Abstract
Background
Improper use of antimicrobials can cause adverse drug events and high costs. The purpose of this study was to investigate the frequency and potential drug–drug interactions associated with antimicrobials among hospitalized patients.
Material/Methods
This study was conducted on the same day in 5 different hospitals in Turkey. We included patients aged ≥18 years who received at least 1 antimicrobial drug and at least 1 of any other drug. The Micromedex® online drug reference system was used to control and describe the interactions. Drug interactions were classified as contraindicated, major, moderate, and minor.
Results
Potential drug–drug interactions with antimicrobials were 26.4% of all interactions. Five (42%) of 12 contraindicated interactions and 61 (38%) of 159 major interactions were with antimicrobials. Quinolones, triazoles, metronidazole, linezolid, and clarithromycin accounted for 173 (25.7%) of 673 prescribed antimicrobials, but were responsible for 141 (92.1%) of 153 interactions. In multivariate analysis, number of prescribed antimicrobials (odds ratio: 2.3001, 95% CI: 1.6237–3.2582), number of prescribed drugs (odds ratio: 1.2008, 95% CI: 1.0943–1.3177), and hospitalization in the university hospital (odds ratio: 1.7798, 95% CI: 1.0035–3.1564) were independent risk factors for developing drug interactions.
Conclusions
Due to risk of drug interactions, physicians should be more cautious when prescribing antimicrobials, particularly when prescribing quinolones, linezolid, azoles, metronidazole, and macrolides.
MeSH Keywords: Anti-Infective Agents, Drug Interactions, Polypharmacy
Background
The use of antimicrobials in hospitals has been increasing in recent years, and over a third of antibiotics are not prescribed compliant with guidelines [1]. Physicians should be aware of the benefits and risks of prescribing antimicrobials. They should know whether it is beneficial to prescribe an antimicrobial, which antimicrobial to prescribe, and the dosage and treatment duration of it. Improper use of antimicrobials may cause undesired adverse drug events (ADEs) and high costs [2].
Potential drug–drug interactions (PDDIs) are among the leading preventable causes of ADEs. In hospitalized patients, it was estimated that 17% of all preventable ADEs were caused by DDI and that approximately 1% of hospitalized patients experienced an ADE due to DDI [3]. PDDIs may also cause treatment failure besides the ADE, which is an important cause of morbidity, mortality, and high health care costs [4,5]. Polypharmacy, many prescribers, and advanced age are the defined risk factors for occurrence of PDDIs [6]. PDDIs may occur with antibiotics, and physicians should control the PDDIs when prescribing antibiotics, just as with other medicines. Antimicrobials are among the leading drug groups in the general PDDIs studies, and the status of the PDDIs with antimicrobials is not very clear [6,7]. Because of insufficient data, in the present study we investigated the frequency and type of PDDIs with only antimicrobials in hospitalized patients.
Material and Methods
Setting and study population
This multicenter, observational, point-prevalence study was conducted on the same day (15 July 2016) in 5 different hospitals in Turkey:
Cukurova University Hospital (CUH), a 1200-bed tertiary care hospital located in the Mediterranean region.
Adana Numune Training and Research Hospital (ANTRH), a 910-bed tertiary care hospital located in the Mediterranean region.
Zonguldak Bulent Ecevit University Hospital (ZBEUH), a 527-bed tertiary care hospital located in the West-Black Sea region.
Kahta State Hospital (KSH), a 150-bed secondary care hospital located in the Southeast Anatolia region.
Kirikkale University Hospital (KUH), a 200-bed tertiary care hospital located in the Middle Anatolia region.
Hospitalized patients who were aged ≥18 and received at least 1 administration of intravenous or oral antimicrobials and at least 1 of any other drug were included in the study (Figure 1). All medications and clinical data for patients were collected from the electronic hospital data management system and treatment charts of the patients. Demographic and clinical characteristics of patients, administered antimicrobials, and other drugs with generic names were recorded. Ethics Committee approval was obtained from Cukurova University Medical Faculty.
Figure 1.
Flowchart of patient selection.
Potential drug–drug Interactions
A PDDI was defined as 2 potentially interacting drugs that were administered concomitantly. Micromedex® online drug reference was used to control and define the types of PDDIs [8,9]. Drug interactions were classified into 4 main levels based on the severity:
Contraindicated: The drugs are contraindicated for concurrent use.
