Corals are responsible for creating the immense structures that are essential to reef ecosystems; unfortunately, pathogens like the bacterium Vibrio coralliilyticus can cause fatal infections of reef-building coral species. However, compared to related human pathogens, the mechanisms by which V. coralliilyticus initiates infections and locates new coral hosts are poorly understood. This study investigated the effects of chemotaxis, the directional swimming in response to chemical signals, and bacterial swimming patterns on infection of the coral Montipora capitata. Infection experiments with different mutant strains suggested that a smooth-swimming pattern resulted in hypervirulence. These results demonstrate that the role of chemotaxis in coral infection may not be as straightforward as previously hypothesized and provide valuable insight into V. coralliilyticus pathogenesis.
KEYWORDS: Vibrio coralliilyticus, chemotaxis, coral disease
ABSTRACT
Chemotaxis, the directed movement toward or away from a chemical signal, can be essential to bacterial pathogens for locating hosts or avoiding hostile environments. The coral pathogen Vibrio coralliilyticus chemotaxes toward coral mucus; however, chemotaxis has not been experimentally demonstrated to be important for virulence. To further examine this, in-frame mutations were constructed in genes predicted to be important for V. coralliilyticus chemotaxis. Most Vibrio genomes contain multiple homologs of various chemotaxis-related genes, and two paralogs of each for cheB, cheR, and cheA were identified. Based on single mutant analyses, the paralogs cheB2, cheR2, and cheA1 were essential for chemotaxis in laboratory assays. As predicted, the ΔcheA1 and ΔcheR2 strains had a smooth-swimming pattern, while the ΔcheB2 strain displayed a zigzag pattern when observed under light microscopy. However, these mutants, unlike the parent strain, were unable to chemotax toward the known attractants coral mucus, dimethylsulfoniopropionate, and N-acetyl-d-glucosamine. The ΔcheB2 strain and an aflagellate ΔfliG1 strain were avirulent to coral, while the ΔcheA1 and ΔcheR2 strains were hypervirulent (90 to 100% infection within 14 h on average) compared to the wild-type strain (66% infection within 36 h on average). Additionally, the ΔcheA1 and ΔcheR2 strains appeared to better colonize coral fragments than the wild-type strain. These results suggest that although chemotaxis may be involved with infection (the ΔcheB2 strain was avirulent), a smooth-swimming phenotype is important for bacterial colonization and infection. This study provides valuable insight into understanding V. coralliilyticus pathogenesis and how this pathogen may be transmitted between hosts.
IMPORTANCE Corals are responsible for creating the immense structures that are essential to reef ecosystems; unfortunately, pathogens like the bacterium Vibrio coralliilyticus can cause fatal infections of reef-building coral species. However, compared to related human pathogens, the mechanisms by which V. coralliilyticus initiates infections and locates new coral hosts are poorly understood. This study investigated the effects of chemotaxis, the directional swimming in response to chemical signals, and bacterial swimming patterns on infection of the coral Montipora capitata. Infection experiments with different mutant strains suggested that a smooth-swimming pattern resulted in hypervirulence. These results demonstrate that the role of chemotaxis in coral infection may not be as straightforward as previously hypothesized and provide valuable insight into V. coralliilyticus pathogenesis.
INTRODUCTION
An immense area of coral reefs is threatened or has perished from increasing water temperatures associated with global climate change (1–6), which can be further exacerbated by infections from pathogenic microorganisms (7–12). Infections by bacterial pathogens can result in tissue loss diseases that can lead to the complete mortality of coral colonies previously formed by decades of growth (13–19). After mass coral mortalities, the three-dimensional coral structure degenerates, followed by a phase shift to an environment dominated by algae and then a drop in biodiversity (20, 21). Furthermore, coral larval recruitment is inhibited by environments dominated by algae, which creates a negative feedback loop preventing reef regeneration (20, 22, 23). Therefore, climate change and infectious diseases pose serious threats to the health of coral reefs and the subsequent stability of the marine environment.
One important coral pathogen, the bacterium Vibrio coralliilyticus, has been implicated in fatal infections of multiple coral species (16, 19, 24, 25). Some of the afflicted corals are important reef-building species, such as Acropora and Montipora spp., that can make up large proportions of Pacific reefs (16, 19). Infections by V. coralliilyticus result in coral bleaching (24, 26), which is the loss of the intracellular photosynthetic algae (Symbiodinium spp.) that serve as the major energy source for many corals, or tissue lysis, which can lead to rapid mortality (16, 19, 25). A number of putative and known virulence factors used by V. coralliilyticus during the infectious process have been identified (16, 19, 26); however, less is known about what occurs during the colonization of hosts and how this pathogen initiates an infection.
Like numerous other marine bacteria, V. coralliilyticus is believed to navigate microenvironments and possibly locate new hosts through chemotaxis (27–32), the directed movement toward or away from chemical attractants or repellents, respectively. V. coralliilyticus accomplishes chemotaxis and general motility using a single polar flagellum to propel it through the marine environment (30, 33). Chemoattractants bind to chemoreceptors on the cell surface, called methyl-accepting chemotaxis proteins (MCPs), which causes the flagellum to alter between a clockwise (CW) and counterclockwise (CCW) rotation. A three-step pattern, used by monotrichous bacteria, consists of a smooth-swimming run, followed by a reverse in direction, and then a flick that changes orientation by approximately 90° caused by the flagellum alternating between a CCW and CW rotation (30, 34). The flick randomizes the new swimming direction for the constant search of nutrients without having to depend on Brownian motion to alter direction (29). However, bacteria do not usually maintain a “normal” three-step cycle, because binding of a chemoattractant, for example, will cause a signal cascade that modulates the frequency of these steps to achieve an overall movement toward a chemical source by increasing the frequency of the smooth-swimming run. Therefore, if a bacterium like V. coralliilyticus consistently senses the presence of a chemoattractant, such as coral mucus, it will maintain an overall smooth-swimming pattern until the signal diminishes.
The polar flagellum is essential for V. coralliilyticus infection, while chemotaxis has only been hypothesized to be involved. Meron et al. in 2009 demonstrated that an aflagellate V. coralliilyticus flhA mutant is unable to colonize or infect a coral host (33). Further studies have demonstrated that coral mucus and mucus components such as dimethylsulfoniopropionate (DMSP) are chemoattractants for V. coralliilyticus (31, 33). Additionally, at elevated water temperatures, the overall chemotactic response and swimming speed increased for V. coralliilyticus strain ATCC BAA-450 (35), which becomes more virulent as temperatures rise (26). Consequently, V. coralliilyticus was hypothesized to utilize chemotaxis to locate available coral hosts; however, no causal relationship between chemotaxis and host infection has yet been demonstrated.
This study examines the relationship between chemotaxis and the swimming pattern with the virulence of the coral pathogen V. coralliilyticus strain OCN008, here termed OCN008. Targeted deletions were constructed in OCN008 to create nonchemotactic (Che−) mutants that have their flagellum biased toward a CCW rotation or CW rotation or are aflagellate and which have a smooth-swimming pattern, a zigzag swimming pattern, or are nonmotile, respectively. Using the Hawaiian rice coral Montipora capitata as an infection model, the virulence of these Che− mutants were compared to that of the wild-type strain. If chemotaxis alone is important for infection, then all mutant strains should have attenuated virulence. Conversely, if the swimming pattern is more important for infection than chemotaxis, then virulence should vary between the different mutants. Finally, the aflagellate, nonmotile mutant should demonstrate that a functional flagellum is required for infection, which has been shown in other studies.
RESULTS
Identification and mutation of chemotaxis- and motility-associated genes in OCN008.
Analysis of the OCN008 genome (36) revealed that there are two paralogs for each of the genes that encode the chemotaxis-related proteins CheR, CheB, and CheA, as well as the flagellar motor switch complex protein FliG, based upon predicted protein homology. The chemotaxis- and motility-related gene paralogs were designated cheB1 (MF996552), cheB2 (MF996553), cheR1 (MF996550), cheR2 (MF996551), cheA1 (MF996554), cheA2 (MF996555), fliG1 (MF996556), and fliG2 (MF996557). BLAST analyses and protein alignments of the predicted amino acid sequences of the V. coralliilyticus paralogs revealed that CheB2, CheR2, CheA1, and FliG1 had the highest similarity (greater than 70% identity) to the CheB2, CheR2, CheA2, and FliG paralogs in Vibrio cholerae (Table 1; see Fig. S1 in the supplemental material). The OCN008 CheB1, CheR1, CheA2, and FliG2 proteins shared less than 62% homology with their respective paralogs identified in V. cholerae or Escherichia coli. Between the paralog pairs in OCN008, the CheB1-CheB2, CheR1-CheR2, CheA1-CheA2, and FliG1-FliG2 pairs shared 39%, 28%, 44%, and 30% amino acid homology between each other, respectively (Table 1). We also examined the arrangement of the corresponding genes in the genome of V. coralliilyticus. According to the OCN008 draft genome (36) and the completed genome of strain OCN014 (37), there are three general clusters of chemotaxis-related genes (arbitrarily labeled clusters I, II, and III). The paralogs cheB2 and cheA1 are in cluster I, cheR2 is in cluster II, and cheB1, cheR1, and cheA2 are in cluster III.
TABLE 1.
