Abstract
Oxidative stress and inflammation are interlinked processes. The aim of the study was to perform a phytochemical analysis and to evaluate the antioxidant and anti-inflammatory activities of ethanolic Mahonia aquifolium flower (MF), green fruit (MGF), and ripe fruit (MRF) extracts. Plant extract chemical composition was evaluated by HLPC. A DPPH test was used for the in vitro antioxidant activity. The in vivo antioxidant effects and the anti-inflammatory potential were tested on a rat turpentine oil-induced inflammation, by measuring serum nitric oxide (NOx) and TNF-alpha, total oxidative status (TOS), total antioxidant reactivity (TAR), oxidative stress index (OSI), 3-nitrothyrosine (3NT), malondialdehyde (MDA), and total thiols (SH). Extracts were administrated orally in three dilutions (100%, 50%, and 25%) for seven days prior to inflammation. The effects were compared to diclofenac. The HPLC polyphenol and alkaloid analysis revealed chlorogenic acid as the most abundant compound. All extracts had a good in vitro antioxidant activity, decreased NOx, TOS, and 3NT, and increased SH. TNF-alpha was reduced, and TAR increased only by MF and MGF. MDA was not influenced. Our findings suggest that M. aquifolium has anti-inflammatory and antioxidant effects that support the use in primary prevention of the inflammatory processes.
1. Introduction
The relation between antioxidants and degenerative diseases is a topic that focuses the attention of many researchers nowadays [1]. Reactive oxygen species (ROS) result from the oxidative processes in every living organism, as part of the aerobic metabolism. They are represented by superoxide anion, hydrogen peroxide, and hydroxyl radicals [2]. In small doses, they are useful and play physiological roles and are also involved in signalling processes [3]. When the antioxidant system is overloaded, ROS will damage proteins, DNA, and lipids [4]. Therefore, it is essential to identify exogenous sources of antioxidants which can reduce ROS effects [5].
Plants represent an important source of protective agents, due to their content of polyphenols, vitamins, fiber, phytosterols, and carotenoids [6]. Polyphenols have both antioxidant and prooxidant properties. The antioxidant activity is due to the scavenging effect of free radicals [7] and ensures the protection of intracellular structures against oxidative stress, favouring cell viability [8].
As prooxidants, polyphenols may stimulate apoptosis and inhibit tumour growth [8]. Polyphenols have good effects on degenerative diseases like cancer, cardiovascular diseases, diabetes, and osteoporosis [9]. As for their effect on the cardiovascular system, polyphenols reduce blood pressure, inflammation, and oxidative markers, they prevent endothelial dysfunction [10], they are antithrombotic, and they act as vasodilators [11]. They also inhibit the proinflammatory activity of cyclooxygenase (COX), lipooxygenase (LOX), and inducible nitric oxide synthase (iNOS) [12]. As protectors for the endothelial function, polyphenols act in the early stages of the atherosclerotic process by reducing LDL oxidation [12].
Genus Mahonia is the second largest one from the Berberidaceae family. The plants from this genus were used in traditional medicine as a treatment for psoriasis, dermatitis, fungal infections, tuberculosis, dizentheria, and wounds [13]. From all Mahonia species, Mahonia aquifolium is the most cultivated in Turkey [14]. Due to its high content in alkaloids, M. aquifolium has antioxidant, anti-inflammatory, [15, 16], hypoglycemic, hepatoprotective, and hypotensive properties [17]. In the cardiovascular system, M. aquifolium alkaloids induce vasodilatation by blocking Ca2+ entrance in the cells [18] and act as alpha-1 adrenoreceptor antagonists [19].
A variety of fruits are known for having anti-inflammatory and vasodilatation properties [5]. Fruits from M. aquifolium are light yellow and bloom in April, but less information is known about their effects [14]. However, the fruits from a Mahonia were used in the treatment of insomnia, tinnitus, and dizziness [20].
Considering all these previous findings, the present work aimed at performing a phytochemical analysis and investigating the antioxidant and anti-inflammatory activity of the ethanolic M. aquifolium flower and fruit extracts.
2. Materials and Methods
2.1. Plant Material
Fresh Mahonia aquifolium (Pursh) Nutt. flowers and fruits were purchased from the A. Borza Botanical Garden “Babes-Bolyai” University of Cluj-Napoca, Romania between April and June 2015 and extracted in the Mycology Laboratory of “Babes-Bolyai” University, Cluj-Napoca, Romania, by a modified Squibb repercolation method with 70% ethanol (Merck, Bucuresti, Romania), producing the following extracts of M. aquifolium: green fruit extract 1 : 1 (g : mL) (MGF), ripe fruit extract 1 : 1 (g : mL) (MRF), and flower extract 1 : 1 (g : mL) (MF) [21]. The plants were taxonomically identified and authenticated, and voucher specimens (number 665978) were deposited in the Herbarium of “A. Borza” Botanical Garden, “Babes-Bolyai” University of Cluj-Napoca, Romania.