Major: The interaction may be life-threatening and/or require medical intervention to minimize or prevent serious adverse effects.
Moderate: The interaction may result in exacerbation of the patient’s condition and/or require an alteration therapy.
Minor: The interaction would have limited clinical effects. Manifestations may include an increase in the frequency or severity of the adverse effects, but generally would not require a major alteration in therapy.
Statistical analysis
Statistical analysis was performed using SPSS 20.0. Descriptive statistics are presented using percentages, median, min-max values, means, and standard deviations. The variables were investigated using Kolmogorov-Smirnov test to determine whether they were normally distributed. The patients were divided into 2 groups due to whether they had PDDIs with antimicrobials or not. The t test or Mann-Whitney U test for continuous variables and chi-square or Fischer exact test for discrete variables were used for univariate analysis between these 2 groups. For the logistic regression analysis, the possible factors identified with univariate analysis were further entered into the logistic regression analysis to determine independent predictors of PDDIs risk. A p-value of less than 0.05 was considered to be statistically significant.
Results
We included 427 patients in the study, with a mean age of 57±18 years and 208 (48.7%) were males. Number of patients, mean ages, sex, number of the patients in internal medicine clinics, and surgical clinics according to different hospitals are shown in Table 1. There were 108 patients (25.3%) hospitalized in intensive care units (ICU).
Table 1.
Characteristics of the patients in different hospitals.
| Hospital | Patients, n | Age, (mean ±SD) | Male, n (%) | Internal Medicine Clinics n (%) | Surgical Clinics n (%) |
|---|---|---|---|---|---|
| CUH | 168 | 55±16 | 77 (45.8) | 85 (50.6) | 83 (49.4) |
| ANTRH | 97 | 58±18 | 53 (54.6) | 48 (49.5) | 49 (50.5) |
| ZBEUH | 56 | 64±13 | 26 (46.4) | 36 (64.3) | 20 (35.7) |
| KSH | 55 | 57±22 | 26 (47.3) | 21 (38.2) | 34 (61.8) |
| KUH | 51 | 58±17 | 26 (51) | 34 (66.7) | 17 (33.3) |
| Total | 427 | 57±18 | 208 (48.7) | 224 (52.5) | 203 (47.5) |
CUH – Cukurova University Hospital; ANTRH – Adana Numune Training and Resarch Hospital; ZBEUH – Zonguldak Bulent Ecevit University Hospital; KSH – Kahta State Hospital; KUH – Kırıkkale University Hospital.
Drugs were administered a total of 2799 times, and 673 (24.0%) of them were antimicrobials. The median number of drugs and antimicrobials per patient were 6 (min: 1, max: 16) and 1 (min: 1, max: 6), respectively. There were 579 PDDIs detected in 229 patients (53.6%).
There were 153 PDDIs detected in 97 (22.7%) patients, considering only antimicrobial drug interactions. PDDIs with antimicrobials were 26.4% of all PDDIs. Five (42.0%) of 12 contraindicated PDDIs and 61 (38.0%) of 159 major PDDIs were with antimicrobials (Table 2).
Table 2.
Number of contraindicated, major, moderate and minor PDDIs with antimicrobials and other drugs.
| Contraindicated | Major | Moderate | Minor | Total (%) | |
|---|---|---|---|---|---|
| PDDIs with antimicrobials | 5 | 61 | 78 | 9 | 153 (26.4) |
| Other PDDIs | 7 | 159 | 229 | 31 | 426 (73.6) |
| Total (%) | 12 (2.0) | 220 (38.0) | 307 (53.0) | 40 (7.0) | 579 (100.0) |
PDDIs – potential drug–drug interactions.
While cephalosporins and carbapenems were the most commonly prescribed antimicrobials (23.0% and 19.3% respectively), there were no PDDIs with these antimicrobials. On the other hand, quinolones, triazoles, metronidazole, linezolid, and clarithromycin were 173 of 673 prescribed antimicrobials (25.7%), but they were responsible for 141 (92.1%) of 153 PDDIs (Table 3, Figure 2). The most common PDDIs (contraindicated, major, moderate, minor) with antimicrobials are shown in Table 4.
Table 3.