Percent amino acid homology between V. coralliilyticus OCN008, V. cholerae N16961, and E. coli K-12 MG1655
| Strain and protein | Accession no. | % amino acid homology with indicated protein | |||||
|---|---|---|---|---|---|---|---|
| OCN008 CheB1 | OCN008 CheB2 | N16961 CheB1 | N16961 CheB2 | N16961 CheB3 | K-12 MG1655 CheB | ||
| OCN008 CheB1 | MF996552 | 39 | 37 | 40 | 62 | 50 | |
| OCN008 CheB2 | MF996553 | 39 | 29 | 80 | 42 | 41 | |
| N16961 CheB1 | AAF94558 | 37 | 29 | 33 | 35 | 80 | |
| N16961 CheB2 | AAF95208 | 40 | 80 | 33 | 39 | 34 | |
| N16961 CheB3 | DQ899637 | 62 | 42 | 35 | 39 | 45 | |
| K-12 MG1655 CheB | AAC74953 | 50 | 41 | 80 | 34 | 45 | |
| OCN008 CheR1 | OCN008 CheR2 | N16961 CheR1 | N16961 CheR2 | N16961 CheR3 | K-12 MG1655 CheR | ||
| OCN008 CheR1 | MF996550 | 28 | 50 | 33 | 53 | 40 | |
| OCN008 CheR2 | MF996551 | 28 | 31 | 90 | 31 | 34 | |
| N16961 CheR1 | AAF94556 | 50 | 31 | 29 | 36 | 36 | |
| N16961 CheR2 | AAF95346 | 33 | 90 | 29 | 39 | 35 | |
| N16961 CheR3 | AAF96983 | 53 | 31 | 36 | 39 | 39 | |
| K-12 MG1655 CheR | AAC74954 | 40 | 34 | 36 | 35 | 39 | |
| OCN008 CheA1 | OCN008 CheA2 | N16961 CheA1 | N16961 CheA2 | N16961 CheA3 | K-12 MG1655 CheA | ||
| OCN008 CheA1 | MF996554 | 44 | 40 | 71 | 45 | 35 | |
| OCN008 CheA2 | MF996555 | 44 | 50 | 43 | 60 | 45 | |
| N16961 CheA1 | AAF94554 | 40 | 50 | 38 | 45 | 42 | |
| N16961 CheA2 | AAF95209 | 71 | 43 | 38 | 45 | 52 | |
| N16961 CheA3 | AAF96987 | 45 | 60 | 45 | 45 | 39 | |
| K-12 MG1655 CheA | AAC74958 | 35 | 45 | 42 | 52 | 39 | |
| OCN008 FliG1 | OCN008 FliG2 | N16961 FliG | K-12 MG1655 FliG | ||||
| OCN008 FliG1 | MF996556 | 30 | 86 | 30 | |||
| OCN008 FliG2 | MF996557 | 30 | 34 | 9 | |||
| N16961 FliG | AAF95277 | 86 | 34 | 33 | |||
| K-12 MG1655 FliG | AAC75006 | 30 | 9 | 33 | |||
To characterize the function of these chemotaxis- and motility-related genes, in-frame deletions were created for each gene in OCN008 to minimize the introduction of frameshift mutations or polar effects. We introduced the mutations singly, and the characterization of the resulting deletion mutant strains is described below.
Only one paralog out of each gene pair is required for chemotaxis in laboratory assays.
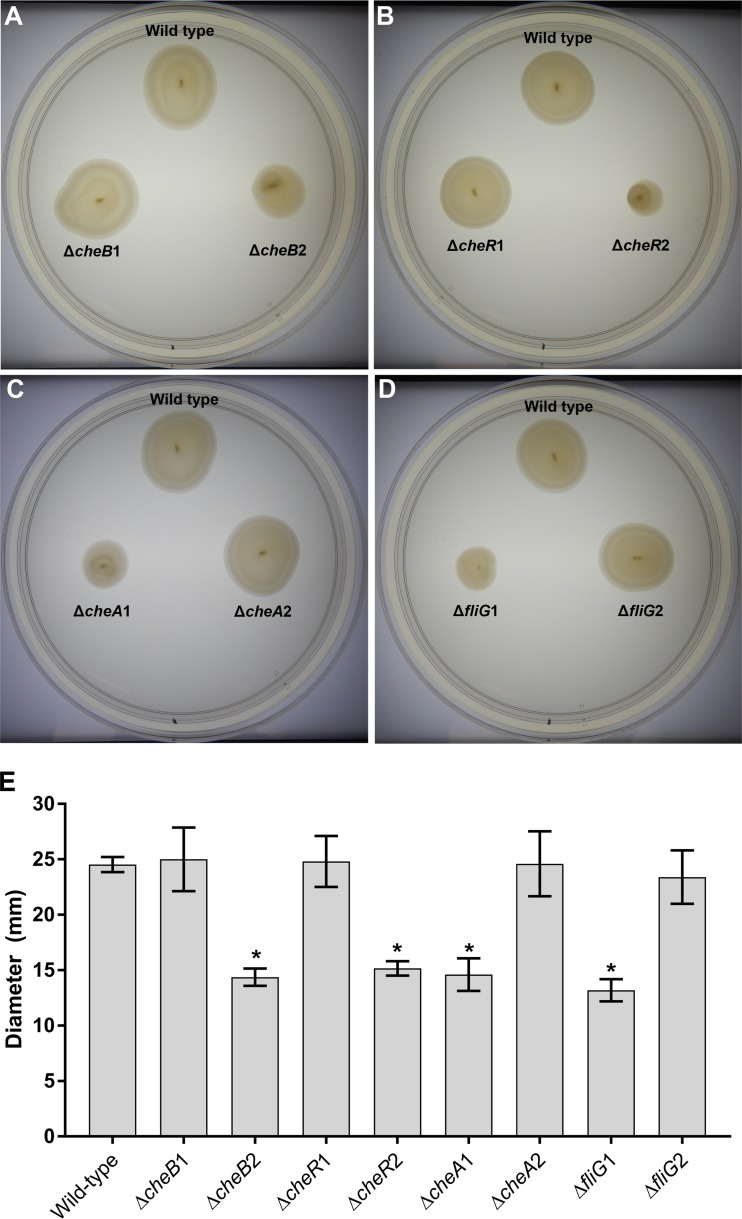
Soft glycerol artificial seawater (GASW) agar assays were used to examine the mutants for general chemotactic ability in a “rich” medium and to determine which of the V. coralliilyticus paralogs out of every pair is required for chemotaxis in this assay. Chemotaxis was observed as larger-diameter circles of bacteria swimming out of a point of inoculation forming concentric rings that resemble a distinctive bullseye pattern (38–40). The ΔcheB2, ΔcheR2, ΔcheA1, and ΔfliG1 strains all had swim circles without the bullseye pattern that were consistently smaller in diameter than wild-type strain circles (one-way analysis of variance [ANOVA], P < 0.001, n = 3), indicating that they were nonchemotactic (Che−) mutants (Fig. 1). Genetically complemented ΔcheB2, ΔcheR2, ΔcheA1, and ΔfliG1 strains (carrying the relevant wild-type copy of the gene on a plasmid) all showed restored chemotaxis (Che+) phenotypes on soft GASW agar (Fig. S2). In contrast, the ΔcheB1, ΔcheR1, ΔcheA2, and ΔfliG2 strains did not display any chemotactic defect in this assay (one-way ANOVA, P > 0.05, n = 3) (Fig. 1).
FIG 1.
Soft GASW agar assays. (A) Comparison of OCN008 to the cheB1 and cheB2 deletion mutants on soft agar; (B) comparison of OCN008 to the cheR1 and cheR2 deletion mutants on soft agar; (C) comparison of OCN008 to the cheA1 and cheA2 deletion mutants on soft agar; (D) comparison of OCN008 to the fliG1 and fliG2 deletion mutants on soft agar; (E) average diameter of swim circles of all the mutant strains tested from three independent trials. All photographs and measurements were taken after incubation at 27°C for 24 h. Error bars represent the standard deviation of the results of three independent trials, and an asterisk indicates strains with swim diameters that are significantly different from that of the wild-type strain (ANOVA, P < 0.05, n = 3 for each strain).
