Spreading of vibrios throughout the world correlates with increased global temperatures. As they spread, they find new niches in which to survive, proliferate, and invade. Therefore, genetic manipulation of vibrios is of the utmost importance for studying these species. Here, we have delineated and validated a rapid method to create genetic deletions in Vibrio parahaemolyticus. This study provides insightful methodology for studies with other Vibrio species.
KEYWORDS: EspD, T2SS, Vibrio parahaemolyticus, natural transformation systems
ABSTRACT
The Gram-negative bacterium Vibrio parahaemolyticus is an opportunistic human pathogen and the leading cause of seafood-borne acute gastroenteritis worldwide. Recently, this bacterium was implicated as the etiologic agent of a severe shrimp disease with consequent devastating outcomes to shrimp farming. In both cases, acquisition of genetic material via horizontal transfer provided V. parahaemolyticus with new virulence tools to cause disease. Dissecting the molecular mechanisms of V. parahaemolyticus pathogenesis often requires manipulating its genome. Classically, genetic deletions in V. parahaemolyticus are performed using a laborious, lengthy, multistep process. Here, we describe a fast and efficient method to edit this bacterium's genome based on V. parahaemolyticus natural competence. Although this method is similar to one previously described, V. parahaemolyticus requires counterselection for curing of acquired plasmids due to its recalcitrant nature of retaining extrachromosomal DNA. We believe this approach will be of use to the Vibrio community.
IMPORTANCE Spreading of vibrios throughout the world correlates with increased global temperatures. As they spread, they find new niches in which to survive, proliferate, and invade. Therefore, genetic manipulation of vibrios is of the utmost importance for studying these species. Here, we have delineated and validated a rapid method to create genetic deletions in Vibrio parahaemolyticus. This study provides insightful methodology for studies with other Vibrio species.
INTRODUCTION
Vibrio parahaemolyticus is a halophilic, Gram-negative bacterial pathogen, widely established as a causative agent of acute gastroenteritis associated with contaminated seafood (1, 2). Some of these infections, primarily in patients with preexisting conditions, lead to septicemia with fatal outcomes (3). Recently, this pathogen has been recognized as the leading cause of a devastating shrimp disease, acute hepatopancreatic necrosis disease (AHPND) (4). In light of recent findings, such as V. parahaemolyticus' adaptation to an intracellular life cycle and its ability to rewire host signaling pathways, there is a need to further understand the molecular mechanisms involved in its pathogenesis (5–7). Moreover, V. parahaemolyticus has emerged as a shrimp pathogen with consequent massive economic burden to the shrimp industry (8). Many of these pathogenic qualities can be attributed to the acquisition of new genetic material (4).
Investigating the various mechanisms involved in bacterial pathogenesis at the genetic level most commonly involves genetic engineering. The standard and most widely used technique for achieving genetic modifications in V. parahaemolyticus employs a multistep process, including cloning of a gene-flanking region into a nonreplicating suicide vector, such as pDM4, electroporation into an S17-1 (λpir) Escherichia coli strain for propagation, transfer of the plasmid into V. parahaemolyticus by conjugation, mutant selection in antibiotics, and final curing of plasmid (1, 9–14). This process is laborious and can take up to 2 to 3 weeks before mutant strains are validated and useful for downstream applications.
Natural competence is the ability of a bacterium to take up foreign DNA from the surroundings, and natural transformation is the ability to integrate the foreign DNA into the genome based on homologous recombination. Meibom et al. first reported natural competence induced by chitin in Vibrio cholerae, proposing a transformation model based on chitin, cell density, and nutrient limitation for natural competence (15). Recently, other members of the Vibrionaceae family have been shown to be naturally competent and transformable in the presence of chitin (16, 17).
In this study, we have investigated the use of the natural competence phenomenon and here show that natural transformation can be successfully used to create genetic modifications in V. parahaemolyticus. However, the method for V. parahaemolyticus is unlike the one used for Vibrio natriegens, as it requires plasmid curing via counterselection because of V. parahaemolyticus' recalcitrant nature of retaining extrachromosomal plasmid DNA (18).
RESULTS AND DISCUSSION
Chitin does not induce natural transformation in V. parahaemolyticus.
