Abstract
In the developing hypothalamus, the fat-derived hormone leptin stimulates the growth of axons from the arcuate nucleus of the hypothalamus (ARH) to other regions that control energy balance. These projections are significantly reduced in leptin deficient (Lepob/ob) mice and this phenotype is largely rescued by neonatal leptin treatments. However, treatment of mature Lepob/ob mice is ineffective, suggesting that the trophic action of leptin is limited to a developmental critical period. To temporally delineate closure of this critical period for leptin-stimulated growth, we treated Lepob/ob mice with exogenous leptin during a variety of discrete time periods, and measured the density of Agouti-Related Peptide (AgRP) containing projections from the ARH to the ventral part of the dorsomedial nucleus of the hypothalamus (DMHv), and to the medial parvocellular part of the paraventricular nucleus (PVHmp). The results indicate that leptin loses its neurotrophic potential at or near postnatal day 28. The duration of leptin exposure appears to be important, with 9- or 11- day treatments found to be more effective than shorter (5-day) treatments. Furthermore, leptin treatment for 9 days or more was sufficient to restore AgRP innervation to both the PVHmp and DMHv in Lepob/ob females, but only to the DMHv in Lepob/ob males. Together, these findings reveal that the trophic actions of leptin are contingent upon timing and duration of leptin exposure, display both target and sex specificity, and that modulation of leptin-dependent circuit formation by each of these factors may carry enduring consequences for feeding behavior, metabolism, and obesity risk.
Keywords: agouti-related peptide (AgRP), arcuate nucleus of the hypothalamus (ARH), Critical period, leptin, sexual dimorphism, RRID:IMSR_JAX:000632, RRID:AB_2313908, RRID:IMSR_JAX:007914, RRID:IMSR_JAX:006417, RRID:SCR_002668
1 INTRODUCTION
The arcuate nucleus of the hypothalamus (ARH) represents a critical interface between peripheral hormonal cues and neural circuits that control body weight (Hetherington & Ranson, 1940; Hewson, Tung, Connell, Tookman, & Dickson, 2002; Mirshamsi et al., 2004; Myers, MUnzberg, Leinninger, & Leshan, 2009; Saper, Chou, & Elmquist, 2002; Schwartz, Woods, Porte, Seeley, & Baskin, 2000; Sternson, Atasoy, Betley, Henry, & Xu, 2016). Within the ARH, neurons that co-express neuropeptide Y (NPY) and agouti-related peptide (AgRP) are activated or inhibited by signals that convey information about energy balance (Atasoy, Betley, Su, & Sternson, 2012; Betley, Cao, Ritola, & Sternson, 2013; Garfield et al., 2016; Gropp et al., 2005; Hahn, Breininger, Baskin, & Schwartz, 1998; Zarjevski, Cusin, Vettor, Rohner Jeanrenaud, & Jeanrenaud, 1993). The adipocyte-derived hormone, leptin, is required for central nervous system regulation of energy homeostasis and directly impacts the activity of AgRP neurons to rapidly alter feeding behavior and energy metabolism through connections to a distributed network of nuclei controlling downstream neuroendocrine and autonomic output (Atasoy et al., 2012; Betley et al., 2013, _S1_Reference7,2015; Bouyer & Simerly, 2013; Campfield, Smith, Guisez, Devos, & Burn, 1995; Carter, Soden, Zweifel, & Palmiter, 2013; Garfield et al., 2016; Grill et al., 2002; Halaas et al., 1995; Huo, Maeng, Bj∅rbæk, & Grill, 2007; Scott, Williams, Rossi, Lee, & Elmquist, 2011; Zhang et al., 1994).
The projections of ARH neurons are established primarily during the first two postnatal weeks, concomitant with a naturally occurring surge in leptin secretion (Ahima, Prabakaran, & Flier, 1998; Bouret, 2004). During this time, leptin signals through the b-form of its receptor (LepRb) at the level of the ARH to stimulate growth of axonal projections from AgRP neurons (Bouret, 2004; Bouret, Draper, & Simerly, 2004; Bouyer & Simerly, 2013). Thus, in the leptin deficient (Lepob/ob) mouse, AgRP projections to several key nuclei controlling energy balance are severely diminished, including projections to the paraventricular (PVH) and dorsomedial (DMH) nuclei of the hypothalamus (Bouret, 2004; Bouret et al., 2004; Bouyer & Simerly, 2013). This diminished innervation is largely rescued by treatment with exogenous leptin during the postnatal period (P4-P14) when the surge in leptin secretion normally occurs (Atasoy et al., 2012; Betley et al., 2013; Bouret et al., 2004; Bouyer & Simerly, 2013; Garfield et al., 2016; Gropp et al., 2005; Hahn et al., 1998; Zarjevski et al., 1993). Moreover, impairments in food intake, adiposity, and certain aspects of autonomic function are improved when Lepob/ob mice are treated with leptin only during this restricted postnatal period. However, leptin treatment in mature Lepob/ ob animals is ineffective at restoring normal patterns of ARH innervation (Atasoy et al., 2012; Betley et al., 2013, 2015; Bouret et al., 2004; Bouyer & Simerly, 2013; Campfield et al., 1995; Carter et al., 2013; Garfield et al., 2016; Grill et al., 2002; Halaas et al., 1995; Huo et al., 2007; Scott et al., 2011; Zhang et al., 1994), suggesting that the trophic actions of leptin are restricted to a discrete developmental critical period.
Postnatal leptin also appears to influence targeting of ARH neurons to specific parts of the PVH. AgRP neurons project to two broad subgroups of neurons within the PVH: neuroendocrine neurons that send projections to the median eminence and posterior pituitary to mediate hormonal responses, and preautonomic neurons that project to the brainstem, spinal cord, limbic system, and other hypothalamic sites to coordinate autonomic and goal-directed behaviors (Ahima et al., 1998; Biag et al., 2011; Bouret, 2004; Bouyer & Simerly, 2013; Cowley et al., 1999; Saper et al., 2002; Swanson, 2000). In Lepob/ob mice, deficits in innervation from the ARH to preautonomic regions of the PVH are rescued by treatment with exogenous leptin during the postnatal period (P4–14) when the surge in leptin secretion normally occurs (Bouret, 2004; Bouret et al., 2004; Bouyer & Simerly, 2013). However, leptin treatment from P4–14 is insufficient to restore AgRP projections to neuroendocrine regions of the PVH in males, suggesting that the growth of AgRP projections to individual target regions is not impacted uniformly by leptin (Bouret, 2004; Bouret et al., 2004; Bouyer & Simerly, 2013).
In order to understand how environmental stimuli presented at different times during development impact programming of CNS architecture, it is important to first define when circuits are capable of responding to leptin. To probe the site-specificity and temporal constraints for the neurotrophic action of leptin on development of ARH projections, we evaluated the density of AgRP projections to the PVH and DMH of Lepob/ob mice that received exogenous leptin treatment during several discrete postnatal time periods. The ability of leptin to rescue growth of AgRP projections was dependent on both duration and timing, with the period of effective leptin treatment closing around the 28th postnatal day. Furthermore, the neurotrophic actions of postnatal leptin appear to exhibit both target specificity and sexual dimorphism. Together, these findings reveal that the neurotrophic action of leptin does not extend past the fourth postnatal week, and exhibits anatomical and sex specific characteristics, that have long-term physiological consequences for metabolic function and obesity risk.