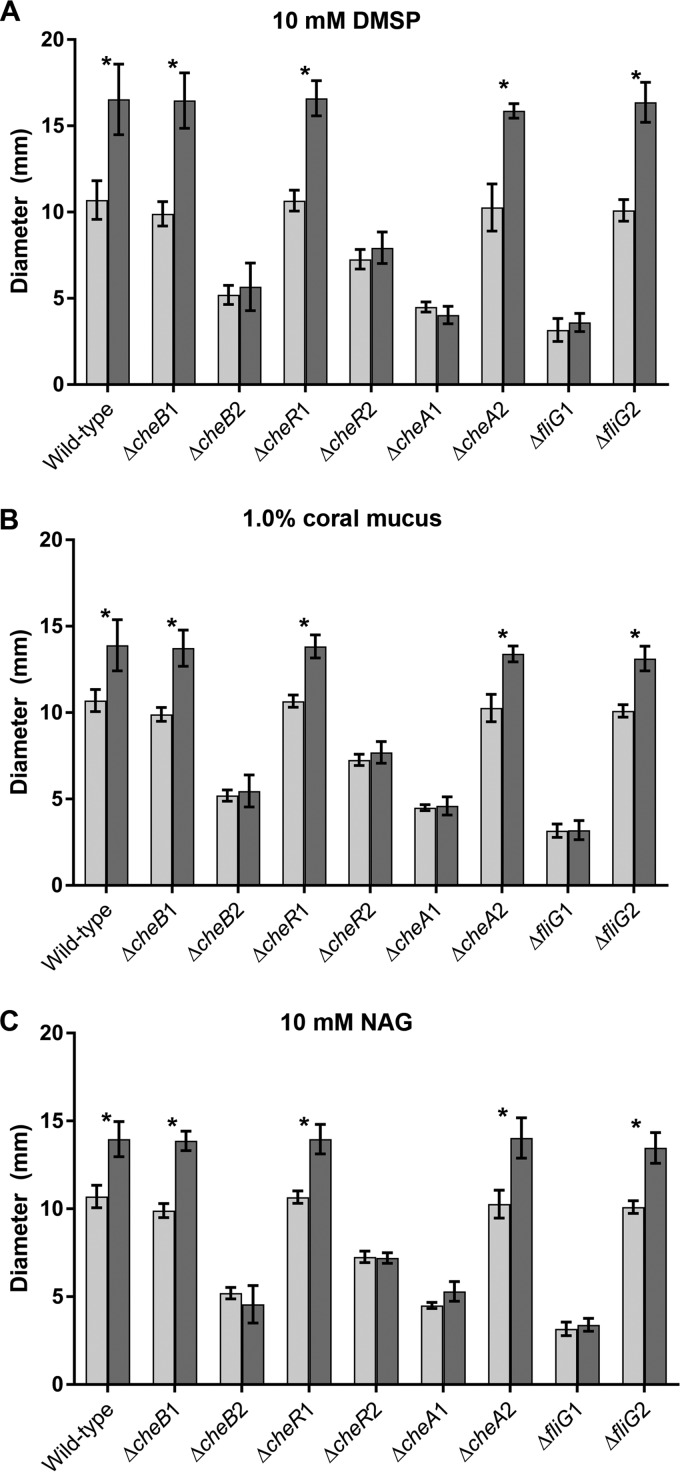
To investigate the response to select chemoattractants, additional chemotaxis assays in more defined conditions used artificial seawater (ASW) soft agar with 50 mM succinate as the sole carbon source and supplemented with 10 mM DMSP, 1.0% (vol/vol) coral mucus, or 10 mM N-acetyl-d-glucosamine (NAG) as a chemoattractant. The wild-type strain and the ΔcheB1, ΔcheR1, ΔcheA2, and ΔfliG2 phenotypically Che+ mutants all had significantly larger swim circles in soft agar supplemented with the chemoattractants than in ASW with succinate alone (Fig. 2). However, the ΔcheB2, ΔcheR2, ΔcheA1, and ΔfliG1 strains did not appear to respond to the presence of chemoattractants in the media, as indicated by the consistently small swim diameters in all medium types.
FIG 2.
Soft ASW agar assays supplemented with dimethylsulfoniopropionate (DMSP), M. capitata mucus, or N-acetyl-d-glucosamine (NAG). (A) Average diameters of the swim circles of OCN008 and investigated mutants on soft ASW agar with 50 mM succinate (light gray bars) and soft ASW agar with 50 mM succinate and 10 mM DMSP (dark gray bars); (B) average diameters of the swim circles on soft ASW agar with 50 mM succinate (light gray bars) and soft ASW agar with 50 mM succinate and 1.0% (vol/vol) coral mucus (dark gray bars); (C) average diameters of the swim circles on soft ASW agar with 50 mM succinate (light gray bars) and soft ASW agar with 50 mM succinate and 10 mM NAG (dark gray bars). Error bars represent the standard deviations of the results of three independent trials, and an asterisk indicates strains with swim circles that are significantly larger on medium with a chemoattractant than on medium with succinate only (ANOVA, P < 0.05, n = 3 for each strain).
All the mutants, except for the ΔfliG1 strain, were determined to be motile (Mot+) using light microscopy (data not shown). As predicted, the ΔcheR2 and ΔcheA1 strains displayed a smooth-swimming phenotype that was almost indistinguishable from that of the wild-type strain in rich medium, which should have a bias toward a CCW flagellar rotation. In contrast, the ΔcheB1 strain had a distinctive zigzag swimming pattern that was identical to what was described for V. cholerae CW-biased mutants (41). The CW flagellar rotation seemed to prohibit net taxis in a single direction, although this strain was still motile.
Scanning electron microscopy (SEM) confirmed that the ΔfliG2, but not the ΔfliG1, strain produces a polar flagellum, whereas the three other Che− Mot+ mutants (ΔcheB2, ΔcheR2, and ΔcheA1) had a polar flagellum and gross cellular morphologies similar to those of the wild-type strain and the V. coralliilyticus type strain ATCC BAA-450 (Fig. S3). In addition, all the mutants grew at rates comparable to that of the wild-type strain in GASW, Zobell marine broth 2216, or LBS (LB with 2% NaCl) when incubated at 27°C (data not shown).
V. coralliilyticus cheB and fliG deletion mutants are avirulent to coral.
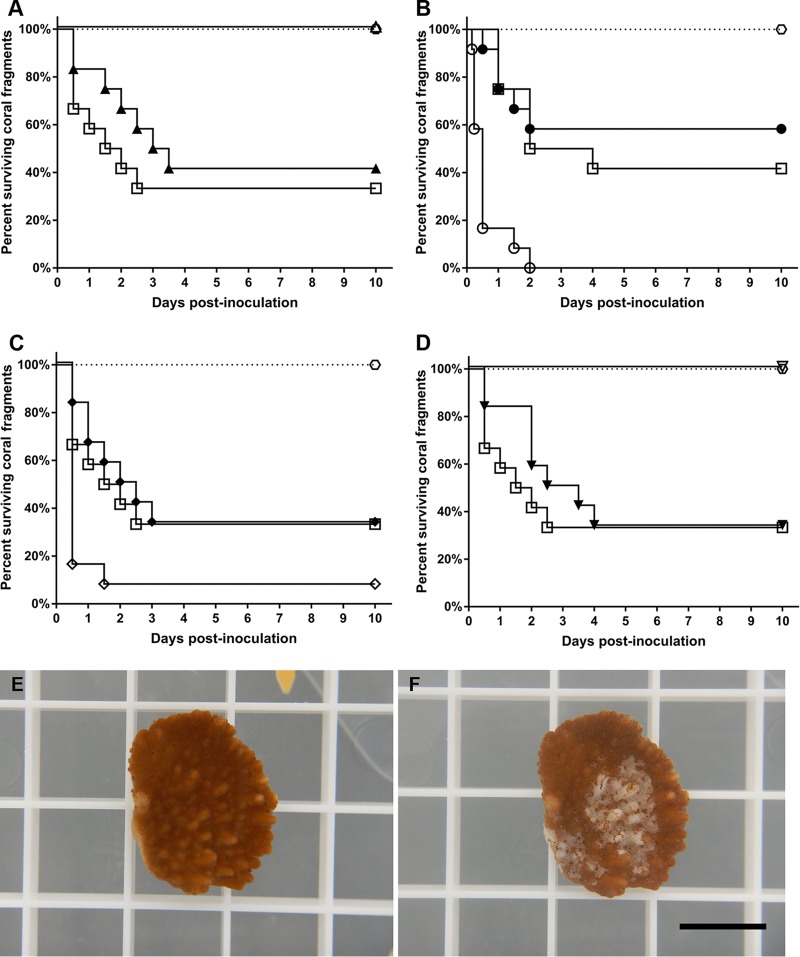
To examine the role of these chemotaxis- and motility-related genes in the virulence of OCN008, all of the deletion mutants were compared to the wild-type strain in an established infection model with the coral M. capitata (25). After exposure to the Che+ Mot+ ΔcheB1 and ΔfliG2 strains, 58% and 67% of the coral fragments developed tissue loss, respectively, which was not significantly different from the wild-type strain, which had a 67% infection rate (Mantel-Cox test, P > 0.05, n = 12) (Fig. 3A and D). In contrast, the Che− Mot+ ΔcheB2 strain was completely avirulent to the M. capitata fragments (Mantel-Cox test, P < 0.01, n = 12) (Fig. 3A). Similarly, the nonmotile ΔfliG1 mutant was avirulent (Mantel-Cox test, P < 0.01, n = 12) (Fig. 3D), consistent with previous studies with an aflagellate V. coralliilyticus flhA mutant (33). The bacterial controls, Alteromonas sp. strain OCN004 and Vibrio sp. strain HMSC5, as well as the filtered seawater (FSW) control, did not elicit signs of disease in any of the exposed M. capitata fragments over the course of the 10-day experiment (data not shown). The genetically complemented ΔcheB2 and ΔfliG1 strains caused tissue loss in 66% and 83% of the coral fragments (Fig. S4A and D), respectively, which was not significantly different from the wild-type strain, which had an infection rate of 83% (Mantel-Cox test, P > 0.05, n = 6). As expected, the ΔcheB2 and ΔfliG1 strains carrying the complementation vector with no insert (pBU246) were still avirulent to coral (Fig. S4A and D).
FIG 3.
Coral infection trials with the Che+ and Che− mutants. (A to D) Kaplan-Meier survival curves of M. capitata fragments exposed to OCN008 and mutant strains. Squares represent coral fragments exposed to the wild-type strain, while hexagons represent control corals exposed to FSW, OCN004, or HMSC5 (n = 10 for each treatment). (A) Trials with the ΔcheB1 strain (closed triangles) and the ΔcheB2 strain (open triangles); (B) trials with the ΔcheR1 strain (closed circles) and the ΔcheR2 strain (open circles); (C) trials with the ΔcheA1 strain (open diamonds) and the ΔcheA2 strain (closed diamonds); (D) trials with the ΔfliG1 strain (open triangles) and the ΔfliG2 strain (closed triangles). (E and F) Representative photo of M. capitata tissue loss from OCN008 exposure. (E) An M. capitata fragment before inoculation; (F) the same fragment as shown in panel E but 24 h postinoculation with OCN008. Scale bar, 1 cm.