To test whether chitin can induce natural transformation in V. parahaemolyticus, we attempted to delete the epsD gene (VP0133). epsD encodes secretin, an integral part of the type 2 secretion system (T2SS) in V. parahaemolyticus. For these experiments we used CAB2, a V. parahaemolyticus strain derived from the clinical isolate RIMD 2210633, containing deletions in TDH hemolysins (lacking tdhA and tdhS) and in the first type III secretion system (T3SS1) transcriptional regulator (exsA mutant) (7). A linear PCR construct, consisting of a chloramphenicol acetyltransferase gene (cat) cassette flanked by sequences homologous to upstream and downstream regions of epsD, was used to replace the epsD gene with cat via homologous recombination (Fig. 1A). Transformation reactions were plated onto minimal marine medium (MMM) plates with chloramphenicol to select for transformants. Chitin-induced natural transformation resulted in no CAB2 transformants when using increasing concentrations of transforming DNA (tDNA) with either 1-kb (ΔepsD::cat 1kb HA) or 3-kb (ΔepsD::cat 3kb HA) homology regions (Table 1).
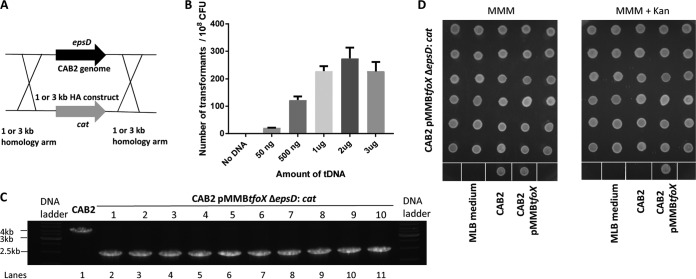
FIG 1.
Natural transformation of V. parahaemolyticus expressing V. cholerae tfoX improves transformation frequency; however, plasmid curing is inefficient. (A) Schematic showing the homologous recombination between the 1-kb or 3-kb HA construct and the epsD locus in the CAB2 genome. (B) V. parahaemolyticus CAB2 strain expressing V. cholerae tfoX gene via pMMBtfoX was used in transformation with various concentrations of transforming DNA (tDNA). (C) PCR analysis using primers A and B confirms the deletion of epsD gene in 10 randomly chosen CAB2 pMMBtfoX ΔepsD::cat transformants using primers designed to amplify the region spanning 1 kb upstream to 1 kb downstream of epsD with the amplification of a 2.8-kb band (0.8-kb cat plus 2-kb flanking epsD) in all of the transformants (lanes 2 to 11) and a 4-kb band in CAB2 (2-kb epsD and 2-kb flanking, lane 1). (D) CAB2 pMMBtfoX ΔepsD::cat transformants were subcultured 10 times in MLB medium without the antibiotic to cure the plasmid pMMBtfoX and further plated to obtain single colonies. Thirty of these colonies were chosen randomly and patched onto MMM plates with kanamycin (right) and MMM plain plates (left) to check for curing. CAB2 and CAB2pMMBtfoX serve as negative and positive controls, respectively, for growth on MMM+ kanamycin plates.
TABLE 1.
Chitin does not induce natural transformation in V. parahaemolyticusa
| Amt of tDNA (ng) | No. of transformants/108 total CFU |
|||
|---|---|---|---|---|
|
V. parahaemolyticus |
V. cholerae |
|||
| 1-kb homology | 3-kb homology | 1-kb homology | 3-kb homology | |
| 0 | 0 | 0 | 0 | 0 |
| 50 | 0 | 0 | 4.0 × 103 | 1.4 × 105 |
| 500 | 0 | 0 | 1.7 × 104 | 4.9 × 105 |
| 1,000 | 0 | 0 | 2.0 × 105 | 2.2 × 106 |
| 2,000 | 0 | 0 | 2.1 × 105 | 3.8 × 106 |
| 3,000 | 0 | 0 | 2.7 × 105 | 7.7 × 106 |
V. parahaemolyticus CAB2 strain was used in chitin-induced natural transformation with increasing concentrations of tDNA, consisting of either 1-kb or 3-kb homology flanking regions. V. cholerae TND 0266 strain was used as a positive control.
To confirm that chitin transformation was working in our hands, we used as a positive control, a ΔCTX (cholera toxin-deficient) derivative of V. cholerae strain E7946 (TND 0266), which was previously shown to be transformable by chitin (19). By following the same protocol as that described above, we replaced the transposase pseudogene VC1807 with a kanamycin resistance cassette and observed 105 transformants with 1 kb of flanking DNA (Table 1). Although a previous study reported successful natural transformation of V. parahaemolyticus using chitin, we were not able to reproduce these results (16).
Natural transformation of V. parahaemolyticus by overexpression of V. cholerae TfoX improves transformation frequency.