2 METHODS
2.1 Animals
All animal care and experimental procedures were performed in accordance with the Institutional Animal Care and Usage Committee of Children's Hospital Los Angeles. Mice were housed at 22°C on a 13:11-hr light:dark cycle (lights on at 0600h:lights off at 1900h) with ad libitum access to a standard chow diet (PicoLab Rodent Diet 20, #5053). Mice expressing Cre recombinase under the control of the leptin receptor promoter (LRbcre mice) were provided by Dr. Martin Myers, University of Michigan (Leshan, Björnholm, MUnzberg, & Myers, 2006). Mice expressing the Cre dependent reporter, tdTomato (stock 007914), mice expressing GFP under the control of the neuropeptide Y (NPY) promoter (NPYGFP mice; stock 006417), and mice heterozygous for a mutation in the leptin gene (Lepob stock 000632) were obtained from The Jackson Laboratory.
Mice heterozygous for a mutation in the leptin gene were bred to generate homozygous, leptin deficient (Lepob/ob) mice, and control lit- termates (WT) with normal leptin expression. Lepob/ob mice were maintained on a C57/BL6J background. On postnatal day (P) 1, litter size was adjusted to 6–8 pups per litter to standardize nutrition during the lactation period. Male and female, Lepob/ob and WT littermates were treated daily (between 11:00 and 13:00 hours) by intraperitoneal (i.p.) injection of either leptin (10 mg/kg body weight; Peprotech Inc.) or vehicle (5 mM sodium citrate) during one of seven postnatal time periods. Thus, genotyped male offspring were assigned to a treatment group (leptin or vehicle) corresponding to one of the following time periods: P4–8, P6–10, P8–12, P12–16, P4–14, P16–26, P28–38. In addition, groups of female mice were genotyped and treated with either leptin or vehicle during the following intervals: P4–14, P16–24, or P25–33. Mice from all experimental groups were weaned on postnatal day 22 onto an ad libitum standard chow diet. On P60-P70 mixed groups of leptin or vehicle treated mice were perfused and processed for immunohistochemistry together with Lepob/ob and WT littermates.
2.2 Immunohistochemistry
Mice were anesthetized with tribromoethanol and perfused transcar- dially with saline followed by fixative (4% paraformaldehyde in borate buffer, pH 9.5). Brains were postfixed in a solution of 20% sucrose in fixative, cryoprotected overnight in 20% sucrose in 0.2M potassium phosphate buffered saline (KPBS), and frozen in powdered dry ice. Four series of 20 μm-thick frozen sections were collected using either a sliding microtome or cryostat for immunohistochemical staining. Tissue sections were incubated overnight in blocking buffer (0.2M KPBS, 2% normal goat or donkey serum, 0.3% Triton X-100) at 4°C with constant agitation, followed by incubation for 72 hr at 4°C in blocking buffer containing combinations of antibodies directed against AgRP (Phoenix Pharmaceuticals Cat# H-003–53, RRID:AB_2313908), HuC/ D (Molecular Probes Cat# A21271,RRID:AB_221448), and/or estrogen receptor alpha (EMD Millipore Cat# 06–935, RRID:AB_310305). After several rinses in KPBS, sections were incubated for 2 hr at room temperature in blocking buffer containing a cocktail of species-specific Alexa Fluor conjugated secondary antibodies (Thermo Fisher Scientific Cat# A10042, RRID:AB_2534017; Thermo Fisher Scientific Cat# A- 31571,RRID:AB_2536181; Thermo Fisher Scientific Cat# A-21206, RRID:AB_2535792). Sections were rinsed in KPBS, mounted onto gelatin-subbed slides and cover-slipped using Fluoromount G mounting medium (Southern Biotech, Birmingham, AL).
Immunohistochemical labeling of androgen receptor (EMD Millipore 06–080, AB_310214) or pSTAT3 (Cell Signaling Technology Cat# 9131L, RRID:AB_331587) was performed as described above, with slight modification, as described previously (Levin, Dunn- Meynell, & Banks, 2004). Briefly, sections were treated with 0.5% NaOH, 0.5% H2O2, in KPBS for 20 min, rinsed several times in KPBS, then incubated in 0.3% glycine in KPBS for 10 min. Following several rinses in KPBS, sections were then treated with 0.03% sodium dodecyl sulfide, and rinsed again several times in KPBS before blocking for 20 min in 4% normal goat serum (NGS), 0,4% triton X-100,1% bovine serum albumin (BSA, fraction V) in KPBS. Sections were incubated in anti-pSTAT3 or androgen receptor antibody diluted to 1:1,000 in 1% NGS, 0.4% triton X-100, 1% BSA in KPBS for 48 hr at 4°C. Sections were rinsed several times in KPBS before incubating in secondary goat anti-rabbit Alexa 488 (Molecular Pres Cat# A-11008, RRID:AB_143165) (1:500) in 1% NGS, 0.3% triton X- 100 in KPBS for 2 hr at room temperature. Sections were rinsed several times in KPBS, and a coverslip applied using Fluoromount G mounting medium (Southern Biotech).
2.3 Antibody characterization
Antibodies used in this study are summarized in Table 1, and described in greater detail by corresponding number below.
Table 1.
Primary antibodies
| Antibody | Immunogen | Manufacturer, Catalog #, RRID, Host Species, Monoclonal/Polyclonal | Concentration used |
|---|---|---|---|
| 1. Agouti Related Peptide (AgRP) | Synthetic peptide sequence N’- TRSCPNMATGRCYCFANFFR- CACPDCCPVQQGSEHLRVCRRSS-C’ | Phoenix Pharmaceuticals, H-003–53, AB_2313908, Rabbit Polyclonal | 1:2,500 |
| 2. Androgen Receptor (AR) | KLH-conjugated linear peptide corresponding to the N-terminus of rat Androgen Receptor (MEVQLGLGRVYPRPPSKTYRGC) | EMD Millipore, 06–680, AB_310214, Rabbit Polyclonal | 1:1,000 |
| 3. Estrogen Receptor alpha (ERα) | KLH-conjugated linear peptide corresponding to the C-terminus of rat Estrogen Receptor alpha (TYYIPPEAEGFPNTI) | EMD Millipore, 06–935, AB_310305, Rabbit Polyclonal | 1:10,000 |
| 4. Human HuC/HuD neuronal protein (HuC/D) | Human HuC/HuD neuronal protein | Molecular Probes, A21271, AB_221448, Mouse Monoclonal |
1:500 |
| 5. phospho-Signal Transducer and Activator of Transcription 3 (pSTAT3) | Synthetic phosphopeptide corresponding to residues surrounding Tyr705 of mouse Stat3 (ADPGSAAPyLKTKFIC) | Cell Signaling Technology, 9131L, AB_331587, Rabbit Polyclonal | 1:1,000 |
The polyclonal rabbit anti-AgRP antibody (AB_91683) was generated against synthetic peptide sequence N’-TRSCPNMATGRCYCF- ANFFRCACPDCCPVQQGSEHLRVCRRSS-C’. Specificity of this antibody has been demonstrated by immunolabeling in mouse hypothalamic tissue sections, which was blocked completely by preadsorption with AgRP peptide (83–132) (Nilsson, Lindfors, Fetissov, Hökfelt, & Johansen, 2007). A single band between 14–21kDa was recognized following SDS-PAGE in duck total brain and hypothalamic tissue samples (Mirabella, Esposito, Squillacioti, De Luca, & Paino, 2004). Furthermore, patterns of immunoreactivity in histological staining with AB_91683 are consistent with in situ hybridization data from mouse ARH (Hahn et al., 1998), as well as our own staining for AgRP in the hypothalamus in developing and adult mice.