V. coralliilyticus cheR and cheA deletion mutants are hypervirulent to coral.
The Che+ Mot+ ΔcheR1 and ΔcheA2 strains each had an infection rate of 67% (Fig. 3B and C), which was identical to that of the wild-type strain (Mantel-Cox test, P > 0.05, n = 12). Conversely, the Che− Mot+ ΔcheR2 and ΔcheA1 strains were both hypervirulent and caused tissue loss in 100% and 92% of the exposed coral fragments, respectively (Fig. 3B and C), which was significantly higher than that of the wild type (Mantel-Cox test, P < 0.01, n = 12). Additionally, the average time needed to initiate disease for the ΔcheR1 and ΔcheA2 strains was shorter than that for the wild-type strain. The average time from inoculation until the fragment was considered diseased (≥30% surface tissue loss) was on average 14 h for fragments exposed to the ΔcheR1 and ΔcheA2 strains, compared to 36 h for the fragments exposed to the wild-type strain. Coral fragments treated with the bacterial controls OCN004 and HMSC5 or with the FSW control did not display any obvious signs of disease (Fig. 3). The genetically complemented ΔcheR2 and ΔcheA1 strains caused tissue loss in 66% and 83% of the coral fragments (Fig. S4B and C), respectively, which was not significantly different from the wild-type strain, which had an infection rate of 83% (Mantel-Cox test, P > 0.05, n = 6). The ΔcheR2 and ΔcheA1 strains carrying the no-insert complementation vector were more virulent than the wild-type strain with regard to both the number of diseased fragments and the incubation time (Fig. S4B and C).
Virulence correlates with ability to colonize the coral host.
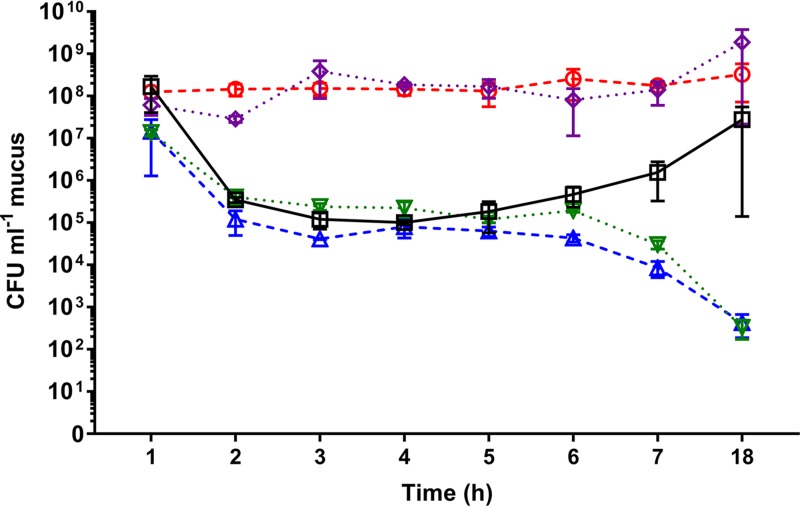
To further examine the ability of the described Che− mutants to colonize coral, the inoculum abundance of OCN008 or the chemotaxis/motility mutants in the mucus of inoculated coral fragments was measured over time using plate counts with selective medium and genetically tagged strains (Fig. 4). The tagged OCN008 strain did not seem to replicate in the tank water and stayed at a constant concentration of approximately 108 CFU/ml of tank water throughout the 18-h experiment (data not shown). The concentration of tagged OCN008 was 1.67 × 108 CFU/ml of mucus by 1 h postinoculation and then decreased over the next 4 h to 1.01 × 105 CFU/ml. After this point, the concentration of OCN008 started to increase over time and was calculated to be 2.78 × 107 CFU/ml by 18 h postinoculation. The tagged Che− mutants did not follow this “inverse bell curve” colonization pattern. Concentrations of the avirulent ΔcheB2 and ΔfliG1 strains steadily declined over time and by 18 h postinoculation had decreased to approximately 102 CFU/ml. In contrast, the ΔcheR2 and ΔcheA1 strains maintained a consistent concentration of at least 108 CFU/ml over the course of the 18-h experiment. By 18 h postinoculation, the remaining fragments that were exposed to the ΔcheR2 and ΔcheA1 strains had developed tissue loss or had no remaining healthy tissue.
FIG 4.
Colonization assays of Che− mutants and M. capitata. The average numbers of CFU per milliliter of coral mucus calculated for the wild-type, ΔcheB2, ΔcheR2, ΔcheA1, and ΔfliG1 strains reisolated at different time points postinoculation from M. capitata fragments are shown. Squares, OCN008; triangles, ΔcheB2 strain; circles, ΔcheR2 strain; diamonds, ΔcheA1 strain; inverted triangles, ΔfliG1 strain. All strains were genetically tagged with the replicative vector pBU235 for growth on selective medium. The CFU counts are averages of results from three independent trials with three replicates for each trial, and error bars represent the standard errors of the mean.
DISCUSSION
The work presented here is the first study to identify and describe chemotaxis-related genes in V. coralliilyticus and to demonstrate their role during bacterial infection of the coral M. capitata. The V. coralliilyticus genome contains multiple cheB, cheR, cheA, and fliG paralogs, and mutagenesis determined that only one paralog for each gene appears to be important for chemotactic responses to chemoattractants, such as DMSP, coral mucus, or NAG, in traditional chemotaxis soft agar assays. Furthermore, the resulting Che− mutants were either avirulent (ΔcheB2 and ΔfliG1) or hypervirulent (ΔcheR2 and ΔcheA1) in a coral infection model. Additionally, a higher concentration of the ΔcheR2 and ΔcheA1 strains than of the wild type could be reisolated from inoculated corals during early time points, whereas concentrations of the ΔcheB2 and ΔfliG1 strains diminished over time in mucus samples collected from exposed fragments.
The presence of multiple paralogs of chemotaxis-related genes in V. coralliilyticus is similar to what was found in the human pathogen Vibrio cholerae (42, 43), where Vibrio chemotaxis has been more extensively studied. In V. cholerae, there are multiple paralogs of chemotaxis-related proteins arranged in three gene clusters distributed between the organism's two chromosomes (clusters I and II on chromosome I and cluster II on chromosome II) (44). As in V. cholerae, only certain V. coralliilyticus paralogs seem to be required for chemotaxis in standard laboratory assays, which are mostly located in cluster II. For example, there are three cheA paralogs in V. cholerae, but cheA2, and not cheA1 or cheA3, is important for laboratory chemotaxis assays (39). Interestingly, all of the V. coralliilyticus paralogs required for chemotaxis in laboratory assays share the highest homology to paralogs in V. cholerae cluster II (Table 1). In contrast to V. cholerae, not all of the “functional” V. coralliilyticus paralogs are found in the same gene cluster. The V. coralliilyticus paralogs cheB2 and cheA1 are in cluster I, and cheR2 is in cluster II, while the remaining “nonfunctional” paralogs (cheB1, cheR1, and cheA2) are found in cluster III. Though the arrangements of the chemotaxis gene clusters are different, the amino acid sequence similarity and soft agar assays suggest conserved functionality between the two pathogens regarding these chemotaxis proteins. Future experiments expressing the respective paralogs in the different Vibrio species (i.e., V. cholerae paralogs expressed in V. coralliilyticus and vice versa) would shed further light on this.
Chemotaxis and swimming pattern may play a role in V. coralliilyticus infections of coral that is analogous to that in V. cholerae infections in an infant mouse or rabbit model. In previous studies on V. cholerae, mutants with a bias toward a CCW flagellar rotation, which are unable to chemotax but had a smooth-swimming phenotype, outcompeted the wild-type strain by a magnitude of 70-fold (41, 45, 46). In contrast, a V. cholerae mutant with a “locked” CW flagellar rotation was unable to chemotax, and although it was motile, its zigzag pattern of swimming prohibited net progress in any direction and this strain was found to have attenuated virulence (41). So, chemotaxis may hinder V. cholerae colonization of the gut, while the smooth-swimming phenotype benefits infection. These results mirror what was observed with V. coralliilyticus, where the cheA1 and cheR2 mutants were hypervirulent and more effectively colonized a coral host than the wild-type strain, while a cheB2 mutant was avirulent. Lastly, the nonmotile V. coralliilyticus fliG1 mutant, and the previously described flhA mutant (33), demonstrated attenuated virulence similar to that of a V. cholerae flaA mutant (45). However, it should be noted that although the data gathered on V. cholerae and V. coralliilyticus are similar, the mouse and rabbit cholera models are vastly different from the coral infection model. For V. cholerae, after this pathogen is introduced into an organism, it then must navigate its way through the gastrointestinal tract. In contrast, it is unclear if V. coralliilyticus must invade specific regions of a coral (e.g., the epidermal cells versus the gastrodermis) after initial colonization. However, the similarities between these two infection systems do invite speculation that V. coralliilyticus may cause a gastrointestinal infection as opposed to a topical infection of the epidermal tissue. Taken together, these results suggest that, as for V. cholerae, motility and a smooth-swimming pattern (CCW flagellar rotation) are important for V. coralliilyticus infections.
