Abstract
Bone is a metabolically active tissue that renews itself throughout one's life. Cytokines along with several hormonal, nutritional, and growth factors are involved in tightly regulated bone remodeling. Accordingly, vitamin K as a multifunctional vitamin has been recently deemed appreciable as a topic of research as it plays a pivotal role in maintenance of the bone strength, and it has been proved to have a positive impact on the bone metabolism. Vitamin K exerts its anabolic effect on the bone turnover in different ways such as promoting osteoblast differentiation, upregulating transcription of specific genes in osteoblasts, and activating the bone-associated vitamin k dependent proteins which play critical roles in extracellular bone matrix mineralization. There is also credible evidence to support the effects of vitamin k2 on differentiation of other mesenchymal stem cells into osteoblast. The main objective of the present paper is to comprehensively outline the preclinical studies on the properties of vitamin K and its effects on the bone metabolism. The evidence could shed light on further clinical studies to improve osteogenesis in bone graft surgeries.
1. Introduction
Bone is a metabolically active tissue that undergoes constant remodeling throughout one's life. Old bones are regularly resorbed by osteoclasts and constantly replaced with newly formed ones. However, the process of bone remodeling in some parts of the body may reduce the bone mass and result in bone deformation. After tooth extraction, for instance, there is profound resorption often observed in the alveolar crestal bone [1]. This may later interfere with the ideal rehabilitation of the edentulous site with dental implants. Bone adequacy around dental implants has been well documented as a prerequisite for implant osseointegration and its survival in long run [2]. Different bone grafting techniques and bone substitutes have been suggested for bone augmentation [3, 4]. In this regard, various growth factors [5, 6] (such as bone morphogenetic proteins [7]), drugs (such as simvastatin [8]), and nutrients (such as vitamin D [9]) have been evaluated to promote bone formation.
Attempts are ongoing to find materials and techniques to stimulate bone cells and their progenitors to produce native bone at desired sites. Vitamin K is a multifunctional vitamin, which has gained the spotlight for its efficacy for enhancing bone turnover. Vitamin K promotes bone formation by stimulating the osteoblast differentiation, increasing the level of some bone formation markers (e.g., alkaline phosphatase and insulin-like growth factor [10]), and regulating the extracellular matrix mineralization through Y-glutamyl carboxylation [11]. Additionally, vitamin K prevents bone resorption via its anticatabolic activities, namely, decreasing osteoclast differentiation and inhibiting osteoblast apoptosis [10].
This review article aims to comprehensively review the properties of vitamin K and its effect on bone metabolism in preclinical studies. Such evidence may serve as a basis for clinical studies to enhance osteogenesis in bone graft surgeries.
2. What Is Vitamin k?
Vitamins are essential to boost physical well-being, and doubtlessly vitamin deficiency can have serious health consequences. Vitamin K is a fat-soluble vitamin first identified in a study on blood coagulation by Carl Peter Henrik in Denmark. The letter “K” stands for “Koagulation”, a Danish term for coagulation. Vitamin K is significantly at play in a wide range of biological activities including regulation of calcium metabolism in tissues, cell growth and proliferation, oxidative stress, inflammatory reactions, and blood coagulation and hemostasis. Vitamin K naturally falls in two types: vitamin K1 and vitamin K2. Although it is fat-soluble, its synthetic analogue, known as menadione or K3, is water-soluble, and it is converted to vitamin K2 in the liver. Vitamin K1, also known as phylloquinone or phytonadione, enjoys an herbal origin and is an inseparable part of human diet. Vitamin K is found in many fruits and vegetables (Kiwifruit, avocado, broccoli, green grapes, and lettuce) as well as oils (canola, soybean, and olive oil). A type of this vitamin, dihydrophylloquinone, that is present in some hydrogenated oils, is less prominent in the body than phylloquinone [12, 13].
Vitamin K2 (menaquinone) refers to a group of chemical compounds with a specific formulation. Such compounds share a naphthoquinone ring and a side chain with variable lengths. The chemical formulation of vitamin K2 is MK_n (MK-2 to MK-14) where “n” is the number of remaining chains of isoprenoid. Although most of these isoprene residues are unsaturated, some forms of menaquinones which are produced by bacteria have saturated prenyl units [14]. Menaquinones, except for MK-4, are synthesized by bacteria. Anaerobic bacteria present in the colon are capable of synthesizing MK-10 to MK-13. Found in fish, liver, milk, vegetables, and eggs, MK-4 is the dominant form of vitamin K in human body where it is primarily produced by conversion of menadione (vitamin k3), directly synthesized from the dietary phylloquinone [15, 16]. As demonstrated by Suhara et al. human osteoblasts could apply the same procedure to produce MK-4 [17]. It has been suggested that the types of vitamin K2 (e.g., Mk-7) with longer chains can be converted to MK-4 as well [18].
Absorbed in small quantities, vitamin K commences the cycle of absorption in the small intestine and it is delivered to the liver and other tissues via the lymphatic system. The major portion of vitamin K1 is stored in the liver and the rest will join the vitamin K2 to be transferred by the low density lipoproteins to other tissues [12]. In human body, MK-4 to MK-10 vitamins are absorbed in greater amounts and show a higher biological activity than K1. Mainly stored in the liver, vitamin K is found in small quantities in body. Liver stores vitamin K1 and long-chain forms of vitamin K2. Brain as well as glands such as pancreas and genital organs are among the other sites storing MK-4 [19]. Therefore, vitamin K deficiency does not equally affect all tissues. For instance, liver, the main reservoir of vitamin K is the last organ to be affected in case of insufficiency or deficiency [20]. There is a body of evidence linking the shortage of vitamin K with increased risk of cancer, cardiovascular disease, soft tissue calcification, and osteoporosis [21–24].