Number of PDDIs due to different antimicrobial groups.
| Antibiotic groups | No. of used antimicrobials (%) | No. of contraindicated PDDIs | No. of major PDDIs | No. of moderate PDDIs | No. of minor PDDIs |
|---|---|---|---|---|---|
| Cephalosporins | 155 (23.0) | – | – | – | – |
| Carbapenems | 130 (19.3) | – | – | – | – |
| Penicillins | 75 (11.1) | – | 2 | – | – |
| Quinolones | 69 (10.3) | – | 27 | 28 | 5 |
| Metronidazole | 62 (9.2) | – | 17 | 14 | – |
| Tigecycline | 34 (5.1) | – | – | 2 | – |
| Glycopeptides | 24 (3.6) | – | – | – | – |
| Colistin | 18 (2.7) | – | – | – | – |
| Triazoles | 17 (2.5) | 2 | 2 | 16 | – |
| Linezolid | 16 (2.4) | 3 | 10 | 4 | – |
| Anti-tuberculous* | 14 (2.1) | – | – | 8 | – |
| Daptomycin | 11 (1.6) | – | – | – | – |
| Clarithromycin | 9 (1.3) | – | 7 | 7 | 4 |
| Tetracycline | 9 (1.3) | – | – | – | – |
| Other** | 30 (4.5) | – | 1 | 1 | – |
| Total, n (%) | 673 (100.0) | 5 | 66*** | 80**** | 9 |
PDDIs – potential drug–drug interactions.
Isoniazid, Rifampicin, Pyrazinamide, Ethambutol;
Acyclovir, Valacyclovir, Entecavir, Amikacin, Gentamicin, Clindamycin, Fusidic Acid, Co-Trimoxosazole, Liposomal Amphotericin B, Anidulafungin, Caspofungin, Terbinafine.
Actual major interaction number was 61, but it was shown in the table 66 because ciprofloxacin and metronidazole interacted with each other five times.
Actual moderate interaction number was 78, but it was shown in the table 80 because Linezolid and Rifampicin interacted with each other two times.
Figure 2.
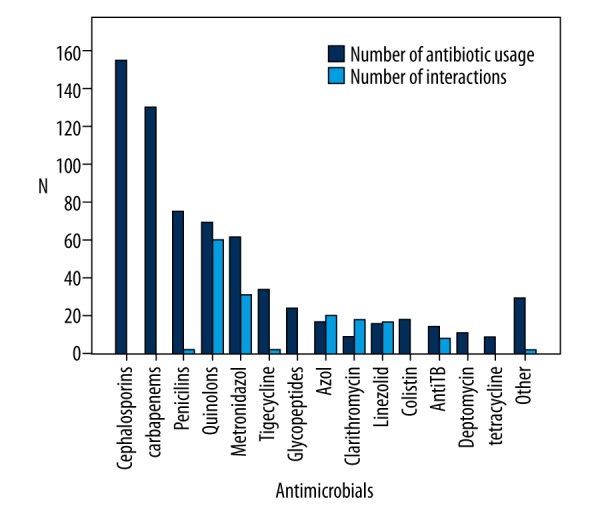
Quantity of antimicrobials and PDDIs with these antimicrobials.
Table 4.
Drug interactions and potential adverse drug events.
