Abstract
Background
Chemoradiotherapy (CRT) is widely accepted and is considered a standard treatment, particularly for unresectable and inoperable esophageal squamous cell carcinoma (ESCC). However, the optimal use of the combined modalities of chemotherapy (CT) and radiotherapy (RT) remains controversial. In addition, no consensus has been reached regarding the exact efficacy of consolidation chemotherapy (CCT) and the most appropriate radiotherapy dose.
Material/Methods
Clinical data from 262 ESCC patients treated with CRT (n=165) or RT alone (n=97) were collected and reviewed. The long-term outcomes were analyzed, and treatment related acute toxicity reactions were compared.
Result
The 1-year, 3-year, and 5-year overall survival (OS) rates were 75.3%, 35.6%, and 25.3%, respectively, for the CRT group and 61.5%, 26.7%, and 17.6% for the RT-alone group (P=0.015). The concurrent chemoradiotherapy (CCRT) and sequential chemoradiotherapy (SCRT) groups exhibited similar survival outcomes (for OS, P=0.568; for progression-free survival (PFS,) P=0.145). CCT after CCRT did not influence OS (P=0.236) but was associated with a more favorable PFS (P=0.020). In addition, high-dose of 60–65 Gy tended to prolong OS compared with low-dose (<60 Gy) or excessive-dose (>65 Gy). The incidence of adverse reactions, such as esophagitis and leukopenia, in the CRT group were significantly higher than in the RT-alone group (P=0.019, P=0.001, respectively), and no significant difference was observed between patients treated with CCRT and CCT after CCRT.
Conclusions
Treating non-surgical ESCC patients with CCRT conferred a significant survival benefit compared with RT alone. CCT after CCRT prolongs PFS but does not increase acute toxicity. High-dose (60–65 Gy) CCRT could generate more favorable survival outcomes.
MeSH Keywords: Carcinoma, Squamous Cell; Chemoradiotherapy; Esophageal Neoplasms; Treatment Outcome
Background
Esophageal cancer (EC) is a fatal upper gastrointestinal malignancy with high morbidity and mortality in East Asia, especially in China, and the global burden of EC is expected to rise in the future [1,2]. Most EC patients are diagnosed at advanced stages. Radical surgery is possible in only 15–20% cases as a result of tumor sites, comorbidities, or poor performance status [3]. Radiotherapy (RT) has played an important part in the management of unresectable esophageal carcinoma, but this approach alone can rarely achieve sustained remission and long-term survival. Some studies have reported that combined chemotherapy (CT) and RT appear to be as effective as esophagectomy for localized EC [4–6]. At present, neoadjuvant chemoradiotherapy (CRT) followed by surgery is commonly used for locally advanced EC, and for patients with locally advanced unresectable disease; or medically fit patients who decline surgery, definitive CRT has been a standard treatment [7–10]. The RTOG 8501 trial showed that concurrent chemoradiotherapy (CCRT) is superior to radiation alone and generates a favorable long-term survival rate for non-surgical EC patients [7]. In addition, the histopathology of EC varies based on geographic regions; more than 90% of EC cases are squamous cell carcinoma (ESCC) in China. According to some studies in Japan, ESCC shows a higher locoregional recurrence rate than adenocarcinoma and may require a higher RT dose (≥60 Gy) [11–13]. Although CCRT has been recommended by the National Comprehensive Cancer Network (NCCN) as one of the standard treatments for locally advanced esophageal carcinoma [14], the role of CCRT and the optimal radiation dose remain controversial in China. Therefore, we retrospectively analyzed the survival of ESCC patients who were not candidates for surgery and received CCRT, sequential chemoradiotherapy (SCRT) or RT alone, and compared the safety and efficacy of different radiation doses. In addition, we considered the benefits of CCT following CCRT.
Material and Methods
Patients
Previously untreated patients with histopathologically confirmed primary ESCC at the Affiliated Tumor Hospital of Guangxi Medical University from 2004 to 2013 were retrospectively analyzed. Patients enrolled were restaged according to the 6th edition of the 2002 UICC staging system. The hematological and biochemical parameters of the patients were suitable for RT or CT and patients had an ECOG status ≤1. All the patients were considered unsuitable for a radical surgery. Pre-treatment evaluations included a detailed clinical history, physical examination, barium swallow x-ray, upper gastrointestinal tract endoscopy, and computed tomography scans of the thorax and abdomen. None of the patients had distant metastasis, tracheoesophageal fistula, signs of bleeding or other primary cancers before and during the treatment.
Treatment regimens
Radiotherapy (RT)
2DRT
Patients who received 2DRT were irradiated using megavoltage photons (6 MV) in 2 phases. In the first phase, the treatment was performed using an anterior/posterior opposed field with a dose of 34–40 Gy. The radiation field included a 3 cm to 5 cm margin with the primary tumor craniocaudal and extended 2 cm to 3 cm beyond the radial margins. In the second phase, the treatment field was changed to lateral or oblique fields to avoid the spinal cord. Total RT doses ranged from 56 Gy to 74 Gy with a 1.8–2.0 Gy daily fraction.
