are unbiased, epitope identification involves testing many peptides, rendering this approach costly and tedious. Predictive bioinformatics approaches, on the other hand, are high throughput and economical as only the predicted epitopes with high confidence would be tested in functional biological assays to validate its activity. The bioinformatics approach has been used heavily in predicting T cell epitopes in dengue. The first step of in silico T cell epitope prediction is to predict peptide binding to HLA alleles and supertypes. Several prediction methods are available, based on the method of machine learning used. These include Position Specific Scoring Matrices (PSSM) such as RANKpep [80, 82], SYFPEITHI [73, 78, 82], BIMAS [73], Support Vector Machines (SVM) [127, 128] and Artificial Neural Network (ANN) (e.g. netMHC [129]). The previously listed algorithms identify peptide binding to individual HLA alleles. Now, it is possible to predict promiscuous peptide binding to HLA, based on its binding grove, termed panHLA binding. Several pan-specific algorithms exists such as MULTIPRED [130] netMHCpan [70, 131], KISS [132], TEPITOPEpan [133], Pick-Pocket [134] and ADT [135]. Once the HLA-binding peptide is predicted, other computational algorithms that model the MHC class I antigen processing, such as proteosomal cleavage, transport associated protein (TAP) transport (e.g. NetCTL [79, 136] and MHC-PATHWAY [137]) and hydopathy [138] may be applied to improve the prediction stringency. Finally, selected predicted epitopes should be tested using functional biological assays to validate its biological function. The predictive approaches have its own limitations - identification of false positive epitopes, and the poor discrimination between MHC-binding peptides which are T cell epitopes or non-epitopes.
7. Peptide length affects antigenicity
Immunogenicity has been shown to be dependent on peptide length [139, 140]. Short peptides (8 -11 amino acids) are able to bind directly to the MHC-I molecule, without the need of processing by professional APCs [141, 142]. As a consequence, such short peptides containing the minimal Tc epitopes are capable of binding to MHC-I molecules on any cell, including non-professional APCs, which lack the co-stimulatory molecules required to generate effector Tc cells, resulting in CD8+ T cell tolerance [143, 144]. On the other hand, longer peptides (more than 17 amino acids) have been reported to require proteosomal processing by APCs prior to its presentation by MHC molecules [145]. Synthetic peptides that were extended so that they were now sufficiently long to be processed by DCs were able to trigger the expansion of effector Tc [146]. Several studies that performed direct comparisons between minimal Tc epitopes and long peptides containing the Tc epitopes in different antigens (human papillomavirus, ovalbumin or adenovirus) have shown that longer peptides were able to elicit more robust and effective Tc responses [140, 147].
8. Improving the efficacy of synthetic peptides – adding the CD4+ T cell epitope
Activation of cytotoxic T cells requires two signals. The first signal is the recognition of the antigen via its presentation on the MHC–I molecules by the T cell receptor on the naïve cytotoxic T cells. The second signal involves the interaction between co-stimulatory molecules which are expressed on the surfaces of APC and the naïve cytotoxic T cell. T-helper cells are among the APCs that express co-stimulatory molecules. Experiments performed by co-injecting the minimal epitopes of Tc and Th were successful in preventing Tc tolerance [140], and augmenting the Tc response [148-150] as both signal 1 and 2 were present, allowing Tc cells to mature into effector cells. The delivery of multi-epitope peptide vaccines by linking both the minimal epitopes of Tc and Th synthetically as a single peptide appears to be more efficient than co-injecting a mixture of each epitope [151-153]. As the multi-epitope vaccine is now a longer peptide, it is highly likely that its increased potency could be attributed to it being processed by the APCs, ensuring the accurate Tc epitopes presented on MHC-I, as well as the activation via co-stimulatory molecules. In fact, a 15-mer peptide which was initially reported to be able to induce Tc responses in the absence of any adjuvants, was later identified to also contain a Th epitope, which likely aided this response [154].
As in MHC-I, MHC-II genes are also highly polymorphic, and the recognition of Th epitopes are restricted by its HLA alleles. To overcome this, an artificial pan HLA-DR helper T-lymphocyte epitope (PADRE) peptide was constructed by introducing anchor residues for different DR motifs within a polyalanine backbone to generate a ‘universal’ T-helper epitope [10]. PADRE binds to majority of the human HLA-DR receptors with moderate to high affinity, hence it is able to overcome the HLA class II restriction by providing ‘universal’ immune stimulation in a MHC-heterologous population [101, 155]. PADRE has been shown to increase the immune response to associated peptide antigens. In a study using HBV-transgenic mice, a construct of HBV-specific Tc epitope and PADRE could break the Tc tolerance [156]. Potent HPV-16 E7-specific CD8+ T cell immune responses were generated when C57BL/6 mice were injected with HPV-16 E749-57 (9 aa-long peptide) in combination with PADRE and polyriboinosinic: polyribocytidylic acid [poly (I:C)] [157]. Ghaffari-Nazari et al. (2015) demonstrated that the addition of the PADRE peptide and CpG adjuvant to synthetic, multi-epitope, long Her2/neu CTL peptide, P5+435 (44 aa-long peptide, inclusive of the RR linker) enhanced Tc immune response and anti-tumor effects [145]. In dengue vaccine development, a multi-epitope vaccine was designed containing a T cell epitope (E345-359), a B cell epitope (E383-397), both originating from the envelope domain III (EDIII) of DENV-2 and PADRE. Mice immunized with this multi-epitope vaccine generated specific antibodies against EDIII, and were able to induce in vitro lymphoproliferation and Th1-dominated cytokine release [158].
Instead of manually selecting dominant Tc and Th epitopes to design a peptide vaccine, an alternative is to use either very long synthetic peptides (approximately 100 amino acids long) [159], or a mixture containing long overlapping synthetic peptides spanning the antigen of choice [160]. In both cases, the long synthetic peptide used will contain the naturally occurring antigen-specific Tc and Th epitopes. Due to its length, these synthetic peptides have to be processed by the professional APCs, prior to presentation on MHC-I or MHC-II receptors. This approach therefore offers the advantage of not biasing the Tc or Th epitope selection. In addition, as the APC is required to proteolytically digest and present the epitopes on HLA-I and HLA-II molecules, the in vivo cellular machinery would select the correct HLA-restricted epitopes, eliminating the laborious (and perhaps erroneous) pre-selection of epitopes.
9. Other co-stimulAtory molecules
The first signal alone (TCR recognition of antigen epitope presented via MHC) is insufficient for T cell activation and maturation, and often leads to anergy. The second signal of activation is provided upon interaction of co-stimulatory molecules expressed on the surfaces of T cells and APC. However, T cell activation and differentiation has been shown to occur in the absence of APC, if the co-stimulatory molecule was introduced together with the first signal. This was demonstrated in a study by den Boer et al. (2001) where mice were injected subcutaneously with a Tc epitope derived from the human adenovirus type 5 E1A in incomplete Freud’s adjuvant (IFA) with or without CD40-activating antibodies [161]. They observed that the Tc peptide alone was not able to generate E1A-specific Tc responses, but mice that received the Tc peptide and CD40 treatment were completely protected against tumor outgrowth [161]. In another study performed in murine models, co-injection of a nine-amino acid CD8+ T cell epitope peptide originating from HPV-16 E7 protein with soluble 4-1BBL, a costimulatory molecule, showed higher efficacy in eradicating established TC-1 tumors compared to injection of the peptide alone [162]. In general, co-stimulatory receptors can be broadly classified into two families; the CD28 family (e.g. CD28, CTLA-4, ICOS, PD-1 and BTLA) and the TNFR family (e.g. CD27, 4-1BB, CD40, TRAIL, CD30 and OX40). Each member appears to be important in different phases of the immune response, and has been described in detail in a review by Duttagupta et al. [163].
10. Adjuvants and carriers for peptide vaccines
10.1. Inclusion of Inflammatory Cytokines (Third Signal)
Adjuvants are agents that can stimulate the immune system, and when administered together with vaccines, improve the antigenicity of the vaccine. Various molecules have been used as adjuvants, including (but not limited to) inflammatory cytokines, TLR agonists, emulsions, bacterial cell components, carbohydrates, mineral salts or combinations of several molecules. In addition to signal 1 (recognition of MHC presented epitopes by TCR) and signal 2 (co-stimulation), optimal T-cell activation and maturation requires the correct inflammatory cytokine milieu (signal 3) [164, 165]. The types of cytokines present in the microenvironment of the CD4+ T cells after activation and co-stimulation determine the differentiation pathway taken by the cell to become Th1, Th2 or Th17 cells [165].
One of the early experiments to demonstrate the requirement of the third signal was performed in an APC-free environment, using naïve T cells from TCR transgenic mice and artificial MHC peptide/protein antigen complexes immobilized on inert latex microspheres [166]. It was observed that CD8+ T cells required the presence of IL-12 in addition to the antigen peptide and IL-2 to have optimal differentiation and clonal expansion, while for CD4+ T cells, IL-1 was the necessary third signal [166]. More recent studies have demonstrated that CD8+ T cells require IL-12 or type I IFN to support the expansion of this cell type in vivo. In experiments where cells lacked the receptors for IL-12, type I IFN, or both, the dependency on the third signal changed when different infections were introduced. The CD8+ T cell expansion was shown to be dependent on type I IFN for choriomeningitis virus (LCMV) infection [164]. In vesicular stomatitis virus (VSV) infection, type I IFN was able to substitute the function of IL-12 in CD8+ T cell expansion [150, 167]. Neither IL-12 nor type I IFN was required for cell expansion during vaccinia virus (VV) infection [164]. On one hand, it has been reported that high amounts of the co-stimulatory signal IL-2 supported CD8+ T cell cytolytic activity and its differentiation into effector Tc, [147, 168] and that IL-2 was able to support T cell activation, but the question remains if IL-2 is able to compensate for IL-12 and type I IFN during vaccinia virus infection.