Although the results of V. coralliilyticus and V. cholerae chemotaxis studies are analogous, in comparison to other vibrios, they can be perceived as counterintuitive. For the fish pathogen Vibrio anguillarum and the squid symbiont Vibrio fischeri, chemotaxis is utilized by these species to find/recognize their respective hosts in the marine environment (47, 48). For example, V. anguillarum and V. fischeri cheR mutants are unable to chemotax toward host-produced mucus and are outcompeted by wild-type strains during colonization experiments in their respective systems (47, 48). Furthermore, a V. anguillarum cheR mutant had a reduction in virulence similar to that of nonmotile mutants, suggesting that chemotaxis was important for infection of fish (47). However, host-produced mucus is also a chemoattractant for V. cholerae and V. coralliilyticus (31, 33, 47), so why would certain Che− mutants have increased virulence? For V. cholerae, this pathogen is inadvertently consumed by humans from contaminated water (49), so chemotaxis is not initially involved with infection. Once inside a host, V. cholerae localizes to the lower jejunum and ileum and then, through chemotaxis, penetrates the mucus layer and localizes in intestinal crypts at the base of the villi; in contrast, Che− CCW-biased mutants accumulate in the mucus throughout the small intestine (50–53). Host defensins released in the intestinal crypts kill a large proportion of the bacteria that localize there, giving Che− strains an advantage over the cells following a chemoattractant gradient to the crypts (54). Therefore, following chemoattractant gradients in an animal host can lead to the death of the wild-type strain, while the Che− mutants are safer in the upper mucus layer and go on to more effectively colonize the length of the small intestine.
The virulence of the V. coralliilyticus ΔcheA1 and ΔcheR2 strains suggests that a smooth-swimming phenotype, as with V. cholerae, benefits infection of their specific hosts, while the role of chemotaxis is still unclear. There is some evidence that V. coralliilyticus infections may require a fomite or vector rather than free-swimming bacteria from the environment colonizing coral surfaces, which would circumvent the need for chemotaxis to locate a host. First, the infectious dose required for V. coralliilyticus, 106 to 108 CFU/ml, is magnitudes higher than one would expect of free-swimming bacteria in the ocean (16, 19, 24, 25). However, a vector/fomite could be colonized with a high concentration of V. coralliilyticus, similar to what occurs with V. cholerae colonizing zooplankton vectors that are infectious when ingested by humans (55–57). Captive M. capitata polyps have been observed to capture and ingest zooplankton like Artemia nauplii (B. Ushijima, unpublished data), which are susceptible to V. coralliilyticus infection (58–60). Second, recent experiments demonstrated that V. coralliilyticus accumulates in the polyp's gastrovascular cavity, suggesting that this pathogen may be ingested inadvertently (81, 82). Conversely, the attenuated virulence of the cheB2 mutant implies that chemotaxis might play a role in the infection process. The high inoculum concentrations used in this coral infection system do not seem to remove the need for chemotaxis, because the cheB2 mutant, which is still motile but nonchemotactic, is avirulent and unable to colonize coral effectively. However, this does highlight the complexity of this system by potentially indicating dominance of the smooth-swimming phenotype over the ability to chemotax. However, this is currently all speculation, because it is not yet understood where these different strains localize on the coral surface or if ingestion is a more ecologically relevant mode of infection by V. coralliilyticus.
The hypervirulence of the V. coralliilyticus ΔcheA1 and ΔcheR2 strains could result from an upregulation of virulence factors, from a physical advantage that the ΔcheA1 and ΔcheR2 strains have over the wild-type strain, or because chemotaxis is detrimental to colonization and results in the demise or removal of the pathogen from corals. Follow-up transcriptomic studies are required to determine if the previous scenario is plausible. Instead, the bias toward a smooth-swimming phenotype by the ΔcheA1 and ΔcheR2 strains could bestow an intrinsic physical advantage to these mutants. There is an approximately 1,000-fold decrease in the concentration of the wild-type strain at 4 h postinoculation that gradually increases after this point, while the hypervirulent mutants maintain a relatively consistent concentration over time (Fig. 4). This decrease in bacterial concentration could be attributed to the mucus sloughing and ciliary actions of the coral (62, 63); however, this would also have caused a decrease in the concentration of the ΔcheA1 and ΔcheR2 strains, which was not observed. This could indicate that these smooth-swimming strains can somehow overcome the host's ability to shed potential pathogens. There were no obvious differences in swimming speed between the ΔcheA1, ΔcheR2, and wild-type strains when viewed under light microscopy (data not shown), although it is not known if speed confers a competitive advantage to the hypervirulent mutants. However, Shapiro et al. (63) demonstrated that the cilia of the coral Pocillopora damicornis could generate vorticial flows exceeding 1 mm/s at the coral surface and extending up to 2 mm into the surrounding seawater. Furthermore, the top swimming speed of Vibrio alginolyticus, a monotrichous bacterium with a run-reverse-flick swimming pattern like V. coralliilyticus, is approximately 60 μm/s (64), roughly 16 times slower than the flows generated by P. damicornis. It is possible that the persistent run of the hypervirulent V. coralliilyticus mutants allows the cells to overcome the coral ciliary flow or that M. capitata is not able to generate flows as strong as those of P. damicornis. Nonetheless, more sophisticated quantitative measurements are required before any conclusive statements can be made.
An alternative scenario is that V. coralliilyticus cells that follow chemoattractant gradients are killed off by either host defensins or the coral microflora. Multiple studies have postulated that constituents of the healthy coral microflora produce compounds that inhibit the growth of V. coralliilyticus or may prevent the pathogen from colonizing their coral host (65–71). It is tempting to speculate that V. coralliilyticus and the coral microflora may follow the same chemoattractant gradients causing interspecific competition over the microniches present on a coral. However, there is not yet direct evidence for host-derived or microflora-produced coral defensins killing off V. coralliilyticus cells that follow a chemotactic gradient. In conclusion, this work has provided several key insights into the pathogenesis of V. coralliilyticus as well as the development of several genetic tools that are valuable for future investigations.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains are listed in Table 2. Marine bacteria were grown in a modified GASW medium (17) and incubated at 27°C, unless otherwise stated. For solid medium, 15 g/liter of agar was added prior to autoclaving. For plates or overnight growth, the GASW medium was supplemented with 50 mM Tris base (GASW-Tris), and the pH was adjusted to 8.3 using HCl prior to autoclaving to prevent acidification of the medium. All E. coli strains were grown in LB-Miller (10 g/liter tryptone, 5 g/liter yeast extract, and 10 g/liter NaCl) at 37°C, unless otherwise stated. For selection with E. coli, the following antibiotics were used at the indicated concentrations unless otherwise stated: kanamycin, 50 μg/ml; streptomycin, 25 μg/ml; spectinomycin, 50 μg/ml; and chloramphenicol, 15 μg/ml. For selection with V. coralliilyticus, the following antibiotics were used at the indicated concentrations unless otherwise stated: ampicillin, 200 μg/ml; streptomycin, 60 μg/ml; spectinomycin, 100 μg/ml; and chloramphenicol, 10 μg/ml. Growth media for E. coli auxotrophic strains were supplemented with deoxythymidine (DT) or diaminopimelate (DAP) at a final concentration of 0.3 mM as required. Arabinose-inducible expression of the ccdB gene was achieved by the addition of 0.3% l-arabinose to GASW-Tris (GASW-ARA), and expression was repressed by the addition of 1% d-glucose to LB (LB-DEX) or GASW-Tris (GASW-DEX) (72). Bacterial cultures were washed with either ASW (GASW lacking glycerol, tryptone, and yeast extract) or phosphate-buffered saline (PBS) for Vibrio and E. coli strains, respectively.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| V. coralliilyticus strains | ||
| OCN008 | Wild type; pathogen of M. capitata, isolated in Hawaii; Apr | |
| OCN008 ΔcheR1 | OCN008 with a clean, in-frame deletion of cheR1 (MF996550); Apr | This study |
| OCN008 ΔcheR2 | OCN008 with a clean, in-frame deletion of cheR2 (MF996551); Apr | This study |
| OCN008 ΔcheB1 | OCN008 with a clean, in-frame deletion of cheB1 (MF996552); Apr | This study |
| OCN008 ΔcheB2 | OCN008 with a clean, in-frame deletion of cheB2 (MF996553); Apr | This study |
| OCN008 ΔcheA1 | OCN008 with a clean, in-frame deletion of cheA1 (MF996554); Apr | This study |
| OCN008 ΔcheA2 | OCN008 with a clean, in-frame deletion of cheA2 (MF996555); Apr | This study |
| OCN008 ΔfliG1 | OCN008 with a clean, in-frame deletion of fliG1 (MF996556); Apr | This study |
| OCN008 ΔfliG2 | OCN008 with a clean, in-frame deletion of fliG2 (MF996557); Apr | This study |
| Other marine bacteria | ||
| Alteromonas sp. strain OCN004 | Nonpathogenic, negative bacterial control for coral infection experiments | 17 |
| Vibrio sp. strain HMSC5 | Nonpathogenic, negative bacterial control used for infection experiments (provided by Chris Langdon at the Hatfield Marine Science Center) | This study |
| E. coli strains | ||
| β3914 | Conjugation strain; ΔdapA::(erm-pir) Kmr Emr Tcr | 72 |
| π3813 | Conjugation strain; ΔthyA::(erm-pir) Emr | 72 |
| Plasmids | ||
| pACT3 | Ptac expression vector; Cmr | 78 |
| pEVS87 | Replicative vector; Cmr | 76 |
| pRL1383a | Replicative vector; source of Spr/Smr cassette; Spr Smr | 77 |
| pSW44226T | Suicide vector; Cmr Spr Smr | 72 |
| pBU125 | Suicide vector used to delete cheR1 in OCN008; Cmr Spr Smr | This study |
| pBU201 | Suicide vector used to delete cheR2 in OCN008; Cmr Spr Smr | This study |
| pBU219 | Suicide vector used to delete cheB1 in OCN008; Cmr Spr Smr | This study |
| pBU220 | Suicide vector used to delete cheB2 in OCN008; Cmr Spr Smr | This study |
| pBU221 | Suicide vector used to delete fliG1 in OCN008; Cmr Spr Smr | This study |
| pBU222 | Suicide vector used to delete fliG2 in OCN008; Cmr Spr Smr | This study |
| pBU224 | Suicide vector used to delete cheA1 in OCN008; Cmr Spr Smr | This study |
| pBU225 | Suicide vector used to delete cheA2 in OCN008; Cmr Spr Smr | This study |
| pBU235 | Replicative vector for tagging OCN008 strains; Cmr Spr Smr | This study |
| pBU246 | Ptac expression vector for complementing deletion mutants | This study |
| pBU300 | Vector used to complement the ΔcheR2 mutant; Cmr Spr Smr | This study |
| pBU301 | Vector used to complement the ΔcheB2 mutant; Cmr Spr Smr | This study |
| pBU302 | Vector used to complement the ΔfliG1 mutant; Cmr Spr Smr | This study |
| pBU303 | Vector used to complement the ΔcheA1 mutant; Cmr Spr Smr | This study |
Apr, resistance to ampicillin; Cmr, resistance to chloramphenicol; Spr, resistance to spectinomycin; Smr, resistance to streptomycin; Kmr, resistance to kanamycin. NCBI accession numbers for indicated genes are given in parentheses.