| Drug–drug combination | Potential Adverse Drug Events | No. of patients |
|---|---|---|
| Contraindicated | ||
| Linezolid-Carbamezepine | Increased risk of serotonin syndrome | 1 |
| Linezolid-Venlafaxin | Increased risk of serotonin syndrome | 1 |
| Linezolid-Citalopram | Increased risk of serotonin syndrome | 1 |
| Fluconazole-Granisetron | Increased risk of QT interval prolongation | 1 |
| Voriconazole-Carbamazepine | Reduced systemic exposure to voriconazole | 1 |
| Major* | ||
| Ciprofloxacin-Metronidazole | Increased risk of QT interval prolongation | 5 |
| Linezolid-Tramadol | Increased risk of serotonin syndrome | 4 |
| Clarithromycin-Tramadol | Increased risk of serotonin syndrome,seizure | 3 |
| Ciprofloxacin-Insulin | Changes in blood glucose (hypo/hyperglycemia) | 3 |
| Linezolid-Fentanyl | Increased risk of serotonin syndrome | 3 |
| Linezolid-Morphine | Potentiation of the CNS and respiratory depressant effects of morphine | 2 |
| Metronidazole-Famotidine | Increased risk of QT interval prolongation | 2 |
| Metronidazole-Quetiapine | Increased risk of QT interval prolongation | 2 |
| Moxifloxacin-Insulin | Changes in blood glucose (hypo/hyperglycemia) | 2 |
| Metronidazole-Trazodone | Increased risk of QT interval prolongation | 2 |
| Moderate* | ||
| Metronidazole-Diclofenac | Increased level or effect of diclofenac | 14 |
| Moxifloxacin-Methylprednisolone | Increased risk for tendon rupture | 9 |
| Fluconazole-Pantoprazole | Increased plasma concentration of pantoprazol | 5 |
| Moxifloxacin-Budesonide | Increased risk for tendon rupture | 5 |
| Clarithromycin-Budesonide | Increased budesonide plasma conconcentrations | 4 |
| Ciprofloxacin-Budesonide | Increased risk for tendon rupture | 3 |
| Clarithromycin-MethylPrednisolone | Increased risk of metilprednisolone side effects | 3 |
| Ciprofloxacin-Diclofenac | Increased ciprofloxacin plasma conconcentrations | 2 |
| Linezolid-Rifampicin | Subtherapeutic linezolid serum concentrations | 2 |
| Tigecycline-Warfarine | Increased warfarin exposure | 2 |
| Minor | ||
| Ciprofloxacin-Metoprolol | Bradycardia, hypotension | 3 |
| Ciprofloxacin-Propranolol | Bradycardia, hypotension | 2 |
| Clarithromycin-Theophylline | Theophylline toxicity | 2 |
| Clarithromycin-Lansoprazol | Glossitis, stomatitis, or black tongue | 2 |
Most prevalent ten interactions are shown.
In univariate analysis (Table 5), comparing PDDI and non-PDDI groups in terms of age (p=0.465), sex (p=0.133), and patients hospitalized in ICUs or in other clinics (p=0.348), no statistically significant difference was found between the groups. The patients hospitalized in the internal medicine clinics and university hospitals were more likely to be exposed to PDDIs than the patients hospitalized in surgical clinics and non-university hospitals (p=0.01 and p=0.005, respectively). Additionally, the median number of prescribed antimicrobials was 2, and the median number of other prescribed drugs was 8 in the PDDI group, whereas these medians were 1 and 6, respectively, in the non-PDDI group, and there were statistically significant differences between the groups (p<0.0001 and p<0.0001).
Table 5.
Comparisons of patients whether they have PDDI with antimicrobials or not.
| Characteristics | PDDIs with antimicrobial (n=97) | None-PDDIs with antimicrobial (n=330) | p |
|---|---|---|---|
| Age (mean±SD) | 58.7±15.1 | 56.8±18.1 | 0.465 |
| Male, n (%) | 54 (55.7) | 154 (46.7) | 0.133 |
| Female, n (%) | 43 (44.3) | 176 (53.3) | |
| ICU, n (%) | 21 (21.7) | 87 (26.3) | 0.348 |
| Non-ICU, n (%) | 76 (78.3) | 243 (73.7) | |
| University Hospital, n (%) | 76 (78.3) | 199 (60.3) | 0.001 |
| Non-university Hospital, n (%) | 21 (21.7) | 131 (39.7) | |
| Internal Medicine Clinics, n (%) | 63 (64.9) | 161 (48.8) | 0.005 |
| Surgical Clinics, n (%) | 37 (38.1) | 169 (51.2) | |
| Number of drugs, [median, (min–max)] | 8 (4–14) | 6 (1–16) | <0.0001 |
| Number of antimicrobials [median, min–max)] | 2 (1–6) | 1 (1–4) | <0.0001 |
PDDIs – potenntial drug–drug interactions; ICU – Intensive Care Unit.
In multivariate analysis, number of prescribed antimicrobials (odds ratio: 2.3001, 95% CI: 1.6237–3.2582), number of prescribed drugs (odds ratio: 1.2008, 95% CI: 1.0943–1.3177), hospitalization in a university hospital (odds ratio: 1.7798, 95% CI: 1.0035–3.1564) were independent risk factors for developing PDDIs. The logistic regression model predicted occurrence of PDDIs with sensitivity of 78% (the area under the ROC curve was 82%).
