Vibrio cholerae is a natural inhabitant of aquatic ecosystems. Some strains of V. cholerae can colonize human hosts and cause cholera, a profuse watery diarrhea.
KEYWORDS: Vibrio cholerae, virulence, environment, emergence, pathogen ecology, evolution, virulence factors
ABSTRACT
Vibrio cholerae is a natural inhabitant of aquatic ecosystems. Some strains of V. cholerae can colonize human hosts and cause cholera, a profuse watery diarrhea. The major pathogenicity factors and virulence regulators of V. cholerae are encoded either in mobile genetic elements acquired in the environment (e.g., pathogenicity islands or lysogenic phages) or in the core genome. Several lines of evidence indicate that the emergence of numerous virulence traits of V. cholerae occurred in its natural environment, due to biotic and abiotic pressures. Here, we discuss the connections between the human host and the potential ecological roles of these virulence traits. Elucidating these connections will help us understand the emergence of this organism and other facultative bacterial pathogens.
TEXT
Facultative pathogens do not rely on their human hosts for survival and long-term persistence. Some members of the family of aquatic bacteria Vibrionaceae represent several distinct paradigms of facultative and emergent pathogens. Although some species, such as Vibrio vulnificus, Vibrio parahaemolyticus, or Vibrio cholerae, can cause disease in humans, they are natural inhabitants of estuarine and brackish environments and most strains are nonpathogenic (1–4). V. cholerae, the etiological agent of the severe diarrheal disease cholera, is the most widely studied pathogenic species of the Vibrionaceae. Cholera remains a major scourge in places with limited access to clean drinking water and with poor sanitation (5, 6). There have been cholera outbreaks in places as diverse as South America, the Caribbean, South Asia, Africa, and the Middle East. Although cholera cases are often unreported, there are an estimated 3 to 5 million cases per year globally (5, 6). The largest epidemic in the world is currently taking place in Yemen, where there have been over 1,000,000 suspected cholera cases (7–10).
Among the >200 known serogroups of V. cholerae, only the O1 and O139 serogroups have been associated with cholera symptoms (5, 6). Both serogroups belong to a clade of phylogenetically confined strains of V. cholerae, the pandemic genome (PG) group (11–13). To date, only strains from this group have been found to cause cholera in humans; however, other strains of V. cholerae (non-O1/non-O139) can cause gastrointestinal infections (14, 15). Numerous virulence factors of V. cholerae are encoded within mobile genetic elements and were horizontally acquired by pathogenic strains (16). For instance, cholera toxin (CT), the source of profuse watery diarrhea, is encoded within the CTXϕ lysogenic phage (17) and toxin-coregulated pilus (TCP), an essential colonization factor (18), is encoded within Vibrio pathogenicity island 1 (VPI-1) (19). However, other factors, such as N-acetylglucosamine-binding protein A (GbpA), an adhesin involved in attachment to intestinal epithelial cells, and the inner membrane-localized virulence regulator ToxR, are encoded in the core genome of both clinical and environmental strains (20, 21).
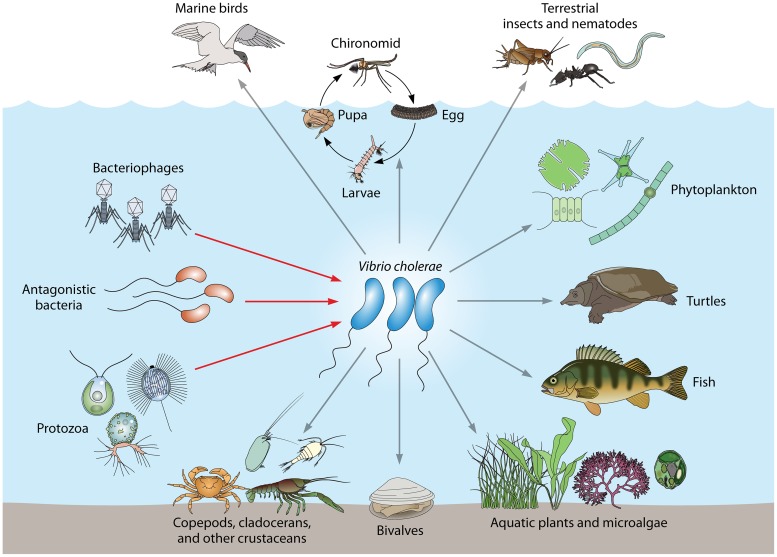
In its natural environment, V. cholerae is frequently found in association with other aquatic organisms, such as copepods and crustaceans (22–25), arthropods and chironomid egg masses (26–28), cyanobacteria (29, 30), shellfish (31, 32), waterfowl (33), and fish (34–36) (Fig. 1). In addition, V. cholerae generally faces a wide range of abiotic and biotic stressors that pose threats to its survival, such as nutrient limitations, pH changes, temperature and salinity fluctuations, grazing by protozoa, and phage predation (Fig. 1) (37–44). It appears that some of the mechanisms that allow the bacteria to colonize and to persist in their natural environment provide preadaptations for virulence in human hosts (Fig. 2).
FIG 1.
Vibrio cholerae interactions in its natural environment. The associations of V. cholerae with reservoirs and antagonistic organisms that shape its virulence potential are shown. Gray arrows indicate reservoirs, such as crustaceans, copepods, chironomid egg masses, phytoplankton, fish, turtles, aquatic birds, shellfish, and protozoa. Red arrows indicate antagonistic relationships with protists, bacteriophages, and predatory bacteria.
FIG 2.
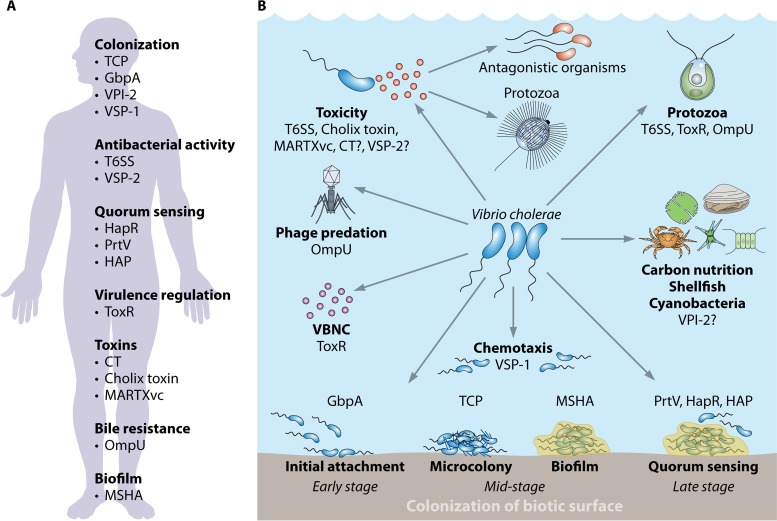
Convergence of the aquatic environment and the human host. Factors involved in Vibrio cholerae colonization, survival, and toxicity in the human host (A) and the aquatic environment (B) are shown. MSHA, mannose-sensitive hemagglutinin; TCP, toxin-coregulated pilus; GbpA, N-acetylglucosamine-binding protein A; VPI-2, Vibrio pathogenicity island 2; VSP-1, Vibrio seventh pandemic island I; HAP, hemagglutinin protease; PrtV, Vibrio metalloprotease; CT, cholera toxin; MARTXvc, multifunctional autoprocessing repeats-in-toxin; T6SS, type VI secretion system; VSP-2, Vibrio seventh pandemic island II; VBNC, viable but nonculturable. Question marks indicate hypothetical roles or connections.