Another frequently used cytokine adjuvant is the granulocyte-monocyte colony stimulating factor (GM-CSF). GM-CSF is able to augment both humoral and cellular immunity by upregulating the surface expression of MHC-II and co-stimulatory molecules, CD40 and CD86 [169], enhancing antigen processing and presentation [170]. GM-CSF has been used successfully as an adjuvant with the commercialized HBV vaccine, Engerix-B® [171] and with several peptide vaccines in clinical trials [172, 173]. In dengue vaccination, the use of GM-CSF has so far been restricted to DNA vaccines where it was capable of eliciting stronger immune responses [174, 175]. While GM-CSF is safe and effective, it’s only disadvantage seems to be its short circulating half-life [176], which can be overcome by either attaching a polyethylene glycol moiety to GM-CSF [177], or by encapsulation in a micro or nanoparticle [178, 179].
10.2. Toll-Like Receptor (TLR) Agonists
TLRs are transmembrane proteins that are localized either on the cell surface or intracellular components such as endosomes. TLRs of the cell surface (TLR1, 2, 4, 5, and 6) are primarily involved in detection of extracellular bacterial products, while TLRs of the endosomes (TLR 3, 7, 8 and 9) target the identification of viral and bacterial nucleic acids [180]. TLRs are able to identify pathogen-associated microbial patterns (PAMPs) from microorganisms, and initiate the signaling cascade of early innate immune responses. Agonists of TLR3, 4, 7 and 9 have been shown to enhance the efficacy and potency of anti-viral or anti-cancer peptide vaccines in mouse models [147, 181-186]. In fact, the success of early vaccines is due to its accidental contamination with bacterial components that were able to elicit TLR activation, providing it the additional “adjuvant effect”. Some examples are the lesions from cowpox patients that contained vaccinia virus and TLR 9 agonists, and the presence of TLR 2, 4 and 9 in the heat-killed Mycobacterium tuberculosis, which is a major component of complete Freund’s adjuvant [180].
TLRs are able to act as adjuvants of the poorly immunogenic peptide vaccines, as they can activate Dendritic Cells (DCs) [187]. When DCs are activated, the expression of co-stimulatory molecules such as CD80/86 and CD40 get upregulated, and are key in subsequent T-cell activation and differentiation [188]. In addition, TLR ligands are able to recruit MHC molecules from the endosomal recycling compartments, and thus enhancing antigen cross-presentation [189]. TLRs are often used in viral vaccine formulation owing to their strong adjuvant properties. Imidazoquinoline, a TLR-7/8 agonist, was able to act as an adjuvant and broaden responses to the avian influenza virus H5N1 HA based antigens in related viral clades, conferring a wider protection against this virus [190]. A highly selective TLR7 agonist, GS-9620, when administered together with a HIV peptide showed increased CD8+ T cell degranulation, cytolytic activity and cytokine secretion [190]. Some studies performed in humans and mice propose using several TLR agonists in combination for a more potent adjuvant effect [191, 192]. A combination of three TLRs (TLR 2/6, TLR 3 and TLR 9) increased protective efficacy of HIV envelope peptide vaccination in mice, compared to when only 2 TLR ligands were used. Increased vaccine efficacy was most likely due to higher production of IL-15 and its receptor IL-15Rα which contributed to the high avidity of T cells to the HIV antigen, enhancing viral clearance [191]. In dengue vaccine development, a group from Taiwan has evaluated the use of CpG oligodeoxynucleotides (a TLR-9 agonist) in a subunit vaccine preparation containing dengue-1 envelope protein domain III and water-in-oil-in-water emulsion [193]. This vaccine preparation was able to induce anti-DENV-1 neutralizing antibodies and increased IFN-γ secretion from splenocytes following in vitro stimulation, unlike another preparation formulated using aluminium phosphate [193]. The applications of TLR agonists are vast, and has also been shown to be efficacious in the areas of cancer and infection immunotherapy [180, 194, 195].
10.3. Alum
Alum (aluminium hydroxide) is a safe, well-known, and US FDA (US Food and Drug Administration)-approved adjuvant for human use [196]. Antigens bind to alum by adsorption, and the resulting alum-antigen complex has enhanced antigen uptake and presentation by APCs [197]. Despite the wide use of alum in different vaccine formulations, it is a poor activator of DCs, and induces poor Th1-associated immune response essential for anti-viral vaccines [198].
10.4. Emulsion Based Carriers
Complete Freund’s Adjuvant (CFA) is one of the most commonly used adjuvants in animal research. CFA is not approved for human application as it is too toxic, exhibiting high reactogenicity and has reported to cause local neurotic ulcers. It is made up of a combination of bacterial and emulsion adjuvants, specifically heat killed bacillus Calmette-Guérin (BCG) (Mycobacterium bovis strain) and mineral oils. The mode of action of CFA is two-pronged: the mineral oil encapsulation of antigen provides a depot for slow antigen release, favoring phagocytosis. The heat killed bacterium activates DCs and the Th1 pathway via its adjuvant effect. In animal immunizations, CFA is usually only used during the first antigen injection, as it induces a strong Th1 dominated inflammatory response at the site of injection. For subsequent injections, antigen is injected with the less toxic IFA, which lacks the Mycobacterium component.
As synthetic peptide vaccines are poorly immunogenic, encapsulation of peptide vaccines are helpful as it provides what is known as the “depot effect”, which is a prolonged exposure, and slow release from the emulsion to ensure a complete immune response. In addition, encapsulation protects peptide vaccines against proteolytic digestion. However, in some cases, the depot effect has resulted in prolonged local chronic infection [10, 140, 199]. Additionally, in the absence of the second signal, the depot effect can risk promoting CD8+ T cell tolerance, which has been observed in vaccination carried out with minimal T cell peptides [10, 140, 200, 201].
Encapsulated carriers are customizable, and are either delivered as water-in-oil emulsions, or multi-loaded with other adjuvants such as inflammatory cytokines or TLR agonists. Two of the newer emulsion based carriers that have been used in human vaccinations include MF59 and Montanide. MF59 is a microfluidized water-in-oil emulsion made up of squalene, polysorbate 80 and sorbitan trioleate [202]. It is the first adjuvant to be used in FLUAD®, a seasonal influenza vaccine [203, 204]. Montanide™ is another family of water-in-oil emulsion adjuvants, of which two formulations, Montanide ISA51 (IFA) and Montanide ISA720 are specific for human use. Its applications are vast and has been used in about 300 clinical trials involving vaccines of malaria, AIDS, cancer and autoimmune diseases. Commercially, Montanide ISA51 has been used in the formulation of CimaVax-EGF, a vaccine against non-small-cell lung carcinoma [205]. Other squalene-based emulsions such as AF03, AS03 and AS04 have also been approved for human applications, but are disease and country specific.
10.5. Liposomes
Liposomes are self-assembling vesicular structures composed of phospholipid bilayer shell with an aqueous core, and have been used extensively as drug delivery vehicles [206]. They can be generated as either multilameller vesicles which consist of several concentric phospholipid shells separated by layers of water, or unilameller vesicles made of a single phospholipid bilayer [207, 208]. Liposomes are biocompatible and completely biodegradable, making them advantageous over other vaccine carriers because of their lack of toxicity. Liposome are chemically and structurally flexible, and can encapsulate or conjugate to either a hydrophilic antigen, or a lipophilic component which can intercalate between the lipid molecules, promoting a better uptake by APCs due to improved antigen accessibility [206]. In addition, surfaces of liposomes can be designed to target immune cells and co-deliver immune-stimulatory agents, to enhance the immune response [124, 209]. For example, liposomes have been used in the delivery of diphtheria toxoid in combination with poly (I:C) [209]. It has also been approved in vaccine formulations for human use, such as Inflexal® (influenza vaccine) and Epaxal V® (hepatitis A vaccine) [210, 211]. In the field of dengue vaccine research, a novel vaccine candidate was formulated using truncated recombinant envelope protein from all 4 serotypes (DEN-80E) and ionisable cationic lipid nanoparticles [212]. Immunization of mice, rodents and non-human primates with this vaccine formulation was able to elicit high titers of DENV neutralizing antibodies, and induced DEN-80E specific CD4+ and CD8+ T cell responses [212].
10.6. Virus-Like Particles (VLPs)
VLPs are self-assembling nanoparticle structures that resemble viruses, composed of viral structural proteins (such as capsid and envelope proteins). However, they are non-infectious and are unable to replicate as they do not contain any viral genetic material. Hence, VLPs are a safer alternative to LAVs, having the same external viral protein assembly that is capable of inducing strong humoral and cell-mediated immunity, without the adverse effects associated with LAVs [208, 213, 214]. The use of VLPs as a vaccination strategy in dengue has also been explored. Recombinant VLPs containing prM and E proteins of each dengue serotype were generated in mammalian cells, and evaluated for their humoral and cell-mediated immune responses in mice [215]. Both monovalent and tetravalent VLPs demonstrated specific antibody production and neutralizing antibody activity. Splenocytes harvested from mice vaccinated with either dengue VLPs or virions were then stimulated in vitro with VLPs, and both groups showed comparable IFN-γ release, indicating that the VLPs were capable of stimulating cell-mediated immunity [215]. In another similar study, the secretion of TNF-α and IL-10 were reported in mice immunized with tetravalent VLPs containing the prM and E proteins [216]. Although the VLP approach for dengue vaccination has been shown to be efficacious in mice, its effectiveness in humans is yet to be tested. These experiments would be important to do, as the current knowledge of epitopes point to the fact that dengue virus structural proteins (and NS1) mainly elicit the humoral response, while the cell mediated-immunity is primarily generated against T cell epitopes from the non-structural regions, and is mostly concentrated at NS3, NS4B and NS5 [26].
10.7. Other Nanoparticles
Several other nanoparticle strategies for the delivery of peptide vaccines exist, and are summarized in Table 3. Among these nanoparticles, some have been investigated in relation to dengue vaccines such as polymeric nanoparticles (PLGA and chitosan) and inorganic nanoparticles (carbon nanotubes) (Table 3)
Table 3.