Polyclonal anti-AR antibody used in this study was generated using KLH-conjugated linear peptide corresponding to the N-terminus of rat androgen receptor as the immunogen according to the manufacturer. Specificity of this antibody was confirmed by SDS-PAGE in African Cichlid fish demonstrating that it recognizes two bands between 70 and 100 kDa corresponding to alpha and beta forms of the receptor (Munchrath & Hofmann, 2010). In addition, preadsorption with the immunogen followed by immunofluorescence staining in tissue from rat brain sections completely abolished all signal (Munchrath & Hofmann, 2010).
According to the information provided by the manufacturer, polyclonal rabbit anti-estrogen receptor alpha antisera (AB_310305) recognizes the C-terminus amino acids of human, rat, and mouse. Specificity of this antibody, then referred to as C1355, has been demonstrated by western blot, which revealed that the antibody recognizes several bands of molecular weight 64–66 kDa, 50–55 kDa, 40–45 kDa, and 20–24 kDa, corresponding to different ERα forms (Friend, Resnick, Ang, & Shupnik, 1997). Immunodetection of all bands was eliminated by preadsorption with excess antigen (Friend et al., 1997). Furthermore, immunostaining in sensory and autonomic ganglia using AB_310305 is consistent with other antiestrogen receptor alpha antisera, and signal is not diminished by preabsorption with estrogen receptor beta protein (Papka et al., 2001).
Antibodies directed against HuC/D in this study have been widely cited in published literature used in human, mouse, rat, pig, reptile, non-human primate, sheep, zebrafish, chicken, horse, xenopus, and guinea pig according to the manufacturers information. The mouse monoclonal antibody was described by Marusich, et al. as binding several Hu/Elav neuronal proteins visualized by western blot around 40 kDa in human, avian, and mouse neural tissue (Marusich, Fur- neaux, Henion, & Weston, 1994). Preadsorbtion with HuD protein abolished antibody labeling in western blot (Marusich et al., 1994). The antibodies bind exclusively to neuronal antigens and are used in this study to identify the cellular boundaries of the PVH and DMH.
Polyclonal pSTAT3 antibody recognizes both alpha and beta forms revealed as 79 and 86 kDa molecular weight bands following western blot, according to the manufacturer information. Specificity of the antibody has been demonstrated in tissue from transgenic animals in which Tyr 1183 of the leptin receptor was mutated disrupting STAT3 signaling, referred to as ‘s/s’ mice (Bates et al., 2003). Robust pSTAT3 signal is observed in wild-type mice following leptin administration, but no immunoreactive signal was detected in tissue from s/s mice by either western blot or immunohistochemistry (Bates et al., 2003).
2.4 Image acquisition and analysis
The density of immunolabeled neuronal fibers was measured in discrete regions of interest (ROI) in the medial parvicellular region of the PVH (PVHmp) or ventral DMH (DMHv). Preautonomic regions of the PVH were not assessed due to inherent difficulties in visualization without the use of retrograde tracer injections to label specific populations, a technique that is not feasible given the size of cohorts necessary for this study. Anatomical features were identified cytoarchitecturally, using the neuronal marker HuC/D and nuclear regions classified as defined in Dong, 2008 (DongThe Allen Institute for Brain Science, 2008). All images were obtained using a laser scanning confocal microscope (Zeiss LSM 710) equipped with high N.A. objectives. Confocal image stacks were collected through the z-axis of each ROI at a frequency of 0.4 μm using a high-magnification, 63X oil-corrected objective (NA 1,4). Using Volocity software (Perkin Elmer), axonal fibers containing AgRP or tdTomato fluorescence were visualized in each image stack by using a thresholded intensity value. Identified fibers were then skeletonized to one-pixel thickness and the total length of each line measured and summed. The resulting values represent fiber density in a defined volume, which closely correspond to other methods used to measure axonal density such as use of the lipophilic tracer, 1,1’- dioctadecyl-3,3,3’3’-tetramethylindocarbocyanine perchlorate (DiI) (Bouret et al., 2004, 2008). While expression of AgRP peptide is, itself, leptin dependent (Morrison, 2005; Ollmann, 1997), statistical evaluation of AgRP fiber density following leptin treatment was made through comparisons between leptin-deficient Lepob/ob mice weeks after leptin treatment cessation, such that hormonal effects on gene expression would be comparable between groups.
To confirm effective leptin signaling in LRbcre-expressing neurons, the number of pSTAT3 immunoreactive cells colocalized with LRbcretdTomato endogenous fluorescence was measured in sections containing the ARH. Confocal images were collected in a defined ROI through a 1:4 series of sections through the ARH (unilaterally). The number of cells labeled for pSTAT3 immunoreactivity, tdTomato, or both were counted manually, aided by Volocity software. The total number of labeled cells in the ARH was estimated by multiplying the number of counted cells by 2, to account for bilateral ARH cell numbers, and then multiplied by 4, to account for the use of a single series of tissue sections (of 4 series collected) for quantification. This calculation approximates the total number of neurons in the ARH positive for pSTAT3, tdTomato, and colocalized populations.
For analysis of AgRP projections derived from neurons that express LRb, the density of fibers doubly labeled for AgRP and tdTomato was measured in LRbcretdTomato mice using Volocity software. The density of fibers containing both AgRP and tdTomato signals was measured and expressed as a percentage of singly labeled AgRP containing fibers in order to estimate the relative penetrance of LRb expression in AgRP projections to each target.
2.5 Statistical analyses
Statistical significance was analyzed using GraphPad Prism software, for which data are expressed as mean values ± SEM. Normality of the data was assessed using the D’Agostino Pearson test. For multiple comparisons with one independent variable, a one-way ANOVA followed by Holm-Sidak post hoc test was used. Comparisons included were between leptin treatments of equal duration. p values less than .05 were considered significant.
3 RESULTS
3.1The trophic effect of leptin on AgRP axonal growth is temporally restricted to a discrete developmental period
To evaluate temporal constraints of leptin’s trophic action on ARH outgrowth, the density of AgRP projections to the DMHv was measured on P60 in male Lepob/ob mice and WT littermates treated daily with either vehicle or leptin during several discrete time periods designed to test the timing and duration of effective leptin exposure. Consistent with previous findings (Bouret et al., 2004), the density of AgRP innervation in the DMHv was significantly decreased in vehicle injected Lepob/ob animals (Figure 1b,e) compared to vehicle injected WT animals, which displayed robust AgRP innervation of the DMHv (Figure 1a,d). Treatment of Lepob/ob mice with leptin from P4–14 or P16–26 significantly increased the density of AgRP labeled fibers in the DMHv compared to vehicle injected Lepob/ob mice. However, leptin treatment from P28–38 was not effective in increasing the density of AgRP projections to the DMHv (Figure 1c,f,g), suggesting that the neurotrophic actions of leptin on the development of ARH projections does not extend beyond the 4th week of postnatal life in mice.