Humans play an unquestionable role in the emergence and evolution of pathogenic V. cholerae, by selecting and amplifying virulent clones and their traits (44–46). In recent years, however, several virulence and colonization factors of V. cholerae have been found to play roles in the survival and persistence of the bacteria in their natural environment (Fig. 2). In this review, we discuss the environmental roles of several V. cholerae virulence factors that are involved in a wide variety of functions, such as colonization, motility, adhesion, biofilm formation, quorum sensing (QS), and toxin secretion. Overall, we highlight some of the factors that, together with host selective pressures, could have led to the emergence of pathogenic traits in V. cholerae.
TYPE VI SECRETION SYSTEM
Some non-O1/non-O139 strains of V. cholerae can cause gastrointestinal infections (14, 15). V. cholerae V52, a strain that belongs to the O37 serogroup, encodes a nanosyringe-like system termed the type VI secretion system (T6SS), which induces inflammatory diarrhea, facilitates replication of V. cholerae within the rabbit intestine, and plays a role in competing against the gut microbiota (Fig. 2A) (15, 47, 48). Since the seminal discovery by Pukatzki et al. (15), T6SSs have been described in V. cholerae O1 strains and other bacterial species (48–52). It was recently shown that T6SS inactivation attenuates V. cholerae pathogenesis in Drosophila melanogaster (53). Interestingly, the T6SS can be reactivated in the presence of commensal gut bacteria such as Acetobacter pasteurianus (53). The roles of the T6SS in intestinal colonization, virulence, and antagonistic interactions with gut microbes are governed by diverse regulatory mechanisms such as QS or carbon utilization and chitin-induced natural competency pathways (50, 52, 54, 55). Recent findings show a direct regulatory relationship between the T6SS and QS; however, the possible contribution of the T6SS to the virulence regulatory cascade needs further elucidation (see below) (48). Besides its critical role in the host, the T6SS plays a major role in the environmental survival of V. cholerae (15, 49–52). In the environment, the T6SS confers protection against predators, aids in competition against antagonistic microorganisms, and facilitates gene acquisition and horizontal gene transfer (48). The T6SS secretes self-protecting proteins (TsiV1, TsiV2, and TsiV3) and toxic effector proteins (VasX, TseL, and VgrG-3), which provide a competitive advantage over other bacterial species in the natural environment and mediate cytotoxicity to both mammalian cell lines and the soil-living amoeba Dictyostelium discoideum (Fig. 2B) (15, 49–51). Secretion of toxins and effectors by the T6SS provides a selective advantage during interspecies competition against numerous species, such as Escherichia coli and Salmonella enterica serovar Typhimurium (47). Interestingly, besides serving as a predatory killing device, the T6SS is part of the competence regulon in V. cholerae (56, 57). Borgeoud and colleagues showed that the T6SS-encoding gene cluster is under the positive control of the competence regulators TfoX and QstR and fosters horizontal gene transfer by making exogenous DNA accessible to V. cholerae cells (56, 57). All of these findings highlight the critical roles of the T6SS both in the host and in the natural environment, allowing V. cholerae to prey on other microorganisms and also acquire novel genetic traits (Fig. 2).
QUORUM SENSING
QS is a phenomenon by which bacteria monitor their cell population density through the extracellular accumulation of signaling molecules called autoinducers (58–62). Expression of hapR, a negative regulator of virulence, is repressed at low cell densities; however, during the late stages of colonization, when cell numbers are high, hapR becomes derepressed, thus negatively affecting virulence gene expression (Fig. 2A) (59, 62). The signaling molecules produced from QS at high cell densities also facilitate cellular processes that cause increased motility, repression of Vibrio polysaccharide (VPS) production, downregulation of TCP and CT, upregulation of the T6SS, and protease secretion (58–64). At high cell densities, quorum regulatory small RNAs become activated by HapR to activate T6SS genes, a phenomenon that is conserved across V. cholerae strains (65). Zheng et al. reported that the activity of the T6SS in V. cholerae is controlled by the combined actions of LuxO, a QS response regulator, and TsrA, a global regulator of V. cholerae (54). The authors found that TsrA represses the production of the T6SS substrate Hcp (54). Disruption of LuxO and TsrA activates the T6SS, thus increasing intestinal colonization in the mouse model and inflammatory diarrhea in infant rabbits (54). The influence of QS on the survivability and persistence of V. cholerae in aquatic habitats has been discussed previously (66–68). The production of HapR in the natural environment plays a role in preventing the bacteria from protozoal grazing through secretion of PrtV and, at high cell densities, regulates the transcription of hapA, which encodes a hemagglutinin protease (HAP) that cleaves biofilm proteins (58–62). PrtV plays a role in bacterial survivability against predators such as phages, protozoa, and bacteriovorous organisms such as Cafeteria roenbergensis and Tetrahymena pyriformis (69, 70). In the human host, PrtV mediates degradation of the epithelial extracellular matrix and blood components and induces an inflammatory response (Fig. 2A) (69, 71). HAP is a HapR-regulated metalloprotease that cleaves proteins in the biofilm matrix when the cell density increases, thus possibly facilitating bacterial cell dispersal in the late stages of colonization (58, 59, 72, 73). In the aquatic environment, HAP digests the gelatinous matrix of chironomid egg masses, mediates associations with cyanobacteria, and aids in dissolving organic matter, thereby releasing nutrients for V. cholerae cells (Fig. 2B) (74, 75). Recently, Kamareddine et al. reported a direct relationship between QS and the intestinal colonization of an arthropod host by V. cholerae (76). They showed that QS-mediated intestinal colonization promotes Drosophila melanogaster survival and reduction of succinate uptake by the bacteria (76).
N-ACETYLGLUCOSAMINE-BINDING PROTEIN A
In its natural environment, V. cholerae can be typically found in association with the chitinaceous exoskeleton of crustaceans (22, 37, 38). GbpA is a chitin-binding protein that is highly conserved on the core genome of members of the family Vibrionaceae (20, 77, 78). GbpA promotes adherence, colonization, and interactions with various environmental biotic surfaces, such as crustacean shells, mussel hemocytes, and bivalves and their hepatopancreatic cells (Fig. 2B) (20, 77–79). Chitin is one of the most abundant carbon sources in the aquatic environment; therefore, binding to and degrading chitin provide a competitive advantage for V. cholerae outside the human host (80, 81). Recently, Wang et al. showed active interactions of GbpA during the intestinal colonization of soft-shelled turtles (Fig. 1) (82). These findings prompted the authors to propose the turtle gut as an alternative model system for V. cholerae colonization (82). In addition, GbpA has been shown to mediate attachment to human intestinal epithelial cells and is required for successful gut colonization, which provides a direct link between environmental and host colonization of V. cholerae (20).