Discussion
The prevalence of PDDIs in hospitalized patients is approximately 60% [10]. Despite this high prevalence, a small proportion (<5%) of PDDIs cause clinically important ADEs [3]. However, the absolute number of patients affected is high, representing a considerable proportion of ADEs, and these ADEs may be very simply prevented by physician awareness, monitoring, and drug dosage adjustment [11]. There are many studies in the medical literature about PDDIs in general or specific populations, including geriatric, pediatric, and cardiac patients [4–7,10,12,13]. However, studies related to drug interactions with antimicrobials are very limited. In this study, we focussed only on the PDDIs related to antimicrobials.
Polypharmacy is defined as the use of many drugs at the same time. Various studies have shown that polypharmacy is an important risk factor for the occurrence of PDDIs [6,14–17]. Similarly, we found that patients with PDDIs were using more drugs and antimicrobials than were the other patients. Occurrence of PDDIs with antimicrobials is increasing with the number of administered antibiotics. Simplification of the antibiotic treatments should be considered, and, when possible, monotherapy should be used to avoid PDDIs.
In a recently published study conducted on 54 549 pediatric ICU patients, 75.2% were exposed to at least 1 PDDI [7]. In another study, Uijtendaal et al. assessed prevalence of PDDIs as 54% of all ICU patients [5]. In a study conducted among elderly patients, prevalence of PDDIs was 62.2% [18]. The prevalence of PDDIs in the present study is in line with the findings of the aforementioned studies. We found that 53.6% of the patients exposed to at least 1 PDDI and also 22.7% of the patients exposed to at least 1 PDDI were related to antimicrobials.
Linezolid is a reversible, nonselective inhibitor of monoamine oxidase and prevents the breakdown of serotonin. Linezolid generally interacted with the serotonergic (e.g., antidepressants and anti-epileptics) and adrenergic (e.g., sympathomimetic, vasopressive, and dopaminergic agents) drugs [19]. Dai et al. found that linezolid was responsible for 3 of the most prevalent 10 contraindicated interactions [7]. We also found that linezolid was one of drugs most often contraindicated and most often responsible for major PDDIs. Serotonin toxicity, a potentially fatal status with a wide range of severity, has been reported to be associated with use of linezolid when concomitantly used with serotonergic agents. Selective serotonin reuptake inhibitors (SSRI), opioid analgesics, and anti-epileptic drugs commonly interact with linezolid [20]. Use of these risky co-medications should be carefully considered to avoid serotonin syndrome. In the present study we noticed that physicians were not aware of the risk when prescribing linezolid with other serotonergic agents. Patients who were hospitalized in certain departments (e.g., neurology, neurosurgery, psychiatry, and ICU) are more likely to be treated with this type of serotonergic medication. Physicians should be more cautious while prescribing linezolid, especially in these departments.
All triazole antifungal agents are inhibitors of 1 or more phase 1 (cytochrome p450) biotransformation enzymes and may also be the inhibitors or substrates of a phase 2 biotransformation enzyme or transporter protein. Because of these properties, triazoles frequently interact with other drugs and may cause severe clinical conditions [21]. In our study, 17 patients who were treated with triazoles had 20 PDDIs. Two of them were contraindicated and 2 of them were major PDDIs. Despite the lower number of triazole prescriptions, PDDIs were relatively high. In a study conducted in patients treated with mold-active triazoles, 82% of voriconazole, 61% of itraconazole, and 83% of posaconazole hospitalizations had at least 1 severe drug interaction [22]. Due to higher PDDI risk, triazoles should also be prescribed cautiously.
In our study, macrolides and quinolones were also commonly prescribed drugs associated with PDDIs, as were triazoles and linezolid. We found that macrolides and quinolones were responsible for 34 (56%) of 61 major PDDIs, 35 (45%) of 78 moderate PDDIs, and all of the minor PDDIs. Similar to our findings, Fantaye et al. found that ciprofloxacin and clarithromycin were the only drugs responsible for contraindicated PDDIs and are among the leading antimicrobials responsible for major PDDIs [18]. Some quinolones and macrolides are associated with QT interval prolongation and may cause a life-threatening arrhythmia called torsades de pointes [23,24]. There are many commercially available drugs, including antimicrobials, antidepressants, and cardiovascular drugs, that can cause QT interval prolongation. Co-medication with QT interval-prolonging drugs and quinolone or macrolide antimicrobials should be avoided.