Examples of other nanoparticles used for peptide vaccine delivery.
| Nanoparticle | Use in Dengue Vaccine | Example of Use |
|---|---|---|
| Immunostimulatory complexes (ISCOMs) | No | HBV, HSV type I, influenza [208] |
| Self-assembling peptide nanoparticles | No | Cancer [217], avian influenza [218] and HIV [219] |
| Polymeric nanoparticles (PLGA) | Yes [220] | H1N1 [221] |
| Polymeric nanoparticles (Chitosan) | Yes [222-224] | HPV [155] |
| Inorganic nanoparticles | Yes [225] | Influenza A [226], Hepatitis B core proteins [227] |
| Ligand Epitope Antigen Presentation System (L.E.A.P.S) | No | Influenza, HSV [228] |
| CEL-1000 | No | Malaria [229] |
11. Creative strategies in designing multi-epitope peptide vaccines
Designing a multi-epitope peptide vaccine allows for customization of the molecule, in order to stimulate the appropriate immune response desired. Using the multi-epitope strategy, it is possible to “hand-pick” epitopes of interest, thereby combining epitopes of interest from different protein subunits in one long synthetic peptide. This was elegantly demonstrated in a vaccine candidate of hepatitis C virus, where the peptide vaccine contained three immunodominant CD8+ T cell epitopes from different protein subunits, a CD4+ epitope and a B cell epitope [230]. When tested in Balb/c mice, this vaccine candidate was able to elicit specific HCV IgG1 and IgG2a antibodies, increased IFN-γ levels, and a stable and long lasting immune response when tested 9 weeks after the last immunization [230]. A similar approach was employed in designing an anti-foot and mouth disease virus (FMDV) vaccine candidate, consisting of tandem repeats of five B cell epitopes from different FMDV variants and one T cell epitope [231].
Another study combined the approaches of multi-epitope peptides and subunit vaccine. The Multi-Epitope Assembly Peptide (MEAP) against Herpes Simplex Virus Type 2 (HSV-2) was constructed by inserting 12 different epitopes (six B cell epitopes, four CD4+ T cell epitopes and two CD8+ T cell epitopes) into the extracellular fragments of HSV-2 glycoprotein D [232]. This MEAP construct could be a potential vaccine candidate against HSV-2, as it was able to completely protect immunized mice against HSV-2 infection by eliciting high titers of neutralizing antibodies and cell-mediated immune responses [232].
The peptide vaccine approaches described so far have used different epitopes from a single protein. Creative designing of vaccines using multiple peptides derived from multiple proteins have been gaining attention of late. In cancer, as Tumor Associated Antigen (TAA) expression is limited to a proportion of tumors, the use of multi-protein peptide vaccine is more advantageous compared to traditional single-protein peptide vaccine. Multiple peptides originating from different proteins have the possibility of targeting higher amounts of Tc, and have shown to be effective in studies involving myeloma [233], esophageal cancer [234] and non-small cell lung cancer [235]. A multi-protein peptide vaccine for renal cancer, IMA901, is currently undergoing phase III trial [236], and has shown to be clinically beneficial. This approach has a potential to be explored in the area of dengue, given that Tc epitopes have been identified in multiple proteins of a particular dengue virus serotype, and that epitopes of all four virus serotypes must be included in the final vaccine formulation to confer complete protection.
HLA restriction of epitopes is one of the major stumbling blocks in selecting the appropriate epitopes to design a successful peptide vaccine. Two different strategies could be used to overcome this issue. First, a mixture epitopes specific to different HLA molecules could be co-delivered during vaccination. This approach has been used successfully in a phase II trial for melanoma [237]. In this study, either 4 or 12 defined melanoma peptides from different proteins, melanocytic differentiation proteins (tyrosinase and gp100), cancer testis antigens (MAGE-A1, -A3, -A10 and NY-ESO-1) and five other novel peptides, that were restricted to HLA-A1, HLA-A2 and HLA-A3 were co-delivered with tetanus toxoid peptide, GM-CSF and Montanide ISA51 [237]. The authors observed that the more complex 12-peptide mixture induced a broader and more robust immune response. In addition, despite competition due to additional peptides specific to MHC molecules that did not match MHC of the patients, immunogenicity of individual peptides were maintained [237]. The second approach entails the use of T cell epitopes that are promiscuous in their binding to HLA. The HLA-DR restricted CD4+ T cell epitope of cancer-testis antigen HCA587 was shown to behave as a promiscuous epitope, as it was able to stimulate T cells in the context of multiple HLA-II alleles [238]. In dengue research, the group of Ernesto Marques identified DENV-3 T cell epitopes that were highly conserved among all four dengue serotypes, and demonstrated that these epitopes were also promiscuous in their HLA binding, some able to bind to as many as 14 HLA molecules [93], making these epitopes promising candidates to be used in dengue vaccination.
12. Future outlooks in designing peptide vaccines against dengue
In the last few years, there have been concentrated research efforts in identifying T- and B- cell epitopes against dengue virus. This was reflected in the number of dengue-specific epitopes deposited in the immune epitope database (IEDB). In May 2017, there were 825 HLA-I restricted epitopes and 884 HLA-II restricted epitopes, which was a significant increase from what was previously reported [38]. The DENV proteome is made up of 10 proteins, three structural and seven non-structural. The results of multiple studies point out that the humoral response is mostly directed against the structural proteins (pre-membrane and envelope) and NS1 [239, 240], CD8+ T-cell epitopes are concentrated in the non-structural region [76] (mainly NS3 and NS5) [19, 26, 69, 78] and CD4+ T cell epitopes recognize the capsid, envelope and NS3 regions [19, 26, 77, 83, 84]. An exception is DENV3, where the CD8+ T cell epitopes were found in both structural and non-structural proteins [241].
Dengvaxia®, the only licensed vaccine to date is not effective against DENV-1 and -2. As prM and E proteins were the only dengue proteins in Dengvaxia® which was built on a YFV backbone, it was not surprising that it only elicited anti-prM and anti-E antibody responses in vaccinated individuals, while no cell-mediated immunity was reported, likely due to the absence of dengue NS proteins in the vaccine construct. The other two most advanced vaccines in clinical trials, DENVax (Takeda) and TV003/TV005 (NIAID) is made up entirely of dengue proteins, and were therefore able to elicit both humoral and T-cell responses against DENV. As these vaccines are LAVs, there are two main disadvantages - the significant risks associated with its use, and the high cost factor of producing tetravalent LAVs.
Finding a good dengue vaccine has been difficult as the pathogenesis of the disease is complexed by the four co-circulating serotypes, and the worsening of the symptoms during secondary heterologous infections. An ideal dengue vaccine must mimic the natural immune response against the natural virus- that is having both humoral and cell-mediated immune responses against all four serotypes, while being safe, and ideally affordable. HLA-restriction of CD8+ and CD4+ T cell epitopes must also be factored in the vaccine design. As shown from multiple studies (Table 2), the HLA-restriction observed in different populations (with regards to the protective and susceptible HLA molecules) were heterogeneous. Studies done to investigate peptide binding to cell lines expressing single HLA molecules or purified HLA proteins showed that as several HLA molecules shared peptide binding specificities, only a small number of HLA needs to be targeted in order to cover the restriction profile of the majority of a population [106, 107]. In relation to dengue vaccines, once the major protective HLA molecules for a certain population have been identified, these HLA-restrictions should be taken into account to select the appropriate Tc epitopes in the multi-epitope vaccine design.
One of the hallmarks of a successful vaccine candidate is its ability to protect against viral infections. In the case of peptide vaccines against dengue, this was evaluated by first immunizing the mice with the peptides in relevant adjuvants, followed by infection using a mouse-adapted viral strain of dengue. Two groups have shown that different formulations of multi-epitope peptide vaccines were able to protect vaccinated mice against dengue viral challenge, likely via a combination of humoral and cell-mediated pathways [69, 158]. Similar promising outlooks were reported in peptide vaccines developed against other viruses such as influenza [242, 243], vaccinia and variola viruses [244] and foot-and-mouth disease virus [245] that were able to confer protection when challenged with virus.
An effective anti-dengue vaccine should be capable of eliciting both neutralizing antibodies and activating CD8+ cytotoxic T cells to kill virus-infected cells, eventually leading to viral clearance from the host. However, it is insufficient for a peptide vaccine candidate to elicit high levels of peptide-specific IgG antibodies without sufficient neutralizing activity [90], as this could be potentially cause ADE [246, 247], subsequently leading to severe dengue. The live attenuated tetravalent vaccine Dengvaxia® was able to elicit high levels of neutralizing antibodies [range: 47% (DENV2) to 83% (DENV4)] against all four dengue virus serotypes, but still reported poor efficacy against DENV1 (<58%) and DENV2 (<47%) [31]. This suggests that complete immune protection cannot be conferred solely by neutralizing antibodies, and should be complemented by the cellular immunity. Initial clinical trial data of the three other dengue vaccines, two from NIAID [42, 43], one from Takeda [46], showed that they were able to stimulate both neutralizing antibodies and cytotoxic T cell response against dengue virus. However, protective efficacies of both vaccines will be known based on the phase III clinical trial outcomes.
The multi-epitope peptide vaccine based strategy appears to be an attractive route to pursue as it is safe, reproducible and cost-effective. The vaccine will be able to elicit both humoral and cellular immunity to mimic the natural immune response against dengue as it is comprised of B- and T- cell epitope sequences that are highly conserved in all four serotypes. Immunogenicity of the peptide vaccine may be improved by the addition of adjuvants, and its delivery to lymphocyte cells targeted using nanoparticles.