TOXIN-COREGULATED PILUS
TCP, a type IV pilus, is an essential colonization factor that mediates microcolony formation in the intestine (18). Microcolonies are clusters of V. cholerae cells that confer numerous properties to the bacteria (83). For instance, TCP enhances attachment to intestinal epithelial cells, facilitates bacterium-bacterium interactions by tethering cells together, mediates secretion of the colonization factor TcpF, and provides protection against antimicrobial agents (84–86). The ability to form microcolonies correlates with the ability to colonize infant mice and humans (Fig. 2A) (18, 84–86). In addition, TCP also acts as the receptor of the CTXϕ phage (17). In aquatic environments, together with other pili such as mannose-sensitive hemagglutinin (MSHA) and chitin-regulated pilus (ChiRP), TCP mediates attachment to and colonization of the chitinaceous surface of copepods (Fig. 2B) (80, 87). Furthermore, it has been shown that mutant strains that do not secrete TCP are unable to form differentiated biofilms on those surfaces, which leads to increased sensitivity to stressors (87). Overall, it appears that the ability of V. cholerae to colonize crustaceans provides the bacteria with the ability to form microcolonies in the human gut.
CHOLERA TOXIN
The production of CT in the intestine is directly responsible for the severity of the profuse diarrhea associated with cholera (5, 6). CT constitutively activates adenylate cyclase by ADP-ribosylating a coupled G-protein, which leads to increased intracellular cAMP levels (5, 6). This prompts the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel to be constitutively opened, Cl− to be effluxed with sodium, and water to follow passively (5, 6). Although a direct environmental role of CT has yet to be reported, it has been shown that, due to the lysogenic nature of the CTXϕ phage, the insertion and deletion of this phage can enable gene recombination, which leads to diversity within the pandemic strains (17, 88–90). This serves as an opportunity to increase the pathogenic potential of pandemic strains (17, 88–90). Intriguingly, V. cholerae secretes CT while associated with the cyanobacterium Rhizoclonium fontanum; the biological reason behind this remains unknown (91). Furthermore, studies have shown that CT causes protein trafficking and death of D. melanogaster (28). CT also causes disruption of exocyst trafficking, which induces the breakdown of intestinal adherens junctions in both D. melanogaster and mammalian intestines in a manner dependent on Rab11, a conserved G protein (92). These unresolved associations indicate that CT plays a role in the environment; however, more research needs to be conducted in order to establish an evolutionary origin of the toxin. It was previously hypothesized that, given its inherent function, CT might act as an osmoregulator when produced in the gills of crustaceans, providing an advantage to the crustaceans as they move into environments of increasing salinity (22, 23, 25, 93, 94). It is tempting to speculate that V. cholerae might establish a symbiotic relationship with those crustaceans, obtaining a suitable place to attach and to feed while providing the host with a powerful osmoregulator.
ToxR AND OUTER MEMBRANE PORIN U
The transmembrane transcriptional activator ToxR is encoded in the core genome of every sequenced member of the family Vibrionaceae (21, 95). It influences the expression of numerous genes (∼150 genes) involved in diverse cellular functions (96–99). In association with TcpP, ToxR is required for transcription of the gene encoding ToxT, which regulates the expression of the major pathogenicity factors of V. cholerae (e.g., TCP and CT) (21, 100–105). When V. cholerae cells are exposed to nutrient limitations at alkaline pH, ToxR is proteolyzed via a process that involves the site 2 protease RseP and is dependent on the sigma E-dependent envelope stress response (106–109). The proteolysis of ToxR is associated with the entry of V. cholerae into a dormant state called viable but nonculturable (VBNC) (106, 107). When conditions are not suitable for growth, V. cholerae enters a dormant state (VBNC) in which it loses culturability and adopts a viable coccoid form, which appears to facilitate its survival and persistence in the environment (106, 107). It seems possible that ToxR evolved as a nutrient sensor in the Vibrionaceae and was adopted by the virulence cascade as a means to detect the presence of the host, as it is intrinsically associated with nutrient abundance.
In response to the nutritional status of the cell, ToxR also reciprocally regulates the expression of the outer membrane porin genes ompU and ompT (96, 109–112). It has been shown that OmpU provides resistance to bile and organic acids and confers an advantage in intestinal colonization (113–115). OmpU also confers resistance against phage predation, facilitates survival inside the amoebal lysosome, and is involved in biofilm formation (44, 116, 117). These traits provide an evolutionary advantage in the natural environment of V. cholerae that likely led to the emergence of virulence traits (Fig. 2B) (44, 116, 117).
VIBRIO SEVENTH PANDEMIC ISLANDS
El Tor strains are responsible for the seventh and current pandemic of cholera. There are numerous traits that distinguish El Tor from classic as strains, among them the presence of two gene clusters, i.e., Vibrio seventh pandemic (VSP) islands I and II (118). Although the phenotypic functions provided by these clusters are not completely understood, recent work has revealed some roles of the VSP islands (119). Davies et al. showed that VspR, a transcriptional factor encoded in VSP-I, is regulated by the master regulator of virulence in V. cholerae, ToxT, through the small RNA TarB (119). Repression of VspR by TarB is associated with lower levels of intestinal colonization as well as decreased chemotaxis (119). Interestingly, VSP-I was also found in nonpandemic strains of V. cholerae, and it has been suggested to have an environmental role related to chemotaxis (120). It has also been reported that the presence of VSP-II in clinical and environmental strains might be associated with environmental survival and fitness of the bacteria (121–123). Comparative genomic analysis of V. cholerae El Tor N16961 and a group of V. cholerae strains that caused an outbreak in Florida associated with oyster consumption revealed the presence of a novel bacteriocin and a pyocin protein in the VSP-II elements of the V. cholerae Florida group (123). Numerous microorganisms secrete bacteriocin and other antimicrobial peptides in order to protect themselves from other microorganisms (124). Furthermore, pyocin mediates cytotoxicity toward other inhabitants of its natural environment, such as the fish pathogen Vibrio anguillarum (123, 125). Overall, these findings indicate that VSP-II might provide a competitive advantage to V. cholerae El Tor versus other microbial marine dwellers.
VIBRIO PATHOGENICITY ISLAND 2
VPI-2 is a 57.3-kb horizontally acquired region present in pandemic strains of V. cholerae (16, 126). VPI-2 includes genes for sialic acid (N-acetylneuraminic acid) utilization (16, 126). Sialic acids or nonulosonic acids constitute a family of 9-carbon amino sugars that are prevalent in mucus-rich environments (127). VPI-2 includes the genes necessary for the scavenging, transport, and catabolism of sialic acid (127, 128). NanH, a neuraminidase that allows for the scavenging of sialic acid, converts higher-order gangliosides found in the intestinal mucus into GM1 gangliosides, thus unmasking the CT receptors (129, 130). The capacity to utilize sialic acid as a carbon and energy source provides V. cholerae with a competitive advantage in the mucus-rich environment of the gut, where sialic acid availability is extensive (131). The ability to use sialic acid likely confers a competitive advantage in the natural ecosystem of V. cholerae, as the molecule is present in the mucilaginous sheath of cyanobacteria, the guts of fish, and the mucus-rich gills of oysters (31, 35, 132, 133). Furthermore, the catabolic pathways of sialic acid and N-acetylglucosamine (the monomer of chitin) converge, suggesting a synergistic relationship between the two pathways and the different hosts of V. cholerae.