FIGURE 1.
The trophic effect of leptin on AgRP axonal growth is restricted to the first 4 postnatal weeks. On P60, the density of AgRP projections to the DMHv was measured in male WT and Lepob/ob mice treated with either vehicle or leptin during one of several discrete time periods in early postnatal life. Representative images of AgRP innervation of the DMH in (a) WT vehicle injected, (b) Lepob/ob vehicle injected, and (c) Lepob/ob leptin treated mice in the treatment group spanning from P4–14. Dashed lines indicate the anatomical borders of the DMH. d, dorsal; c, central; v, ventral subregion of the DMH. 3V denotes the third ventricle. Scale bar, 70 μm. Square indicates the ROI in the DMHv used for quantification further illustrated in d-f using a high-magnification 63X oil corrected lens. Scale bar, 13 μm. (g) Quantification of AgRP axonal fiber density in the DMHv for all treatment groups revealed that after postnatal day 28, leptin no longer stimulates axonal growth from AgRP neurons. OB, Lepob/ob; Veh, Vehicle; Lep, Leptin. (WT Veh, n = 32, OB Veh, n = 22, OB Lep P4–8, n = 5, OB Lep P6–10, n = 4, OB Lep P8–12, n = 3, OB Lep P12–16, n = 4, OB Lep P4–14, n = 4, OB Lep P16–26, n = 5, OB Lep P28–38, n = 4) Data are expressed as mean ± SEM of the density of AgRP-containing axonal fibers within the DMHv in a set volume. Significance between groups was determined by one-way ANOVA and Holm-Sidak’s multiple comparisons test; * indicates p < .0001 compared to WT vehicle, ˆ indicates p < .05 compared to Lepob/ob vehicle
In addition to these 11-day treatment groups, several shorter (5-day) treatment groups were included in the study, including daily leptin injections from P4–8, P6–10, P8–12, and P12–16. None of the Lepob/ob mice in the 5-day leptin treatment groups exhibited a statistically significant increase in the density of AgRP containing projections to the DMHv compared to that of Lepob/ob animals injected with vehicle (Figure 1g).
3.2 Neurotrophic action of leptin treatment displays target specificity
In contrast to the effects of leptin on AgRP projections to the DMHv, previous studies have demonstrated that leptin treatment from P4–14 does not rescue AgRP innervation to the medial parvocellular compartment of the PVH (PVHmp), in male Lepob/ob mice (Bouyer & Simerly, 2013). Here, we confirm and extend this observation demonstrating that leptin treatment from P4–8, P6–10, P8–12, P12–16, P4–14, P16–26, or P28–38 was insufficient to rescue AgRP innvervation of the PVHmp in male Lepob/ob mice (Figure 2). Innervation of the PHVmp was examined in the same cohort of animals used to study rescue of ARH projections to the DMHv in Lepob/ob mice, thus confirming that that the inability to rescue AgRP innervation in the PVHmp is not due to technical issues, such as poor leptin lot efficacy, because leptin treatments were sufficient to stimulate growth of AgRP projections to the DMHv in the same animals.
FIGURE 2.
Leptin treatment is not sufficient to restore AgRP innervation to the PVHmp in leptin-deficient males. Representative images of AgRP innervation of the PVH in (a) WT mice injected with vehicle, (b) Lepob/ob mice injected with vehicle, and (c) Lepob/ob mice treated with leptin from P4–14. Dashed lines indicate the anatomical borders of the PVH. PVHmp, medial parvicellular part of the PVH. 3V, third ventricle. Scale bar, 40 μm. Square indicates the ROI in the PVHmp used for quantification, further illustrated in (d-f). Scale bar, 13 μm. (g) Quantification of AgRP axonal fiber density in the PVHmp for all treatment groups. OB, Lepob/ob; Veh, Vehicle; Lep, Leptin. (WT Veh, n = 32, OB Veh, n = 31, Lep P4–8, n = 5, OB Lep P6–10, n = 4, OB Lep P8–12, n = 3, OB Lep P12–16, n = 4, OB Lep P4–14, n = 4, OB Lep P16–26, n = 5, OB Lep P28–38, n = 4) Data are expressed as mean ± SEM of the density of AgRP-containing axonal fibers within each target region in a set volume. Significance between groups was determined by one-way ANOVA and Holm-Sidek multiple comparisons test; * indicates p < .0001 compared to WT vehicle
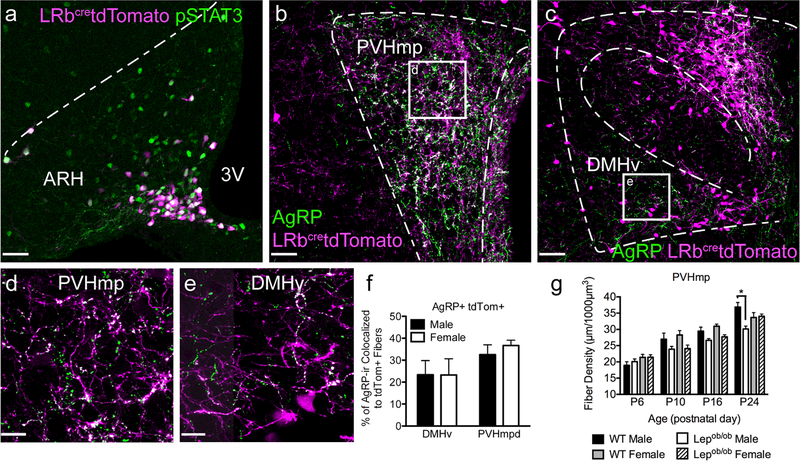
AgRP neurons do not appear to project equally to all nuclei innervated by AgRP neurons. Subpopulations of AgRP neurons provide inputs to distinct subsets of ARH targets and LepRb does not appear to be expressed in AgRP neurons that project to hypothalamic targets in adult mice (Betley et al., 2013). Therefore, it is possible that LepRb may be expressed transiently by subsets of ARH cells that project to either the PVHmp or DMHv. To test whether AgRP neurons that project to the DMHv or PVHmp have the capacity to respond to leptin during development, LepRbcretdTomato mice were used to visualize neurons that express LepRb in neonatal mice (P10). Following leptin injection, most (89%) tdTomato labeled ARH neurons displayed pSTAT3 immunoreactivity, indicating that the tdTomato reporter faithfully labels neurons expressing leptin receptors in neonatal mice, and that these neurons are capable of initiating downstream signaling (Figure 3a). To determine whether the AgRP neurons that project to the PVHmp and DMHv are differentially sensitive to leptin during development, the degree of colocalization between tdTomato and AgRP was measured in axonal projections labeled by immunofluorescence staining in P10 mice. Furthermore, this analysis was done in both males and females to determine whether a sexual dimorphism exists in the expression of leptin receptor by neurons projecting to either the PVHmp or DMHv. The data indicate that approximately 30% of AgRP immunoreactive fibers in the PVHmp contained the tdTomato label (i.e., are derived from leptin receptor expressing neurons) in both male and female mice on P10 (Figure 3b, d, f). In the DMHv, more than 20% of AgRP immunoreactive fibers colocalized tdTomato labeling in both males and females (Figure 3c, e, f). Therefore, functional LepRb signaling appears to be intact in AgRP neurons of male and female neonates that project to either the PVHmp or DMHv.