Conclusion
Dengue remains a threat in modern society and is endemic in more than 100 countries. Although some advances have been made in terms of dengue vaccines, there are still areas of improvements in terms of efficacy and safety profiles. Peptide-based vaccine holds potential promise as it is safe, highly biocompatible and is cost-effective. In addition, peptide vaccines are customizable and can be engineered to target both the cell-mediated and humoral responses against dengue. The use of biological or chemical based adjuvants, as well as carriers (emulsions, lipids, nanoparticles) may significantly improve the immunogenicity of peptide vaccines.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of Sunway University Grant INTM-2017-RCBS-01.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Beatty M.E., Stone A., Fitzsimons D.W., et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl. Trop. Dis. 2010;4(11):e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S., Gething P.W., Brady O.J., et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman M.G., Halstead S.B., Artsob H., et al. Dengue: a continuing global threat. Nat. Rev. Microbiol. 2010;8(12) Suppl.:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin H.E., Tsai W.Y., Liu I.J., et al. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl. Trop. Dis. 2012;6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver S.C., Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 2009;9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swain S.L., McKinstry K.K., Strutt T.M. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin. Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosendahl Huber S., van Beek J., de Jonge J., Luytjes W., van Baarle D. T cell responses to viral infections - opportunities for Peptide vaccination. Front. Immunol. 2014;5:171. doi: 10.3389/fimmu.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliks S.C., Nisalak A., Brandt W.E., Wahl L., Burke D.S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 12.Dejnirattisai W., Jumnainsong A., Onsirisakul N., et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K.R., Zhang S.L., Tan H.C., et al. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc. Natl. Acad. Sci. USA. 2011;108:12479–12484. doi: 10.1073/pnas.1106568108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongkolsapaya J., Dejnirattisai W., Xu X.N., et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 15.Bashyam H.S., Green S., Rothman A.L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 2006;176:2817–2824. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 16.Zompi S., Harris E. Original antigenic sin in dengue revisited. Proc. Natl. Acad. Sci. USA. 2013;110:8761–8762. doi: 10.1073/pnas.1306333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thein S., Aung M.M., Shwe T.N., et al. Risk factors in dengue shock syndrome. Am. J. Trop. Med. Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 18.Rivino L. T cell immunity to dengue virus and implications for vaccine design. Expert Rev. Vaccines. 2016;15:443–453. doi: 10.1586/14760584.2016.1116948. [DOI] [PubMed] [Google Scholar]
- 19.Duangchinda T., Dejnirattisai W., Vasanawathana S., et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. USA. 2010;107:16922–16927. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friberg H., Bashyam H., Toyosaki-Maeda T., et al. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci. Rep. 2011;1:51. doi: 10.1038/srep00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangada M.M., Rothman A.L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 22.Mongkolsapaya J., Duangchinda T., Dejnirattisai W., et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J. Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 23.Rivino L., Kumaran E.A., Thein T.L., et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci. Transl. Med. 2015;7:278ra35. doi: 10.1126/scitranslmed.aaa0526. [DOI] [PubMed] [Google Scholar]
- 24.Inyoo S., Suttitheptumrong A., Pattanakitsakul S.N. Synergistic Effect of TNF-alpha and Dengue Virus Infection on Adhesion Molecule Reorganization in Human Endothelial Cells. Jpn. J. Infect. Dis. 2017;70:186–191. doi: 10.7883/yoken.JJID.2016.123. [DOI] [PubMed] [Google Scholar]
- 25.Dung N.T., Duyen H.T., Thuy N.T., et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 2010;184:7281–7287. doi: 10.4049/jimmunol.0903262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiskopf D., Angelo M.A., de Azeredo E.L., et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2013;110:E2046–E2053. doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham R.R., Juffrie M., Tan R., et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995-1996. Am. J. Trop. Med. Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 28.Sangkawibha N., Rojanasuphot S., Ahandrik S., et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 29.Capeding M.R., Tran N.H., Hadinegoro S.R., et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson N.M., Rodriguez-Barraquer I., Dorigatti I., Mier Y.T-R.L., Laydon D.J., Cummings D.A. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016;353:1033–1036. doi: 10.1126/science.aaf9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadinegoro S.R., Arredondo-Garcia J.L., Capeding M.R., et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 32.Sabchareon A., Wallace D., Sirivichayakul C., et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 33.Osorio J.E., Huang C.Y., Kinney R.M., Stinchcomb D.T. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29:7251–7260. doi: 10.1016/j.vaccine.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguiar M., Stollenwerk N., Halstead S.B. The Impact of the Newly Licensed Dengue Vaccine in Endemic Countries. PLoS Negl. Trop. Dis. 2016;10:e0005179. doi: 10.1371/journal.pntd.0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew M.H., Rahman M.M., Hussin S. Molecular epidemiology and phylogenetic analysis of Dengue virus type-1 and 2 isolated in Malaysia. Pak. J. Med. Sci. 2015;31:615–620. doi: 10.12669/pjms.313.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng L.C., Chem Y.K., Koo C., et al. 2013 dengue outbreaks in Singapore and Malaysia caused by different viral strains. Am. J. Trop. Med. Hyg. 2015;92:1150–1155. doi: 10.4269/ajtmh.14-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanoa A., Hassan S.S., Ngim C.F., et al. Impact of dengue virus (DENV) co-infection on clinical manifestations, disease severity and laboratory parameters. BMC Infect. Dis. 2016;16:406. doi: 10.1186/s12879-016-1731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiskopf D., Sette A. T-cell immunity to infection with dengue virus in humans. Front. Immunol. 2014;5:93. doi: 10.3389/fimmu.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz L.M., Halloran M.E., Durbin A.P., Longini I.M., Jr The dengue vaccine pipeline: Implications for the future of dengue control. Vaccine. 2015;33:3293–3298. doi: 10.1016/j.vaccine.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev. Vaccines. 2016;15:509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas S.J. Developing a dengue vaccine: progress and future challenges. Ann. N. Y. Acad. Sci. 2014;1323:140–159. doi: 10.1111/nyas.12413. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick B.D., Durbin A.P., Pierce K.K., et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J. Infect. Dis. 2015;212:702–710. doi: 10.1093/infdis/jiv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkpatrick B.D., Whitehead S.S., Pierce K.K., et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016;8:330ra36. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 44.Weiskopf D., Angelo M.A., Bangs D.J.S., et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015;89:120–128. doi: 10.1128/JVI.02129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osorio J.E., Velez I.D., Thomson C., et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2014;14:830–838. doi: 10.1016/S1473-3099(14)70811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirivichayakul C., Barranco-Santana E.A., Esquilin-Rivera I., et al. Safety and Immunogenicity of a Tetravalent Dengue Vaccine Candidate in Healthy Children and Adults in Dengue-Endemic Regions: A Randomized, Placebo-Controlled Phase 2 Study. J. Infect. Dis. 2016;213:1562–1572. doi: 10.1093/infdis/jiv762. [DOI] [PubMed] [Google Scholar]
- 47.Chu H., George S.L., Stinchcomb D.T., Osorio J.E., Partidos C.D. CD8+ T-cell Responses in Flavivirus-Naive Individuals Following Immunization with a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J. Infect. Dis. 2015;212:1618–1628. doi: 10.1093/infdis/jiv258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brewoo J.N., Kinney R.M., Powell T.D., et al. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine. 2012;30:1513–1520. doi: 10.1016/j.vaccine.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torresi J., Ebert G., Pellegrini M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum. Vaccin. Immunother. 2017;•••:1–14. doi: 10.1080/21645515.2016.1261770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durbin A.P., Kirkpatrick B.D., Pierce K.K., Schmidt A.C., Whitehead S.S. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29:7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy B.R., Whitehead S.S. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 52.Coller B.A., Barrett A.D., Thomas S.J. The development of Dengue vaccines. Introduction. Vaccine. 2011;29:7219–7220. doi: 10.1016/j.vaccine.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez S., Thomas S.J., De La Barrera R., et al. An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques. Am. J. Trop. Med. Hyg. 2015;92:698–708. doi: 10.4269/ajtmh.14-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Comber J.D., Karabudak A., Huang X., Piazza P.A., Marques E.T., Philip R. Dengue virus specific dual HLA binding T cell epitopes induce CD8+ T cell responses in seropositive individuals. Hum. Vaccin. Immunother. 2014;10:3531–3543. doi: 10.4161/21645515.2014.980210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Testa J.S., Shetty V., Sinnathamby G., et al. Conserved MHC class I-presented dengue virus epitopes identified by immunoproteomics analysis are targets for cross-serotype reactive T-cell response. J. Infect. Dis. 2012;205:647–655. doi: 10.1093/infdis/jir814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagnon S.J., Zeng W., Kurane I., Ennis F.A. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 1996;70:141–147. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zivny J., DeFronzo M., Jarry W., et al. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J. Immunol. 1999;163:2754–2760. [PubMed] [Google Scholar]