3DRT and IMRT
The gross tumor volume (GTV) consisted of primary tumor (GTVnx) and enlarged mediastinal lymph nodes (GTVnd). Contrast-enhanced chest computed tomography scan, gastrointestinal barium x-ray, and esophageal endoscopy were performed to delineate the GTVnx and the GTVnd. The clinical target volume (CTV) included the primary tumor with 3–5 cm craniocaudal and 0.8–1 cm lateral margins and metastatic lymph nodes with a 2 cm radial margin. CTV plus 5 cm to 8 mm margins for uncertainty represented the planning target volume (PTV). RT plans were designed using the 5 to 7 therapeutic field techniques, which limit the radiation dose to some organs at risk (OARs) such as the heart, lungs and spinal cord. The total prescribed RT dose ranged from 50 Gy to 70.4 Gy, and the daily fraction ranged from 1.8 Gy to 2.2 Gy (5 fractions/week).
Chemotherapy (CT)
Patients who received CT underwent 1 of 2 regimens, a cisplatin-based regimen combined with fluorouracil (5-FU) or a paclitaxel regimen. 5-FU (500 mg/m2 per day) was infused for the first 5 days, paclitaxel (135/175 mg/m2) on day 1, and cisplatin (75–80 mg/m2) was administered for 3 days in a 21-day cycle. For frail and elderly (≥80 years) patients, 75% of the standard dose was used. All regimens were administered in 1 to 3 cycles during or after RT. An additional 2 to 4 cycles of platinum-based CCT were administered depending on patients’ conditions after CCRT.
Endpoints and toxicity assessments
The primary endpoints were overall survival (OS) and progression-free survival (PFS). OS referred to the duration from the date of treatment to the date of death from any cause or the date of the last visit. In addition, PFS was the duration from the date of treatment to the date of death or tumor progression. The Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) was applied for assessing treatment responses 1–3 weeks after all treatments were completed. Acute radiation toxicities were assessed by using the toxicity criteria of the RTOG, and the chemotherapeutic toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE, V 4.0).
Follow-up
All patients enrolled were followed by outpatient review and telephone follow-up after treatment completion. Patients were rechecked every 3 months for the first year and every 6 months thereafter. The main inspection items included careful physical examination of lymph nodes, esophageal endoscopy, and computed tomography scanning. Bone scans were applied when necessary.
Statistical analysis
Continuous data conforming to a normal distribution are presented as the median ±SD, and the differences were analyzed using Student’s t-test. Categorical variables were analyzed by the χ2 test or Fisher’s exact test. OS and PFS rates were calculated by the Kaplan-Meier method, and differences in survival curves were compared by log-rank tests. Multivariate analysis of prognostic factors was measured by a Cox proportional hazards model, and toxic reactions were compared using the non-parametric Kruskal-Wallis test or the Mann-Whitney U test. A P value <0.05 indicated a significant difference. All statistical analyses were performed with IBM SPSS 17.0.
Results
Patient characteristics and follow-up results
Among 262 EC patients, 124 cases received CCRT, 41 received SCRT, and 97 received RT alone. In the CCRT group, 65 received CCT, and 59 cases did not. Detailed characteristics of the patients are shown in Table 1. The endpoint of the follow-up was September 2017, and the median follow-up time was 18.5 months (range, 3 to 106 months). The follow-up rate was 96.9%, with 8 cases lost to follow-up.
Table 1.
Baseline patient characteristics.
| RT alone (n=97) | CRT (n=165) | P-value | |
|---|---|---|---|
| Gender | 0.007 | ||
| Male | 68 | 139 | |
| Female | 29 | 26 | |
| Age, years | 0.000 | ||
| Range | 41–84 | 40–80 | |
| Median | 64 | 55 | |
| Primary tumor site | 0.516 | ||
| Cervical | 6 | 20 | |
| Upper thoracic | 33 | 46 | |
| Middle thoracic | 44 | 71 | |
| Low thoracic | 11 | 22 | |
| Diffuse | 3 | 6 | |
| Primary tumor length | 0.806 | ||
| Range | 1.7–12 | 1.0–15.2 | |
| Median | 6 | 5.7 | |
| T stage | 0.220 | ||
| T1 | 2 | 7 | |
| T2 | 15 | 34 | |
| T3 | 46 | 58 | |
| T4 | 34 | 66 | |
| N stage | 0.200 | ||
| N0 | 35 | 47 | |
| N1 | 62 | 118 | |
| M stage | 0.278 | ||
| M0 | 72 | 112 | |
| M1a | 25 | 53 | |
| Clinical stage | 0.188 | ||
| I + II | 22 | 26 | |
| III | 52 | 85 | |
| IV | 23 | 54 | |
| Pathology differentiation | 0.817 | ||
| Well | 13 | 22 | |
| 6Moderate | 29 | 56 | |
| Poor | 42 | 67 | |
| Radiotherapy technique | 0.062 | ||
| 2DRT | 20 | 17 | |
| 3DRT | 32 | 57 | |
| IMRT | 45 | 91 | |
| Radiation dose for GTV (Gy) | 0.176 | ||
| <60 Gy | 15 | 24 | |
| 60–65 Gy | 31 | 71 | |
| >65 Gy | 51 | 70 |
RT – radiotherapy; CRT – concurrent chemoradiotherapy; 2DRT – conventional radiotherapy; 3DRT – three-dimensional conformal radiotherapy; IMRT – intensity-modulated radiation therapy; GTV – gross tumor volume.