Approximately 90% of the drugs are metabolized by 6 main enzymes: CYP 1A2, 2C9, 2C19, 2D6, 2E1, and 3A4/5 [25]. Ciprofloxacin is a well-known hepatic CYP 1A2 inhibitor [26]. CYP1A2 inhibition can cause alteration of the metabolism of many important drugs like theophylline, clozapine, olanzapine, and caffeine [27]. In the present study, quinolones were most commonly responsible for PDDIs. Due to its well-known CYP1A2 inhibition, when prescribing ciprofloxacin, other drugs that are potential substrates of this enzyme should be used with caution.
In our study, while cephalosporins and carbapenems were the most commonly prescribed antimicrobials, we did not detect any PDDIs with these antimicrobials. Cephalosporins and carbapenems are generally safe antimicrobials for PDDIs and should usually be preferred to quinolones, macrolides, or linezolid.
Drug interaction studies show that polypharmacy is a well-known risk factor for PDDIs. The level of risk increases with the number of concomitantly used drugs [28,29]. Advanced age and multiple prescribers are also other risk factors [30]. In our study, we found that the number of antimicrobials and number of other drugs used are associated with higher risk of PDDIs. as As a precaution, safer drugs such as cephalosporins and carbapenems should also usually be preferred at this patient group using many medications.
We also found that the patients hospitalized in the university hospitals were at higher risk of PDDIs with antimicrobials than the patients in the non-university hospitals. This could be due to the hospitalization of more severe and complicated patients to the university hospitals, as well as the training and less experienced physicians working. Education on PDDIs in training programs may be solution to at least part of this problem.
Our study has the advantages of being a multicenter study in hospitals of various sizes. The study also has some limitations. An important limitation is its evaluation of the potential interactions and not reporting the actual clinical occurrence of interactions. One other limitation is that, because it is a point-prevalence study, it reflects the situation at a particular moment, and wider data could be obtained if the study was conducted over a period of time. Another limitation is the lack of information about indications of drugs. Further data characterizing the patients who were prescribed these antimicrobials vs. other antimicrobials (such as B-lactams) could be useful in identifying ways to decrease use of these problematic medications.
In our opinion, awareness about PDDIs was increased among the physicians who participated in our study, and larger, longitudinal studies could be helpful in raising this awareness. Finally, a center’s individual data and the whole output can be shared in meetings and further spread by this dissemination of this article.
Conclusions
Antimicrobials are one of the leading drug groups involved in general PDDIs, and all antimicrobials should be checked for PDDIs before prescribing. Physicians should be more cautious when prescribing antimicrobials, particularly quinolones, linezolid, azoles, metronidazole, and clarithromycin, and mobile device applications can be practical and helpful when prescribing. Warnings from integrated commercial PDDI databases to the hospital information management systems also can raise awareness among prescribers, and input from clinical pharmacists could also be helpful.
Acknowledgements
We thank the doctors and nurses of the Infectious Diseases Department for their valuable help in collecting patient data.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Zarb P, Amadeo B, Muller A, et al. Identification of targets for quality improvement in antimicrobial prescribing: the web-based ESAC Point Prevalence Survey 2009. J Antimicrob Chemother. 2011;66:443–49. doi: 10.1093/jac/dkq430. [DOI] [PubMed] [Google Scholar]
- 2.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;30(4):CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Krahenbuhl-Melcher A, Schlienger R, Lampert M, et al. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 5.Uijtendaal EV, van Harssel LL, Hugenholtz GW, et al. Analysis of potential drug–drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34(3):213–19. doi: 10.1002/phar.1395. [DOI] [PubMed] [Google Scholar]
- 6.Bjerrum L, Lopez-Valcarcel BG, Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract. 2008;14:23–29. doi: 10.1080/13814780701815116. [DOI] [PubMed] [Google Scholar]