3. Vitamin K Cycle
As described below, vitamin K is recycled through a cycle referred to as the vitamin K cycle. This enables the body to recycle and reuse vitamin k as many times as required to obviate the need for dietaries [25]. The cycle operates as follows.
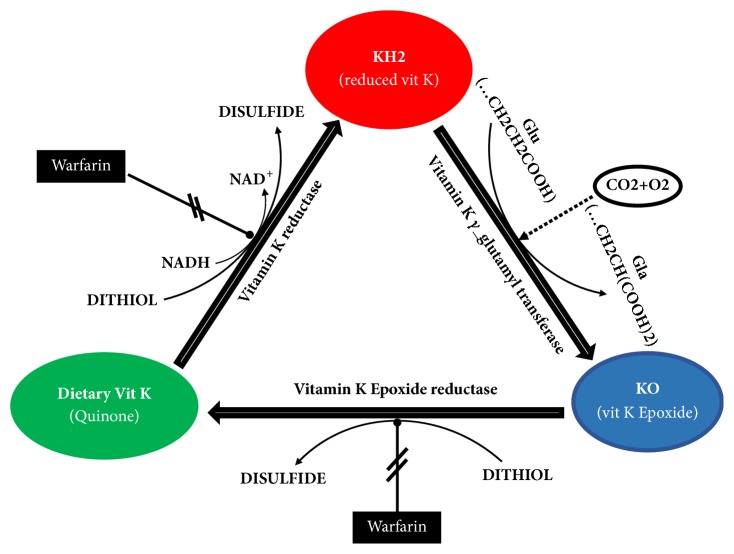
Vitamin K enters the cells and functions as the cofactor of the endoplasmic reticulum resident γ-glutamyl carboxylase (GGCX), which carboxylates any selected glutamate residues on the target proteins and enables these proteins to bind to calcium [11, 26]. Subsequently, vitamin K reductase enzyme converts dietary vitamin K to its reduced form (hydroquinone) which allows GGCX to load a –COOH group on specific proteins. After carboxylation, vitamin K epoxide reductase converts back the resultant oxidized form of vitamin k to hydroquinone (Figure 1).
Figure 1.
The scheme showing the steps of the vitamin K cycle, the enzymes involved, and the inhibitory action of warfarin.
Worth to be mentioned is the fact that Coumarin (i.e., warfarin), an anticoagulant drug, is the antagonist of vitamin K epoxide reductase and interferes with the vitamin K recycling.
4. Biological Activity
Different types of vitamin K vary in their biological activities. This is triggered by the discrepancies in enzyme affinity and tissue distribution. Vitamin K1 is mainly stored in the liver; thus, it plays a greater role in production of coagulation proteins, while vitamin K2 is extensively distributed in the human body [27]. Vitamin K2 enjoys a higher affinity to γ-glutamyl carboxylase than that of vitamin K1. Different subtypes of vitamin K2 also differ in the levels of bioactivity and enzyme affinity. For example, MK-7 has the greater bioavailability and plasma half-life than do vitamin K1 and MK-4 [15]. Furthermore, MK-7 overtakes MK-4 and vitamin K1 in the level of anti-NF-κB activity; however, MK-7 cannot induce growth differentiation factor and stanniocalcin genes, identified as the target genes for MK-4 in osteoblasts [28].
5. Vitamin K Dependent Proteins
The main function of vitamin K is to serve as a cofactor of GGCX in production of vitamin K dependent proteins (VKDP). To date, 14 VKDPs have been identified, albeit with a shallow account of their functions. Vitamin K dependent proteins include seven proteins (II, IIV, IX, X, protein S, protein X, and protein Z) involved in blood coagulation and synthesized in the liver. There are also four proteins of transmembrane Gla family. Found in bones, the other VKDPs could be listed as Osteocalcin, matrix Gla protein, growth arrest specific 6 protein (Gas 6), and protein S [14, 29, 30].
One of the main noncollagenous proteins found in the bones is Osteocalcin (OC). Also known as bone-Gla-protein, OC is secreted by osteoblasts and some other cells [31]. OC binds to calcium ions and hydroxyapatite crystals. This way, OC seems to be able to exert its regulatory effects on the organization of the bone extracellular matrix and modulates the size and shape of the hydroxyapatite crystals [11, 32]. Transcription and translation of OC gene are regulated by 1,25(OH)2 D3 [33], but its ability to bind to calcium ions depends on the vitamin K that is responsible for γ-carboxylation of three glutamic acid residues in positions 17, 21, and 24 in OC molecule [11].
Evidence supports the role of OC in different physiological activities other than bone metabolism such as glucose metabolism [32], energy metabolism, fertility [34], and ectopic calcification [35]. Based on their degree of carboxylation, various forms of OC differ in their affinity to Ca ions. Since uncarboxylated and undercarboxylated forms of OC demonstrate low affinity to hydroxyapatite, they are easily released into the blood circulation. The circulating OC is used as a good biomarker of bone formation. Irrespective of the concentration of vitamin K, the plasma concentration of OC is correlated to bone turnover and metabolism. However, uncarboxylated OC level is vitamin K dependent [32, 36], and it is considered as an indicator of vitamin K status [37].
Matrix Gla protein (MGP), an extensively studied extra-hepatic Gla proteins, is synthetized by chondrocytes, osteoclasts, and vascular smooth muscle cells. According to the findings in both animal and human studies, MGP inhibits the calcification of arterial media and cartilages, while facilitating normal bone metabolism. It has been demonstrated that MGP attains optimal biological activity just after posttranslational carboxylation [38, 39].