Treatment response
For patients treated with CRT (CCRT+SCRT), 37.6% patients (62 out of 165) achieved CR (complete response), 52.7% patients (87 out of 165) achieved PR (partial response), 6.7% (11 out of 165) achieved stable disease, and 1.2% (2 out of 165) developed PD (progressive disease). For the patients who received RT alone, 29.9% (29 out of 97) achieved CR, 43.3% (42 out of 97) achieved PR, 19.6% (19 out of 97) achieved stable disease, and 3.1% (3 out of 97) developed PD. The CRT group demonstrated a significantly higher overall response rate (CR+PR) than the RT-alone group (90.3% vs. 73.2%, P<0.05).
Survival outcomes
For the entire group of patients, the 1-year, 3-year, and 5-year OS were 69.6%, 32.1%, and 22.5%, respectively. The median OS was 20 months (95% CI, 16.9 to 23.1 months). As shown in Figure 1, patients treated with CRT exhibited a significantly better OS than patients treated with RT alone (1-year OS 75.3% vs. 61.5%; 3-year OS 35.6% vs. 26.7%; 5-year OS 25.3% vs. 17.6%, P=0.015). The median OS was 22 months (95% CI, 18.2 to 25.8 months) and 16 months (95% CI, 13.2 to 18.8 months), respectively.
Figure 1.
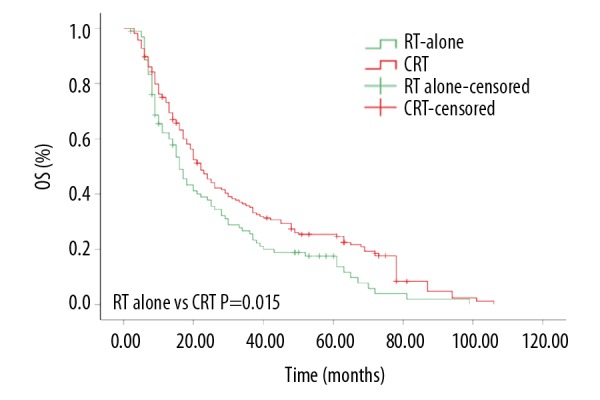
The overall survival rates of esophageal squamous cell carcinoma patients treated with chemoradiotherapy or radiotherapy alone.
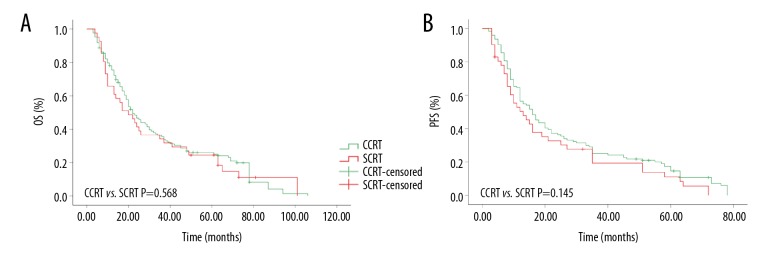
For CCRT group, 1-year, 3-year, and 5-year OS rates were 78.2%, 36.1%, and 26.1%, respectively, while 65.6%, 34.4%, and 23.7% for SCRT group, respectively. The median OS was 22 months (95% CI, 17.4 to 26.6 months) and 20 months (95% CI, 11.2 to 28.8 months), respectively. The 1-year, 3-year, and 5-year PFS rates were 63.9%, 24.9%, and 17.4%, respectively, for the CCRT group and 52.8%, 19.9%, and 11.2%, respectively, for the SCRT group. The median OS for these 2 groups were 16 months (95% CI, 11.9 to 20.1 months) and 13 months (95% CI, 6.8 to 19.2 months), respectively. No obvious difference was found between the 2 groups in relation to either OS (P=0.568) or PFS (P=0.145). The results are shown in Figure 2.
Figure 2.
Kaplan-Meier curves of esophageal squamous cell carcinoma patients treated with concurrent chemoradiotherapy or sequential chemoradiotherapy. (A) Overall survival rate; (B) progress-free survival rate.