MANNOSE-SENSITIVE HEMAGGLUTININ AND BIOFILM FORMATION
V. cholerae O1 El Tor and O139 strains produce a second type IV pilus, MSHA (134–137). MSHA promotes attachment of V. cholerae to abiotic surfaces and the exoskeleton of crustaceans and mediates biofilm formation (134–137). Strains with functional MSHA are able to adhere to and colonize both abiotic and biotic surfaces, independent of the surface chemistry (77, 78, 137). MSHA provides a major advantage for persistence of V. cholerae in its natural environment, due to its role in attachment to various substrates (Fig. 2B) (37, 38, 77, 78, 137). Furthermore, biofilm acts as a reservoir of VBNC Vibrio cholerae O1 cells between epidemics and promotes long-term survivability of the bacterium in the ecological niches it colonizes (138, 139). Interestingly, the role of MSHA and biofilm formation in human pathogenesis remains puzzling (140). V. cholerae cells that are ingested as part of a biofilm can successfully survive the low pH of the stomach (141). Furthermore, while forming biofilm, V. cholerae can be found in a hyperinfectious physiological state that reduces its infectious dose (142, 143). However, the inability of V. cholerae cells to repress MSHA biosynthesis prevents colonization of the mouse intestine in the presence of secretory IgA (144). Furthermore, TcpJ, a prepilin peptidase encoded within the TCP operon, cleaves the primary structural pilin of MSHA, indicating that TCP and MSHA play antagonistic roles in vivo (145). Thus, it appears that biofilm formation and MSHA biosynthesis have a precise spatiotemporal pattern that provides advantages at some specific stages during host and environmental colonization (Fig. 2) (140).
OTHER TOXINS
Cholix toxin.
Cholix toxin has been found to be cytotoxic toward eukaryotic cell lines (146, 147). The cytotoxic effect is caused by protein synthesis inhibition in the cytoplasm of the host cells (146). The inhibition can potentially damage cellular functions due to a modification of translational elongation factor 2 in the eukaryotic ribosome (146). The diversity of cholix toxin genes is high among different strains that have been isolated from both the environment and patients (148). Cholix toxin also plays a role in the environmental survivability and fitness of V. cholerae strains, as it is cytotoxic toward yeast cells, Artemia nauplii, and other crustaceans (147, 149).
Multifunctional autoprocessing repeats-in-toxin.
Multifunctional autoprocessing repeats-in-toxin (MARTXvc) has been found to enhance the colonization ability of V. cholerae in vivo (150–152). MARTXvc inhibits phagocytosis and intestinal clearance of the bacterial cells (150–152). MARTXvc has also been hypothesized to play a part in niche adaptation and to be involved in the pathogenesis of various marine organisms (150). Some members of the repeats-in-toxin (RTX) family play a defensive role in the environment as bacteriocins, indicating that these effectors evolved as a natural defense mechanism for bacteria (150, 153, 154).
CONCLUSIONS
Humans play an undisputable role in the emergence and selective amplification of virulence traits in V. cholerae (44–46). As discussed above, however, the environmental roles of some virulence factors of V. cholerae appear to confer prolonged survivability of the bacterium in the aquatic environment and also increase its the ability to colonize and infect the human host (e.g., GbpA) or express virulence factors (e.g., ToxR) (Fig. 2). It remains to be determined which other abiotic and biotic factors have driven the emergence of virulence traits of V. cholerae in its natural environment. We recently discovered that pandemic V. cholerae strains encode allelic variations in core genes in the form of virulence adaptive polymorphisms (VAPs) that enhance their pathogenic potential (117). VAPs confer preadaptations to virulence prior to the acquisition of virulence genes such as CT or TCP and are also encoded by environmental strains (117). Since some of the virulence traits of V. cholerae appear to have evolved prior to host colonization, we speculate that VAPs circulate in nonpathogenic environmental populations of V. cholerae and are selected for and enriched in the environment. Combined with the presence of selective pressures such as grazing, phage predation, and environmental fluctuations, it is possible that the bacteria are prompted to regularly adapt and to develop novel defensive strategies, which might drive the emergence of virulence properties (155–157). Multidisciplinary approaches that integrate fields such as genomics, evolutionary ecology, and pathogenesis might provide us with the knowledge and tools to understand the sets of conditions and the environmental drivers that lead to the emergence and acquisition of virulent traits in bacterial populations.
ACKNOWLEDGMENTS
We thank the reviewers for their thoughtful comments. We are grateful to C. Brandon Ogbunugafor and Trudy-Ann Grant for their insightful suggestions and for critically revising the manuscript. We apologize to our colleagues whose work was not cited due to space limitations.
This work was supported by startup funds from the Burnett School of Biomedical Sciences and funds from the Binational Science Foundation (grant BSF 2016319) to S.A.-M.
Biographies

S. Nazmus Sakib graduated with a bachelor’s degree in biotechnology and genetic engineering from Khulna University, Bangladesh. His research focused on understanding the predator-prey interactions of Vibrio cholerae with marine prokaryotic and eukaryotic organisms. Currently, he is pursuing an M.S. in biotechnology at the University of Central Florida and is interested in understanding the evolution of the pathogenesis of facultative pathogens such as Vibrio cholerae. Specifically, he studies the role and ecological dynamics of virulence adaptive polymorphisms in Vibrio cholerae and the evolution of its pathogenicity factors.

Geethika Reddi received her bachelor's degree in biomedical sciences from the University of Central Florida. Since then, she has been working as a research technician in the Moreno laboratory. Currently, her research focuses on the molecular mechanisms involved in entry into and exit from dormancy in Vibrio cholerae. She was recently accepted into the biotechnology graduate program at the University of Central Florida for fall 2018. Her future interests lie in studying infectious diseases, specifically ones that affect the human gastrointestinal tract.

Salvador Almagro-Moreno is an assistant professor of medicine in the Burnett School of Biomedical Sciences and the National Center for Integrated Coastal Research at the University of Central Florida. He was the E. E. Just Postdoctoral Fellow at Dartmouth College, in Ronald Taylor's laboratory. He obtained his Ph.D. from the National University of Ireland and wrote his dissertation in the laboratory of E. Fidelma Boyd, on the evolution of pathogenic Vibrio cholerae. The primary scientific interest of his laboratory lies at the interface between ecology and pathogenesis. The laboratory's work focuses on the emergence and evolution of pathogenic bacteria, investigating how environmental factors affect their pathogenic potential, which genetic traits are prerequisites for colonizing a new niche such as the human host, how the bacteria acquire and regulate virulence genes, and what their ecological relationships with other members of their natural environment are.