- 58.Kurane I., Okamoto Y., Dai L.C., Zeng L.L., Brinton M.A., Ennis F.A. Flavivirus-cross-reactive, HLA-DR15-restricted epitope on NS3 recognized by human CD4+ CD8- cytotoxic T lymphocyte clones. J. Gen. Virol. 1995;76(Pt 9):2243–2249. doi: 10.1099/0022-1317-76-9-2243. [DOI] [PubMed] [Google Scholar]
- 59.Mathew A., Kurane I., Green S., et al. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J. Virol. 1998;72:3999–4004. doi: 10.1128/jvi.72.5.3999-4004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurane I., Zeng L., Brinton M.A., Ennis F.A. Definition of an epitope on NS3 recognized by human CD4+ cytotoxic T lymphocyte clones cross-reactive for dengue virus types 2, 3, and 4. Virology. 1998;240:169–174. doi: 10.1006/viro.1997.8925. [DOI] [PubMed] [Google Scholar]
- 61.Zivny J., Kurane I., Leporati A.M., et al. A single nine-amino acid peptide induces virus-specific, CD8+ human cytotoxic T lymphocyte clones of heterogeneous serotype specificities. J. Exp. Med. 1995;182:853–863. doi: 10.1084/jem.182.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto Y., Kurane I., Leporati A.M., Ennis F.A. Definition of the region on NS3 which contains multiple epitopes recognized by dengue virus serotype-cross-reactive and flavivirus-cross-reactive, HLA-DPw2-restricted CD4+ T cell clones. J. Gen. Virol. 1998;79(Pt 4):697–704. doi: 10.1099/0022-1317-79-4-697. [DOI] [PubMed] [Google Scholar]
- 63.Zeng L., Kurane I., Okamoto Y., Ennis F.A., Brinton M.A. Identification of amino acids involved in recognition by dengue virus NS3-specific, HLA-DR15-restricted cytotoxic CD4+ T-cell clones. J. Virol. 1996;70:3108–3117. doi: 10.1128/jvi.70.5.3108-3117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Livingston P.G., Kurane I., Dai L.C., et al. Dengue virus-specific, HLA-B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones. Recognition of NS3 amino acids 500 to 508 by CTL clones of two different serotype specificities. J. Immunol. 1995;154:1287–1295. [PubMed] [Google Scholar]
- 65.Imrie A., Meeks J., Gurary A., et al. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J. Virol. 2007;81:10081–10091. doi: 10.1128/JVI.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zivna I., Green S., Vaughn D.W., et al. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J. Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]
- 67.Chang C.X., Tan A.T., Or M.Y., et al. Conditional ligands for Asian HLA variants facilitate the definition of CD8+ T-cell responses in acute and chronic viral diseases. Eur. J. Immunol. 2013;43:1109–1120. doi: 10.1002/eji.201243088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathew A., Kurane I., Rothman A.L., Zeng L.L., Brinton M.A., Ennis F.A. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J. Clin. Invest. 1996;98:1684–1691. doi: 10.1172/JCI118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elong Ngono A., Chen H.W., Tang W.W., et al. Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine. 2016;13:284–293. doi: 10.1016/j.ebiom.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi J., Sun J., Wu M., et al. Inferring Protective CD8+ T-Cell Epitopes for NS5 Protein of Four Serotypes of Dengue Virus Chinese Isolates Based on HLA-A, -B and -C Allelic Distribution: Implications for Epitope-Based Universal Vaccine Design. PLoS One. 2015;10:e0138729. doi: 10.1371/journal.pone.0138729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakraborty S., Chakravorty R., Ahmed M., et al. A computational approach for identification of epitopes in dengue virus envelope protein: a step towards designing a universal dengue vaccine targeting endemic regions. In Silico Biol. 2010;10:235–246. doi: 10.3233/ISB-2010-0435. [DOI] [PubMed] [Google Scholar]
- 72.Zhong H., Zhao W., Peng L., Li S-F., Cao H. Bioinformatics analysis and characteristics of envelop glycoprotein E epitopes of dengue virus. J. Biomed. Sci. Eng. 2009;2:123. [Google Scholar]
- 73.Sierra B., Alegre R., Perez A.B., et al. HLA-A, -B, -C, and -DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: advantages of the Cuban population for HLA studies of dengue virus infection. Hum. Immunol. 2007;68:531–540. doi: 10.1016/j.humimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Appanna R., Huat T.L., See L.L., Tan P.L., Vadivelu J., Devi S. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia. Clin. Vaccine Immunol. 2007;14:969–977. doi: 10.1128/CVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rivino L., Tan A.T., Chia A., et al. Defining CD8+ T cell determinants during human viral infection in populations of Asian ethnicity. J. Immunol. 2013;191:4010–4019. doi: 10.4049/jimmunol.1301507. [DOI] [PubMed] [Google Scholar]
- 76.Weiskopf D., Angelo M.A., Sidney J., Peters B., Shresta S., Sette A. Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J. Virol. 2014;88:11383–11394. doi: 10.1128/JVI.01108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiskopf D., Yauch L.E., Angelo M.A., et al. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 2011;187:4268–4279. doi: 10.4049/jimmunol.1101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan Z., Guo J., Huang X., et al. Identification of cytotoxic T lymphocyte epitopes in dengue virus serotype 1. J. Med. Virol. 2015;87:1077–1089. doi: 10.1002/jmv.24167. [DOI] [PubMed] [Google Scholar]
- 79.Lund O., Nascimento E.J., Maciel M., Jr, et al. Human leukocyte antigen (HLA) class I restricted epitope discovery in yellow fewer and dengue viruses: importance of HLA binding strength. PLoS One. 2011;6:e26494. doi: 10.1371/journal.pone.0026494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen J.S., Jiang L.F., Zhou J.M., Yan H.J., Fang D.Y. Computational prediction and identification of dengue virus-specific CD4(+) T-cell epitopes. Virus Res. 2008;132:42–48. doi: 10.1016/j.virusres.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yauch L.E., Zellweger R.M., Kotturi M.F., Qutubuddin A., Sidney J., Peters B., Prestwood T.R., Sette A., Shresta S. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wen J., Duan Z., Jiang L. Identification of a dengue virus-specific HLA-A*0201-restricted CD8+ T cell epitope. J. Med. Virol. 2010;82:642–648. doi: 10.1002/jmv.21736. [DOI] [PubMed] [Google Scholar]
- 83.Simmons C.P., Dong T., Chau N.V., et al. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 2005;79:5665–5675. doi: 10.1128/JVI.79.9.5665-5675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivino L., Kumaran E.A., Jovanovic V., et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013;87:2693–2706. doi: 10.1128/JVI.02675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malavige G.N., McGowan S., Atukorale V., et al. Identification of serotype-specific T cell responses to highly conserved regions of the dengue viruses. Clin. Exp. Immunol. 2012;168:215–223. doi: 10.1111/j.1365-2249.2012.04566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Townsley E., Woda M., Thomas S.J., et al. Distinct activation phenotype of a highly conserved novel HLA-B57-restricted epitope during dengue virus infection. Immunology. 2014;141:27–38. doi: 10.1111/imm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan A.M., Miotto O., Nascimento E.J.M., et al. Conservation and Variability of Dengue Virus Proteins: Implications for Vaccine Design. PLoS Negl. Trop. Dis. 2008;2:e272. doi: 10.1371/journal.pntd.0000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loke H., Bethell D.B., Phuong C.X., et al. Strong HLA class I--restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J. Infect. Dis. 2001;184:1369–1373. doi: 10.1086/324320. [DOI] [PubMed] [Google Scholar]
- 89.Leclerc C., Deriaud E., Megret F., Briand J.P., Van Regenmortel M.H., Deubel V. Identification of helper T cell epitopes of dengue virus E-protein. Mol. Immunol. 1993;30:613–625. doi: 10.1016/0161-5890(93)90072-j. [DOI] [PubMed] [Google Scholar]
- 90.Rocha R.P., Livonesi M.C., Fumagalli M.J., et al. Evaluation of tetravalent and conserved synthetic peptides vaccines derived from Dengue virus Envelope domain I and II. Virus Res. 2014;188:122–127. doi: 10.1016/j.virusres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Vazquez S., Guzman M.G., Guillen G., et al. Immune response to synthetic peptides of dengue prM protein. Vaccine. 2002;20:1823–1830. doi: 10.1016/s0264-410x(01)00515-1. [DOI] [PubMed] [Google Scholar]
- 92.Roehrig J.T., Risi P.A., Brubaker J.R., et al. T-helper cell epitopes on the E-glycoprotein of dengue 2 Jamaica virus. Virology. 1994;198:31–38. doi: 10.1006/viro.1994.1005. [DOI] [PubMed] [Google Scholar]
- 93.Nascimento E.J., Mailliard R.B., Khan A.M., Sidney J., Sette A., Guzman N., Paulaitis M., de Melo A.B., Cordeiro M.T., Gil L.V., Lemonnier F., Rinaldo C., August J.T., Marques E.T., Jr Identification of conserved and HLA promiscuous DENV3 T-cell epitopes. PLoS Negl. Trop. Dis. 2013;7:e2497. doi: 10.1371/journal.pntd.0002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanchez-Burgos G., Ramos-Castaneda J., Cedillo-Rivera R., Dumonteil E. Immunogenicity of novel Dengue virus epitopes identified by bioinformatic analysis. Virus Res. 2010;153:113–120. doi: 10.1016/j.virusres.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 95.Kutubuddin M., Kolaskar A.S., Galande S., Gore M.M., Ghosh S.N., Banerjee K. Recognition of helper T cell epitopes in envelope (E) glycoprotein of Japanese encephalitis, west Nile and Dengue viruses. Mol. Immunol. 1991;28:149–154. doi: 10.1016/0161-5890(91)90098-5. [DOI] [PubMed] [Google Scholar]
- 96.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide Vaccine: Progress and Challenges. Vaccines (Basel) 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platt L.R., Estivariz C.F., Sutter R.W. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014;210(Suppl. 1):S380–S389. doi: 10.1093/infdis/jiu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boot H.J., Kasteel D.T., Buisman A.M., Kimman T.G. Excretion of wild-type and vaccine-derived poliovirus in the feces of poliovirus receptor-transgenic mice. J. Virol. 2003;77:6541–6545. doi: 10.1128/JVI.77.11.6541-6545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eibl M.M., Wolf H.M. Vaccination in patients with primary immune deficiency, secondary immune deficiency and autoimmunity with immune regulatory abnormalities. Immunotherapy. 2015;7:1273–1292. doi: 10.2217/IMT.15.74. [DOI] [PubMed] [Google Scholar]