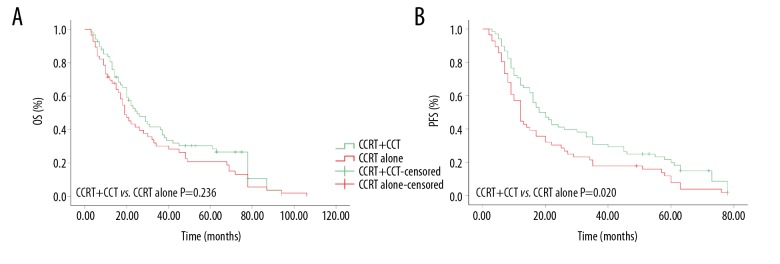
We also evaluated the efficacy of CCT. Patients who underwent CCRT (n=124) were divided into 2 groups: patients who received CCT after CCRT (n=65) and those who did not (n=59). As is shown in Figure 3, for CCRT with CCT (CCRT+CCT), 1-year, 3-year, and 5-year OS were 83.7%, 41.9%, and 30.5%, respectively, while 71.2%, 30.4%, and 21% for CCRT without CCT (CCRT alone), respectively (P=0.236). However, the CCRT+CCT group achieved better PFS (70.5%, 31.2%, and 21.8 vs. 57.6%, 17.7%, and 12.1%, P=0.020). In addition, the median OS and PFS for the CCRT alone group were 19 months (95% CI, 14.9 to 23.4 months) and 12 months (95% CI, 9.9 to 14.1 months), respectively, while for the CCRT+CCT group, the median OS and PFS were 25 months (95% CI, 17.4 to 32.6 months) and 18 months (95% CI, 12.6 to 23.4 months).
Figure 3.
Kaplan-Meier curves of esophageal squamous cell carcinoma patients treated with or without consolidation chemotherapy after concurrent chemoradiotherapy. (A) Overall survival rate; (B) progress-free survival rate.
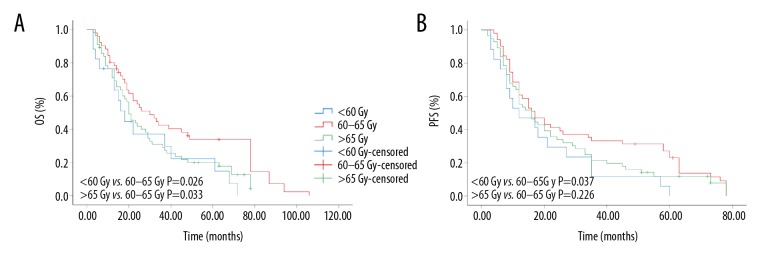
As no consensus has been reached on the appropriate dose of RT for CCRT, in the current study, we compared the efficacies of different doses. Among the patients who received CCRT, 17 received CCRT <60 Gy (median: 56.0 Gy, low-dose group), 51 received 60–65 Gy CCRT (median: 63.0 Gy, high-dose group), and 56 received >65 Gy CCRT (median: 66.6 Gy, excessive-dose group). Our results indicated that high dose CCRT was significantly associated with higher OS (P=0.026) and PFS (P=0.037) compared with low-dose CCRT. Compared with the excessive-dose group, high-dose CCRT tended to prolong the OS (P=0.033), as shown in Figure 4.
Figure 4.
Kaplan-Meier curves of patients received different radiotherapy dose. (A) Overall survival rate; (B) progress-free survival rate.
Toxicity of treatment
The toxicity profile is presented in Tables 2 and 3. The most frequently observed acute toxicities included radiation esophagitis, radiation pneumonitis, leukopenia and thrombocytopenia. There was no significant difference for CRT or RT alone between the low-dose (<60 Gy) and high-dose (60–65 Gy) groups with respect to radiation esophagitis, radiation pneumonitis and thrombocytopenia (all P>0.05). Almost all grade 4 acute toxicities were observed in patients who received excessive-dose (> 65 Gy). However, patients who received CRT developed significantly more leukopenia above grade 2 (<60 Gy: 41.7% vs. 6.7%, P=0.006; 60–65 Gy: 45.7% vs. 22.6%, P=0.014; >65 Gy: 54.3% vs. 29.4%, P=0.001). There was no significant difference in radiation-esophagitis ≥ grade 2 among the high- and low-dose groups (P=0.739), but the excessive-dose group showed significantly more radiation-esophagitis ≥ grade 2 than the high-dose group (P=0.021). Furthermore, no significant difference in toxicities ≥ grade 2 was observed between patients treated with CCRT alone and CCRT with CCT.
Table 2.