REFERENCES
- 1.Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, Sack DA, Nair GB, Mekalanos JJ. 2004. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci U S A 101:2123–2128. doi: 10.1073/pnas.0308485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen ALV, Oliver JD, DePaola A, Feil EJ, Fidelma Boyd E. 2007. Emergence of a virulent clade of Vibrio vulnificus and correlation with the presence of a 33-kilobase genomic island. Appl Environ Microbiol 73:5553–5565. doi: 10.1128/AEM.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Y, Yang X, Didelot X, Guo C, Li D, Yan Y, Zhang Y, Yuan Y, Yang H, Wang J, Wang J, Song Y, Zhou D, Falush D, Yang R. 2015. Epidemic clones, oceanic gene pools, and eco-LD in the free living marine pathogen Vibrio parahaemolyticus. Mol Biol Evol 32:1396–1410. doi: 10.1093/molbev/msv009. [DOI] [PubMed] [Google Scholar]
- 4.Rivera IN, Chun J, Huq A, Sack RB, Colwell RR. 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl Environ Microbiol 67:2421–2429. doi: 10.1128/AEM.67.6.2421-2429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 7.Qadri F, Islam T, Clemens JD. 2017. Cholera in Yemen: an old foe rearing its ugly head. N Engl J Med 377:2005–2007. doi: 10.1056/NEJMp1712099. [DOI] [PubMed] [Google Scholar]
- 8.von Seidlein L, Sack D, Azman AS, Ivers LC, Lopez AL, Deen JL. 2017. Cholera outbreak in Yemen. Lancet Gastroenterol Hepatol 2:777. doi: 10.1016/S2468-1253(17)30287-X. [DOI] [PubMed] [Google Scholar]
- 9.The Lancet Gastroenterology and Hepatology. 2017. Health catastrophe: the toll of cholera in Yemen. Lancet Gastroenterol Hepatol 2:619. doi: 10.1016/S2468-1253(17)30224-8. [DOI] [PubMed] [Google Scholar]
- 10.The Lancet. 2017. Yemen and cholera: a modern humanity test. Lancet 390:626. doi: 10.1016/S0140-6736(17)32210-9. [DOI] [PubMed] [Google Scholar]
- 11.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon Y-S, Kim DW, Lee J-H, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, Croucher NJ, Choi SY, Harris SR, Lebens M, Niyogi SK, Kim EJ, Ramamurthy T, Chun J, Wood JLN, Clemens JD, Czerkinsky C, Nair GB, Holmgren J, Parkhill J, Dougan G. 2011. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477:462–465. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher Y, Orata FD, Alam M. 2015. The out-of-the-delta hypothesis: dense human populations in low-lying river deltas served as agents for the evolution of a deadly pathogen. Front Microbiol 6:L19401. doi: 10.3389/fmicb.2015.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, Rahman MH, Heidelberg JF, Decker J, Li L, Montgomery KT, Grills G, Kucherlapati R, Mekalanos JJ. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci U S A 102:3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almagro-Moreno S, Murphy RA, Boyd EF. 2011. How genomics has shaped our understanding of the evolution and emergence of pathogenic Vibrio cholerae, p 85–99. In Fratamico P, Liu Y, Kathariou S (ed), Genomes of foodborne and waterborne pathogens. American Society of Microbiology, Washington, DC. [Google Scholar]
- 17.Waldor MK, Mekalanos JJ. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A 84:2833–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaolis DK, Johnson JA, Bailey CC, Boedeker EC, Kaper JB, Reeves PR. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci U S A 95:3134–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirn TJ, Jude BA, Taylor RK. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438:863–866. doi: 10.1038/nature04249. [DOI] [PubMed] [Google Scholar]
- 21.Miller VL, Taylor RK, Mekalanos JJ. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 22.Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huq A, West PA, Small EB, Huq MI, Colwell RR. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol 48:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Magny GC, Mozumder PK, Grim CJ, Hasan NA, Naser MN, Alam M, Sack RB, Huq A, Colwell RR. 2011. Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl Environ Microbiol 77:6125–6132. doi: 10.1128/AEM.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol 56:1977–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpern M, Broza YB, Mittler S, Arakawa E, Broza M. 2004. Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb Ecol 47:341–349. doi: 10.1007/s00248-003-2007-6. [DOI] [PubMed] [Google Scholar]
- 27.Broza M, Halpern M. 2001. Pathogen reservoirs: chironomid egg masses and Vibrio cholerae. Nature 412:40. doi: 10.1038/35083691. [DOI] [PubMed] [Google Scholar]
- 28.Purdy AE, Watnick PI. 2011. Spatially selective colonization of the arthropod intestine through activation of Vibrio cholerae biofilm formation. Proc Natl Acad Sci U S A 108:19737–19742. doi: 10.1073/pnas.1111530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam MS, Miah MA, Hasan MK, Sack RB, Albert MJ. 1994. Detection of non-culturable Vibrio cholerae O1 associated with a cyanobacterium from an aquatic environment in Bangladesh. Trans R Soc Trop Med Hyg 88:298–299. doi: 10.1016/0035-9203(94)90085-X. [DOI] [PubMed] [Google Scholar]
- 30.Epstein PR. 1993. Algal blooms in the spread and persistence of cholera. Biosystems 31:209–221. doi: 10.1016/0303-2647(93)90050-M. [DOI] [PubMed] [Google Scholar]
- 31.Twedt RM, Madden JM, Hunt JM, Francis DW, Peeler JT, Duran AP, Hebert WO, McCay SG, Roderick CN, Spite GT, Wazenski TJ. 1981. Characterization of Vibrio cholerae isolated from oysters. Appl Environ Microbiol 41:1475–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hood MA, Ness GE, Rodrick GE. 1981. Isolation of Vibrio cholerae serotype O1 from the eastern oyster, Crassostrea virginica. Appl Environ Microbiol 41:559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halpern M, Senderovich Y, Izhaki I. 2008. Waterfowl: the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog 4:e1000173. doi: 10.1371/journal.ppat.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senderovich Y, Izhaki I, Halpern M. 2010. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. 2014. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80:1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messelhäusser U, Colditz J, Thärigen D, Kleih W, Höller C, Busch U. 2010. Detection and differentiation of Vibrio spp. in seafood and fish samples with cultural and molecular methods. Int J Food Microbiol 142:360–364. doi: 10.1016/j.ijfoodmicro.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Lutz C, Erken M, Noorian P, Sun S, McDougald D. 2013. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front Microbiol 4:375. doi: 10.3389/fmicb.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almagro-Moreno S, Taylor RK. 2013. Cholera: environmental reservoirs and impact on disease transmission. Microbiol Spectrum 1:OH-0003-2012. doi: 10.1128/microbiolspec.OH-0003-2012. [DOI] [PubMed] [Google Scholar]
- 39.Sandström G, Saeed A, Abd H. 2010. Acanthamoeba polyphaga is a possible host for Vibrio cholerae in aquatic environments. Exp Parasitol 126:65–68. doi: 10.1016/j.exppara.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Thom S, Warhurst D, Drasar BS. 1992. Association of Vibrio cholerae with fresh water amoebae. J Med Microbiol 36:303–306. doi: 10.1099/00222615-36-5-303. [DOI] [PubMed] [Google Scholar]