Chitin has been shown to induce natural competence in V. cholerae by upregulating expression of the competence master regulator TfoX (15). Additional studies have elucidated the link between chitin sensing and induction of competence in Vibrio species via TfoX (15, 20–22). Chitin upregulates TfoX by interacting with TfoS, resulting in exposure of the Shine-Dalgarno sequence of the TfoX mRNA to positively regulate TfoX translation, and this in turn activates the genes necessary for competence (23). Overexpression of TfoX was shown to bypass the chitin requirement for competence, allowing natural transformation in Vibrio species (18, 24).
Based on these observations, we next set out to induce natural transformation in V. parahaemolyticus by overexpressing the highly conserved V. cholerae TfoX, which has 81% similarity and 69% identity to the V. parahaemolyticus homologue and was shown to function in other vibrios. The V. cholerae TfoX was ectopically expressed in CAB2 using the kanamycin-resistant pMMBtfoX plasmid. This strain was then transformed using the previously described ΔepsD::cat 1kb HA and ΔepsD::cat 3kb HA constructs. While transformation deploying the ΔepsD::cat 1kb HA construct resulted in marginal improvement of transformation frequency (a single transformant for every 108 total CFU at 500 ng tDNA), the use of its 3-kb counterpart significantly improved transformation efficiency, with 274 transformants per 108 total CFU using 2 μg tDNA. Ten transformants were randomly picked, and PCR analysis was carried out to confirm the deletion of the epsD gene. PCR amplification using the primers A and B (see Fig. 3A; see also Table S1 in the supplemental material), designed to amplify the region spanning 1 kb upstream to 1 kb downstream of epsD, resulted in a 4-kb band in CAB2 (2 kb of epsD and 2 kb of flanking region) and in a 2.8-kb band (0.8 kb of cat and 2 kb of flanking region of epsD) in all transformants tested, thus confirming epsD deletion (Fig. 1C, lanes 2 to 11).
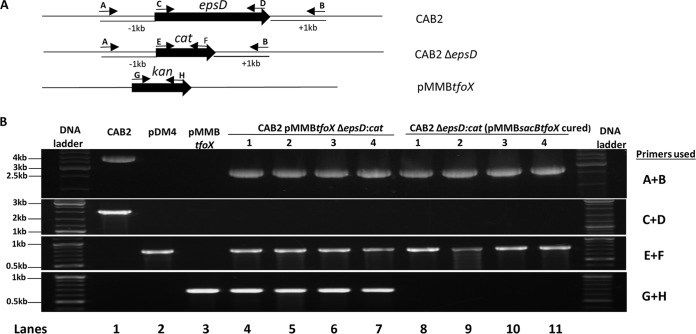
FIG 3.
PCR analysis confirms the replacement of epsD gene with cat gene in the transformants and the absence of kanamycin resistance cassette in the cured transformants. (A) Schematic of the epsD locus in CAB2 and CAB2 ΔepsD strains and the location of primers used for PCR amplification. (B, top row) Primers A and B, designed to amplify the region spanning 1 kb upstream to 1 kb downstream of epsD, were used, which resulted in a 4-kb band in CAB2 (lane 1; 2-kb epsD gene plus 2-kb flanking region) and 2.8-kb band (0.8-kb cat plus 2-kb flanking epsD) in all transformants (both uncured CAB2 pMMBtfoX ΔepsD::cat strains 1 to 4 [lanes 4 to 7] and cured CAB2 ΔepsD::cat strains 1 to 4 [lanes 8 to 11]). Gene-specific primers C and D were used to amplify the epsD gene in the second row, confirming the deletion of the epsD gene in all transformants (both uncured CAB2 pMMBtfoX ΔepsD::cat strains 1 to 4 and cured CAB2 ΔepsD::cat strains 1 to 4). The third row, using primers E and F, shows the replacement of the epsD gene with the cat gene in all transformants (both uncured CAB2 pMMBtfoX ΔepsD::cat strains 1 to 4 and cured CAB2 ΔepsD::cat strains 1 to 4). (Bottom) Using primers G and H confirms the absence of Kan cassette in the cured CAB2 ΔepsD::cat strains 1 to 4 compared to its amplification in the uncured CAB2 pMMBtfoX ΔepsD::cat strains 1 to 4. CAB2, pDM4, and pMMBtfoX were used as positive controls for the epsD gene, and cat and Kan cassettes, respectively.