- 7.Dai D, Feinstein JA, Morrison W, et al. Epidemiology of polypharmacy and potential drug–drug interactions among pediatric patients in ICUs of U.S. children’s hospitals. Pediatr Crit Care Med. 2016;17(5):e218–28. doi: 10.1097/PCC.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrons R. Evaluation of personal digital assistant software for drug interactions. Am J Health Syst Pharm. 2004;61:380–85. doi: 10.1093/ajhp/61.4.380. [DOI] [PubMed] [Google Scholar]
- 9.MICROMEDEX Healthcare Series. Vol. 118. MICROMEDEX; Greenwood Village, Colorado: [Google Scholar]
- 10.Egger SS, Drewe J, Schlienger RG. Potential drug–drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58( 11):773–78. doi: 10.1007/s00228-002-0557-z. [DOI] [PubMed] [Google Scholar]
- 11.Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94. doi: 10.1517/14740338.2012.631910. [DOI] [PubMed] [Google Scholar]
- 12.Mateti U, Rajakannan T, Nekkanti H, et al. Drug–drug interactions in hospitalized cardiac patients. J Young Pharm. 2011;3(4):329–33. doi: 10.4103/0975-1483.90246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler GI, Bode-Boger SM, Busse R, et al. Drug–drug interactions in medical patients: Effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 14.Chatsisvili A, Sapounidis I, Pavlidou G, et al. Potential drug–drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm World Sci. 2010;32(2):187–93. doi: 10.1007/s11096-010-9365-1. [DOI] [PubMed] [Google Scholar]
- 15.Cruciol-Souza JM, Thomson JC. A pharmacoepidemiologic study of drug interactions in a Brazilian teaching hospital. Clinics. 2006;61(6):515–20. doi: 10.1590/s1807-59322006000600005. [DOI] [PubMed] [Google Scholar]
- 16.Gagne J, Maio V, Rabinowitz C. Prevalence and predictors of potential drug–drug interactions in Regione Emilia-Romagna, Italy. J Clin Pharm Ther. 2008;33(2):141–51. doi: 10.1111/j.1365-2710.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 17.Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially. Postgrad Med. 1989;86(8):179–86. doi: 10.1080/00325481.1989.11704506. [DOI] [PubMed] [Google Scholar]
- 18.Fantaye T, Gebrehiwot T, Eskindeir A, Terefe T. Potential drug–drug interactions among elderly patients admitted to medical ward of Ayder Referral Hospital, Northern Ethiopia: A cross sectional study. BMC Res Notes. 2016;9(1):431. doi: 10.1186/s13104-016-2238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douros A, Grabowksi K, Stahlmann R. Drug–drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol. 2015;11(12):1849–59. doi: 10.1517/17425255.2015.1098617. [DOI] [PubMed] [Google Scholar]
- 20.Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother. 2013;47(3):388–97. doi: 10.1345/aph.1R386. [DOI] [PubMed] [Google Scholar]
- 21.Nivoix Y, Ubeaud-Sequier G, Engel P, et al. Drug–drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab. 2009;10(4):395–409. doi: 10.2174/138920009788499012. [DOI] [PubMed] [Google Scholar]
- 22.Andes D, Azie N, Yang H, et al. Drug–drug interaction associated with mold-active triazoles among hospitalized patients. Antimicrob Agents Chemother. 2016;60(6):3398–406. doi: 10.1128/AAC.00054-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abo-Salem E, Fowler JC, Attari M, et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther. 2014;32(1):19–25. doi: 10.1111/1755-5922.12054. [DOI] [PubMed] [Google Scholar]
- 24.Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: Infrequent side effects from commonly used medications. Cardiology. 2011;120(2):103–10. doi: 10.1159/000334441. [DOI] [PubMed] [Google Scholar]
- 25.Glue P, Clement RP. Cytochrome P450 enzymes and drug metabolism basic concepts and methods of assessment. Cell Mol Neurobiol. 1999;19:309–23. doi: 10.1023/A:1006993631057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravi PR, Vats R, Kora UR. Effect of ciprofloxacin and grapefruit juice on oral pharmacokinetics of riluzole in Wistar rats. J Pharm Pharmacol. 2013;65(3):337–44. doi: 10.1111/j.2042-7158.2012.01604.x. [DOI] [PubMed] [Google Scholar]
- 27.Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–34. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- 28.Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–96. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- 29.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamblyn RM, McLeod PJ, Abrahamowicz M, Laprise R. Do too many cooks spoil the broth? Multiple physician involvement in medical management of elderly patients and potentially inappropriate drug combinations. CMAJ. 1996;154:1177–84. [PMC free article] [PubMed] [Google Scholar]