Gla-rich protein and periostin are two other vitamin K dependent proteins, supposed to regulate extracellular matrix mineralization of the bones [32]. Last but not least, a VKDP, protein S, is mainly synthetized in the liver and involved in the anticoagulation pathway. It is also secreted by osteoblasts and involved in the bone turnover in an unclear mechanism [40].
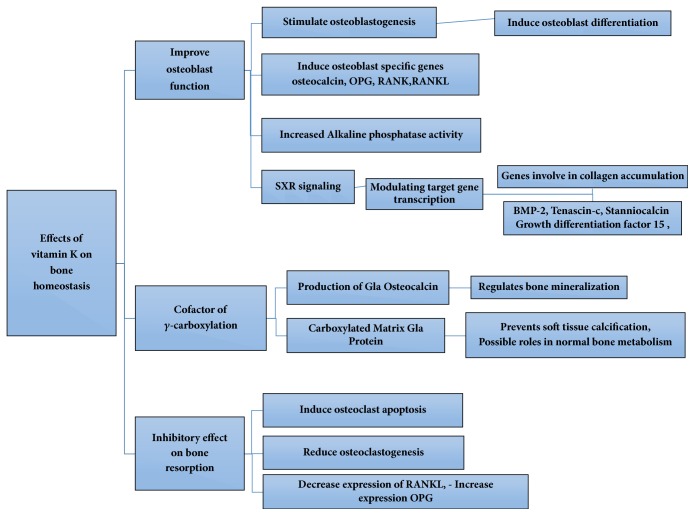
What follows is a more detailed account of the effects of vitamin K on bone cells behaviors. The various effects of vitamin k on bone are also summarized in Figure 2.
Figure 2.
Mechanisms of action of vitamin k on bone homeostasis.
6. Effects of Vitamin K on Osteoblast Function
Vitamin K affects the proliferation and differentiation of osteoblasts. It prevents the induction of apoptosis in osteoblasts and inhibits Fass-mediated apoptosis in a dose-dependent manner [41, 42], but more effectively improves the osteoblast function [28]. As suggested in the literature, vitamin K2 treatment of osteoblasts could increase both the alkaline phosphatase activity [42–44] and the level of bone anabolic markers such as OC [45, 46] in the cell medium. The more the alkaline phosphatase activity is, the more the formation of the organic matrix and mineral part of the bone is, and so is the deposition of OC and hydroxyapatite in the bone.
Essential role of vitamin K in osteoblastic function through γ carboxylation pathway is well established. However, vitamin K2 performs some of its osteoprotective functions by upregulating bone marker genes. Vitamin K2 activates the steroid and xenobiotic receptor (SXR) [28, 47–50] and operates as a transcriptional regulator of the number of osteoblastic biomarker genes and extracellular matrix related genes. The SXR, also known as the pregnane X receptor (PXR), is a nuclear receptor which modulates gene transcription [51] and its protective role in bone metabolism has been shown in PXR-knockout mice study [52]. Research has the fact that vitamin K2 can induce upregulation of CYP3A4 (target gene of SXR) and activation of MSX2 (target gene for PXR) [28]. Menaquinone-7 upregulates Tenascin C and bone morphogenetic protein-2 (BMP-2) genes expression [47]. The effects of vitamin K are not still limited to these pathways. Ichikawa et al. (2007) observed that MK-4, through a pathway independent of SXR and γ carboxylation, resulted in activation of two genes, namely, growth differentiation factor 15 and Stanniocalcin. Induction of these genes is exclusive to MK-4, and vitamin K1 and MK-7 do not have such effects. The authors suggested that MK-4 regulates the expression of its target gene through a mechanism dependent on phosphorylation of protein kinase A [53].
Moreover, vitamin K2 supports bone formation and suppresses bone resorption by stimulating the expression of cytokines such as osteoprotegerin (OPG) and inhibiting the expression of receptor activator of nuclear factor kappa-B ligand (RANKL) on osteoblasts/osteoclasts and by this way improves osteoblast differentiation [42, 45]. In the same vein, Yamagushi et al. (2011) observed that vitamin K2 induced downregulating NF-κB (cytokine-induced nuclear factorκ) activation in osteoblasts, which is a process independent of γ carboxylation mechanism [54].
As illustrated by the results of the studies in vitro, vitamin K (K2 in particular) improves the function of osteoblasts by inducing their proliferation, decreasing their apoptosis, and increasing the expression of osteogenic genes. It also has positive effects on the bone turnover and accordingly regulates bone metabolism.
Effect of vitamin K on osteocyte function also investigated in some in vitro and animal studies. Two osteoporotic rat models demonstrated that vitamin K2 ameliorates adverse effects of glucocorticoid treatment and/or sciatic neurectomy on osteocyte density and lacunar occupancy and had an additive effect on cortical porosity [55, 56]. In a cell culture study Atkins et al. provided the evidence that vitamin K promotes osteoblast transition to osteocyte. They observed that incubation of human osteoblasts with vitamin K2 in a collagen gel medium increased the number of osteocyte-like cells with elongated cytoplasmic processes. This effect seems to be independent to γ carboxylation pathway [57]. Vitamin K2 also has regulatory effect on the transcription of bone markers in murine osteocytes [50].