Although transformation is complete at this stage, it is necessary to eliminate the V. cholerae TfoX plasmid. We next set out to cure the plasmid pMMBtfoX from V. parahaemolyticus transformants. Five randomly picked clones from the above-confirmed ΔepsD::cat transformants were subcultured 10 times in MLB medium (Luria-Bertani [LB] medium supplemented with 3% NaCl) without kanamycin (pMMBtfoX-borne antibiotic resistance marker) to cure the V. parahaemolyticus transformants of the plasmid pMMBtfoX, followed by plating onto MMM plates to obtain single colonies. Thirty of these single colonies (CAB2 pMMBtfoX ΔepsD::cat) were randomly picked and patched onto MMM plates with and without kanamycin to check for plasmid curing. Unfortunately, all of the 30 tested colonies grew on both MMM and MMM-plus-kanamycin plates, demonstrating that none of the clones were cured of the plasmid (Fig. 1D). Therefore, although ectopic expression of V. cholerae TfoX allows for natural transformation of V. parahaemolyticus strains, this Vibrio species appears to be resistant to curing of the TfoX plasmid.
Addition of sacB gene to pMMBtfoX plasmid allows for curing of V. parahaemolyticus transformants.
SacB-mediated sucrose sensitivity is a popular method for counterselection in genetic editing of bacterial genomes (25). The Bacillus subtilis sacB gene encoding levansucrase is not toxic to its native Gram-positive bacteria; however, when expressed in Gram-negative bacteria it is lethal in the presence of sucrose (26). The mechanism of this toxicity is not well understood but is thought to be due to accumulation of the high-molecular-weight fructose polymers synthesized by the levansucrase in the periplasm of Gram-negative bacteria (27).
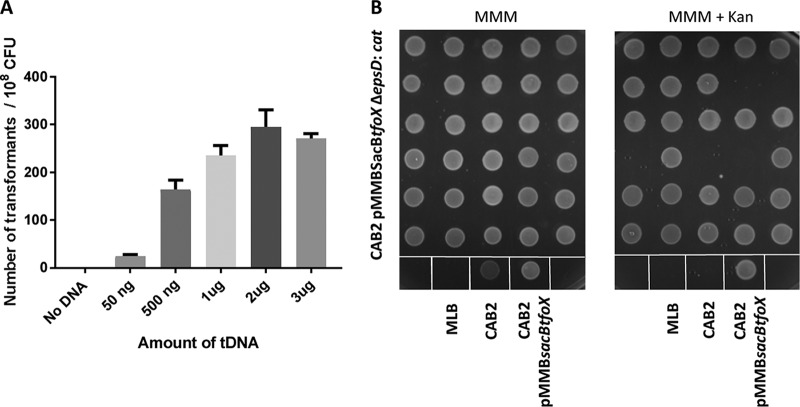
We therefore attempted to use the Bacillus subtilis sacB gene as a counterselection method to remove pMMBtfoX from V. parahaemolyticus. The SacB counterselection marker was incorporated into pMMBtfoX to create pMMBsacBtfoX. This plasmid was used to ectopically express V. cholerae TfoX for natural transformation of V. parahaemolyticus CAB2 along with the tDNA ΔepsD::cat 3kb HA construct. pMMBsacBtfoX-mediated transformation was comparable to that of pMMBtfoX, with a similar number of transformants that increased with increasing amounts of tDNA up to 1 μg (Fig. 2A). Five randomly chosen transformants (CAB2 pMMBsacBtfoX ΔepsD::cat) were then plated in the presence of sucrose for curing of the plasmid. To confirm loss of the plasmid, single colonies were patched onto MMM plates with or without kanamycin plates (plasmid-borne antibiotic resistance marker). Up to 16% (5 out of 30 colonies patched) of the clones were unable to grow on kanamycin plates, suggesting loss of plasmid (Fig. 2B).
FIG 2.
Addition of sacB gene to pMMBtfoX plasmid helps in curing the plasmid from the transformants. (A) V. parahaemolyticus CAB2 strain expressing V. cholerae tfoX gene via pMMBsacBtfoX was used in transformation with various concentrations of transforming DNA (tDNA). (B) Thirty randomly chosen CAB2 pMMBsacBtfoX ΔepsD::cat transformants were patched onto MMM plates with kanamycin (right) and MMM plain plates (left) after growth on MMM plates containing sucrose to cure the plasmid pMMBsacBtfoX. MLB is the medium used for growth. CAB2 and CAB2pMMBsacBtfoX serve as negative and positive controls for growth on MMM+ kanamycin plates, respectively.