- 41.Abd H, Weintraub A, Sandström G. 2005. Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ Microbiol 7:1003–1008. doi: 10.1111/j.1462-2920.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 42.Faruque SM, Naser IB, Islam MJ, Faruque ASG, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ. 2005. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci U S A 102:1702–1707. doi: 10.1073/pnas.0408992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faruque SM, Islam MJ, Ahmad QS, Faruque ASG, Sack DA, Nair GB, Mekalanos JJ. 2005. Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc Natl Acad Sci U S A 102:6119–6124. doi: 10.1073/pnas.0502069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seed KD, Yen M, Shapiro BJ, Hilaire IJ, Charles RC, Teng JE, Ivers LC, Boncy J, Harris JB, Camilli A, Cossart P. 2014. Evolutionary consequences of intra-patient phage predation on microbial populations. Elife 3:e03497. doi: 10.7554/eLife.03497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. 2016. Within-host evolution of bacterial pathogens. Nat Rev Microbiol 14:150–162. doi: 10.1038/nrmicro.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levade I, Terrat Y, Leducq J-B, Weil AA, Mayo-Smith LM, Chowdhury F, Khan AI, Boncy J, Buteau J, Ivers LC, Ryan ET, Charles RC, Calderwood SB, Qadri F, Harris JB, LaRocque RC, Shapiro BJ. 2017. Vibrio cholerae genomic diversity within and between patients. Microb Genom doi: 10.1099/mgen.0.000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A 107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi A, Kostiuk B, Rogers A, Teschler J, Pukatzki S, Yildiz FH. 2017. Rules of engagement: the type VI secretion system in Vibrio cholerae. Trends Microbiol 25:267–279. doi: 10.1016/j.tim.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A 106:4154–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J, Ho B, Mekalanos JJ. 2011. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS One 6:e23876. doi: 10.1371/journal.pone.0023876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van der Henst C, Scrignari T, Maclachlan C, Blokesch M. 2016. An intracellular replication niche for Vibrio cholerae in the amoeba Acanthamoeba castellanii. ISME J 10:897–910. doi: 10.1038/ismej.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa T, Sabharwal D, Bröms J, Milton DL, Sjöstedt A, Uhlin BE, Wai SN. 2012. Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains. Infect Immun 80:575–584. doi: 10.1128/IAI.05510-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fast D, Kostiuk B, Foley E, Pukatzki S. 2018. Commensal-pathogen competition impacts host viability. bioRxiv doi: 10.1101/245324. [DOI] [PMC free article] [PubMed]
- 54.Zheng J, Shin OS, Cameron DE, Mekalanos JJ. 2010. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 107:21128–21133. doi: 10.1073/pnas.1014998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watve SS, Thomas J, Hammer BK. 2015. CytR is a global positive regulator of competence, type VI secretion, and chitinases in Vibrio cholerae. PLoS One 10:e0138834. doi: 10.1371/journal.pone.0138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borgeaud S, Metzger LC, Scrignari T, Blokesch M. 2015. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science 347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 57.Veening J-W, Blokesch M. 2017. Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat Rev Microbiol 15:621–629. doi: 10.1038/nrmicro.2017.66. [DOI] [PubMed] [Google Scholar]
- 58.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu J, Mekalanos JJ. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell 5:647–656. doi: 10.1016/S1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 60.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 61.Bassler BL. 2002. Small talk: cell-to-cell communication in bacteria. Cell 109:421–424. doi: 10.1016/S0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 62.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. doi: 10.1016/S0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 63.Hammer BK, Bassler BL. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 64.Lo Scrudato M, Blokesch M. 2012. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet 8:e1002778. doi: 10.1371/journal.pgen.1002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shao Y, Bassler BL. 2014. Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae. Mol Microbiol 92:921–930. doi: 10.1111/mmi.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reidl J, Klose KE. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev 26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 67.Kierek K, Watnick PI. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl Environ Microbiol 69:5079–5088. doi: 10.1128/AEM.69.9.5079-5088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joelsson A, Liu Z, Zhu J. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun 74:1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaitkevicius K, Rompikuntal PK, Lindmark B, Vaitkevicius R, Song T, Wai SN. 2008. The metalloprotease PrtV from Vibrio cholerae. FEBS J 275:3167–3177. doi: 10.1111/j.1742-4658.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaitkevicius K, Lindmark B, Ou G, Song T, Toma C, Iwanaga M, Zhu J, Andersson A, Hammarstrom ML, Tuck S, Wai SN. 2006. A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc Natl Acad Sci U S A 103:9280–9285. doi: 10.1073/pnas.0601754103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ou G, Rompikuntal PK, Bitar A, Lindmark B, Vaitkevicius K, Wai SN, Hammarström M-L. 2009. Vibrio cholerae cytolysin causes an inflammatory response in human intestinal epithelial cells that is modulated by the PrtV protease. PLoS One 4:e7806. doi: 10.1371/journal.pone.0007806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benitez JA, Silva AJ. 2016. Vibrio cholerae hemagglutinin (HA)/protease: an extracellular metalloprotease with multiple pathogenic activities. Toxicon 115:55–62. doi: 10.1016/j.toxicon.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva AJ, Pham K, Benitez JA. 2003. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883–1891. doi: 10.1099/mic.0.26086-0. [DOI] [PubMed] [Google Scholar]
- 74.Islam MS, Goldar MM, Morshed MG, Khan MNH, Islam MR, Sack RB. 2002. Involvement of the hap gene (mucinase) in the survival of Vibrio cholerae O1 in association with the blue-green alga, Anabaena sp. Can J Microbiol 48:793–800. doi: 10.1139/w02-073. [DOI] [PubMed] [Google Scholar]
- 75.Halpern M, Gancz H, Broza M, Kashi Y. 2003. Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl Environ Microbiol 69:4200–4204. doi: 10.1128/AEM.69.7.4200-4204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamareddine L, Wong ACN, Vanhove AS, Hang S, Purdy AE, Kierek-Pearson K, Asara JM, Ali A, Morris JG Jr, Watnick PI. 2018. Activation of Vibrio cholerae quorum sensing promotes survival of an arthropod host. Nat Microbiol 3:243–252. doi: 10.1038/s41564-017-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stauder M, Huq A, Pezzati E, Grim CJ, Ramoino P, Pane L, Colwell RR, Pruzzo C, Vezzulli L. 2012. Role of GbpA protein, an important virulence-related colonization factor, for Vibrio cholerae's survival in the aquatic environment. Environ Microbiol Rep 4:439–445. doi: 10.1111/j.1758-2229.2012.00356.x. [DOI] [PubMed] [Google Scholar]
- 78.Stauder M, Vezzulli L, Pezzati E, Repetto B, Pruzzo C. 2010. Temperature affects Vibrio cholerae O1 El Tor persistence in the aquatic environment via an enhanced expression of GbpA and MSHA adhesins. Environ Microbiol Rep 2:140–144. doi: 10.1111/j.1758-2229.2009.00121.x. [DOI] [PubMed] [Google Scholar]