Protein comparison.
Protein sequences from V. coralliilyticus strain OCN008, V. cholerae El Tor strain N16961, and E. coli strain K-12 substrain MG1655 were compared using BLAST, and all accession numbers are listed in Table 1. Amino acid sequences were aligned using the BLOSUM62 exchange weights matrix within the PRofile ALIgNEment (PRALINE) multiple-sequence alignment application (73, 74).
Plasmid construction.
All plasmids are listed in Table 2, and DNA oligonucleotides are listed in Table S1 in the supplemental material. The plasmids pBU125, pBU201, pBU219, pBU220, pBU221, pBU222, pBU224, and pBU225 are suicide vectors based on pSW4426T that were used to cleanly delete all but the first 18 and last 18 nucleotides of the cheR1, cheR2, cheB1, cheB2, fliG1, fliG2, cheA1, and cheA2 coding regions in OCN008, respectively. Regions up- and downstream of cheR1, cheR2, cheB1, cheB2, fliG1, fliG2, cheA1, and cheA2 were amplified from OCN008 chromosomal DNA by PCR with the primer pairs cheR1-up-F-XbaI and cheR1-up-R-OEX and cheR1-down-F-OEX and cheR1-down-R-XbaI, cheR2-up-BcuI-F and cheR2-up-OEX-R and cheR2-down-OEX-F and cheR2-down-BcuI-Rl, cheB1-up-EcoR1-F and cheB1-up-OEX-R and cheB1-down-OEX-F and cheB1-down-BcuI-R, cheB2-up-EcoR1-F and cheB2-up-OEX-R and cheB2-down-OEX-F and cheB2-down-BcuI-R, fliG1-up-XbaI-F and fliG1-up-OEX-R and fliG1-down-OEX-F and fliG1-down-XbaI-R, fliG2-up-EcoR1-F and fliG2-up-OEX-R and fliG2-down-OEX-F and fliG2-down-XbaI-R, cheA1-up-EcoRI-F and cheA1-up-OEX-R and cheA1-down-OEX-F and cheA1-down-XbaI-R, cheA2-up-EcoRI-F and cheA2-up-OEX-R and cheA2-down-OEX-F and cheA2-down-BcuI-R, respectively. The up- and downstream fragments were fused together by overlap extension (OEX) PCR (75), digested with XbaI, and then cloned into the BcuI site of pSW4426T to create pBU125. The up- and downstream fragments were fused together by overlap extension PCR, digested with BcuI, and then cloned into the BcuI site of pSW4426T to create pBU201. Plasmids were confirmed using PCR and the primer pair pSW4426T-MCS-F and pSW4426T-MCS-R. The up- and downstream fragments were fused together by overlap extension (OEX) PCR, digested with EcoRI and BcuI, and then cloned into the EcoRI/XbaI site of pSW4426T to create pBU219. To create pBU220, the OEX PCR products were digested with EcoRI and BcuI and then cloned into the EcoRI/XbaI site of pSW4426T. To create pBU221, OEX PCR products were digested with XbaI and then cloned into the BcuI site of pSW4426T to create pBU221. To create pBU222, the OEX PCR products were digested with EcoRI and XbaI and then cloned into the EcoRI/XbaI site of pSW4426T. To create pBU222, the OEX PCR products were digested with EcoRI and XbaI and then cloned into the EcoRI/XbaI site of pSW4426T. To create pBU225, the OEX PCR products were digested with EcoRI and BcuI and then cloned into the EcoRI/XbaI site of pSW4426T.
Plasmid pBU235 is a replicative vector derived from pEVS78 (76). The spectinomycin-streptomycin resistance cassette was amplified from pRL1383a (77) by PCR with the primer pair Sp/Sm-EcoRV-F and Sp/Sm-EcoRV-R. The PCR product was digested with EcoRV and cloned into the same site in pEVS78 to create pBU235.
Plasmid pBU246 is a replicative tac promoter (Ptac) expression vector derived from pACT3 (78) to genetically complement the OCN008 deletion mutants. PCR with the primer pair pACT3-Cm-up and pACT3-Cm-down was used to remove the chloramphenicol resistance cassette from pACT3 and to linearize the plasmid. The spectinomycin-streptomycin resistance cassette was amplified from pRL1383a by PCR with the primer pair Sp/Sm-EcoRV-F and Sp/Sm-EcoRV-R. The PCR product was digested with EcoRV and ligated to the linearized pACT3 PCR product to create pBU237. The plasmid pSW4426T was used as a template for PCR with the primers pSW4499-oriT-R and pSW4499-cat-F to amplify the oriT and the chloramphenicol resistance cassette. The PCR product was then cloned into the NruI site of pBU237 to create plasmid pBU246.
The plasmids pBU300, pBU301, pBU302, and pBU303 are expression plasmids to complement the cheR2, cheB2, fliG1, and cheA1 null mutants, respectively, with a wild-type copy of the corresponding gene. OCN008 DNA was used as a template for PCR with the primers cheR2-SacI-F and cheR2-XbaI-R, cheB2-SacI-F and cheB2-XbaI-R, fliG1-SacI-F and fliG1-XbaI-R, and cheA1-SacI-F and cheA1-XbaI-R, respectively. The PCR products was digested with SacI and XbaI and then cloned into the same site in pBU246 to create pBU300, pBU301, pBU302, and pBU303.
Bacterial conjugation and mutant creation.
All V. coralliilyticus suicide vectors were introduced using triparental conjugations with E. coli as previously described but with slight modifications (19, 25). Donor and recipient strains were grown overnight with the appropriate antibiotics and DAP or DT as required. Overnight cultures were diluted 1:100 in fresh culture medium without antibiotics and grown to an optical density at 600 nm (OD600) of 0.4, and then 1 ml was washed three times with either ASW or PBS for Vibrio or E. coli strains, respectively. The strains were then combined, resuspended in ASW to a total volume of 50 μl, and spotted onto GASW-DEX plates supplemented with DAP and DT. Conjugation spots were incubated at 30°C for 15 h before being resuspended in ASW, washed three times with ASW, diluted, and plated onto GASW-DEX supplemented with chloramphenicol but lacking DAP or DT, at 27°C. Chloramphenicol-resistant colonies were streaked for isolation on GASW-DEX with spectinomycin and streptomycin, and then colonies were screened for the presence of the suicide vector integrated into the chromosome using colony PCR and the primers pSW4499-cat-F and pSW4499-oriT-R. Colonies of V. coralliilyticus with the integrated plasmid were grown for 15 h in GASW-DEX broth. Cultures were washed with ASW three times, diluted, and plated onto GASW-ARA to isolate mutants with a clean deletion of the target gene. Mutants were confirmed using PCR and primers specific to the gene being mutated.
Chemotaxis assays.
GASW or ASW soft agar plates were used to assess chemotactic behavior using a modified version of a previously described protocol (43, 79, 80). For soft agar plates, GASW or ASW broth was supplemented with agarose at a final concentration of 0.3% (wt/vol) before autoclaving. ASW soft agar plates were supplemented with succinate as the sole carbon source at a final concentration of 50 mM (43). Some soft agar plates were supplemented with the known V. coralliilyticus chemoattractant DMSP (10 mM final concentration) or coral mucus (1.0%, wt/vol) (31, 33). Coral mucus was collected from M. capitata fragments originating from a different colony for each experimental replicate to control for intraspecies differences. After collection, fragments were placed into plastic sample bags without seawater, transferred to a sterile 50-ml conical centrifuge tube, centrifuged for 15 min at 200 × g, flash frozen in liquid nitrogen, and then stored at −80°C until use. For use, coral mucus was thawed at 30°C and then added to molten medium previously cooled to 40°C before pouring. Additionally, some plates were supplemented with NAG (10 mM, final concentration), a known chemoattractant for multiple Vibrio spp. as a control. Plates were made fresh for each experiment and used 24 h after pouring. Soft agar plates were inoculated with a sterile toothpick used to touch two or three bacterial colonies on a GASW plate and stabbing the toothpick straight down into the agar. Plates were not turned over and were incubated at 25°C for 24 h for GASW-based plates or 48 h for ASW-based plates. Plates were measured by hand after incubation, and measurements were then analyzed in GraphPad Prism (v7.03). Light microscopy of mutants used overnight cultures of each strain diluted 1:100 in fresh medium, and the cultures were observed using a hanging-drop slide. Electron microscopy was performed at the OSU Electron Microscopy Facility (http://emfacility.science.oregonstate.edu/) using their standard protocols for imaging bacterial cells.