2.2. Phytochemical Analysis
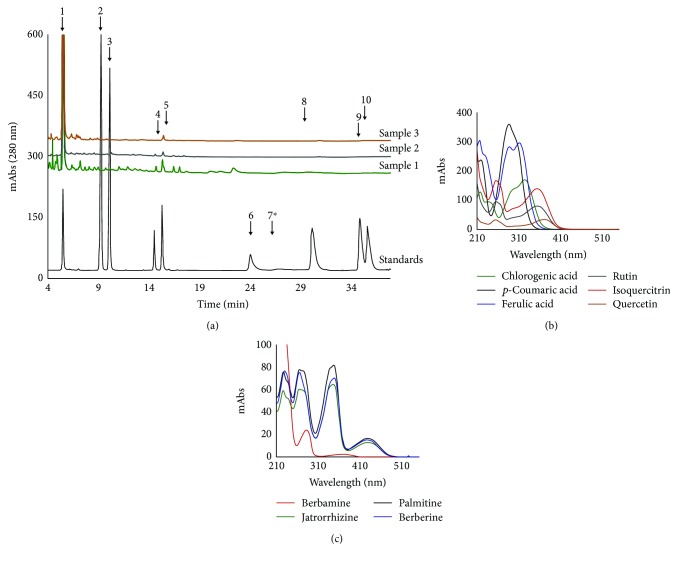
A HPLC-DAD approach was used to separate and quantitatively determine the polyphenols and alkaloids. In the first chromatographic approach, the assays were performed on an Agilent 1200 HPLC system (Waldbronn, Germany) equipped with an online vacuum degasser, quaternary pump, temperature-controlled sample tray, automatic injector, a column thermostat compartment, and a DAD detector. The chromatographic separations were run on a Nucleosil 100 C18 column (240 mm × 4.6 mm, 5 μm particle size) from Macherey-Nagel (Duren, Germany). The injection volume was 5 μL (0.2 μm filtered extract), the column temperature was set at 25°C, and the flow rate was 1.2 mL/min. Several preliminary tests were employed for method optimization by varying the experimental conditions. The optimum method consisted of a gradient elution using solvent A, 10 mM of ammonium acetate pH 5, and solvent B as acetonitrile. The gradient was as follows: 0–15 min from 8 to 30% B, 15–25 min isocratic at 30% B, 25–35 min from 30% to 85% B, 35–38 min from 85% to 95% B, 38–39 min isocratic at 95% B and 39–39.1 min back to 8% B where it was kept until 40 min. As standards, there were chlorogenic acid, p-coumaric acid, ferulic acid, rutin, isoquercitrin, quercetin, berbamine, jatrorrhizine, palmatine, and berberine, all of analytical grade purity from different commercially available sources (Sigma-Aldrich, Germany). A calibration curve was constructed for each compound at 11, 22, 44, 88, 175, and 340 μg/mL using the area of the peak by integration employed by the Agilent software. The limit of quantification (LOQ) and limit of detection (LOD) were determined by the formulas LOQ = (10 × standard deviation of intercept)/calibration curve slope and LOD = (3.3 × standard deviation of intercept)/calibration curve slope, respectively. The UV-Vis detection of the compounds has been accomplished using the DAD detector that measured the entire spectrum in the 210–700 nm region every 1 s, and the chromatograms were monitored at 220, 280, 340, and 425 nm. The identification of the compounds was employed by both chromatographic retention time (with a 0.3 s as tolerance) and spectral similarities (higher than 99.9% was considered as positive) which were done by the built-in software. The chromatograms were exported and the graphs were developed in Excel.
2.3. In Vitro Antioxidant Effects
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay was used for the evaluation of the antioxidant capacity of the investigated extracts. Briefly, in 3 mL of each diluted extract, a 1 mL DPPH and 0.1 mM methanol solution was added. Blanks were included replacing extract volumes for acetone/water. After 30 min in the dark and at room temperature, mixture absorbance was measured at 517 nm against a blank. The percentage of the radical scavenging activity of each extract was calculated using the following formula:
percentage of radical scavenging activity (AA%) = [(OD control − OD sample)/OD control] × 100. AA% was converted to Trolox equivalents using a calibration curve of Trolox standard solutions (0.5–5 μg/mL). The concentration required to scavenge 50% of DPPH free radicals (IC50) was calculated [22].