- 100.Hammerling G.J., Vogt A.B., Kropshofer H. Antigen processing and presentation-towards the millennium. Immunol. Rev. 1999;172:5–9. doi: 10.1111/j.1600-065x.1999.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 101.Skwarczynski M., Toth I. Peptide-based synthetic vaccines. Chem. Sci. (Camb.) 2016;7:842–854. doi: 10.1039/c5sc03892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu. Rev. Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sette A., Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 104.Greenbaum J., Sidney J., Chung J., Brander C., Peters B., Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63:325–335. doi: 10.1007/s00251-011-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang S., Song R., Popov S., Mirshahidi S., Ruprecht R.M. Overlapping synthetic peptides as vaccines. Vaccine. 2006;24:6356–6365. doi: 10.1016/j.vaccine.2006.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barber L.D., Gillece-Castro B., Percival L., Li X., Clayberger C., Parham P. Overlap in the repertoires of peptides bound in vivo by a group of related class I HLA-B allotypes. Curr. Biol. 1995;5:179–190. doi: 10.1016/s0960-9822(95)00039-x. [DOI] [PubMed] [Google Scholar]
- 107.Sidney J., del Guercio M.F., Southwood S., et al. Several HLA alleles share overlapping peptide specificities. J. Immunol. 1995;154:247–259. [PubMed] [Google Scholar]
- 108.Fraser P.A., Moore B., Stein R., et al. Complotypes in individuals of African origin: frequencies and possible extended MHC haplotypes. Immunogenetics. 1990;31:89–93. doi: 10.1007/BF00661218. [DOI] [PubMed] [Google Scholar]
- 109.Tshabalala M., Mellet J., Pepper M.S. Human Leukocyte Antigen Diversity: A Southern African Perspective. J. Immunol. Res. 2015;2015:746151. doi: 10.1155/2015/746151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Appanna R., Ponnampalavanar S., Lum Chai See L., Sekaran S.D. Susceptible and protective HLA class 1 alleles against dengue fever and dengue hemorrhagic fever patients in a Malaysian population. PLoS One. 2010;•••:5. doi: 10.1371/journal.pone.0013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mercado E.S., Espino F.E., Perez M.L., Bilar J.M., Bajaro J.D., Huy N.T., Baello B.Q., Kikuchi M., Hirayama K. HLA-A*33:01 as protective allele for severe dengue in a population of Filipino children. PLoS One. 2015;10:e0115619. doi: 10.1371/journal.pone.0115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xavier Eurico de Alencar L., de Mendonca Braga-Neto U., Jose Moura do Nascimento E., et al. HLA-B *44 Is Associated with Dengue Severity Caused by DENV-3 in a Brazilian Population. J. Trop. Med. 2013;2013:648475. doi: 10.1155/2013/648475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polizel J.R., Bueno D., Visentainer J.E., et al. Association of human leukocyte antigen DQ1 and dengue fever in a white Southern Brazilian population. Mem. Inst. Oswaldo Cruz. 2004;99:559–562. doi: 10.1590/s0074-02762004000600003. [DOI] [PubMed] [Google Scholar]
- 114.Monteiro S.P., Brasil P.E., Cabello G.M., Souza R.V., Brasil P., Georg I., Cabello P.H., De Castro L. HLA-A*01 allele: a risk factor for dengue haemorrhagic fever in Brazil’s population. Mem. Inst. Oswaldo Cruz. 2012;107:224–230. doi: 10.1590/s0074-02762012000200012. [DOI] [PubMed] [Google Scholar]
- 115.Paradoa Perez M.L., Trujillo Y., Basanta P. Association of dengue hemorrhagic fever with the HLA system. Haematologia (Budap.) 1987;20:83–87. [PubMed] [Google Scholar]
- 116.Nguyen T.P., Kikuchi M., Vu T.Q., et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl. Trop. Dis. 2008;2:e304. doi: 10.1371/journal.pntd.0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Falcon-Lezama J.A., Ramos C., Zuniga J., et al. HLA class I and II polymorphisms in Mexican Mestizo patients with dengue fever. Acta Trop. 2009;112:193–197. doi: 10.1016/j.actatropica.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 118.Malavige G.N., Rostron T., Rohanachandra L.T., et al. HLA class I and class II associations in dengue viral infections in a Sri Lankan population. PLoS One. 2011;6:e20581. doi: 10.1371/journal.pone.0020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stephens H.A., Klaythong R., Sirikong M., Vaughn D.W., Green S., Kalayanarooj S., Endy T.P., Libraty D.H., Nisalak A., Innis B.L., Rothman A.L., Ennis F.A., Chandanayingyong D. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens. 2002;60:309–318. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 120.Vejbaesya S., Thongpradit R., Kalayanarooj S., et al. HLA Class I Supertype Associations With Clinical Outcome of Secondary Dengue Virus Infections in Ethnic Thais. J. Infect. Dis. 2015;212:939–947. doi: 10.1093/infdis/jiv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiewsilp P., Scott R.M., Bhamarapravati N. Histocompatibility antigens and dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981;30:1100–1105. doi: 10.4269/ajtmh.1981.30.1100. [DOI] [PubMed] [Google Scholar]
- 122.Fernández-Mestre M., Navarrete C.V., Brown J., Brown C., Correa E., Layrisse Z. HLA Alleles and Dengue Virus Infection in Venezuelan Patients: A Preliminary Study. Inmunologia. 2009;28:96–100. [Google Scholar]
- 123.Brown M.G., Salas R.A., Vickers I.E., Heslop O.D., Smikle M.F. Dengue HLA associations in Jamaicans. West Indian Med. J. 2011;60:126–131. [PubMed] [Google Scholar]
- 124.Kim M-G., Park J.Y., Shon Y., Kim G., Shim G., Oh Y-K. Nanotechnology and vaccine development. Asian Journal of Pharmaceutical Sciences. 2014;9:227–235. [Google Scholar]
- 125.Stone J.D., Demkowicz W.E., Jr, Stern L.J. HLA-restricted epitope identification and detection of functional T cell responses by using MHC-peptide and costimulatory microarrays. Proc. Natl. Acad. Sci. USA. 2005;102:3744–3749. doi: 10.1073/pnas.0407019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cossu D., Masala S., Frau J., et al. Antigenic epitopes of MAP2694 homologous to T-cell receptor gamma-chain are highly recognized in multiple sclerosis Sardinian patients. Mol. Immunol. 2014;57:138–140. doi: 10.1016/j.molimm.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Donnes P., Elofsson A. Prediction of MHC class I binding peptides, using SVMHC. BMC Bioinformatics. 2002;3:25. doi: 10.1186/1471-2105-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wan J., Liu W., Xu Q., Ren Y., Flower D.R., Li T. SVRMHC prediction server for MHC-binding peptides. BMC Bioinformatics. 2006;7:463. doi: 10.1186/1471-2105-7-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calis J.J., Maybeno M., Greenbaum J.A., et al. Properties of MHC class I presented peptides that enhance immunogenicity. PLOS Comput. Biol. 2013;9:e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang G.L., Khan A.M., Srinivasan K.N., August J.T., Brusic V. MULTIPRED: a computational system for prediction of promiscuous HLA binding peptides. Nucleic Acids Res. 2005;33:W172-9. doi: 10.1093/nar/gki452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nielsen M., Lundegaard C., Blicher T., et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2:e796. doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacob L., Vert J.P. Efficient peptide-MHC-I binding prediction for alleles with few known binders. Bioinformatics. 2008;24:358–366. doi: 10.1093/bioinformatics/btm611. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L., Chen Y., Wong H.S., Zhou S., Mamitsuka H., Zhu S. TEPITOPEpan: extending TEPITOPE for peptide binding prediction covering over 700 HLA-DR molecules. PLoS One. 2012;7:e30483. doi: 10.1371/journal.pone.0030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang H., Lund O., Nielsen M. The PickPocket method for predicting binding specificities for receptors based on receptor pocket similarities: application to MHC-peptide binding. Bioinformatics. 2009;25:1293–1299. doi: 10.1093/bioinformatics/btp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jojic N., Reyes-Gomez M., Heckerman D., Kadie C., Schueler-Furman O. Learning MHC I--peptide binding. Bioinformatics. 2006;22:e227–e235. doi: 10.1093/bioinformatics/btl255. [DOI] [PubMed] [Google Scholar]
- 136.Larsen M.V., Lundegaard C., Lamberth K., et al. An integrative approach to CTL epitope prediction: a combined algorithm integrating MHC class I binding, TAP transport efficiency, and proteasomal cleavage predictions. Eur. J. Immunol. 2005;35:2295–2303. doi: 10.1002/eji.200425811. [DOI] [PubMed] [Google Scholar]
- 137.Petrovsky N., Brusic V. Virtual models of the HLA class I antigen processing pathway. Methods. 2004;34:429–435. doi: 10.1016/j.ymeth.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Chowell D., Krishna S., Becker P.D., Cocita C., Shu J., Tan X., Greenberg P.D., Klavinskis L.S., Blattman J.N., Anderson K.S. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc. Natl. Acad. Sci. USA. 2015;112:E1754–E1762. doi: 10.1073/pnas.1500973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wen Y., Collier J.H. Supramolecular peptide vaccines: tuning adaptive immunity. Curr. Opin. Immunol. 2015;35:73–79. doi: 10.1016/j.coi.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bijker M.S., van den Eeden S.J., Franken K.L., Melief C.J., Offringa R., van der Burg S.H. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 141.Nijman H.W., Houbiers J.G., Vierboom M.P., et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur. J. Immunol. 1993;23:1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 142.De Bruijn M.L., Schumacher T.N., Nieland J.D., Ploegh H.L., Kast W.M., Melief C.J. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur. J. Immunol. 1991;21:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 143.Bennett S.R., Carbone F.R., Toy T., Miller J.F., Heath W.R. B cells directly tolerize CD8(+) T cells. J. Exp. Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suhrbier A., Burrows S.R., Fernan A., Lavin M.F., Baxter G.D., Moss D.J. Peptide epitope induced apoptosis of human cytotoxic T lymphocytes. Implications for peripheral T cell deletion and peptide vaccination. J. Immunol. 1993;150:2169–2178. [PubMed] [Google Scholar]
- 145.Ghaffari-Nazari H., Tavakkol-Afshari J., Jaafari M.R., Tahaghoghi-Hajghorbani S., Masoumi E., Jalali S.A. Improving Multi-Epitope Long Peptide Vaccine Potency by Using a Strategy that Enhances CD4+ T Help in BALB/c Mice. PLoS One. 2015;10:e0142563. doi: 10.1371/journal.pone.0142563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toes R.E., van der Voort E.I., Schoenberger S.P., et al. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J. Immunol. 1998;160:4449–4456. [PubMed] [Google Scholar]