- 79.Bhowmick R, Ghosal A, Das B, Koley H, Saha DR, Ganguly S, Nandy RK, Bhadra RK, Chatterjee NS. 2008. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun 76:4968–4977. doi: 10.1128/IAI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. 2004. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A 101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunt DE, Gevers D, Vahora NM, Polz MF. 2008. Conservation of the chitin utilization pathway in the Vibrionaceae. Appl Environ Microbiol 74:44–51. doi: 10.1128/AEM.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Yan M, Gao H, Lu X, Kan B. 2017. Vibrio cholerae colonization of soft-shelled turtles. Appl Environ Microbiol 83:e00713-. doi: 10.1128/AEM.00713-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almagro-Moreno S, Pruss K, Taylor RK. 2015. Intestinal colonization dynamics of Vibrio cholerae. PLoS Pathog 11:e1004787. doi: 10.1371/journal.ppat.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krebs SJ, Kirn TJ, Taylor RK. 2009. Genetic mapping of secretion and functional determinants of the Vibrio cholerae TcpF colonization factor. J Bacteriol 191:3665–3676. doi: 10.1128/JB.01724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirn TJ, Bose N, Taylor RK. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol Microbiol 49:81–92. doi: 10.1046/j.1365-2958.2003.03546.x. [DOI] [PubMed] [Google Scholar]
- 86.Kirn TJ, Lafferty MJ, Sandoe CM, Taylor RK. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol Microbiol 35:896–910. doi: 10.1046/j.1365-2958.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- 87.Reguera G, Kolter R. 2005. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J Bacteriol 187:3551–3555. doi: 10.1128/JB.187.10.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mukhopadhyay AK, Chakraborty S, Takeda Y, Nair GB, Berg DE. 2001. Characterization of VPI pathogenicity island and CTXϕ prophage in environmental strains of Vibrio cholerae. J Bacteriol 183:4737–4746. doi: 10.1128/JB.183.16.4737-4746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das B, Bischerour J, Barre F-X. 2011. Molecular mechanism of acquisition of the cholera toxin genes. Indian J Med Res 133:195–200. [PMC free article] [PubMed] [Google Scholar]
- 90.Faruque SM, Mekalanos JJ. 2012. Phage-bacterial interactions in the evolution of toxigenic Vibrio cholerae. Virulence 3:556–565. doi: 10.4161/viru.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Islam MS. 1990. Increased toxin production by Vibrio cholerae O1 during survival with a green alga, Rhizoclonium fontanum, in an artificial aquatic environment. Microbiol Immunol 34:557–563. doi: 10.1111/j.1348-0421.1990.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 92.Guichard A, Cruz-Moreno B, Aguilar B, van Sorge NM, Kuang J, Kurkciyan AA, Wang Z, Hang S, Pineton de Chambrun GP, McCole DF, Watnick P, Nizet V, Bier E. 2013. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe 14:294–305. doi: 10.1016/j.chom.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huq A, Huq SA, Grimes DJ, O'Brien M, Chu KH, Capuzzo JM, Colwell RR. 1986. Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl Environ Microbiol 52:586–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reen FJ, Almagro-Moreno S, Ussery D, Boyd EF. 2006. The genomic code: inferring Vibrionaceae niche specialization. Nat Rev Microbiol 4:697–704. doi: 10.1038/nrmicro1476. [DOI] [PubMed] [Google Scholar]
- 95.Miller VL, Mekalanos JJ. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A 81:3471–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bina J, Zhu J, Dziejman M, Faruque S, Calderwood S, Mekalanos J. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc Natl Acad Sci U S A 100:2801–2806. doi: 10.1073/pnas.2628026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DiRita VJ, Neely M, Taylor RK, Bruss PM. 1996. Differential expression of the ToxR regulon in classical and E1 Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci U S A 93:7991–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Skorupski K, Taylor RK. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol 25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 99.Kazi MI, Conrado AR, Mey AR, Payne SM, Davies BW. 2016. ToxR antagonizes H-NS regulation of horizontally acquired genes to drive host colonization. PLoS Pathog 12:e1005570. doi: 10.1371/journal.ppat.1005570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DiRita VJ, Mekalanos JJ. 1991. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell 64:29–37. doi: 10.1016/0092-8674(91)90206-E. [DOI] [PubMed] [Google Scholar]
- 101.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 88:5403–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Higgins DE, DiRita VJ. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol 14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 103.Carroll PA, Tashima KT, Rogers MB, DiRita VJ, Calderwood SB. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol 25:1099–1111. doi: 10.1046/j.1365-2958.1997.5371901.x. [DOI] [PubMed] [Google Scholar]
- 104.Häse CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 95:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krukonis ES, Yu RR, DiRita VJ. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol 38:67–84. doi: 10.1046/j.1365-2958.2000.02111.x. [DOI] [PubMed] [Google Scholar]
- 106.Almagro-Moreno S, Kim TK, Skorupski K, Taylor RK. 2015. Proteolysis of virulence regulator ToxR is associated with entry of Vibrio cholerae into a dormant state. PLoS Genet 11:e1005145. doi: 10.1371/journal.pgen.1005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almagro-Moreno S, Root MZ, Taylor RK. 2015. Role of ToxS in the proteolytic cascade of virulence regulator ToxR in Vibrio cholerae. Mol Microbiol 98:963–976. doi: 10.1111/mmi.13170. [DOI] [PubMed] [Google Scholar]
- 108.Mey AR, Butz HA, Payne SM. 2015. Vibrio cholerae CsrA regulates ToxR levels in response to amino acids and is essential for virulence. mBio 6:e01064-. doi: 10.1128/mBio.01064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mey AR, Craig SA, Payne SM. 2012. Effects of amino acid supplementation on porin expression and ToxR levels in Vibrio cholerae. Infect Immun 80:518–528. doi: 10.1128/IAI.05851-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li CC, Merrell DS, Camilli A, Kaper JB. 2002. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae. Mol Microbiol 43:1577–1589. doi: 10.1046/j.1365-2958.2002.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Champion GA, Neely MN, Brennan MA, DiRita VJ. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol 23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 113.Provenzano D, Schuhmacher DA, Barker JL, Klose KE. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun 68:1491–1497. doi: 10.1128/IAI.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merrell DS, Bailey C, Kaper JB, Camilli A. 2001. The ToxR-mediated organic acid tolerance response of Vibrio cholerae requires OmpU. J Bacteriol 183:2746–2754. doi: 10.1128/JB.183.9.2746-2754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mathur J, Waldor MK. 2004. The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect Immun 72:3577–3583. doi: 10.1128/IAI.72.6.3577-3583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valeru SP, Wai SN, Saeed A, Sandström G, Abd H. 2012. ToxR of Vibrio cholerae affects biofilm, rugosity and survival with Acanthamoeba castellanii. BMC Res Notes 5:33. doi: 10.1186/1756-0500-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shapiro BJ, Levade I, Kovacikova G, Taylor RK, Almagro-Moreno S. 2016. Origins of pandemic Vibrio cholerae from environmental gene pools. Nat Microbiol 2:16240. doi: 10.1038/nmicrobiol.2016.240. [DOI] [PubMed] [Google Scholar]
- 118.Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci U S A 99:1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davies BW, Bogard RW, Young TS, Mekalanos JJ. 2012. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grim CJ, Choi J, Chun J, Jeon Y-S, Taviani E, Hasan NA, Haley B, Huq A, Colwell RR. 2010. Occurrence of the Vibrio cholerae seventh pandemic VSP-I island and a new variant. OMICS 14:1–7. doi: 10.1089/omi.2009.0087. [DOI] [PubMed] [Google Scholar]