FIGURE 3.
AgRP neurons in the ARH that project to the PVHmp and DMHv are leptin sensitive during early postnatal life. (a) Representative image of leptin-induced pSTAT3 (green) colocalized to tdTomato expressing neurons (magenta) in the ARH of a LRbcretdTomato animal on P10, 1 hr following leptin injection. 3V, third ventricle. Dotted lines illustrate the boundaries of the nucleus. Scale bar, 40 μm. (b, c) Immunohistochemical labeling of AgRP projections (green) targeting the PVH (b), and DMH (c) in LRbcretdTomato mice. Squares indicate the ROI in the PVHmp and DMHv quantified and illustrated in (d) and (e). Representative high-magnification images of AgRP immunoreactivity (green) in the PVHmpd (d) and DMHv (e) colocalized to axonal projections from leptin receptor expressing neurons (magenta). Scale bar, 13 μm. (f) Quantification of the percentage of AgRP immunoreactivity (ir) colocalized to tdTomato labeled fibers in male and female LRbcretdTomato mice on P10 (male, n = 3; female, n = 3). (g) The density of AgRP projections to the PVHmp measured in male and female, WT and Lepob/ob mice on P6, P10, P16, and P24 are graphed. Data are expressed as mean ± SEM of the density of AgRP-containing axonal fibers within the PVHmp in a defined volume. Significance between groups was determined by one-way ANOVA and Holm-Sidek multiple comparisons test performed at each age; * indicates p < .01 compared to WT Male. Data are expressed as mean ± SEM
3.3 Neurotrophic action of leptin treatment on AgRP projections to the PVHmp is sexually dimorphic
The density of AgRP projections to the PVHmp was significantly reduced in both male and female Lepob/ob mice treated with vehicle, and remained low in male Lepob/ob mice treated with leptin from P4-P14, confirming previous results (Bouyer & Simerly, 2013). In contrast, treatment of female Lepob/ob mice with leptin during the same period was sufficient to restore the density of AgRP inputs to the PVHmp (Figure 4) indicating that this action of leptin is sexually dimorphic.
FIGURE 4.
Postnatal leptin treatment is sufficient to restore AgRP innervation to the PVHmp in leptin-deficient females. Representative images of the PVH in (a) WT vehicle treated, (b) Lepob/ob vehicle treated, and (c) Lepob/ob leptin treated female mice treated from P4–14. Dashed lines indicate the anatomical borders of the PVH. 3V denotes the third ventricle. Scale bar, 40 μm. Square indicates the ROI in the PVHmpd used for quantification, further illustrated in (d-f). Scale bar, 13 μm. (g) Quanification of AgRP axonal fiber density in the PVHmpd and DMHv in WT and Lepob/ob females treated with vehicle or leptin. OB, Lepob/ob; Veh, Vehicle; Lep, Leptin. (WT Veh, n = 18, OB Veh, n = 19, OB Lep P4–14, n = 4, OB Lep Leptin P16–24, n = 4, OB Lep P25–33, n = 5) Data are expressed as mean SEM of the density of AgRP-containing axonal fibers within each target region in a set volume. Significance between groups was determined by one-way ANOVA and Holm-Sidak multiple comparisons test; * indicates p < .0001 compared to WT vehicle, ˆindicates p <.05 compared to Lepob/ob vehicle
The timing of the critical period for AgRP outgrowth was also examined in female Lepob/ob mice and WT littermates treated with either vehicle or leptin from P4–14, P16–24, and P25–33. Despite a shorter (9-day) treatment period, treatment of Lepob/ob females from P16–24 was sufficient to significantly increase the density of AgRP containing fibers in both the PVHmp and DMHv compared to Lepob/ob females treated with vehicle. However, treatment of Lepob/ob females from P25–33 was not sufficient to restore AgRP innervation density in either the PVHmp or DMHv, suggesting that the capacity for leptin to promote growth of ARH projections to hypothalamic targets is lost around P25-P28 in both males and females (Figure 4).
To determine whether the ability of leptin to rescue ARH projections to the PVHmp is due to a sexual dimorphism in the timing of the growth of AgRP projections, we quantified the density of AgRP containing fibers in the PVHmp of male and female WT or Lepob/ob mice during postnatal development. On P6, the density of AgRP containing axonal projections in the PVHmp was not different between sexes or genotypes, and no significant differences in the density of AgRP projections were found between male and female genotype-matched animals at any time point. However, by P24, the density of AgRP fibers was significantly lower in male Lepob/ob mice, when compared with that of WT males (Figure 3g). No statistically significant effect was found in the density of AgRP projections between male and female age- and genotype- matched samples, suggesting that there is not a sexual dimorphism in the timing of the growth of AgRP projections.
The sexually dimorphic response in the ability of leptin treatment to stimulate growth of AgRP projections to the PVHmp could also result from differential sensitivity to gonadal hormones. To determine whether males and females differentially express receptors for gonadal steroids in AgRP neurons, we used immunohistochemistry to examine the expression of the androgen receptor (AR) and estrogen receptor alpha (ERα) in the ARH on P10. Mice expressing GFP in NPY neurons were labeled immunohistochemically for AR and ERα to compare cellular patterns of hormone receptor expression in AgRP/NPY neurons of the ARH. NPY and AgRP have been demonstrated to be co-produced in 94% of NPY neurons in the ARH (Hahn et al., 1998), therefore, GFP was used as a proxy for AgRP neurons in these experiments. While a significant number of ARH neurons express AR or ERα on P10, only a very small percentage of NPY neurons appear to express either gonadal hormone receptor at this age (Figure 5).
FIGURE 5.
AgRP neurons do not express androgen receptor or estrogen receptor alpha during early postnatal development.
Representative images of estrogen receptor alpha (a and c; ERα; magenta) or androgen receptor (b and d; AR; magenta) immunoreactivity in the ARH of male (a and b) and female (c and d) NPYGFP animals on P10. Square in (a) denotes the location of insets. 3V, third ventricle
4 DISCUSSION
The capacity for synaptic plasticity and the impact of environmental influences on brain architecture is not constant throughout life. The periods when the greatest changes occur are often limited to prenatal or early postnatal development. The ability of leptin to promote innervation of hypothalamic nuclei by AgRP neurons in the ARH appears to display such a restricted developmental sensitive period, because the severe deficits in AgRP projections apparent in Lepob/ob mice can be rescued with exogenous leptin treatment only in neonatal mice (Bouret et al., 2004; Bouyer& Simerly, 2013).