- 147.Zwaveling S., Ferreira Mota S.C., Nouta J., Johnson M., Lipford G.B., Offringa R., van der Burg S.H., Melief C.J. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J. Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 148.Casares N., Lasarte J.J., de Cerio A.L., Sarobe P., Ruiz M., Melero I., Prieto J., Borras-Cuesta F. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur. J. Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::aid-immu1780>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 149.Ossendorp F., Mengede E., Camps M., Filius R., Melief C.J. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Utermohlen O., Schulze-Garg C., Warnecke G., Gugel R., Lohler J., Deppert W. Simian virus 40 large-T-antigen-specific rejection of mKSA tumor cells in BALB/c mice is critically dependent on both strictly tumor-associated, tumor-specific CD8(+) cytotoxic T lymphocytes and CD4(+) T helper cells. J. Virol. 2001;75:10593–10602. doi: 10.1128/JVI.75.22.10593-10602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Widmann C., Romero P., Maryanski J.L., Corradin G., Valmori D. T helper epitopes enhance the cytotoxic response of mice immunized with MHC class I-restricted malaria peptides. J. Immunol. Methods. 1992;155:95–99. doi: 10.1016/0022-1759(92)90275-x. [DOI] [PubMed] [Google Scholar]
- 152.Shirai M., Pendleton C.D., Ahlers J., Takeshita T., Newman M., Berzofsky J.A. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 153.Hiranuma K., Tamaki S., Nishimura Y., et al. Helper T cell determinant peptide contributes to induction of cellular immune responses by peptide vaccines against hepatitis C virus. J. Gen. Virol. 1999;80(Pt 1):187–193. doi: 10.1099/0022-1317-80-1-187. [DOI] [PubMed] [Google Scholar]
- 154.Aichele P., Hengartner H., Zinkernagel R.M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J. Exp. Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Correia-Pinto J.F., Csaba N., Schiller J.T., Alonso M.J. Chitosan-Poly (I:C)-PADRE Based Nanoparticles as Delivery Vehicles for Synthetic Peptide Vaccines. Vaccines (Basel) 2015;3:730–750. doi: 10.3390/vaccines3030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Livingston B.D., Alexander J., Crimi C., et al. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J. Immunol. 1999;162:3088–3095. [PubMed] [Google Scholar]
- 157.Wu C.Y., Monie A., Pang X., Hung C.F., Wu T.C. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J. Biomed. Sci. 2010;17:88. doi: 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li S., Peng L., Zhao W., et al. Synthetic peptides containing B- and T-cell epitope of dengue virus-2 E domain III provoked B- and T-cell responses. Vaccine. 2011;29:3695–3702. doi: 10.1016/j.vaccine.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 159.Audran R., Cachat M., Lurati F., et al. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 2005;73:8017–8026. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Welters M.J., Kenter G.G., Piersma S.J., et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 161.den Boer A.T., Diehl L., van Mierlo G.J., et al. Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J. Immunol. 2001;167:2522–2528. doi: 10.4049/jimmunol.167.5.2522. [DOI] [PubMed] [Google Scholar]
- 162.Sharma R.K., Elpek K.G., Yolcu E.S., et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–4326. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Duttagupta P.A., Boesteanu A.C., Katsikis P.D. Costimulation signals for memory CD8+ T cells during viral infections. Crit. Rev. Immunol. 2009;29:469–486. doi: 10.1615/critrevimmunol.v29.i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Keppler S.J., Rosenits K., Koegl T., Vucikuja S., Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One. 2012;7:e40865. doi: 10.1371/journal.pone.0040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Curtsinger J.M., Mescher M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Curtsinger J.M., Schmidt C.S., Mondino A., Lins D.C., Kedl R.M., Jenkins M.K., Mescher M.F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 167.Obar J.J., Jellison E.R., Sheridan B.S., et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Obar J.J., Molloy M.J., Jellison E.R., et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hornell T.M., Beresford G.W., Bushey A., Boss J.M., Mellins E.D. Regulation of the class II MHC pathway in primary human monocytes by granulocyte-macrophage colony-stimulating factor. J. Immunol. 2003;171:2374–2383. doi: 10.4049/jimmunol.171.5.2374. [DOI] [PubMed] [Google Scholar]
- 170.Toubaji A., Hill S., Terabe M., et al. The combination of GM-CSF and IL-2 as local adjuvant shows synergy in enhancing peptide vaccines and provides long term tumor protection. Vaccine. 2007;25:5882–5891. doi: 10.1016/j.vaccine.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 171.Kapoor D., Aggarwal S.R., Singh N.P., Thakur V., Sarin S.K. Granulocyte-macrophage colony-stimulating factor enhances the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J. Viral Hepat. 1999;6:405–409. doi: 10.1046/j.1365-2893.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 172.Ohno S., Okuyama R., Aruga A., Sugiyama H., Yamamoto M. Phase I trial of Wilms’ Tumor 1 (WT1) peptide vaccine with GM-CSF or CpG in patients with solid malignancy. Anticancer Res. 2012;32:2263–2269. [PubMed] [Google Scholar]
- 173.Weber J., Sondak V.K., Scotland R., et al. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected Stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 174.Zheng Q., Fan D., Gao N., et al. Evaluation of a DNA vaccine candidate expressing prM-E-NS1 antigens of dengue virus serotype 1 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in immunogenicity and protection. Vaccine. 2011;29:763–771. doi: 10.1016/j.vaccine.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 175.Raviprakash K., Ewing D., Simmons M., et al. Needle-free Biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology. 2003;315:345–352. doi: 10.1016/s0042-6822(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 176.Byrne J., Russel N.H. 2008. Haemopoiertic growth factors. [Google Scholar]
- 177.Doherty D.H., Rosendahl M.S., Smith D.J., Hughes J.M., Chlipala E.A., Cox G.N. Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor. Bioconjug. Chem. 2005;16:1291–1298. doi: 10.1021/bc050172r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhai Y., Zhou Y., Li X., Feng G. Immune-enhancing effect of nano-DNA vaccine encoding a gene of the prME protein of Japanese encephalitis virus and BALB/c mouse granulocyte-macrophage colony-stimulating factor. Mol. Med. Rep. 2015;12:199–209. doi: 10.3892/mmr.2015.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song R., Liu S., Adams R.J., Leong K.W. Enhancing efficacy of HIV gag DNA vaccine by local delivery of GM-CSF in murine and macaque models. J. Interferon Cytokine Res. 2006;26:380–389. doi: 10.1089/jir.2006.26.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dowling J.K., Mansell A. Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin. Transl. Immunology. 2016;5:e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gerard C.M., Baudson N., Kraemer K., et al. Therapeutic potential of protein and adjuvant vaccinations on tumour growth. Vaccine. 2001;19:2583–2589. doi: 10.1016/s0264-410x(00)00486-2. [DOI] [PubMed] [Google Scholar]
- 182.Fujimoto C., Nakagawa Y., Ohara K., Takahashi H. Polyriboinosinic polyribocytidylic acid [poly(I:C)]/TLR3 signaling allows class I processing of exogenous protein and induction of HIV-specific CD8+ cytotoxic T lymphocytes. Int. Immunol. 2004;16:55–63. doi: 10.1093/intimm/dxh025. [DOI] [PubMed] [Google Scholar]
- 183.Schwarz K., Storni T., Manolova V., et al. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur. J. Immunol. 2003;33:1465–1470. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- 184.Ahonen C.L., Doxsee C.L., McGurran S.M., et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wille-Reece U., Wu C.Y., Flynn B.J., Kedl R.M., Seder R.A. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J. Immunol. 2005;174:7676–7683. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 186.Welters M.J., Bijker M.S., van den Eeden S.J., et al. Multiple CD4 and CD8 T-cell activation parameters predict vaccine efficacy in vivo mediated by individual DC-activating agonists. Vaccine. 2007;25:1379–1389. doi: 10.1016/j.vaccine.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 187.Gnjatic S., Sawhney N.B., Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16:382–391. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 189.Nair-Gupta P., Baccarini A., Tung N., et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Van Hoeven N., Fox C.B., Granger B., et al. A Formulated TLR7/8 Agonist is a Flexible, Highly Potent and Effective Adjuvant for Pandemic Influenza Vaccines. Sci. Rep. 2017;7:46426. doi: 10.1038/srep46426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu Q., Egelston C., Gagnon S., et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Makela S.M., Strengell M., Pietila T.E., Osterlund P., Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 2009;85:664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]
- 193.Chiang C.Y., Huang M.H., Hsieh C.H., et al. Dengue-1 envelope protein domain III along with PELC and CpG oligodeoxynucleotides synergistically enhances immune responses. PLoS Negl. Trop. Dis. 2012;6:e1645. doi: 10.1371/journal.pntd.0001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Toussi D.N., Massari P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines (Basel) 2014;2:323–353. doi: 10.3390/vaccines2020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Steinhagen F., Kinjo T., Bode C., Klinman D.M. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brewer J.M. (How) do aluminium adjuvants work? Immunol. Lett. 2006;102:10–15. doi: 10.1016/j.imlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 197.Mannhalter J.W., Neychev H.O., Zlabinger G.J., Ahmad R., Eibl M.M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin. Exp. Immunol. 1985;61:143–151. [PMC free article] [PubMed] [Google Scholar]
- 198.Sokolova V., Westendorf A.M., Buer J., Uberla K., Epple M. The potential of nanoparticles for the immunization against viral infections. J. Mater. Chem. B Mater. Biol. Med. 2015;3:4767–4779. doi: 10.1039/c5tb00618j. [DOI] [PubMed] [Google Scholar]
- 199.Salerno E.P., Shea S.M., Olson W.C., Petroni G.R., Smolkin M.E., McSkimming C., Chianese-Bullock K.A., Slingluff C.L., Jr Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol. Immunother. 2013;62:1149–1159. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Toes R.E., Blom R.J., Offringa R., Kast W.M., Melief C.J. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J. Immunol. 1996;156:3911–3918. [PubMed] [Google Scholar]