- 121.O'Shea YA, Finnan S, Reen FJ, Morrissey JP, O'Gara F, Boyd EF. 2004. The Vibrio seventh pandemic island-II is a 26.9 kb genomic island present in Vibrio cholerae El Tor and O139 serogroup isolates that shows homology to a 43.4 kb genomic island in V. vulnificus. Microbiology 150:4053–4063. doi: 10.1099/mic.0.27172-0. [DOI] [PubMed] [Google Scholar]
- 122.Taviani E, Grim CJ, Choi J, Chun J, Haley B, Hasan NA, Huq A, Colwell RR. 2010. Discovery of novel Vibrio cholerae VSP-II genomic islands using comparative genomic analysis. FEMS Microbiol Lett 308:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haley BJ, Choi SY, Grim CJ, Onifade TJ, Cinar HN, Ben Tall D, Taviani E, Hasan NA, Abdullah AH, Carter L, Sahu SN, Kothary MH, Chen A, Baker R, Hutchinson R, Blackmore C, Cebula TA, Huq A, Colwell RR. 2014. Genomic and phenotypic characterization of Vibrio cholerae non-O1 isolates from a US Gulf Coast cholera outbreak. PLoS One 9:e86264. doi: 10.1371/journal.pone.0086264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vining LC. 1990. Functions of secondary metabolites. Annu Rev Microbiol 44:395–427. doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- 125.Michel-Briand Y, Baysse C. 2002. The pyocins of Pseudomonas aeruginosa. Biochimie 84:499–510. doi: 10.1016/S0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- 126.Jermyn WS, Boyd EF. 2002. Characterization of a novel Vibrio pathogenicity island (VPI-2) encoding neuraminidase (nanH) among toxigenic Vibrio cholerae isolates. Microbiology 148:3681–3693. doi: 10.1099/00221287-148-11-3681. [DOI] [PubMed] [Google Scholar]
- 127.Almagro-Moreno S, Boyd EF. 2010. Bacterial catabolism of nonulosonic (sialic) acid and fitness in the gut. Gut Microbes 1:45–50. doi: 10.4161/gmic.1.1.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Almagro-Moreno S, Boyd EF. 2009. Insights into the evolution of sialic acid catabolism among bacteria. BMC Evol Biol 9:118. doi: 10.1186/1471-2148-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holmgren J, Lönnroth I, Månsson J, Svennerholm L. 1975. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A 72:2520–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galen JE, Ketley JM, Fasano A, Richardson SH, Wasserman SS, Kaper JB. 1992. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect Immun 60:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Almagro-Moreno S, Boyd EF. 2009. Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infect Immun 77:3807–3816. doi: 10.1128/IAI.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halpern M, Izhaki I. 2017. Fish as hosts of Vibrio cholerae. Front Microbiol 8:282. doi: 10.3389/fmicb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Islam MS, Drasar BS, Bradley DJ. 1990. Long-term persistence of toxigenic Vibrio cholerae 01 in the mucilaginous sheath of a blue-green alga, Anabaena variabilis. J Trop Med Hyg 93:133–139. [PubMed] [Google Scholar]
- 134.Watnick PI, Fullner KJ, Kolter R. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J Bacteriol 181:3606–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marsh JW, Taylor RK. 1999. Genetic and transcriptional analyses of the Vibrio cholerae mannose-sensitive hemagglutinin type 4 pilus gene locus. J Bacteriol 181:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yildiz FH, Schoolnik GK. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci U S A 96:4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chiavelli DA, Marsh JW, Taylor RK. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl Environ Microbiol 67:3220–3225. doi: 10.1128/AEM.67.7.3220-3225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 139.Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB, Sack DA, Ahmed KU, Sadique A, Watanabe H, Grim CJ, Huq A, Colwell RR. 2007. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci U S A 104:17801–17806. doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silva AJ, Benitez JA. 2016. Vibrio cholerae biofilms and cholera pathogenesis. PLoS Negl Trop Dis 10:e0004330. doi: 10.1371/journal.pntd.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Faruque SM, Biswas K, Udden SMN, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ. 2006. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A 103:6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tamayo R, Patimalla B, Camilli A. 2010. Growth in a biofilm induces a hyperinfectious phenotype in Vibrio cholerae. Infect Immun 78:3560–3569. doi: 10.1128/IAI.00048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hsiao A, Liu Z, Joelsson A, Zhu J. 2006. Vibrio cholerae virulence regulator-coordinated evasion of host immunity. Proc Natl Acad Sci U S A 103:14542–14547. doi: 10.1073/pnas.0604650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hsiao A, Toscano K, Zhu J. 2008. Post-transcriptional cross-talk between pro- and anti-colonization pili biosynthesis systems in Vibrio cholerae. Mol Microbiol 67:849–860. doi: 10.1111/j.1365-2958.2007.06091.x. [DOI] [PubMed] [Google Scholar]
- 146.Jørgensen R, Purdy AE, Fieldhouse RJ, Kimber MS, Bartlett DH, Merrill AR. 2008. Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. J Biol Chem 283:10671–10678. doi: 10.1074/jbc.M710008200. [DOI] [PubMed] [Google Scholar]
- 147.Lugo M, Merrill A. 2015. The father, son and cholix toxin: the third member of the DT group mono-ADP-ribosyltransferase toxin family. Toxins 7:2757–2772. doi: 10.3390/toxins7082757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Awasthi SP, Asakura M, Chowdhury N, Neogi SB, Hinenoya A, Golbar HM, Yamate J, Arakawa E, Tada T, Ramamurthy T, Yamasaki S. 2013. Novel cholix toxin variants, ADP-ribosylating toxins in Vibrio cholerae non-O1/non-O139 strains, and their pathogenicity. Infect Immun 81:531–541. doi: 10.1128/IAI.00982-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Purdy A, Rohwer F, Edwards R, Azam F, Bartlett DH. 2005. A glimpse into the expanded genome content of Vibrio cholerae through identification of genes present in environmental strains. J Bacteriol 187:2992–3001. doi: 10.1128/JB.187.9.2992-3001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Satchell KJF. 2007. MARTX, multifunctional autoprocessing repeats-in-toxin toxins. Infect Immun 75:5079–5084. doi: 10.1128/IAI.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fullner KJ, Mekalanos JJ. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J 19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Satchell KJF. 2015. Multifunctional-autoprocessing repeats-in-toxin (MARTX) toxins of vibrios. Microbiol Spectr doi: 10.1128/microbiolspec.VE-0002-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Phillips KE, Satchell KJF. 2017. Vibrio vulnificus: from oyster colonist to human pathogen. PLoS Pathog 13:e1006053. doi: 10.1371/journal.ppat.1006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Linhartová I, Bumba L, Mašín J, Basler M, Osička R, Kamanová J, Procházková K, Adkins I, Hejnová-Holubová J, Sadílková L, Morová J, Šebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hahn MW, Höfle MG. 2001. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol Ecol 35:113–121. doi: 10.1111/j.1574-6941.2001.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 156.Sherr EB, Sherr BF. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308. doi: 10.1023/A:1020591307260. [DOI] [PubMed] [Google Scholar]
- 157.Erken M, Lutz C, McDougald D. 2013. The rise of pathogens: predation as a factor driving the evolution of human pathogens in the environment. Microb Ecol 65:860–868. doi: 10.1007/s00248-013-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]