7. Effects of Vitamin K on Bone Resorption and Osteoclast Function
Vitamin K2 treatment has been reported to have an inhibitory effect on osteoclastic bone resorption in murine osteogenic culture [58–60], rabbit model [61], and different rat models [44, 62]. Vitamin K prevents bone resorption via several mechanisms. It prevents osteoclast formation either directly or indirectly; that is, it could interfere with the expression of RANKL and upregulates the expression of OPG on osteoclast precursors. In addition, vitamin K decreases both proliferation of tartrate-resistant acid phosphatase positive (TRAP +) cells and TRAP activity in osteogenic culture medium [42, 44, 61]. Moreover, vitamin K2 inhibits bone resorption, induced by bone resorbing factors such as PGE2, IL1α, and 1, 25(OH) 2D3 in a dose-dependent manner [42, 58–60].
Yamaguchi et al. (2011) observed that vitamin K2 downregulated basal and cytokine-induced NF-κB activation in human and murine monocytic cell lines and thereupon prevented the bone resorption. Activation of NF-κB signal transduction pathway is essential for osteoclast formation. Hara (1995) stated that the inhibitory effect of menaquinones on bone loss was probably independent of the mechanism of action of γ carboxylation and it was triggered by the side chain of vitamin K2 (geranylgeraniol). An interesting finding was that such an inhibitory effect was absent in the side chain of vitamin K1 [59].
Although in vivo [63] and in vitro [61] studies showed the potential of vitamin K2 to induce osteoclast apoptosis, a study on ovariectomized rats revealed that, after MK-4 dietary supplementation (50mg/kg a day), there was a decline in osteoclast bioactivity, yet there was no trace of osteoclast apoptosis [44].
In sum, the current evidence suggests that vitamin K2 reduces osteoclastic activity via different strategies and that it applies an anabolic effect on the bone.
8. Effects of Vitamin K on Different Osteoporotic Rat Models
Several animal models have been used to study the effects of vitamin K on the bone metabolism. The studies on ovariectomized rats [44, 62, 64], on unilaterally sciatic neurectomized rats [65, 66], and in tail-suspended rats [67, 68] found that vitamin K treatment had significant positive effects on the bone health. Histologic and microcomputed tomographic evaluations demonstrated that vitamin K2 supplementation inhibited the loss of bone mass density and trabecular bone, improved osteoblast function, and enhanced the serum level of the bone anabolic markers. Accordingly, vitamin K2 improves bone architecture dose-dependently. The highest concentrations used in animal studies have been 30 and 50 mg/kg, which seem to be close to the pharmacologically effective level of this vitamin.
Vitamin K1 and K2 administration elevated serum level of OC (as an indicator of osteoblast function) in high fat diet mice [45]. Osteoblasts and adipocytes differentiate from the same stem cells. Thus, when the number of adipocytes increases as a result of obesity, the number of osteoblasts decreases and causing bone loss [69]. Kim et al. (2013) observed that vitamin K administration in high fat diet mice resulted in a growth in the indices of the bone formation and a reduction in the indices of the bone resorption; however, vitamin K supplementation had no significant effect on the bone metabolism in normal diet mice [45].
Osteoporosis is a systemic skeletal disease and its prevalence increases with age. It often develops asymptomatically and silently and results in reduction of the bone mineral density and bone strength [70]. Literature has addressed the health-enhancing benefits of vitamin K supplementation in osteoporosis [21, 71]. Some animal studies investigated the effect of coadministration of vitamin K2 and other bone acting drugs on osteoporosis. Coadministration of vitamin K2 and Teriparatide (low dose parathyroid hormone derived peptide) improved the osteoblast function and increased the serum level of Gla-OC. These effects were greater in combined use compared to the occasion when vitamin K2 and TPTD were administered in isolation [64].
Bisphosphonates are among the commonly prescribed medications for osteoporosis. They can effectively decrease the risk of bone fracture, hamper the activity of osteoclasts, and decrease the bone turnover; the result is a desirable increase in the bone mineral density. Bisphosphonate-related osteonecrosis of the jaw is among the adverse complications of bisphosphonates, which is hard to treat and its occurrence should be taken into account in planning the dental treatment especially for the patients who take bisphosphonates for long periods of time [72, 73]. Effect of combined use of vitamin K2 and bisphosphonate on osteoporosis was evaluated in rat models. It was recommended that vitamin K2 could ameliorate the suppressive effect of bisphosphonates on bone turnover and increase the bone volume as well as the bone formation parameters [68, 74]. Other reviews emphasized the potentially positive effect of combined treatment with bisphosphonate and vitamin K2 for preventing the fractures in postmenopausal osteoporotic women [75, 76].
Diabetes mellitus is among the most common metabolic disorders worldwide. Diabetes and obesity have been shown to be associated with osteoporosis. Poon et al. (2015) demonstrated that combined use of vitamin K2 and 1,25(OH)2D3 enhanced calcium deposition and OC expression in osteoblasts of lean and obese diabetic mice; these effects were greater on the obese diabetic group. The results indicated that a single administration of these vitamins could not significantly improve bone anabolic factors such as OC and alkaline phosphatase activity. Thus, combination therapy with vitamin K2 and 1,25(OH)2D3 was suggested as a promising modality for the treatment of diabetes-associated osteoporosis [46]. Human studies also reported coadministration of vitamin D and K to have beneficial impacts on prevention of bone loss [77, 78].
9. MK-7 and Bone Metabolism
MK-4 enjoys the highest bioactivity among different compounds of menaquinones, and it has been the most extensively studied. MK-7, however, has higher bioavailability and a longer half-life than vitamin K1 and MK-4. Natto (fermented soy) is a good source of this vitamin produced by Bacillus subtilis [79] and builds a part of the diet in different cultures around the world. Thus, several studies have exclusively evaluated the effects of this vitamin on the activity of osteoblasts and osteoclasts in human and murine cell culture media.