Corals and infection experiments.
Montipora capitata fragments were collected under special activities permit 2018-03 issued by the Hawaii Department of Land and Natural Resources, Division of Aquatic Resources, by authorized individuals. Coral fragments roughly 3 by 3 cm in size were collected from colonies on the fringing reef surrounding the island Moku o Loe in Kāneohe Bay, Hawaii. Corals were allowed to recover in a flowthrough seawater table at ambient light and temperature for at least 2 days before being used in infection experiments.
Infection experiments were conducted as previously described (25). Briefly, individual coral fragments were housed in 3 liters of seawater filtered previously through a 0.2-μm pore membrane filter (FSW), maintained at 27°C, and with ambient lighting at the University of Hawaii at Mānoa. Infection trials utilized a block design with fragments from the same colony exposed to FSW, control bacterium OCN004, control bacterium HMSC5, the wild-type strain (OCN008), or an experimental treatment.
To prepare inocula for infection trials, an overnight culture of the desired strain in GASW-Tris was diluted to an OD600 of approximately 0.03 in GASW. Cultures were grown to an OD600 of 1.6 to 1.8 and were then washed with FSW. For every inoculum, each tank was inoculated to a final concentration of 108 CFU/ml of tank water. Corals were monitored daily, and individual experiments were run for a maximum of 10 days. For complementation experiments, strains harboring an expression plasmid were grown with the appropriate antibiotics for the overnight culture and then subcultured into GASW without antibiotics but supplemented with isopropyl-β-d-1-thiogalactopyranoside (IPTG) at a final concentration of 5 mM.
Colonization experiments.
Colonization experiments were conducted in a manner similar to that of the infection experiments but with some modifications. For each treatment, eight coral fragments from the same colony were housed in a 9.46-liter aquarium filled with 8.5 liters of FSW. Coral fragments were trimmed to the same approximate size, taking into consideration the number of cut and growing edges, surface morphology, and thickness. All the bacterial strains for the colonization experiments were genetically tagged with the replicative vector pBU235 for selective isolation from coral mucus. At each time point, a fragment was removed from each tank, placed individually into a sterile 50-ml conical centrifuge tube, and then centrifuged at 200 × g for 10 min to remove the mucus. Mucus samples were diluted in autoclaved FSW, and then dilutions were plated onto LB supplemented with NaCl (final concentration, 2.0%), spectinomycin, streptomycin, and ampicillin. Plates were incubated at 27°C for 24 h before colonies were counted to calculate the bacterial concentration. Inoculum concentrations were analyzed using Microsoft Excel and GraphPad Prism (v7.03).
Supplementary Material
ACKNOWLEDGMENTS
We thank Fenny Cox and Silvia Beurmann for coral collections, Kuulei Rodgers for the use of water tables for healthy coral, Stuart Donachie for access to facilities at the University of Hawaii at Mānoa, and the Kewalo Marine Laboratories for the use of their filtered seawater system. The additional control strain was graciously provided by Chris Langdon at the Hatfield Marine Science Center.
This work was supported by internal funds awarded to C.C.H. from the College of Veterinary Medicine.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00791-17.
REFERENCES
- 1.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus A, Overstreet RM, Porter JW, Smith GW, Vasta GR. 1999. Emerging marine diseases—climate links and anthropogenic factors. Science 285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 2.Burge CA, Mark Eakin C, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, Petes LE, Prager KC, Weil E, Willis BL, Ford SE, Harvell CD. 2014. Climate change influences on marine infectious diseases: implications for management and society. Annu Rev Mar Sci 6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- 3.Maynard J, van Hooidonk R, Eakin CM, Puotinen M, Garren M, Williams G, Heron SF, Lamb J, Weil E, Willis B, Harvell CD. 2015. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat Clim Change 5:688–694. doi: 10.1038/nclimate2625. [DOI] [Google Scholar]
- 4.Great Barrier Reef Marine Park Authority. 2016. Interim report on the environmental impacts of the 2016 coral bleaching event. Great Barrier Reef Marine Park Authority, Townsville, Queensland, Australia. [Google Scholar]
- 5.Couch CS, Burns JHR, Liu G, Steward K, Gutlay TN, Kenyon J, Eakin CM, Kosaki RK. 2017. Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS One 12:e0185121. doi: 10.1371/journal.pone.0185121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. 2017. Global warming and recurrent mass bleaching of corals. Nature 543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 7.Harvell D, Kim K, Quirolo C, Weir J, Smith G. 2001. Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). Hydrobiologia 460:97–104. [Google Scholar]
- 8.Miller J, Waara R, Muller E, Rogers C. 2006. Coral bleaching and disease combine to cause extensive mortality on reefs in US Virgin Islands. Coral Reefs 25:418–418. doi: 10.1007/s00338-006-0125-6. [DOI] [Google Scholar]
- 9.Cróquer A, Weil E. 2009. Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Dis Aquat Organ 87:33–43. doi: 10.3354/dao02164. [DOI] [PubMed] [Google Scholar]
- 10.Miller J, Muller E, Rogers C, Waara R, Atkinson A, Whelan KRT, Patterson M, Witcher B. 2009. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 28:925–937. doi: 10.1007/s00338-009-0531-7. [DOI] [Google Scholar]
- 11.Williams GJ, Knapp IS, Work TM, Conklin EJ. 2011. Outbreak of Acropora white syndrome following a mild bleaching event at Palmyra Atoll, Northern Line Islands, Central Pacific. Coral Reefs 30:621. doi: 10.1007/s00338-011-0762-2. [DOI] [Google Scholar]
- 12.Correa AMS, Ainsworth TD, Rosales SM, Thurber AR, Butler CR, Vega Thurber RL. 2016. Viral outbreak in corals associated with an in situ bleaching event: atypical Herpes-like viruses and a new Megavirus infecting symbiodinium. Front Microbiol 7:127. doi: 10.3389/fmicb.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci U S A 99:8725. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson LL, Goldberg WM, Carlton RG, Halas JC. 1998. Coral disease outbreak in the Florida Keys: plague type II. Int J Trop Biol Conserv 46:187–198. [Google Scholar]
- 15.Thompson F, Barash Y, Sawabe T, Sharon G, Swings J, Rosenberg E. 2006. Thalassomonas loyana sp. nov., a causative agent of the white plague-like disease of corals on the Eilat coral reef. Int J Syst Evol Microbiol 56:365–368. doi: 10.1099/ijs.0.63800-0. [DOI] [PubMed] [Google Scholar]
- 16.Sussman M, Willis BL, Victor S, Bourne DG, Ahmed N. 2008. Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3:e2393. doi: 10.1371/journal.pone.0002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ushijima B, Smith A, Aeby GS, Callahan SM. 2012. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One 7:e46717. doi: 10.1371/journal.pone.0046717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown T, Bourne D, Rodriguez-Lanetty M. 2013. Transcriptional activation of c3 and hsp70 as part of the immune response of Acropora millepora to bacterial challenges. PLoS One 8:e67246. doi: 10.1371/journal.pone.0067246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ushijima B, Videau P, Poscablo D, Stengel JW, Beurmann S, Burger AH, Aeby GS, Callahan SM. 2016. Mutation of the toxR or mshA genes from Vibrio coralliilyticus strain OCN014 reduces infection of the coral Acropora cytherea. Environ Microbiol 18:4055–4067. doi: 10.1111/1462-2920.13428. [DOI] [PubMed] [Google Scholar]
- 20.Hughes TP. 1994. Catastrophes, phase shifts. and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 21.McManus JW, Polsenberg JF. 2004. Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr 60:263–279. doi: 10.1016/j.pocean.2004.02.014. [DOI] [Google Scholar]
- 22.McCook L, Jompa J, Diaz-Pulido G. 2001. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19:400–417. doi: 10.1007/s003380000129. [DOI] [Google Scholar]
- 23.Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS. 2006. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117. doi: 10.3354/meps323107. [DOI] [Google Scholar]
- 24.Ben-Haim Y, Rosenberg E. 2002. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar Biol 141:47–55. doi: 10.1007/s00227-002-0797-6. [DOI] [Google Scholar]
- 25.Ushijima B, Videau P, Burger AH, Shore-Maggio A, Runyon CM, Sudek M, Aeby GS, Callahan SM. 2014. Vibrio coralliilyticus strain OCN008 is an etiological agent of acute Montipora white syndrome. Appl Environ Microbiol 80:2102–2109. doi: 10.1128/AEM.03463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Haim Y, Zicherman-Keren M, Rosenberg E. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69:4236–4242. doi: 10.1128/AEM.69.7.4236-4242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altindal T, Xie L, Wu X-L. 2011. Implications of three-step swimming patterns in bacterial chemotaxis. Biophys J 100:32–41. doi: 10.1016/j.bpj.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stocker R. 2012. Marine microbes see a sea of gradients. Science 338:628–633. doi: 10.1126/science.1208929. [DOI] [PubMed] [Google Scholar]