In the current study, we defined the closure of this critical neurodevelopmental period. The neurotrophic effect of leptin on growth of AgRP projections is quite robust in early postnatal life, extending into the fourth postnatal week, but does not extend beyond P28, after which exogenous leptin is no longer able to rescue innervation of the DMHv in leptin deficient mice. In addition to its temporal specificity, leptin does not appear to affect all ARH projections equally, nor does it function during development of AgRP projections identically in males and females. In females, exogenous leptin treatment is sufficient to rescue the density of AgRP projections to both the PVHmp and DMHv. However, in males, postnatal leptin treatment rescues AgRP projections to the DMHv, but not to the PVHmp, suggesting that leptin facilitates growth of these projections in conjunction with another sex specific mechanism. Together, the data identify a developmental window of environmental sensitivity, in which ARH circuits are structurally specified, with lasting consequences for metabolic status that may be distinct for males and females (for summary, see Figure 6).
FIGURE 6.
Schematic model of the early postnatal trophic effects of leptin on development of AgRP neuronal projections. (a) The timing of the naturally occurring early postnatal surge in serum leptin, known to stimulate the growth of ARH projections, is graphed. Treatment of Lepob/ob mice with exogenous leptin during several discrete time periods enabled identification of the closure of a critical period for growth of ARH AgRP projections (Bars below graph indicate each leptin treatment period. Green bars indicate treatment periods that rescued innervation and red bars indicate treatment periods that were not effective). Short duration, 5-day, leptin treatment periods from P4–8, P6–10, P8–12, or P12–16 were not sufficient to rescue AgRP innervation density to the DMHv in Lepob/ob mice. By contrast, longer duration, 11-day, leptin treatment periods from P4–14 and P16–26 were able to rescue innervation deficits in the DMHv of Lepob/ob mice. However, leptin treatment of Lepob/ob mice from P28–38 was no longer effective, suggesting that the critical period for the trophic actions of leptin is closed at this time. (b) Early postnatal leptin treatment also revealed striking sexually dimorphic, target-dependent effects on innervation of the PVHmp. While treatment of Lepob/ob mice with leptin (for 9 or 11 days during the critical period) was sufficient to rescue deficits in projections to the DMHv in both males and females, deficits in innervation to the PVHmp were only rescued in female Lepob/ob mice treated with leptin
4.1 Timing and duration of a critical period for leptin-stimulated ARH outgrowth
Defining the timing of cellular responsiveness to signals that control formation of circuit architecture is essential in the context of obesity and metabolic disease, as it specifies windows of potential vulnerability, or opportunity for circuit manipulation. In the current study, we reveal a critical period of neural development in which leptin (and possibly other stimuli such as nutrition, or other hormones and environmental stimuli) can shape ARH connectivity and function. The use of the leptin deficient mouse allows us to model this manipulation and provides a foundation for elucidation of the mechanisms responsible for developmental plasticity of ARH projections, with implications for circuit programming relevant to childhood metabolic disease.
In the current study, we find that treatment of Lepob/ob mice with exogenous leptin for an 11-day period, as late as P16–26, restored the growth of AgRP projections to the DMHv. By contrast, leptin treatment from P28–38 was not sufficient to rescue the deficits in projections observed in Lepob/ob mice, suggesting that leptin loses its trophic action on AgRP neurons at approximately the fourth postnatal week. There was no discernable difference between early leptin treatments (P4-P14) and treatments during the third postnatal week (P16-P26). This finding implies that leptin sensitivity during development of AgRP projections to the DMHv remains relatively constant during the first four weeks of life. This observation is interesting in view of the rapid fall in leptin levels that occurs near the second to third postnatal week (Ahima et al., 1998), because it suggests that the brain remains responsive to the trophic action of leptin long after leptin levels fall from their peak, which occurs at approximately P8–12. Nevertheless, the ability of AgRP neurons to respond to the developmental actions of leptin extends for at least 1 week beyond the end of the naturally occurring postnatal surge in leptin secretion.
While leptin administration beyond the critical period is not sufficient to rescue large-scale axonal elaboration, synaptic inputs to ARH neurons can be influenced by leptin in adulthood. Acute leptin treatment in adult Lepob/ob mice reduces the ratio of excitatory to inhibitory synaptic inputs onto NPY neurons within 6 hr of treatment (Pinto et al., 2004). It remains to be determined whether these rapid plastic effects are under the control of activity dependent mechanisms, or leptin receptor-driven signaling cascades such as pSTAT3 or pERK, which have been shown to be responsible for the large-scale axonal outgrowth examined in the current study (Bouret, Bates, Chen, Myers, & Simerly, 2012). Nevertheless, it is clear that leptin functions not only to modulate synaptic density and neuronal excitability acutely, but also impacts the organization of ARH projections, which may permanently alter how regulation of these neurons by leptin in adulthood affects other components of metabolic circuitry.
The postnatal critical period for the developmental effects of sex steroid hormones on several populations of hypothalamic neurons displays a temporal organization that is quite different from that of leptin. In rats, a single injection of testosterone during the first few days of life effects a dramatic masculinization of both brain structure and physiology, and the critical period for estrogen's action appears to be restricted largely to the first ten days of life (Arnold & Gorski, 1984; Dohler et al., 1984). However, steroid sensitivity and timing vary for different cell types. For example, treatment of female rats with sex steroid hormones for 24 hr induces caspase-dependent cell death in the anteroventral periventricular nucleus, but not if the hormone treatment is delayed until after the sixth postnatal day (Waters & Simerly, 2009), whereas full masculinization of serotonergic inputs to the medial preoptic nucleus requires prenatal exposure to testosterone (Simerly, Swanson, & Gorski, 1984). The postnatal surge in testosterone in males, known to be responsible for most aspects of sexual differentiation, occurs on the first day of life, so even in the case of sex steroids the capacity of neurons to respond to exogenous hormone treatment extends well beyond the time when hormone exposure normally occurs in vivo.
In contrast to the rapid actions of sex steroids on brain development, we did not detect acute effects of leptin on the growth of AgRP projections to the DMHv in male mice. There also appears to be a minimum duration of leptin exposure required to achieve robust target innervation. While leptin treatment for 11 days, from P4–14 or P16–26, was sufficient to cause significant increases in growth of AgRP projections, leptin treatment for 5 days was ineffective. However, fiber densities in the DMHv following 5 days of leptin treatment tended to be intermediate between those observed for Lepob/ob mice treated with leptin for 11 days, and vehicle treated controls. Thus, the length of leptin exposure appears to be positively correlated with the density of AgRP projections. This finding is consistent with the temporal dynamics of signaling cascades mediating leptin-induced growth of ARH projections. In the mature brain, the leptin receptor signals through three known signal transduction cascades including the STAT3, MAP kinase/ERK, and AKT/PI3 pathways (Bates et al., 2003; Bjorbaek et al., 2001; Björnholm et al., 2007; Goetze et al., 2002; Hill et al., 2008). During development, pSTAT3 and pERK mediated signaling is required for normal growth of AgRP projections (Bouret, et al. 2012). The kinetics of STAT3 and MEK/ERK activation occur on rapid timescales, with signals peaking and returning to basal levels within a few hours (El-Haschimi, Pierroz, Hileman, Bj∅rbæk, & Flier, 2000; Mir- shamsi et al., 2004). Thus, our results are consistent with the notion that these signaling pathways need continued activation in order to couple effectively with growth promoting pathways affecting axon targeting of AgRP neurons.