MK-7 affects bone formation by enhancing the function of osteoblasts. This compound results in upregulation of SXR target gene, i.e., CYP3A4 in osteoblasts [28], and induces the synthesis of OPG and OC in osteoblasts. Both of these compounds are osteogenic markers [47, 80]. Further, MK-7 downregulates NF-κB activation in murine and human osteoblasts and osteoclasts. It was suggested that MK-7 exerts this effect independent of γ-carboxylation pathway [54].
10. Conclusion
There is burden of evidence supporting the osteoprotective effects of vitamin K2 in bone metabolisms. Vitamins K2, especially MK-4, promotes bone formation by stimulating the differentiation of the osteoblast, regulating the mineralization of the extracellular matrix, upregulating the expression of the bone marker genes, and inhibiting the osteoclastogenesis. Based on these anabolic properties of vitamin k, it could be suggested that adding vitamin k as an adjunct to the bone materials may stimulate bone cells and their progenitors to produce native bone with promising results. A recent study has evaluated the behavior of dental pulp stem cells after being exposed to MK-4 in an osteogenic medium. According to the findings, based on ALP activity and extracellular Ca deposition assay, menaquinone 4 can ameliorate differentiation of dental pulp stem cells into osteoblast and may enhance bone regenerative capacity of cell-based bone tissue engineering therapies [81]. Well-designed RCTs are suggested to determine clinical and histological efficacy of vitamin k on the results of bone augmentation surgeries.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Chappuis V., Araújo M. G., Buser D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000. 2017;73(1):73–83. doi: 10.1111/prd.12167. [DOI] [PubMed] [Google Scholar]
- 2.Rokn A., Aslroosta H., Akbari S., Najafi H., Zayeri F., Hashemi K. Prevalence of peri-implantitis in patients not participating in well-designed supportive periodontal treatments: a cross-sectional study. Clinical Oral Implants Research. 2017;28(3):314–319. doi: 10.1111/clr.12800. [DOI] [PubMed] [Google Scholar]
- 3.Khojasteh A., Kheiri L., Motamedian S., Khoshkam V. Guided bone regeneration for the reconstruction of alveolar bone defects. Annals of Maxillofacial Surgery. 2017;7(2):p. 263. doi: 10.4103/ams.ams_76_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rokn A. R., Khodadoostan M. A., Rasouli G. A. A. R., et al. Bone formation with two types of grafting materials: A histologic and histomorphometric study. The Open Dentistry Journal. 2011;5(1):96–104. doi: 10.2174/1874210601105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apostu D., Lucaciu O., Lucaciu G. D. O., et al. Systemic drugs that influence titanium implant osseointegration. Drug Metabolism Reviews. 2017;49(1):92–104. doi: 10.1080/03602532.2016.1277737. [DOI] [PubMed] [Google Scholar]
- 6.Khojasteh A., Behnia H., Naghdi N., Esmaeelinejad M., Alikhassy Z., Stevens M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2013;116(6):e405–e423. doi: 10.1016/j.oooo.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Wessing B., Lettner S., Zechner W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. The International Journal of Oral and Maxillofacial Implants. 2017 doi: 10.11607/jomi.5461. [DOI] [PubMed] [Google Scholar]
- 8.Mansour G., Al Ashwah A., Koura A. Evaluation of simvastatin grafting around immediate dental implants in dogs. Implant Dentistry. 2014;23(2):195–199. doi: 10.1097/ID.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 9.Pimentel S. P., Casarin R. C., Ribeiro F. V., et al. Impact of micronutrients supplementation on bone repair around implants: MicroCT and counter-torque analysis in rats. Journal of Applied Oral Science. 2016;24(1):45–51. doi: 10.1590/1678-775720150293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa J. K. D., Diaz M. A. N., Pizziolo V. R., Martino H. S. D. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Critical Reviews in Food Science and Nutrition. 2017;57(18):3959–3970. doi: 10.1080/10408398.2016.1211616. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi G., Perego S., Luzi L., Banfi G. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine Journal. 2015;48(2):394–404. doi: 10.1007/s12020-014-0401-0. [DOI] [PubMed] [Google Scholar]
- 12.Booth S. L., Suttie J. W. Dietary intake and adequacy of vitamin K. Journal of Nutrition. 1998;128(5):785–788. doi: 10.1093/jn/128.5.785. [DOI] [PubMed] [Google Scholar]
- 13.Hamidi M. S., Gajic-Veljanoski O., Cheung A. M. Vitamin K and Bone Health. Journal of Clinical Densitometry. 2013;16(4):409–413. doi: 10.1016/j.jocd.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Beulens J. W. J., Booth S. L., Van Den Heuvel E. G. H. M., Stoecklin E., Baka A., Vermeer C. The role of menaquinones (vitamin K2) in human health. British Journal of Nutrition. 2013;110(8):1357–1368. doi: 10.1017/S0007114513001013. [DOI] [PubMed] [Google Scholar]