Treatment related toxicities of RT alone and CRT groups.
| Toxicities | RT alone (n=97) | CRT(n=165) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 0–1 | Grade 2 | Grade 3 | Grade 4 | Grade 0–1 | Grade 2 | Grade 3 | Grade 4 | ||
| <60 Gy | |||||||||
| Radiation esophagitis | 13 | 2 | 0 | 0 | 20 | 4 | 0 | 0 | 0.436 |
| Radiation pneumonia | 15 | 0 | 0 | 0 | 23 | 1 | 0 | 0 | 0.546 |
| Leukopenia | 14 | 1 | 0 | 0 | 14 | 6 | 4 | 0 | 0.006 |
| Thrombocytopenia | 15 | 0 | 0 | 0 | 22 | 2 | 0 | 0 | 0.345 |
| 60–65 Gy | |||||||||
| Radiation esophagitis | 26 | 4 | 1 | 0 | 57 | 12 | 2 | 0 | 0.515 |
| Radiation pneumonia | 29 | 1 | 1 | 0 | 65 | 5 | 1 | 0 | 0.680 |
| Leukopenia | 24 | 5 | 2 | 0 | 39 | 17 | 14 | 1 | 0.014 |
| Thrombocytopenia | 28 | 1 | 2 | 0 | 65 | 4 | 2 | 0 | 0.503 |
| >65 Gy | |||||||||
| Radiation esophagitis | 42 | 8 | 1 | 0 | 44 | 21 | 4 | 1 | 0.019 |
| Radiation pneumonia | 45 | 4 | 2 | 0 | 57 | 8 | 4 | 1 | 0.167 |
| Leukopenia | 36 | 11 | 3 | 1 | 32 | 25 | 12 | 1 | 0.001 |
| Thrombocytopenia | 49 | 2 | 0 | 0 | 61 | 6 | 3 | 0 | 0.192 |
RT – radiotherapy; CRT – concurrent chemoradiotherapy.
Table 3.
Treatment related toxicities of CCRT alone and CCRT+CCT groups.
| Toxicities | CCRT alone (n=59) | CCRT+CCT (n=65) | χ2 | P-value | ||
|---|---|---|---|---|---|---|
| Grade 0–1 | Grade 2 | Grade 0–1 | Grade 2 | |||
| Leukopenia | 31 | 28 | 26 | 39 | 1.963 | 0.161 |
| Thrombocytopenia | 52 | 7 | 60 | 5 | 0.616 | 0.432 |
| Radiation esophagitis | 43 | 16 | 44 | 21 | 0.399 | 0.528 |
| Radiation pneumonia | 53 | 6 | 55 | 10 | 0.757 | 0.384 |
CCRT – concurrent chemoradiotherapy; CCT – consolidation chemotherapy.
Prognostic analysis
In the multivariate Cox regression analysis, our results demonstrated that only weight loss and T stage were identified as having prognostic significance for OS (p<0.05), and treatment group (RT alone), T stage (T3–4), N stage (N1) and clinical stage (stage 3–4) were associated with a poor prognosis for PFS (Table 4).
Table 4.
Multivariate analysis of prognostic factors for OS and PFS.
| Factors | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Pathology differentiation | 0.813 (0.609–1.085) | 0.160 | 0.896 (0.682–1.179) | 0.433 |
| Primary tumor site | 1.086 (0.521–2.263) | 0.826 | 0.676 (0.334–1.367) | 0.276 |
| Weight loss | 0.747 (0.561–0.994) | 0.046 | 1.107 (0.856–1.432) | 0.440 |
| Concurrent chemotherapy | 1.208 (0.892–1.637) | 0.221 | 1.405 (1.059–1.864) | 0.018 |
| T stage | 0.584 (0.371–0.920) | 0.020 | 0.635 (0.417–0.968) | 0.035 |
| Nstage | 0.743 (0.530–1.044) | 0.087 | 0.656 (0.480–0.896) | 0.008 |
| M stage | 0.801 (0.575–1.113) | 0.186 | 0.736 (0.538–1.008) | 0.056 |
| Clinical stage | 0.746 (0.444–1.253) | 0.268 | 0.563 (0.343–0.924) | 0.023 |
OS – overall survival; PFS – progression-free survival; HR – hazard ratio; 95% CI – 95% confidence interval.
Discussion
For EC patients treated with RT alone, the 5-year survival rate of EC was only approximately 2–20% [15]. The RTOG 85-01 randomized phase 3 clinical trial compared the effective survival benefits of combined-modality therapy (CCRT) with definitive RT alone for non-surgical EC. In this report, the combined therapy arm showed 5-, 8-, and 10-year survival benefits [16]. RT with a dose of 50 Gy combined with CCRT using cisplatin and 5-FU is recommended as a standard treatment by the NCCN guidelines for patients with resectable EC who refused surgery or for patients at an advanced stage or who are not candidates for radical esophagectomy. Although CCRT has shown improved local control and long-term survival, other studies have reached different conclusions[17–19]. In addition, the radiation dose administered to East Asian patients in series reports was higher than that administered to Western patients [20].