- 201.Toes R.E., Offringa R., Blom R.J., Melief C.J., Kast W.M. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc. Natl. Acad. Sci. USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tsai T.F. Fluad®-MF59®-Adjuvanted Influenza Vaccine in Older Adults. Infect. Chemother. 2013;45:159–174. doi: 10.3947/ic.2013.45.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.O’Hagan D.T., Ott G.S., Nest G.V., Rappuoli R., Giudice G.D. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 204.O’Hagan D.T., Ott G.S., De Gregorio E., Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–4348. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 205.Saavedra D., Crombet T. CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Front. Immunol. 2017;8:269. doi: 10.3389/fimmu.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vartak A., Sucheck S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines (Basel) 2016;•••:4. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Salvador A., Igartua M., Hernandez R.M., Pedraz J.L. An overview on the field of micro- and nanotechnologies for synthetic Peptide-based vaccines. J. Drug Deliv. 2011;2011:181646. doi: 10.1155/2011/181646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gregory A.E., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burke M.L., Veer M., Pleasance J., Neeland M., Elhay M., Harrison P., Meeusen E. Innate immune pathways in afferent lymph following vaccination with poly(I:C)-containing liposomes. Innate Immun. 2014;20:501–510. doi: 10.1177/1753425913501213. [DOI] [PubMed] [Google Scholar]
- 210.Giddam A.K., Zaman M., Skwarczynski M., Toth I. Liposome-based delivery system for vaccine candidates: constructing an effective formulation. Nanomedicine (Lond.) 2012;7:1877–1893. doi: 10.2217/nnm.12.157. [DOI] [PubMed] [Google Scholar]
- 211.Watson D.S., Endsley A.N., Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Swaminathan G., Thoryk E.A., Cox K.S., et al. A Tetravalent Sub-unit Dengue Vaccine Formulated with Ionizable Cationic Lipid Nanoparticle induces Significant Immune Responses in Rodents and Non-Human Primates. Sci. Rep. 2016;6:34215. doi: 10.1038/srep34215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jones L.H. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015;7:952–960. doi: 10.1038/nchem.2396. [DOI] [PubMed] [Google Scholar]
- 214.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C., Middelberg A.P. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 215.Zhang S., Liang M., Gu W., et al. Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice. Virol. J. 2011;8:333. doi: 10.1186/1743-422X-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu Y., Zhou J., Yu Z., et al. Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties. BMC Microbiol. 2014;14:233. doi: 10.1186/s12866-014-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li N., Li N., Yi Q., et al. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials. 2014;35:9529–9545. doi: 10.1016/j.biomaterials.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 218.Babapoor S., Neef T., Mittelholzer C., et al. A Novel Vaccine Using Nanoparticle Platform to Present Immunogenic M2e against Avian Influenza Infection. Influenza Res. Treat. 2011;2011:126794. doi: 10.1155/2011/126794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wahome N., Pfeiffer T., Ambiel I., et al. Conformation-specific display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem. Biol. Drug Des. 2012;80:349–357. doi: 10.1111/j.1747-0285.2012.01423.x. [DOI] [PubMed] [Google Scholar]
- 220.Metz S.W., Tian S., Hoekstra G., et al. Precisely Molded Nanoparticle Displaying DENV-E Proteins Induces Robust Serotype-Specific Neutralizing Antibody Responses. PLoS Negl. Trop. Dis. 2016;10:e0005071. doi: 10.1371/journal.pntd.0005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hiremath J., Kang K-i., Xia M., Elaish M., Binjawadagi B., Ouyang K., Dhakal S., Arcos J., Torrelles J.B., Jiang X., Lee C.W., Renukaradhya G.J. Entrapment of H1N1 Influenza Virus Derived Conserved Peptides in PLGA Nanoparticles Enhances T Cell Response and Vaccine Efficacy in Pigs. PLoS One. 2016;11:e0151922. doi: 10.1371/journal.pone.0151922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 222.Hunsawong T., Sunintaboon P., Warit S., Thaisomboonsuk B., Jarman R.G., Yoon I.K., Ubol S., Fernandez S. A novel dengue virus serotype-2 nanovaccine induces robust humoral and cell-mediated immunity in mice. Vaccine. 2015;33:1702–1710. doi: 10.1016/j.vaccine.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 223.Hunsawong T., Sunintaboon P., Warit S., Thaisomboonsuk B., Jarman R.G., Yoon I.K., Ubol S., Fernandez S. Immunogenic Properties of a BCG Adjuvanted Chitosan Nanoparticle-Based Dengue Vaccine in Human Dendritic Cells. PLoS Negl. Trop. Dis. 2015;9:e0003958. doi: 10.1371/journal.pntd.0003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nantachit N., Sunintaboon P., Ubol S. Responses of primary human nasal epithelial cells to EDIII-DENV stimulation: the first step to intranasal dengue vaccination. Virol. J. 2016;13:142. doi: 10.1186/s12985-016-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Versiani A.F., Astigarraga R.G., Rocha E.S., et al. Multi-walled carbon nanotubes functionalized with recombinant Dengue virus 3 envelope proteins induce significant and specific immune responses in mice. J. Nanobiotechnology. 2017;15:26. doi: 10.1186/s12951-017-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tao W., Hurst B.L., Shakya A.K.U., et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antiviral Res. 2017;141:62–72. doi: 10.1016/j.antiviral.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang Y., Wang Y., Kang N., et al. Construction and Immunological Evaluation of CpG-Au@HBc Virus-Like Nanoparticles as a Potential Vaccine. Nanoscale Res. Lett. 2016;11:338. doi: 10.1186/s11671-016-1554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zimmerman D.H., Steiner H., III, Carmabula R., Talor E., Rosenthal K.S. LEAPS therapeutic vaccines as antigen specific suppressors of inflammation in infectious and autoimmune diseases. J. Vaccines Vaccin. 2012;•••:3. doi: 10.4172/2157-7560.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Charoenvit Y., Brice G.T., Bacon D., et al. A small peptide (CEL-1000) derived from the beta-chain of the human major histocompatibility complex class II molecule induces complete protection against malaria in an antigen-independent manner. Antimicrob. Agents Chemother. 2004;48:2455–2463. doi: 10.1128/AAC.48.7.2455-2463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pishraft Sabet L., Taheri T., Memarnejadian A., et al. Immunogenicity of Multi-Epitope DNA and Peptide Vaccine Candidates Based on Core, E2, NS3 and NS5B HCV Epitopes in BALB/c Mice. Hepat. Mon. 2014;14:e22215. doi: 10.5812/hepatmon.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee H.B., Piao D.C., Lee J.Y., et al. Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate. Microb. Cell Fact. 2017;16:33. doi: 10.1186/s12934-017-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang X., Xie G., Liao J., et al. Design and evaluation of a multi-epitope assembly peptide (MEAP) against herpes simplex virus type 2 infection in BALB/c mice. Virol. J. 2011;8:232. doi: 10.1186/1743-422X-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bae J., Smith R., Daley J., et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin. Cancer Res. 2012;18:4850–4860. doi: 10.1158/1078-0432.CCR-11-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kono K., Mizukami Y., Daigo Y., et al. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci. 2009;100:1502–1509. doi: 10.1111/j.1349-7006.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Suzuki H., Fukuhara M., Yamaura T., et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J. Transl. Med. 2013;11:97. doi: 10.1186/1479-5876-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Walter S., Weinschenk T., Stenzl A., et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 237.Slingluff C.L., Jr, Petroni G.R., Chianese-Bullock K.A., et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin. Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 238.Wen W., Zhang L., Peng J., et al. Identification of promiscuous HLA-DR-restricted CD4(+) T-cell epitopes on the cancer-testis antigen HCA587. Cancer Sci. 2011;102:1455–1461. doi: 10.1111/j.1349-7006.2011.01986.x. [DOI] [PubMed] [Google Scholar]
- 239.Rothman A.L. Dengue: defining protective versus pathologic immunity. J. Clin. Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ramanathan B., Poh C.L., Kirk K., McBride W.J., Aaskov J., Grollo L. Synthetic B-Cell Epitopes Eliciting Cross-Neutralizing Antibodies: Strategies for Future Dengue Vaccine. PLoS One. 2016;11:e0155900. doi: 10.1371/journal.pone.0155900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Weiskopf D., Cerpas C., Angelo M.A., et al. Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated With Distinct Patterns of Protein Targets. J. Infect. Dis. 2015;212:1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ma J.H., Yang F.R., Yu H., et al. An M2e-based synthetic peptide vaccine for influenza A virus confers heterosubtypic protection from lethal virus challenge. J. Virol. 2013;10:227. doi: 10.1186/1743-422X-10-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ichihashi T., Yoshida R., Sugimoto C., Takada A., Kajino K. Cross-protective peptide vaccine against influenza A viruses developed in HLA-A*2402 human immunity model. PLoS One. 2011;6:e24626. doi: 10.1371/journal.pone.0024626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Snyder J.T., Belyakov I.M., Dzutsev A., Lemonnier F., Berzofsky J.A. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cubillos C., de la Torre B.G., Jakab A., et al. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 2008;82:7223–7230. doi: 10.1128/JVI.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Moi M.L., Takasaki T., Kurane I. Human antibody response to dengue virus: implications for dengue vaccine design. Trop. Med. Health. 2016;44:1. doi: 10.1186/s41182-016-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Watanabe S., Chan K.W., Wang J., Rivino L., Lok S.M., Vasudevan S.G. Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. J. Virol. 2015;89:5847–5861. doi: 10.1128/JVI.00216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]