- 15.Myneni V. D., Mezey E. Regulation of bone remodeling by vitamin K2. Oral Diseases. 2017;23(8):1021–1028. doi: 10.1111/odi.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota Y., Tsugawa N., Nakagawa K., et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. The Journal of Biological Chemistry. 2013;288(46):33071–33080. doi: 10.1074/jbc.M113.477356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhara Y., Wada A., Okano T. Elucidation of the mechanism producing menaquinone-4 in osteoblastic cells. Bioorganic & Medicinal Chemistry Letters. 2009;19(4):1054–1057. doi: 10.1016/j.bmcl.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Schurgers L. J., Teunissen K. J. F., Hamulyák K., Knapen M. H. J., Vik H., Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109(8):3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 19.Okano T., Shimomura Y., Yamane M., et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. The Journal of Biological Chemistry. 2008;283(17):11270–11279. doi: 10.1074/jbc.M702971200. [DOI] [PubMed] [Google Scholar]
- 20.McCann J. C., Ames B. N. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? American Journal of Clinical Nutrition. 2009;90(4):889–907. doi: 10.3945/ajcn.2009.27930. [DOI] [PubMed] [Google Scholar]
- 21.Schwalfenberg G. K. Vitamins K1 and K2: The Emerging Group of Vitamins Required for Human Health. Journal of Nutrition and Metabolism. 2017;2017:6. doi: 10.1155/2017/6254836.6254836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlando A., Linsalata M., Tutino V., D'Attoma B., Notarnicola M., Russo F. Vitamin K1 exerts antiproliferative effects and induces apoptosis in three differently graded human colon cancer cell lines. BioMed Research International. 2015;2015:15. doi: 10.1155/2015/296721.296721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palermo A., Tuccinardi D., D'Onofrio L., et al. Vitamin K and osteoporosis: Myth or reality? Metabolism - Clinical and Experimental. 2017;70:57–71. doi: 10.1016/j.metabol.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Mizuta T., Ozaki I., Eguchi Y., et al. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: A pilot study. Cancer. 2006;106(4):867–872. doi: 10.1002/cncr.21667. [DOI] [PubMed] [Google Scholar]
- 25.Berkner K. L. Vitamin K-Dependent Carboxylation. Vitamins & Hormones. 2008;78:131–156. doi: 10.1016/S0083-6729(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 26.Stenflo J., Fernlund P., Egan W., Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proceedings of the National Acadamy of Sciences of the United States of America. 1974;71(7):2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krüger T., Westenfeld R., Schurgers L. J., Brandenburg V. M. Coagulation meets calcification: the vitamin K system. The International Journal of Artificial Organs. 2009;32(2):67–74. doi: 10.1177/039139880903200202. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa T., Horie-Inoue K., Ikeda K., Blumberg B., Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2- activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. The Journal of Biological Chemistry. 2006;281(25):16927–16934. doi: 10.1074/jbc.M600896200. [DOI] [PubMed] [Google Scholar]
- 29.Shearer M. J., Fu X., Booth S. L. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Advances in Nutrition. 2012;3(2):182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferland G. The vitamin K-dependent proteins: An update. Nutrition Reviews. 1998;56(8):223–230. doi: 10.1111/j.1753-4887.1998.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 31.Hauschka P. V., Lian J. B., Cole D. E., Gundberg C. M. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiological Reviews. 1989;69(3):990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 32.Booth S. L., Centi A., Smith S. R., Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nature Reviews Endocrinology. 2013;9(1):43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancela L., Hsieh C.-L., Francke U., Price P. A. Molecular structure, chromosome assignment, and promoter organization of the human matrix Gla protein gene. The Journal of Biological Chemistry. 1990;265(25):15040–15048. [PubMed] [Google Scholar]
- 34.Karsenty G., Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Molecular and Cellular Endocrinology. 2014;382(1):521–526. doi: 10.1016/j.mce.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theuwissen E., Smit E., Vermeer C. The role of vitamin K in soft-tissue calcification. Advances in Nutrition. 2012;3(2):166–173. doi: 10.3945/an.111.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gundberg C. M., Nieman S. D., Abrams S., Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. The Journal of Clinical Endocrinology & Metabolism. 1998;83(9):3258–3266. doi: 10.1210/jc.83.9.3258. [DOI] [PubMed] [Google Scholar]
- 37.Shiraki M., Yamazaki Y., Shiraki Y., Hosoi T., Tsugawa N., Okano T. High level of serum undercarboxylated osteocalcin in patients with incident fractures during bisphosphonate treatment. Journal of Bone and Mineral Metabolism. 2010;28(5):578–584. doi: 10.1007/s00774-010-0167-2. [DOI] [PubMed] [Google Scholar]
- 38.Krueger T., Westenfeld R., Ketteler M., Schurgers L. J., Floege J. Vitamin K deficiency in CKD patients: a modifiable risk factor for vascular calcification? Kidney International. 2009;76(1):18–22. doi: 10.1038/ki.2009.126. [DOI] [PubMed] [Google Scholar]
- 39.Booth S. L., Tucker K. L., Chen H., et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. American Journal of Clinical Nutrition. 2000;71(5):1201–1208. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- 40.Maillard C., Berruyer M., Serre C. M., Dechavanne M., Delmas P. D. Protein-s, a vitamin k-dependent protein, is a bone matrix component synthesized and secreted by osteoblasts. Endocrinology. 1992;130(3):1599–1604. doi: 10.1210/endo.130.3.1531628. [DOI] [PubMed] [Google Scholar]