The timing of the closure of the critical period for leptin dependent stimulation of axonal outgrowth from AgRP neurons is coincident with other indicators of their maturation, including establishment of mature electrophysiological properties of AgRP neurons, and the ability of leptin to inhibit food intake. In mature AgRP neurons, leptin signaling induces membrane hyperpolarization (Spanswick, Smith, Groppi, Logan, & Ashford, 1997; Takahashi & Cone, 2005; van den Top, Lee, Whyment, Blanks, & Spanswick, 2004). By contrast, during hypothalamic development, leptin appears to activate AgRP neurons, inducing membrane depolarization (Baquero et al., 2014). The transition from leptin induced depolarization to hyperpolarization as AgRP neurons mature is complete by P30, commensurate with an increase in the expression of KATP channels, which is known to facilitate membrane hyperpolarization and suppression of activity (Baquero et al., 2014). In addition, leptin itself has no impact on food intake until P28 (Mistry, Swick, & Romsos, 1999), despite the fact that the PVH, DMH, and LHA receive ample innervation from the ARH well before this time (Bouret, 2004). Taken together, these findings suggest that AgRP projections reach functional maturity at approximately 4 weeks of age, when they become refractive to the developmental actions of leptin and achieve adult electrophysiological properties.
4.2 Sexually dimorphic target dependence of leptin action
In our experiments, leptin administration is limited to discrete postnatal periods, but it should be noted that following the postnatal treatments, Lepob/ob mice are completely leptin deficient. Despite the absence of circulating leptin, adult Lepob/ob mice that were treated with leptin postnatally display dense AgRP projections to the DMHv, indicating that sustained exposure to leptin is not required for maintenance of this pathway. Although leptin is required for normal development of AgRP projections to the PVHmp in male Lepob/ob mice, these projections are not rescued by postnatal leptin treatments alone, raising the possibility that these projections may require sustained circulating leptin in order to be maintained. The apparent transient expression of LRb in AgRP projections to the PVH argues against this hypothesis, but may represent a possible mechanism for limiting the developmental actions of leptin to the perinatal period (Betley et al., 2013). In contrast to males, AgRP projections to the PVHmp in female Lepob/ob mice are rescued by postnatal leptin treatment alone. Thus, the developmental actions of leptin during the postnatal critical period display not only target specificity, but there also appear to be sex-dependent mechanisms that impact the trophic actions of leptin to induce ARH growth and connectivity.
Interestingly, no sexual differences were detected in expression of receptors for either leptin or gonadal steroid hormones in AgRP neurons. Furthermore, the growth of AgRP neurons to the PVH of WT males and females display similar developmental timelines. Although, the mechanisms underlying the sex specific effects of leptin on development of AgRP projections remain unknown, they may reflect changes in responses of ARH neurons caused by prior exposure to steroid hormones. The ARH exhibits a sexually dimorphic glial morphology as early as P1 (Mong & McCarthy, 2002), and the number of neuropeptide Y expressing neurons in the caudal ARH is determined by postnatal testosterone exposure (Urban, Bauer-Dantoin, & Levine, 1993). Furthermore, ARH neurons exhibit sexually dimorphic patterns of synaptic inputs (Matsumoto & Arai, 1980). Perhaps these reported sex differences in ARH structure impact the ability of leptin to rescue AgRP projections to the PVHmp. The possibility that sex differences in synaptic and neuronal-glial architecture within the ARH may impact the neurotrophic action of leptin should be explored.
In summary, the results presented here demonstrate that the ability of leptin to stimulate growth of ARH axons to their targets is restricted to a discrete time period that extends until postnatal day 28 in the mouse. Furthermore, the data indicate that the density of AgRP containing projections to the PVHmp is permanently specified by postnatal leptin in females, but requires an additional sex-specific factor in males. While age correlations between mice and humans are only general approximations, these data suggest that the corresponding sensitive period for leptin-mediated ARH growth in humans may extend into early postnatal life, underscoring the importance of proper nutrition and hormonal balance during this important period of human hypothalamic development.
ACKNOWLEDGMENTS
The studies were supported by NIH grants DK105217 and DK089237. AK and CHW were supported by Pre-Doctoral Research Career Development Fellowships from The Saban Research Institute.
REFERENCES
- Ahima RS, Prabakaran D, & Flier JS (1998). Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. Journal of Clinical Investigation, 101(5), 1020-1027. 10.1172/JCI1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, & Gorski RA (1984). Gonadal steroid induction of structural sex differences in the central nervous system. Annual Review of Neuroscience, 7, 413-442. 10.1146/annurev.ne.07.030184.002213 [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, & Sternson SM (2012). Deconstruction of a neural circuit for hunger. Nature, 488, 172-177. 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero AF, de Solis AJ, Lindsley SR, Kirigiti MA, Smith MS, Cowley MA, & Grove KL (2014). Developmental switch of leptin signaling in arcuate nucleus neurons. Journal of Neuroscience, 34 (30), 9982-9994. 10.1523/JNEUROSCI.0933-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AWK, Wang Y & Myers MG (2003). STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature, 421 (6925), 856-859. 10.1038/nature01388 [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, & Sternson SM (2013). Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell, 155(6), 1337-1350. 10.1016/j.cell.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, & Sternson SM (2015). Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature, 521(7551), 180-185. 10.1038/nature14416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, & Dong HW (2011). Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. The Journal of Comparative Neurology, 520(1), 6-33. 10.1002/cne.22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, & Flier JS (2001). Divergent roles of SHP-2 in ERK activation by leptin receptors. Journal of Biological Chemistry, 276(7), 4747-4755. 10.1074/jbc.M007439200 [DOI] [PubMed] [Google Scholar]
- Björnholm M, MUnzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, & Myers MG (2007). Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. The Journal of Clinical Investigation, 117(5), 1354-1360. 10.1172/JCI30688DS1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG (2004). Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. Journal of Neuroscience, 24(11), 2797-2805. 10.1523/JNEUROSCI.5369-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG, & Simerly RB (2012). Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. Journal of Neuroscience, 32 (4), 1244-1252. 10.1523/JNEUROSCI.2277-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, & Simerly RB (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science, 304 (5667), 108-110. 10.1126/science.1095004 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, & Sim- erly RB (2008). Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metabolism, 7(2), 179-185. 10.1016/j.cmet.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer K, & Simerly RB (2013). Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. Journal of Neuroscience, 33(2), 840-851. 10.1523/JNEUROSCI.3215-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, & Burn P (1995). Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science, 269(5223), 546-549. [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, & Palmiter RD (2013). Genetic identification of a neural circuit that suppresses appetite. Nature, 503(7474), 111-114. 10.1038/nature12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, & Cone RD (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron, 24(1), 155-163. [DOI] [PubMed] [Google Scholar]