The results of our research indicated that CRT leads to significantly better 3-year and 5-year OS rates than RT alone (35.6% vs. 26.7%; 5-year OS 25.3% vs. 17.6%, P=0.015), and this seemed consistent with some previous studies [11,16]. Our research found a better 5-year OS than Alsarraf et al. reported (27% and 0%), although this discrepancy might be because the patients in that study were all treated with 2DRT, whereas in our study, the majority of enrolled patients received IMRT or 3DRT. In addition, the 3-year OS and 5-year OS for patients who received CCRT in our study were worse than those reported in a phase II prospective trial in Japan [11]. We suggest that a major reason for this survival difference is differences in disease stage. Our study included 32.7% stage IVa ESCC patients; however, all patients in their trial were stage II to III.
Whether SCRT improves ESCC outcomes remains controversial. Gupta et al. [21] reported that CCRT induced a better CR rate than SCRT (82.4% vs. 35%). Thus, CCRT significantly improved local control of ESCC. Xing et al. [22] compared the efficacy and safety of CCRT and SCRT, and the results indicated response rates (RRs) for CCRT and SCRT of 91.6% and 67.7%, respectively (P=0.023). In addition, patients treated with CCRT exhibited higher degrees of acute toxicity reactions, but no significant difference was observed. A meta-analysis containing including 19 randomized controlled trials revealed that SCRT did not improve local control and might cause more severe toxicity reactions [23]. In this study, SCRT slightly improved the 3-year and 5-year OS and PFS, and CCRT led to a better OS than SCRT, but no significant difference was observed. We suggest 2 major reasons for this survival difference. One may be the short CCRT treatment cycle, which might minimize the accelerated proliferation of tumor cells. Therefore, CCRT significantly improves local control and short-term efficiency. However, the toxic effects of CCRT might supersede these effects, which could offset treatment benefits, resulting in no significant improvement in 5-year OS. The survival difference may also be related to fewer patients in the SCRT group.
CCT after initial treatment has shown efficacy and improved clinical outcomes in some cancers, such as cervical [24] and non-small-cell lung cancer (NSCLC) [25]. For EC patients who have achieved a maximum tumor response from CCRT, many oncologists recommend CCT in an attempt to improve disease control and possible survival benefits. However, the exact role of CCT following CCRT has not been defined. Di Fiore F et al. [26] compared the efficacy of CRT and additional CT after CRT, and their results suggested that patients who received additional CT after CRT experienced less metastatic disease (P=0.03). Kim et al. [3] reported that 2–6 additional cycles of CT as CCT led to better survival outcomes, the median PFS and OS were 25.5 months, 12.3 months (P=0.114) and 13.3 months, 7.4 months in the 2 groups, respectively. However, in a recently published large retrospective study, Chen et al. did not find any OS benefit of consolidation CT. In our present study, 2–4 additional cycles of CCT following CCRT tended to prolong OS (25 months vs 19 months, P=0.236) and PFS (18 months vs. 12 months, P=0.020) compared with no consolidation group. These results underlined the potential benefit of CCT in reducing metastatic occurrence.
According to the RTOG 9405 prospective randomized clinical trial results, NCCN guidelines recommended the standard radical dose for EC as 50.0–50.4 Gy [27]. The trial also demonstrated that high-dose RT (64.80 Gy) might lead to a higher treatment-related mortality rate. However, more than 60% of patients enrolled in the RTOG 9405 trial exhibited early clinical stage disease. The pathological types included both squamous cell carcinoma and adenocarcinoma, and gastroesophageal cancers were also included, all of which might have survival rates that differ from that of ESCC. Indeed, some studies have reported that the survival rate and efficiency differ between esophageal adenocarcinoma and squamous cell carcinoma patients receiving CCRT [13,28]. In addition, because the previous trial was performed earlier, 2DRT treatment was administered. Therefore, the heterogeneous inclusion criteria and the conventional RT techniques might not be sufficient for determining the optimal dose of radiotherapy for modern treatments of ESCC.
In recent years, due to the application of 3DRT and IMRT, higher doses can be administered, and greater biological effects can be achieved at the tumor area. The protection of normal tissue is also better compared with conventional conformal radiotherapy, which improves the curative effect and reduces side effects [29–32]. In a retrospective study of 2061 patients, Chang et al. reported that IMRT-based high-dose CCRT (≥60 Gy) yielded more favorable survival outcomes than standard-dose CCRT (<60 Gy), especially for ESCC patients at advanced stages [33]. Furthermore, a meta-analysis containing 55 randomized controlled trials reported that IMRT-based high-dose CCRT induced significant improvements in 5-year OS, CR and total efficiency [34]. In our current research, the majority of patients (85.9%) received IMRT or 3DRT, and a stratified analysis showed that a total dose of 60–65 Gy prolonged OS (20 vs. 26 months, P=0.124) and PFS (19 vs. 24 months, P=0.168) compared with low-dose (<60 Gy), and an excessive-dose (>65 Gy) radiation, which showed poor outcomes. Cancer type is important on response to multiple EC treatment [35]. Due to a higher local recurrence rate than adenocarcinoma, ESCC might require a higher RT dose (≥60 Gy) [12]. All patients in our study were diagnosed with ESCC, and our results revealed that high-dose RT conferred significant survival benefit, which agreed with the conclusions of the aforementioned studies.