- 41.Urayama S., Kawakami A., Nakashima T., et al. Effect of vitamin K2 on osteoblast apoptosis: Vitamin K2 inhibits apoptotic cell death of human osteoblasts induced by Fas, proteasome inhibitor, etoposide, and staurosporine. Journal of Laboratory and Clinical Medicine. 2000;136(3):181–193. doi: 10.1067/mlc.2000.108754. [DOI] [PubMed] [Google Scholar]
- 42.Koshihara Y., Hoshi K., Okawara R., Ishibashi H., Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. Journal of Endocrinology. 2003;176(3):339–348. doi: 10.1677/joe.0.1760339. [DOI] [PubMed] [Google Scholar]
- 43.Akedo Y., Hosoi T., Inoue S., et al. Vitamin K2 modulates proliferation and function of osteoblastic cells in vitro. Biochemical and Biophysical Research Communications. 1992;187(2):814–820. doi: 10.1016/0006-291X(92)91269-V. [DOI] [PubMed] [Google Scholar]
- 44.Asawa Y., Amizuka N., Hara K., et al. Histochemical evaluation for the biological effect of menatetrenone on metaphyseal trabeculae of ovariectomized rats. Bone. 2004;35(4):870–880. doi: 10.1016/j.bone.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Kim M., Na W., Sohn C. Vitamin K1 (phylloquinone) and K2 (menaquinone-4) supplementation improves bone formation in a high-fat diet-induced obese mice. Journal of Clinical Biochemistry and Nutrition. 2013;53(2):108–113. doi: 10.3164/jcbn.13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poon C. C., Li R. W., Seto S. W., et al. In vitro vitamin K(2) and 1alpha,25-dihydroxyvitamin D(3) combination enhances osteoblasts anabolism of diabetic mice. European Journal of Pharmacology. 2015;767:30–40. doi: 10.1016/j.ejphar.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 47.Katsuyama H., Saijoh K., Otsuki T., Tomita M., Fukunaga M., Sunami S. Menaquinone-7 regulates gene expression in osteoblastic MC3T3E1 cells. International Journal of Molecular Medicine. 2007;19(2):279–284. [PubMed] [Google Scholar]
- 48.Igarashi M., Yogiashi Y., Mihara M., Takada I., Kitagawa H., Kato S. Vitamin K induces osteoblast differentiation through pregnane X receptor-mediated transcriptional control of the Msx2 gene. Molecular and Cellular Biology. 2007;27(22):7947–7954. doi: 10.1128/MCB.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Shearer M. J., Newman P. Metabolism and cell biology of vitamin K. Thrombosis and Haemostasis. 2008;100(4):530–547. doi: 10.1160/TH08-03-0147. [DOI] [PubMed] [Google Scholar]
- 50.Tabb M. M., Sun A., Zhou C., et al. Vitamin K2 Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. The Journal of Biological Chemistry. 2003;278(45):43919–43927. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 51.Kliewer S. A., Goodwin B., Willson T. M. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine Reviews. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 52.Azuma K., Casey S. C., Ito M., et al. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. Journal of Endocrinology. 2010;207(3):257–263. doi: 10.1677/JOE-10-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichikawa T., Horie-Inoue K., Ikeda K., Blumberg B., Inoue S. Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. Molecular Endocrinology. 2007;39(3-4):239–247. doi: 10.1677/JME-07-0048. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M., Weitzmann M. N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-kappaB activation. International Journal of Molecular Medicine. 2011;27(1):3–14. doi: 10.3892/ijmm.2010.562. [DOI] [PubMed] [Google Scholar]
- 55.Iwamoto J., Matsumoto H., Takeda T., Sato Y., Yeh J. K. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcified Tissue International. 2010;87(3):254–262. doi: 10.1007/s00223-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 56.Iwamoto J., Matsumoto H., Takeda T., Sato Y., Liu X., Yeh J. K. Effects of vitamin K2 and risedronate on bone formation and resorption, osteocyte lacunar system, and porosity in the cortical bone of glucocorticoid-treated rats. Calcified Tissue International. 2008;83(2):121–128. doi: 10.1007/s00223-008-9146-1. [DOI] [PubMed] [Google Scholar]
- 57.Atkins G. J., Welldon K. J., Wijenayaka A. R., Bonewald L. F., Findlay D. M. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. American Journal of Physiology-Cell Physiology. 2009;297(6):C1358–C1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- 58.Akiyama Y., Hara K., Tajima T., Murota S.-I., Morita I. Effect of vitamin K2 (menatetrenone) on osteoclast-like cell formation in mouse bone marrow cultures. European Journal of Pharmacology. 1994;263(1-2):181–185. doi: 10.1016/0014-2999(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 59.Hara K., Akiyama Y., Nakamura T., Murota S., Morita I. The inhibitory effect of vitamin K2 (Menatetrenone) on bone resorption may be related to its side chain. Bone. 1995;16(2):179–184. doi: 10.1016/8756-3282(94)00027-W. [DOI] [PubMed] [Google Scholar]
- 60.Hara K., Akiyama Y., Ohkawa I., Tajima T. Effects of menatetrenone on prednisolone-induced bone loss in rats. Bone. 1993;14(6):813–818. doi: 10.1016/8756-3282(93)90309-X. [DOI] [PubMed] [Google Scholar]