To confirm the genetic composition of the naturally transformed and cured V. parahaemolyticus, we analyzed transformants using PCR. Using kanamycin-sensitive clones along with uncured transformants (kanamycin resistant), we analyzed the presence or absence of epsD, cat, and the Kan cassette. Using PCR amplification with primers A and B, we observed a 2.8-kb band (0.8-kb cat and 2-kb flanking region of epsD) in all transformants compared to the 4-kb band (2-kb epsD and 2-kb flanking) in CAB2 and confirmed the deletion of the epsD gene in all transformants (Fig. 3A and B, top row of gels, lanes 4 to 11). Absence of amplification of epsD using the primers C and D further demonstrated the successful deletion of this gene (Fig. 3A and B, second row, lanes 4 to 11). The replacement of epsD with cat was observed in all of the transformants using primers E and F (Fig. 3A and B, third row, lanes 4 to 11). Finally, the absence of amplification of the kanamycin cassette using the primers G and H in the kanamycin-sensitive transformants confirmed curing of pMMBsacBtfoX from these clones (Fig. 3A, bottom row, lanes 8 to 11).
Validation of epsD deletion strains using MS.
Since V. parahaemolyticus epsD encodes secretin, a major component of the T2SS, we predicted that its loss would have an effect on the type 2 secretome (28). To test this hypothesis, we performed mass spectrometry (MS) analysis of the secretome isolated from the CAB2 and newly created CAB2ΔepsD strains. We observed that deletion of epsD impaired secretion of many known T2SS proteins, such as lipases, serine proteases, toxins, and enzymes involved in carbohydrate utilization (29, 30). A selected list of proteins found in the CAB2 but not found in the CAB2ΔepsD secretome is presented in Table 2. One such protein, annotated as putative trypsin (VP1642), is a homologue of V. cholerae VesB, recently identified as a T2SS-secreted protein (30).
TABLE 2.
Absence of T2SS-secreted proteins from CAB2ΔepsD secretomea
| Accession no.b | Name | Gene |
|---|---|---|
| 28901322 | Protease II | VPA1467 |
| 28810041 | Secreted RNase, putative | VPA1639 |
| 28900714 | Lipase | VPA0859 |
| 28806633 | Trypsin, putative | VP1642 |
| 28806328 | Collagenase | VP1340 |
| 28897534 | Chitoporin | VP0760 |
| 28900569 | Collagenase | VPA0714 |
| 28806169 | Lactonizing lipase | VP1181 |
| 28806248 | Outer membrane phospholipase, chain A | VP1260 |
| 28899947 | Spindolin-like protein | VPA0092 |
| 28808615 | Serine proteinase, putative | VPA0449 |
| 28900610 | Metalloproteinase | VPA0755 |
| 28901386 | Serine protease | VPA1531 |
| 28898960 | Bacteriocin production protein | VP2186 |
List of T2SS proteins identified by mass spectrometry that are present in the CAB2 secretome but absent from the CAB2ΔepsD secretome.
Accession numbers were obtained from the NCBI protein database for V. parahaemolyticus RIMD 2210633.
Counterintuitively, further comparison of CAB2 and CAB2ΔepsD secretomes revealed that some proteins were secreted at higher levels in the mutant secretome, and other proteins were found to be secreted by the mutant but not by CAB2 (Table S2). Interestingly, some of the proteins with increased levels or that were exclusively present in the CAB2ΔepsD secretome (e.g., superoxide dismutase and catalases) are known to be involved in combating oxidative stress (Table S2). In V. cholerae, the equivalent epsD deletion mutant was also reported to have increased secretion of proteins involved in oxidative stress, and such effect was attributed to the leaky nature of the cell envelope due to T2SS disruption (29, 30). Altogether, our findings indicate the epsD deletion in V. parahaemolyticus results in an altered secretome, indicative of a disabled T2SS.
V. parahaemolyticus, a pathogenic marine bacterium, while not amenable to chitin-induced transformation, did allow for natural transformation by obviating the chitin requirement with TfoX overexpression. Since a PCR construct can be directly used for homologous recombination through natural transformation, the multistep process involved in conventional protocols, i.e., generation and propagation of a suicide vector containing the homologous construct followed by conjugation, can be bypassed to achieve diverse genetic modifications in V. parahaemolyticus in a timely manner. For example, it takes 2 to 3 weeks to generate a gene deletion in V. parahaemolyticus by conventional methods but only 1 week by the natural transformation method described here.
In summary, we have successfully used natural transformation in V. parahaemolyticus to create genetically modified mutants. This approach will likely be applicable to many other Vibrio species and be beneficial to the Vibrio community as a whole.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The V. parahaemolyticus CAB2 strain used in this study was made by deleting the transcriptional factor ExsA from the POR1 strain (RIMD 2210633 ΔtdhAS strain), generously provided by Tetsuya Iida and Takeshi Honda (31). The clinical isolate RIMD 2210633 contains thermostable direct hemolysins (TDHs) and two type 3 secretion systems, T3SS1 and T3SS2. The CAB2 strain contains deletions in TDH genes and in the transcriptional regulator gene exsA, resulting in an inactive T3SS1 (7). CAB2 strain was grown in MLB medium (Luria-Bertani [LB] medium supplemented with 3% NaCl) at 30°C. V. cholerae strain TND 0266 is a cholera toxin-deficient mutant of an V. cholerae strain (E7946, an El Tor V. cholerae isolate) and was grown in LB medium at 30°C (19). When necessary, the medium was supplemented with 25 μg/ml chloramphenicol or 250 μg/ml kanamycin.