- Dong H-W (2008). The Allen Reference Atlas, (Book + CD-ROM). Hoboken, New Jersey: Wiley [Google Scholar]
- Döhler KD, Hancke JL, Srivastava SS, Hofmann C, Shryne JE, & Gorski RA (1984). Participation of estrogens in female sexual differentiation of the brain; neuroanatomical, neuroendocrine and behavioral evidence. Progress in Brain Research, 61, 99-117. 10.1016/S0079-6123(08)64430-1 [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bj∅rbæk C, & Flier JS (2000). Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. The Journal of Clinical Investigation, 105(12), 1827-1832. 10.1172/JCI9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, & Shupnik MA (1997). Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Molecular and Cellular Endocrinology, 131(2), 147-155. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, & Lowell BB (2016). Dynamic GABAergic afferent modulation of AgRP neurons. Nature Neuroscience, 19(12), 1628-1635. 10.1038/nn.4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze S, Bungenstock A, Czupalla C, Eilers F, Stawowy P, Kintscher U, & Grafe M (2002). Leptin induces endothelial cell migration through Akt, which is inhibited by PPAR -ligands. Hypertension, 40(5), 748-754. 10.1161/01.HYP.0000035522.63647.D3 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, & Baskin DG (2002). Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology, 143 (1), 239-246. 10.1210/endo.143.1.8589 [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, & Brüning JC (2005). Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience, 8(10), 1289-1291. 10.1038/nn1548 [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, & Schwartz MW (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience, 1(4), 271-272. 10.1038/1082 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, & Friedman JM (1995). Weight-reducing effects of the plasma protein encoded by the obese gene. Science, 269(5223), 543-546. [DOI] [PubMed] [Google Scholar]
- Hetherington AW, & Ranson SW (1940). Hypothalamic lesions and adiposity in the rat. The Anatomical Record, 78(2), 149-172. 10.1002/ar.1090780203 [DOI] [Google Scholar]
- Hewson AK, Tung LYC, Connell DW, Tookman L, & Dickson SL (2002). The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes, 51(12), 3412-3419. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, & Elmquist JK (2008). Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of Clinical Investigation, 118(5), 1796-1805. 10.1172/JCI32964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Maeng L, Bj∅rbæk C, & Grill HJ (2007). Leptin and the control of food intake: Neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology, 148(5), 2189-2197. 10.1210/en.2006-1572 [DOI] [PubMed] [Google Scholar]
- Leshan RL, Björnholm M, MUnzberg H, & Myers MG (2006). Lep- tin receptor signaling and action in the central nervous system. Obesity (Silver Spring, Md.), 14 Suppl 5, 208S-212S. 10.1038/oby.2006.310 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, & Banks WA (2004). Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 286(1), R143-50. 10.1152/ajpregu.00393.2003 [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, & Weston JA (1994). Hu neuronal proteins are expressed in proliferating neurogenic cells. Journal of Neurobiology, 25(2), 143-155. 10.1002/neu.480250206 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, & Arai Y (1980). Sexual dimorphism in “wiring pattern” in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Research, 190(1), 238-242. [DOI] [PubMed] [Google Scholar]
- Mirabella N, Esposito V, Squillacioti C, De Luca A, & Paino G (2004). Expression of agouti-related protein (AgRP) in the hypothalamus and adrenal gland of the duck (Anas platyrhynchos). Anatomy and Embryology, 994, 267 10.1007/s00429-004-0431-0 [DOI] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, & Ashford ML (2004). Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neuroscience, 5, 54 10.1186/1471-2202-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry AM, Swick A, & Romsos DR (1999). Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. The American Journal of Physiology, 277(3 Pt 2), R742-7. [DOI] [PubMed] [Google Scholar]
- Mong JA, & McCarthy MM (2002). Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Research. Developmental Brain Research, 139(2), 151-158. [DOI] [PubMed] [Google Scholar]
- Morrison CD (2005). Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH- kinase signaling. AJP: Endocrinology and Metabolism, 289(6), E1051-E1057. 10.1152/ajpendo.00094.2005 [DOI] [PubMed] [Google Scholar]
- Munchrath LA, & Hofmann HA (2010). Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. The Journal of Comparative Neurology, 518(16), 3302-3326. 10.1002/cne.22401 [DOI] [PubMed] [Google Scholar]
- Myers MG Jr, MUnzberg H, Leinninger GM, & Leshan RL (2009). The geometry of leptin action in the brain: More complicated than a simple ARC. Cell Metabolism, 9(2), 117-123. 10.1016/j.cmet.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I, Lindfors C, Fetissov SO, Hökfelt T, & Johansen JE (2007). Aberrant agouti-related protein system in the hypothalamus of theanx/anx mouse is associated with activation of microglia. The Journal of Comparative Neurology, 507(1), 1128-1140. 10.1002/cne.21599 [DOI] [PubMed] [Google Scholar]
- Ollmann MM (1997). Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science, 278(5335), 135-138. 10.1126/science.278.5335.135 [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, & Shupnik M (2001). Estrogen receptor-α and -β immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell and Tissue Research, 304(2), 193-214. 10.1007/s004410100363 [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, & Horvath TL (2004). Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science, 304(5667), 110-115. 10.1126/science.1089459 [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, & Elmquist JK (2002). The need to feed: Homeostatic and hedonic control of eating. Neuron, 36(2), 199-211. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley RJ, & Baskin DG (2000). Central nervous system control of food intake. Nature, 404 (6778), 661-671. 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, & Elmquist JK (2011). Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. Journal of Clinical Investigation, 121(6), 2413-2421. 10.1172/JCI43703DS1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, & Gorski RA (1984). Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. The Journal of Comparative Neurology, 225(2), 151-166. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, & Ashford ML (1997). Leptin inhibits hypothalamic neurons by activation of ATP sensitive potassium channels. Nature, 390(6659), 521-525. 10.1038/37379 [DOI] [PubMed] [Google Scholar]
- Sternson SM, Atasoy D, Betley JN, Henry FE, & Xu S (2016). An emerging technology framework for the neurobiology of appetite. Cell Metabolism, 23(2), 234-253. 10.1016/j.cmet.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Swanson LW (2000). Cerebral hemisphere regulation of motivated behavior. Brain Research, 886(1–2), 113-164. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, & Cone RD (2005). Fasting Induces a large, leptin dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology, 146(3), 1043-1047. 10.1210/en.2004-1397 [DOI] [PubMed] [Google Scholar]
- Urban JH, Bauer-Dantoin AC, & Levine JE (1993). Neuropeptide Y gene expression in the arcuate nucleus: Sexual dimorphism and modulation by testosterone. Endocrinology, 132(1), 139-145. 10.1210/endo.132.1.8419120 [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, & Spanswick D (2004). Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature Neuroscience, 7(5), 493-494. 10.1038/nn1226 [DOI] [PubMed] [Google Scholar]
- Waters EM, & Simerly RB (2009). Estrogen induces caspase dependent cell death during hypothalamic development. Journal of Neuroscience, 29(31), 9714-9718. 10.1523/JNEUROSCI.0135-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, & Jeanrenaud B (1993). Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology, 133(4), 1753-1758. 10.1210/endo.133.4.8404618 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, & Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425-432. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]