- 61.Kameda T., Miyazawa K., Mori Y., et al. Vitamin K2 inhibits osteoclastic bone resorption by inducing osteoclast apoptosis. Biochemical and Biophysical Research Communications. 1996;220(3):515–519. doi: 10.1006/bbrc.1996.0436. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama Y., Hara K., Kobayashi M., Tomiuga T., Nakamura T. Inhibitory effect of vitamin K2 (menatetrenone) on bone resorption in ovariectomized rats: A histomorphometric and dual energy X-ray absorptiometric study. Japanese Journal of Pharmacology. 1999;80(1):67–74. doi: 10.1254/jjp.80.67. [DOI] [PubMed] [Google Scholar]
- 63.Kawata T., Zernik J. H., Fujita T., Tokimasa C., Tanne K. Mechanism in inhibitory effects of vitamin K2 on osteoclastic bone resorption: In vivo study in osteopetrotic (op/op) mice. Journal of Nutritional Science and Vitaminology. 1999;45(4):501–507. doi: 10.3177/jnsv.45.501. [DOI] [PubMed] [Google Scholar]
- 64.Nagura N., Komatsu J., Iwase H., et al. Effects of the combination of vitamin K and teriparatide on the bone metabolism in ovariectomized rats. Biomedical Reports. 2015;3(3):295–300. doi: 10.3892/br.2015.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwasaki-Ishizuka Y., Yamato H., Murayama H., Ezawa I., Kurokawa K., Fukagawa M. Menatetrenone rescues bone loss by improving osteoblast dysfunction in rats immobilized by sciatic neurectomy. Life Sciences. 2005;76(15):1721–1734. doi: 10.1016/j.lfs.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Iwamoto J., Matsumoto H., Takeda T., Sato Y., Yeh J. K. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcified Tissue International. 2010;87(3):254–262. doi: 10.1007/s00223-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 67.Iwasaki Y., Yamato H., Murayama H., et al. Maintenance of trabecular structure and bone volume by vitamin K 2 in mature rats with long-term tail suspension. Journal of Bone and Mineral Metabolism. 2002;20(4):216–222. doi: 10.1007/s007740200031. [DOI] [PubMed] [Google Scholar]
- 68.Iwasaki Y., Yamato H., Murayama H., et al. Combination use of vitamin K2 further increases bone volume and ameliorates extremely low turnover bone induced by bisphosphonate therapy in tail-suspension rats. Journal of Bone and Mineral Metabolism. 2003;21(3):154–160. doi: 10.1007/s007740300024. [DOI] [PubMed] [Google Scholar]
- 69.Young H. E., Mancini M. L., Wright R. P., et al. Mesenchymal stem cells reside within the connective tissues of many organs. Developmental Dynamics. 1995;202(2):137–144. doi: 10.1002/aja.1002020205. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadi Z., Fayyazbakhsh F., Ebrahimi M., et al. Association between vitamin D receptor gene polymorphisms (Fok1 and Bsm1) and osteoporosis: A systematic review. Journal of Diabetes and Metabolic Disorders. 2014;13(1) doi: 10.1186/s40200-014-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Z.-B., Wan S.-L., Lu Y.-J., Ning L., Liu C., Fan S.-W. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporosis International. 2015;26(3):1175–1186. doi: 10.1007/s00198-014-2989-6. [DOI] [PubMed] [Google Scholar]
- 72.de-Freitas N.-R., Lima L.-B., de-Moura M.-B., Veloso-Guedes C.-D., Simamoto-Júnior P.-C., de-Magalhães D. Bisphosphonate treatment and dental implants: A systematic review. Medicina Oral Patología Oral y Cirugía Bucal. 2016;21(5):e644–e651. doi: 10.4317/medoral.20920.20920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexander I. M., Knight K. A. 100 Questions and Answers about Osteoporosis and Osteopenia. Jones and Bartlett Learning; 2009. [Google Scholar]
- 74.Iwamoto J., Takeda T., Sato Y., Shen C.-L., Yeh J. K. Beneficial effect of pretreatment and treatment continuation with risedronate and vitamin K2 on cancellous bone loss after ovariectomy in rats: A bone histomorphometry study. Journal of Nutritional Science and Vitaminology. 2006;52(5):307–315. doi: 10.3177/jnsv.52.307. [DOI] [PubMed] [Google Scholar]
- 75.Iwamoto J., Takeda T., Sato Y. Role of vitamin K2 in the treatment of postmenopausal osteoporosis. Current Drug Safety. 2006;1(1):87–97. doi: 10.2174/157488606775252629. [DOI] [PubMed] [Google Scholar]
- 76.Iwamoto J., Takeda T., Sato Y. Menatetrenone (vitamin K2) and bone quality in the treatment of postmenopausal osteoporosis. Nutrition Reviews. 2006;64(12):509–517. doi: 10.1301/nr.2006.dec.509-517. doi: 10.1301/nr.2006.dec.509-517. [DOI] [PubMed] [Google Scholar]
- 77.Braam L. A. J. L. M., Knapen M. H. J., Geusens P., et al. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcified Tissue International. 2003;73(1):21–26. doi: 10.1007/s00223-002-2084-4. [DOI] [PubMed] [Google Scholar]
- 78.Lanham-New S. A. Importance of calcium, vitamin D and vitamin K for osteoporosis prevention and treatment. Proceedings of the Nutrition Society. 2008;67(2):163–176. doi: 10.1017/S0029665108007003. [DOI] [PubMed] [Google Scholar]
- 79.Kaneki M., Hedges S. J., Hosoi T., et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for hip-fracture risk. Nutrition Journal . 2001;17(4):315–321. doi: 10.1016/S0899-9007(00)00554-2. [DOI] [PubMed] [Google Scholar]
- 80.Katsuyama H., Otsuki T., Tomita M., et al. Menaquinone-7 regulates the expressions of osteocalcin, OPG, RANKL and RANK in osteoblastic MC3T3E1 cells. International Journal of Molecular Medicine. 2005;15(2):231–236. [PubMed] [Google Scholar]
- 81.Rasouli-Ghahroudi A. A., Akbari S., Najafi-Alishah M., Bohloli M. The effect of vitamin K2 on osteogenic differentiation of dental pulp stem cells: an in vitro study. Regeneration, Reconstruction, and Restoration. 2017;2(1):26–29. [Google Scholar]