These results could be explained by the fact that high-dose 3DRT or IMRT-based CCRT could increase the local control rate of patients with ESCC, resulting in improved survival outcomes without causing severe radiotherapy-related severe respiratory complications [6,16]; however, an excessive dose might lead to complications associated with a substantial reduction in survival. Limited by the retrospective nature of the study and the relatively small number of samples, as well as the fact that some patients received 2DRT, these results require further prospective clinical studies to be confirmed.
Due to the better target conformity and normal tissue sparing of 3DRT and IMRT compared with those of conventional RT techniques, the irradiation toxicity reactions, including radiation pneumonitis and esophagitis were reduced [17]. Our results indicated that most of the acute toxicity reactions among all patients were grade 1 and 2, although grade 3 or grade 4 acute toxicity reactions occurred in a few cases, similar to previous relevant reports [21,22,36]. Although more toxicities, especially grade 3 and 4 hematological toxicities, were observed in the CRT group than in the RT alone group, most cases of toxicity were manageable, and no treatment-related death was observed. We suggest that the difference was mainly caused by CT. Favorable toxicity profiles were found for both the low-dose (<60 Gy) and high-dose (60–65 Gy) CCRT groups, and no statistically significant differences were observed between the 2 groups. However, more severe toxicities were found in the excessive dose (>65 Gy) CCRT group.
Conclusions
In conclusion, CRT yields more favorable efficacy and survival outcomes, suggesting the important role of CRT in the treatment of non-surgical ESCC. The CCRT and SCRT groups exhibited similar survival outcomes. CCT after CCRT prolongs PFS but does not increase the incidence rate of acute toxicity. Based on these results, we recommend CCRT as the preferred treatment for relatively healthy patients with non-surgical ESCC. In addition, compared with a standard or excessive CCRT dose, high-dose CCRT generates more favorable survival outcomes.
Abbreviations
- ESCC
esophageal squamous cell carcinoma
- RT
radiotherapy
- CRT
chemoradiotherapy
- SCRT
sequential chemoradiotherapy
- CCRT
concurrent chemoradiotherapy
- RTOG
Radiation Therapy Oncology Group
- OS
overall survival
- PFS
progression-free survival
- CCT
consolidation chemotherapy
- 3DRT
three-dimensional conformal radiotherapy
- IMRT
intensity-modulated radiation therapy
Footnotes
Conflicts of interest
None.
Source of support: Guangxi Natural Science Foundation (GXNSFA018225)
References
- 1.Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol. 2017;115(5):564–79. doi: 10.1002/jso.24592. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. GLOBOCAN 2012: Estimated Cancer Incidence Mortality and Prevalence Worldwide in 2012. 2013 [Google Scholar]
- 3.Dae-Eun K, Uh-Jin K, Won-Young C, et al. Clinical prognostic factors for locally advanced esophageal squamous carcinoma treated after definitive chemoradiotherapy. Cancer Res Treat. 2013;45(4):276–84. doi: 10.4143/crt.2013.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan A, Wong A. Is combined chemotherapy and radiation therapy equally effective as surgical resection in localized esophageal carcinoma? Int J Radiat Oncol Biol Phys. 1999;45(2):265–70. doi: 10.1016/s0360-3016(99)00199-6. [DOI] [PubMed] [Google Scholar]
- 5.Hironaka S, Ohtsu A, Boku N, et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2–3)N(any) M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2003;57(2):425–33. doi: 10.1016/s0360-3016(03)00585-6. [DOI] [PubMed] [Google Scholar]
- 6.Chiu PW, Chan AC, Leung SF, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: Early results from the Chinese University Research Group for Esophageal Cancer (CURE) J Gastrointest Surg. 2005;9(6):794–802. doi: 10.1016/j.gassur.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Guo MD, Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;17(281):1623–27. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 8.Ruppert BN, Watkins JM, Shirai K, et al. Cisplatin/Irinotecan versus carboplatin/paclitaxel as definitive chemoradiotherapy for locoregionally advanced esophageal cancer. Am J Clin Oncol. 2010;33(4):346–52. doi: 10.1097/COC.0b013e3181aaca26. [DOI] [PubMed] [Google Scholar]
- 9.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):615–16. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro J, Lanschot JJBV, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–98. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II–III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81(3):684–90. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi A, Shibamoto Y, Miyakawa A, et al. Definitive concurrent chemotherapy and high-dose (60–70 Gy) radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;81(2):S325. [Google Scholar]