Generation of PCR constructs.
The PCR constructs were generated by splicing-by-overlap extension (SOE) PCR (32). For the generation of ΔepsD::cat 1kb HA constructs, the nucleotide sequences 1 kb upstream and downstream of the gene epsD (VP0133; GenBank sequence accession number NC_004603) were amplified with Phusion polymerase (ThermoFisher Scientific) using the primer pairs EpsD1kb_UpF1-EpsD_UpR1 and EpsD_DownF1-EpsD1kb_DownR1, respectively. The cat cassette was amplified from pDM4, a Cmr Ori6RK suicide plasmid with the primers CatF and CatR. Gel-purified PCR products of 1 kb upstream, cat, and 1 kb downstream were then mixed in equal ratios and used as the template for an SOE PCR with the primers 1kb_UpF1 and 1kb_DownR1 to generate the construct ΔepsD::cat 1kb HA. Similarly, the ΔepsD::cat 3kb HA construct was generated using the 3-kb upstream and downstream sequences of epsD with the primer pairs EpsD3kb_UpF1-EpsD_UpR1 and EpsD_DownF1-EpsD3kb_DownR1, respectively. All of the primers used in this study are listed in Table S1 in the supplemental material.
Chitin-induced natural transformation.
Natural transformation through chitin was performed as described previously (19). Briefly, Vibrio cultures were inoculated into 1 ml defined artificial seawater (DASW; 7 g/liter Instant Ocean; Aquarium Systems) containing 80 mg of autoclaved chitin (AAJ6120622; Fisher) at an optical density at 600 nm (OD600) of 0.1 (∼108 CFU) and allowed to grow statically at 30°C for 16 to 24 h. Transforming DNA was then added by exchanging the supernatant with fresh DASW containing tDNA without disturbing the settled chitin and allowed to incubate for an additional 16 to 24 h at 30°C. One milliliter of MLB was then added, and transformants were allowed to outgrow at 30°C with shaking for 1 to 2 h. Transformation mixtures were then plated on MMM plates with or without chloramphenicol (25 μg/ml) to assess transformation efficiency.
Natural transformation by overexpression of V. cholerae TfoX.
TfoX-induced natural transformation was performed as described previously (18). Briefly, V. parahaemolyticus CAB2 strains harboring pMMBtfoX were obtained via conjugation with S17-1 (λpir) Escherichia coli containing the plasmid pMMBtfoX, and transconjugants were selected for on MMM agar containing 250 μg/ml kanamycin. CAB2 pMMBtfoX strains were induced with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) overnight in MLB plus kanamycin (250 μg/ml) at 30°C. The cultures were then diluted 100-fold in 2× Instant Ocean plus 100 μM IPTG (28 g/liter of Instant Ocean), and tDNA was added. Reaction mixtures were gently mixed and incubated at 30°C statically for 4 to 6 h. One milliliter of MLB was added, transformants were allowed to outgrow at 30°C with shaking for 1 to 2 h, and then they were plated on MMM plates with and without chloramphenicol to determine transformation efficiency.
SacB cloning into pMMBtfoX and curing of pMMBsacBtfoX from the transformants.
The sacB gene along with its endogenous promoter was amplified from the pDM4 plasmid using the primers SacBF and SacBR and cloned into pMMBtfox between the restriction sites BamHI and PstI. The resulting pMMBsacBtfoX was transferred into CAB2 via conjugation with S17-1 (λpir) Escherichia coli. Transconjugants were selected for on MMM agar containing 250 μg/ml kanamycin (pMMBsacBtfoX-borne plasmid resistance marker). Curing of pMMBsacBtfoX from Vibrio strains was achieved by plating the bacterium onto MMM agar plates containing 15% (wt/vol) sucrose once to obtain isolated colonies. These single colonies were subsequently plated on MMM plates with or without kanamycin to check for kanamycin sensitivity, i.e., confirmation of curing of plasmid.
Secretome analysis by mass spectrometry.