- 29.Stocker R, Seymour JR. 2012. Ecology and physics of bacterial chemotaxis in the ocean. Microbiol Mol Biol Rev 76:792–812. doi: 10.1128/MMBR.00029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winn KM, Bourne DG, Mitchell JG. 2013. Vibrio coralliilyticus search patterns across an oxygen gradient. PLoS One 8:e67975. doi: 10.1371/journal.pone.0067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garren M, Son K, Raina J-B, Rusconi R, Menolascina F, Shapiro OH, Tout J, Bourne DG, Seymour JR, Stocker R. 2014. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. ISME J 8:999–1007. doi: 10.1038/ismej.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tout J, Jeffries TC, Petrou K, Tyson GW, Webster NS, Garren M, Stocker R, Ralph PJ, Seymour JR. 2015. Chemotaxis by natural populations of coral reef bacteria. ISME J 9:1764–1777. doi: 10.1038/ismej.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E, Greenberg EP, Banin E. 2009. Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl Environ Microbiol 75:5704–5707. doi: 10.1128/AEM.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Z-Y, Hu C-Q, Chen C, Zhang L-P, Ren C-H. 2005. Investigation of seven Vibrio virulence genes among Vibrio alginolyticus and Vibrio parahaemolyticus strains from the coastal mariculture systems in Guangdong, China. Lett Appl Microbiol 41:202–207. doi: 10.1111/j.1472-765X.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 35.Garren M, Son K, Tout J, Seymour JR, Stocker R. 2016. Temperature-induced behavioral switches in a bacterial coral pathogen. ISME J 10:1363–1372. doi: 10.1038/ismej.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ushijima B, Videau P, Aeby GS, Callahan SM. 2013. Draft genome sequence of Vibrio coralliilyticus strain OCN008, isolated from Kane'ohe Bay, Hawai'i. Genome Announc 1:e00786-13. doi: 10.1128/genomeA.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ushijima B, Videau P, Poscablo D, Vine V, Salcedo M, Aeby G, Callahan SM. 2014. Complete genome sequence of Vibrio coralliilyticus strain OCN014, isolated from a diseased coral at Palmyra Atoll. Genome Announc 2:e01318-14. doi: 10.1128/genomeA.01318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe AJ, Berg HC. 1989. Migration of bacteria in semisolid agar. Proc Natl Acad Sci U S A 86:6973–6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gosink KK, Kobayashi R, Kawagishi I, Hase CC. 2002. Analyses of the roles of the three cheA homologs in chemotaxis of Vibrio cholerae. J Bacteriol 184:1767–1771. doi: 10.1128/JB.184.6.1767-1771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLoney-Marino CR, Wolfe AJ, Visick KL. 2003. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl Environ Microbiol 69:7527–7530. doi: 10.1128/AEM.69.12.7527-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler SM, Camilli A. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc Natl Acad Sci U S A 101:5018–5023. doi: 10.1073/pnas.0308052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boin MA, Häse CC. 2007. Characterization of Vibrio cholerae aerotaxis. FEMS Microbiol Lett 276:193–201. doi: 10.1111/j.1574-6968.2007.00931.x. [DOI] [PubMed] [Google Scholar]
- 44.Boin MA, Austin MJ, Häse CC. 2004. Chemotaxis in Vibrio cholerae. FEMS Microbiol Lett 239:1–8. doi: 10.1016/j.femsle.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Butler SM, Camilli A. 2001. Selection for in vivo regulators of bacterial virulence. Proc Natl Acad Sci U S A 98:6889–6894. doi: 10.1073/pnas.111581598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol 3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Toole R, Milton D, Wolf-Watz H. 1996. Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol Microbiol 19:625–637. doi: 10.1046/j.1365-2958.1996.412927.x. [DOI] [PubMed] [Google Scholar]
- 48.DeLoney-Marino CR, Visick KL. 2012. Role for cheR of Vibrio fischeri in the Vibrio-squid symbiosis. Can J Microbiol 58:29–38. doi: 10.1139/w11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reidl J, Klose KE. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev 26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 50.Freter R, O'Brien PC, Macsai MS. 1979. Effect of chemotaxis on the interaction of cholera vibrios with intestinal mucosa. Am J Clin Nutr 32:128–132. doi: 10.1093/ajcn/32.1.128. [DOI] [PubMed] [Google Scholar]
- 51.Freter R, O'Brien PC. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun 34:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freter R, O'Brien PC, Macsai MS. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun 34:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freter R, Allweiss B, O'Brien PC, Halstead SA, Macsai MS. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun 34:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouellette AJ. 2004. Defensin-mediated innate immunity in the small intestine. Best Pract Res Clin Gastroenterol 18:405–419. doi: 10.1016/j.bpg.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Kaneko T, Colwell RR. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl Microbiol 29:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rawlings TK, Ruiz GM, Colwell RR. 2007. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the copepods Acartia tonsa and Eurytemora affinis. Appl Environ Microbiol 73:7926–7933. doi: 10.1128/AEM.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin B, Austin D, Sutherland R, Thompson F, Swings J. 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol 7:1488–1495. doi: 10.1111/j.1462-2920.2005.00847.x. [DOI] [PubMed] [Google Scholar]
- 59.Høj L, Bourne DG, Hall MR. 2009. Localization, abundance and community structure of bacteria associated with Artemia: effects of nauplii enrichment and antimicrobial treatment. Aquaculture 293:278–285. doi: 10.1016/j.aquaculture.2009.04.024. [DOI] [Google Scholar]
- 60.Neu AK, Månsson M, Gram L, Prol-García MJ. 2014. Toxicity of bioactive and probiotic marine bacteria and their secondary metabolites in Artemia sp. and Caenorhabditis elegans as eukaryotic model organisms. Appl Environ Microbiol 80:146–153. doi: 10.1128/AEM.02717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Garren M, Azam F. 2012. Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J 6:1159–1165. doi: 10.1038/ismej.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shapiro OH, Fernandez VI, Garren M, Guasto JS, Debaillon-Vesque FP, Kramarsky-Winter E, Vardi A, Stocker R. 2014. Vortical ciliary flows actively enhance mass transport in reef corals. Proc Natl Acad Sci U S A 111:13391–13396. doi: 10.1073/pnas.1323094111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Son K, Guasto JS, Stocker R. 2013. Bacteria can exploit a flagellar buckling instability to change direction. Nat Phys 9:494–498. doi: 10.1038/nphys2676. [DOI] [Google Scholar]
- 65.Shnit-Orland M, Kushmaro A. 2009. Coral mucus-associated bacteria: a possible first line of defense. FEMS Microbiol Ecol 67:371–380. doi: 10.1111/j.1574-6941.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 66.Nissimov J, Rosenberg E, Munn CB. 2009. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiol Lett 292:210–215. doi: 10.1111/j.1574-6968.2009.01490.x. [DOI] [PubMed] [Google Scholar]
- 67.Rypien KL, Ward JR, Azam F. 2010. Antagonistic interactions among coral-associated bacteria. Environ Microbiol 12:28–39. doi: 10.1111/j.1462-2920.2009.02027.x. [DOI] [PubMed] [Google Scholar]
- 68.Kvennefors EC, Sampayo E, Kerr C, Vieira G, Roff G, Barnes AC. 2012. Regulation of bacterial communities through antimicrobial activity by the coral holobiont. Microb Ecol 63:605–618. doi: 10.1007/s00248-011-9946-0. [DOI] [PubMed] [Google Scholar]
- 69.Krediet CJ, Ritchie KB, Alagely A, Teplitski M. 2013. Members of native coral microbiota inhibit glycosidases and thwart colonization of coral mucus by an opportunistic pathogen. ISME J 7:980–990. doi: 10.1038/ismej.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raina J-B, Tapiolas D, Motti CA, Foret S, Seemann T, Tebben J, Willis BL, Bourne DG. 2016. Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 4:e2275. doi: 10.7717/peerj.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welsh RM, Rosales SM, Zaneveld JR, Payet JP, McMinds R, Hubbs SL, Vega Thurber RL. 2017. Alien vs. predator: bacterial challenge alters coral microbiomes unless controlled by Halobacteriovorax predators. PeerJ 5:e3315. doi: 10.7717/peerj.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Roux F, Binesse J, Saulnier D, Mazel D. 2007. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl Environ Microbiol 73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simossis VA, Heringa J. 2005. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res 33:W289–W294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simossis VA. 2005. Homology-extended sequence alignment. Nucleic Acids Res 33:816–824. doi: 10.1093/nar/gki233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higuchi R, Krummel B, Saiki R. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stabb EV, Ruby EG. 2002. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol 358:413–426. doi: 10.1016/S0076-6879(02)58106-4. [DOI] [PubMed] [Google Scholar]
- 77.Wolk CP, Fan Q, Zhou R, Huang G, Lechno-Yossef S, Kuritz T, Wojciuch E. 2007. Paired cloning vectors for complementation of mutations in the cyanobacterium Anabaena sp. strain PCC 7120. Arch Microbiol 188:551–563. doi: 10.1007/s00203-007-0276-z. [DOI] [PubMed] [Google Scholar]
- 78.Dykxhoorn DM, St Pierre R, Linn T. 1996. A set of compatible tac promoter expression vectors. Gene 177:133–136. doi: 10.1016/0378-1119(96)00289-2. [DOI] [PubMed] [Google Scholar]
- 79.Ames P, Parkinson JS. 1988. Transmembrane signaling by bacterial chemoreceptors: E. coli transducers with locked signal output. Cell 55:817–826. doi: 10.1016/0092-8674(88)90137-7. [DOI] [PubMed] [Google Scholar]
- 80.Bibikov SI, Biran R, Rudd KE, Parkinson JS. 1997. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol 179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavish AR, Shapiro OH, Kramarsky-Winter E, Vardi A. 2018. Microscale tracking of coral disease reveals timeline of infection and heterogeneity of polyp fate. bioRxiv doi: 10.1101/302778. [DOI]
- 82.Gibbin E, Gavish A, Domart-Coulon I, Kramarsky-Winter E, Shapiro O, Meibom A, Vardi A. 2018. Using NanoSIMS coupled with microfluidics to visualize the early stages of coral infection by Vibrio coralliilyticus. BMC Microbiol 18:39. doi: 10.1186/s12866-018-1173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.