- 13.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17(1):38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, D’Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(2):194–227. doi: 10.6004/jnccn.2015.0028. [DOI] [PubMed] [Google Scholar]
- 15.Zhou CH, Wang XY, Chen HJ, et al. Radiotherapy combined with chemotherapy for the treatment of local advanced esophageal cancer. Chin J Radiol. 2004;(03):8–9. [Google Scholar]
- 16.Alsarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15(1):277–84. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 17.Smit JK, Muijs CT, Burgerhof JG, et al. Survival after definitive (chemo)radiotherapy in esophageal cancer patients: A population-based study in the north-East Netherlands. Ann Surg Oncol. 2013;20(6):1985–92. doi: 10.1245/s10434-012-2824-2. [DOI] [PubMed] [Google Scholar]
- 18.Han J, Wang L, Han C, et al. Clinical observation of concurrent chemoradiotherapy for esophageal carcinoma. Chin J Cancer Prevent Treat. 2011;23:1859–63. [Google Scholar]
- 19.Liu J, Lu CX, Wang JM. Concurrent chemoradiotherapy for patients with inoperable esophageal cancer. Chin J Radiol. 2006;15(3):185–87. [Google Scholar]
- 20.Suh YG, Lee IJ, Koom WS, et al. High-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II–III esophageal cancer. Jpn J Clin Oncol. 2014;44(6):534–40. doi: 10.1093/jjco/hyu047. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Roy S, Majumdar A, et al. A randomized study to compare sequential chemoradiotherapy with concurrent chemoradiotherapy for unresectable locally advanced esophageal cancer. Indian J Med Paediatr Oncol. 2014;35(1):54–59. doi: 10.4103/0971-5851.133722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing L, Liang Y, Zhang J, et al. Definitive chemoradiotherapy with capecitabine and cisplatin for elder patients with locally advanced squamous cell esophageal cancer. J Cancer Res Clin Oncol. 2014;140(5):867–72. doi: 10.1007/s00432-014-1615-5. [DOI] [PubMed] [Google Scholar]
- 23.Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2006;6(1):117–18. doi: 10.1002/14651858.CD002092.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Mabuchi S, Isohashi F, Okazawa M, et al. Chemoradiotherapy followed by consolidation chemotherapy involving paclitaxel and carboplatin and in FIGO stage IIIB/IVA cervical cancer patients. J Gynecol Oncol. 2017;28(1):e15. doi: 10.3802/jgo.2017.28.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Bi N, Ji Z, et al. Consolidation chemotherapy may improve survival for patients with locally advanced non-small-cell lung cancer receiving concurrent chemoradiotherapy – retrospective analysis of 203 cases. BMC Cancer. 2015;15(1):1–9. doi: 10.1186/s12885-015-1710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiore FD, Lecleire S, Galais MP, et al. Impact of radiation schedule and chemotherapy duration in definitive chemoradiotherapy regimen for esophageal cancer. Gastroenterol Clin Biol. 2006;30(6–7):845–51. doi: 10.1016/s0399-8320(06)73331-0. [DOI] [PubMed] [Google Scholar]
- 27.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 28.Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20(2):231–38. doi: 10.1093/annonc/mdn622. [DOI] [PubMed] [Google Scholar]
- 29.Fenkell L, Kaminsky I, Breen S, et al. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiother Oncol. 2008;89(3):287–91. doi: 10.1016/j.radonc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77(3):247–53. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Wu VWC, Kwong DLW, Sham JST. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiother Oncol. 2004;71(2):201–6. doi: 10.1016/j.radonc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Fu WH, Wang LH, Zhou ZM, et al. Comparison of conformal and intensity-modulated techniques for simultaneous integrated boost radiotherapy of upper esophageal carcinoma. World J Gastroenterol. 2004;10(8):1098–102. doi: 10.3748/wjg.v10.i8.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CL, Tsai HC, Lin WC, et al. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017;125(1):73–79. doi: 10.1016/j.radonc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Song T, Liang X, Fang M, Wu S. High-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: A systematic review and pooled analysis. Expert Rev Anticancer Ther. 2015;15(10):1157–69. doi: 10.1586/14737140.2015.1074041. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, Zhao F, Zeng Y, Yi C. Risks and benefits of multimodal esophageal cancer treatments: A meta-analysis. Med Sci Monit. 2017;23:889–910. doi: 10.12659/MSM.903328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh HY, Yeh HL, Hsu CP, et al. Feasibility of intensity-modulated radiotherapy for esophageal cancer in definite chemoradiotherapy. J Chin Med Assoc. 2016;79(7):375–81. doi: 10.1016/j.jcma.2016.01.013. [DOI] [PubMed] [Google Scholar]