The secretomes from V. parahaemolyticus strains were isolated from 50 ml of Vibrio cultures grown in MLB. Briefly, 50 ml MLB was inoculated with overnight-grown V. parahaemolyticus culture to an OD600 of 0.18/ml and allowed to grow for 3 h at 30°C with shaking. The cultures were then centrifuged at 4,000 rpm for 20 min at 4°C to pellet the bacteria, and the supernatant was passed through a 0.22-μm syringe filter. Secreted proteins were precipitated from this cell-free supernatant by the addition of trichloroacetic acid (TCA) at 8% (vol/vol) and incubation at 4°C overnight. Precipitated proteins were pelleted by centrifugation for 50 min at 16,000 × g at 4°C using a Beckman-Coulter centrifuge. The protein precipitate was washed twice in 4 ml of chilled acetone, air dried, and resuspended in 1 ml of cold 10 mM Tris, pH 8.0, buffer. A second round of TCA precipitation was carried out at 4°C for 1 h. The final protein precipitate was once again washed twice with 400 μl chilled acetone, air dried, resuspended in 20 μl of SDS sample buffer, and then analyzed by mass spectrometry.
Sample preparation for mass spectrometry analysis included the excision of proteins from polyacrylamide gels via SDS-PAGE. Proteins were reduced and alkylated using dithiothreitol (DTT) and iodoacetamide, respectively. In-gel overnight digestion of proteins with trypsin followed, and the samples were desalted via solid-phase extraction (SPE) prior to analysis. Liquid chromatography-tandem MS (LC-MS/MS) experiments were performed on a Thermo Scientific EASY-nLC 1200 liquid chromatography system coupled to a Thermo Scientific Orbitrap Fusion Lumos mass spectrometer. MS1 spectra were acquired in the Orbitrap mass analyzer with a resolution of 120,000. Subsequent peptide fragmentation took place via high-energy collision-induced dissociation (HCD), generating MS2 spectra which were acquired in the ion trap. Resulting MS/MS spectral data were searched using Proteome Discoverer 2.1 software (Thermo Scientific) against entries included in the V. parahaemolyticus RIMD 2210633 protein database (NCBI). Carbamidomethylation of cysteine residues (+57.021 Da) was set as a static modification, while oxidation of methionine (+15.995 Da) and acetylation of peptide N termini (+42.011 Da) were set as dynamic modifications. The precursor ion tolerance was set to 10 ppm, and the product ion tolerance was set to 0.6 Da for all searches. Peptide spectral matches were adjusted to a 1% false discovery rate.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Orth laboratory for their helpful discussions and advice.
This work was funded by the Welch Foundation, grant I-1561 (K.O.), and the Once Upon a Time Foundation (K.O.). This work was also supported by U.S. National Institutes of Health grant AI118863 to A.B.D. K.O. is a Burroughs Welcome Investigator in Pathogenesis of Infectious Disease, a Beckman Young Investigator, and a W. W. Caruth, Jr., Biomedical Scholar and has an Earl A. Forsythe Chair in Biomedical Science.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00032-18.
REFERENCES
- 1.Broberg CA, Zhang L, Gonzalez H, Laskowski-Arce MA, Orth K. 2010. A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity. Science 329:1660–1662. doi: 10.1126/science.1192850. [DOI] [PubMed] [Google Scholar]
- 2.O'Boyle N, Boyd A. 2014. Manipulation of intestinal epithelial cell function by the cell contact-dependent type III secretion systems of Vibrio parahaemolyticus. Front Cell Infect Microbiol 3:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 4.Lee CT, Chen IT, Yang YT, Ko TP, Huang YT, Huang JY, Huang MF, Lin SJ, Chen CY, Lin SS, Lightner DV, Wang HC, Wang AH, Wang HC, Hor LI, Lo CF. 2015. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci U S A 112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Nisco NJ, Kanchwala M, Li P, Fernandez J, Xing C, Orth K. 2017. The cytotoxic type 3 secretion system 1 of Vibrio rewires host gene expression to subvert cell death and activate cell survival pathways. Sci Signal 10:eaal4501. doi: 10.1126/scisignal.aal4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza Santos M, Orth K. 2014. Intracellular Vibrio parahaemolyticus escapes the vacuole and establishes a replicative niche in the cytosol of epithelial cells. mBio 5:e01506-. doi: 10.1128/mBio.01506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Krachler AM, Broberg CA, Li Y, Mirzaei H, Gilpin CJ, Orth K. 2012. Type III effector VopC mediates invasion for Vibrio species. Cell Rep 1:453–460. doi: 10.1016/j.celrep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV. 2013. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- 9.de Souza Santos M, Salomon D, Orth K. 2017. T3SS effector VopL inhibits the host ROS response, promoting the intracellular survival of Vibrio parahaemolyticus. PLoS Pathog 13:e1006438. doi: 10.1371/journal.ppat.1006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Chen S. 2015. A novel adhesive factor contributing to the virulence of Vibrio parahaemolyticus. Sci Rep 5:14449. doi: 10.1038/srep14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura M, Fujii T, Hiyoshi H, Makino F, Inoue H, Motooka D, Kodama T, Ohkubo T, Kobayashi Y, Nakamura S, Namba K, Iida T. 2015. A repeat unit of Vibrio diarrheal T3S effector subverts cytoskeletal actin homeostasis via binding to interstrand region of actin filaments. Sci Rep 5:10870. doi: 10.1038/srep10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. 2009. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Li L, Zhao Z, Peng D, Zhou X. 2016. Polar flagella rotation in Vibrio parahaemolyticus confers resistance to bacteriophage infection. Sci Rep 6:26147. doi: 10.1038/srep26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Shah DH, Konkel ME, Call DR. 2008. Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol Microbiol 69:747–764. doi: 10.1111/j.1365-2958.2008.06326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Dai J, Morris JG Jr, Johnson JA. 2010. Genetic analysis of the capsule polysaccharide (K antigen) and exopolysaccharide genes in pandemic Vibrio parahaemolyticus O3:K6. BMC Microbiol 10:274. doi: 10.1186/1471-2180-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulig PA, Tucker MS, Thiaville PC, Joseph JL, Brown RN. 2009. USER friendly cloning coupled with chitin-based natural transformation enables rapid mutagenesis of Vibrio vulnificus. Appl Environ Microbiol 75:4936–4949. doi: 10.1128/AEM.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalia TN, Hayes CA, Stolyar S, Marx CJ, McKinlay JB, Dalia AB. 2017. Multiplex Genome Editing by Natural Transformation (MuGENT) for Synthetic Biology in Vibrio natriegens. ACS Synth Biol 6:1650–1655. doi: 10.1021/acssynbio.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalia AB, McDonough E, Camilli A. 2014. Multiplex genome editing by natural transformation. Proc Natl Acad Sci U S A 111:8937–8942. doi: 10.1073/pnas.1406478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonova ES, Bernardy EE, Hammer BK. 2012. Natural competence in Vibrio cholerae is controlled by a nucleoside scavenging response that requires CytR-dependent anti-activation. Mol Microbiol 86:1215–1231. doi: 10.1111/mmi.12054. [DOI] [PubMed] [Google Scholar]
- 21.Lo Scrudato M, Blokesch M. 2012. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet 8:e1002778. doi: 10.1371/journal.pgen.1002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo Scrudato M, Blokesch M. 2013. A transcriptional regulator linking quorum sensing and chitin induction to render Vibrio cholerae naturally transformable. Nucleic Acids Res 41:3644–3658. doi: 10.1093/nar/gkt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalia AB, Lazinski DW, Camilli A. 2014. Identification of a membrane-bound transcriptional regulator that links chitin and natural competence in Vibrio cholerae. mBio 5:e01028-13. doi: 10.1128/mBio.01028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack-Berti A, Wollenberg MS, Ruby EG. 2010. Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ Microbiol 12:2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyrat JM, Pelicic V, Gicquel B, Rappuoli R. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect Immun 66:4011–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gay P, Le Coq D, Steinmetz M, Ferrari E, Hoch JA. 1983. Cloning structural gene sacB, which codes for exoenzyme levansucrase of Bacillus subtilis: expression of the gene in Escherichia coli. J Bacteriol 153:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinmetz M, Le Coq D, Djemia HB, Gay P. 1983. [Genetic analysis of sacB, the structural gene of a secreted enzyme, levansucrase of Bacillus subtilis Marburg]. Mol Gen Genet 191:138–144. doi: 10.1007/BF00330901. [DOI] [PubMed] [Google Scholar]
- 28.Douzi B, Filloux A, Voulhoux R. 2012. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci 367:1059–1072. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikora AE, Beyhan S, Bagdasarian M, Yildiz FH, Sandkvist M. 2009. Cell envelope perturbation induces oxidative stress and changes in iron homeostasis in Vibrio cholerae. J Bacteriol 191:5398–5408. doi: 10.1128/JB.00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikora AE, Zielke RA, Lawrence DA, Andrews PC, Sandkvist M. 2011. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem 286:16555–16566. doi: 10.1074/jbc.M110.211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park KS, Ono T, Rokuda M, Jang MH, Okada K, Iida T, Honda T. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun 72:6659–6665. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton RM, Cai Z, Ho SM, Pease LR. 2013. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 54:129–133. doi: 10.2144/000114017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.