2.4. Experimental Design
For the present study, 12 groups (n = 5) of male albino Wistar rats with body weights between 200 and 250 g were used. They were purchased from the Animal Facility of “Iuliu Hațieganu” University of Medicine and Pharmacy. The rats were kept in common polypropylene cages under controlled conditions (12 h light/dark cycles, at an average temperature of 21-22°C), with free access to a standard pellet diet (Cantacuzino Institute, Bucharest, Romania) and water ad libitum. Three ethanolic extracts of M. aquifolium were tested: ripe fruits (MRF), green fruits (MGF), and flowers (MF). For seven days, the mentioned extracts were administered orally by gavage (1 mL/animal) in three different dilutions, respectively: 100%, 50%, and 25%. Tap water (1 mL/animal) was administrated by gavage for seven days to the animals from the negative control group (CONTROL) and for the positive inflammation group (INFLAM). An anti-inflammatory control group was treated by gavage for seven days with diclofenac (10 mg/kg b.w.) (DICLO) [23]. Inflammation was induced with turpentine oil (6 mL/kg b.w.) administered intramuscularly, in the animals treated with the extracts, as well as in the INFLAM and DICLO groups [24]. To the CONTROL animals, 0.9% saline was injected intramuscularly (i.m.). One day after the inflammation induction, 60 mg/kg b.w. ketamine and 15 mg/kg b.w. xylazine were used to anesthetize the rats [25], blood was withdrawn by retroorbital puncture, and serum was stored at −80°C until use. The experiments were performed in triplicate. All the animals were used only once, and they were killed by cervical dislocation immediately after the assay.
2.5. The Anti-Inflammatory Effect Evaluation
To evaluate the anti-inflammatory effects of the ethanolic plant extracts, nitric oxide (NO) and TNF-alpha were measured.
For NO synthesis, the Griess reaction was used as an indirect method which measures total nitrites and nitrates (NOx) as previously described [26]. The concentration of serum NOx was expressed as nitrite μmol/L [27].
Serum TNF-alpha was measured using a rat ELISA kit (MBS175904) that applies the quantitative sandwich enzyme immunoassay technique.
2.6. The Antioxidant Effect Evaluation
The total oxidative status (TOS) of the serum was measured using a colorimetric assay [26, 28]. The assay results are expressed in μmol H2O2 equiv./L.
The total antioxidant response (TAR) was measured in serum using a colorimetric assay [26, 29]. The results are expressed as μmol Trolox equiv./L.
The oxidative stress index (OSI) is the ratio of the TOS to the TAR and it is an indicator of the oxidative stress level [26, 30]: OSI (arbitrary unit) = TOS (μmol H2O2 equiv./L)/TAR (μmol Trolox equiv./L).
Peroxynitrite formation was assessed indirectly by measuring serum 3-nitrotyrosine (3NT) using a rat ELISA kit (MBS732683) that applies the quantitative sandwich enzyme immunoassay technique.
Malondialdehyde (MDA) was assessed using thiobarbituric acid, as previously described [26, 31]. Serum MDA concentration was expressed as nmol/mL of serum.
Total thiols (SH) were measured using Ellman's reagent [26, 32]. Serum SH concentration was expressed as mmol GSH/mL.
All of the spectroscopic measurements were performed using a Jasco V-530 UV-Vis spectrophotometer (Jasco International Co. Ltd., Tokyo, Japan).
2.7. Statistical Analysis
Data are expressed as mean ± SD, averaged over at least three independent experiments for normally distributed data. Otherwise, the median, first quartile (Q1), and third quartile (Q3) were reported. Comparisons among groups, in all studied parameters, were analyzed by using the one-way analysis of variance (ANOVA) test and Bonferroni-Holm post hoc test. p < 0.05 was considered statistically significant. Correlations among data obtained were calculated using Pearson's correlation coefficient (r). All analyses were performed using the program “Statistical Package for Social Sciences (SPSS) version 16” (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Phytochemical Analysis
In the present study, we measured six polyphenols (chlorogenic acid, p-coumaric acid, ferulic acid, rutin, isoquercitrin, and quercetin) and four alkaloids (berbamine, jatrorrhizine, palmatine, and berberine) (Table 1, Figure 1). We identified and quantified chlorogenic acid, ferulic acid, and p-coumaric acid as hydroxycinnamic acid derivates (Table 1). Chlorogenic acid was found in the highest concentration in MF (2013 ± 2 μg/mL), followed by MGF (1763 ± 7 μg/mL) and MRF (944 ± 22 μg/mL). We could quantify p-coumaric acid only in MF (7.2 ± 0.1 μg/mL) and MRF (4.5 ± 2.0 μg/mL). MF was richer in p-coumaric acid (10.0 ± 0.3 μg/mL) than MRF (7.8 ± 0.0 μg/mL) and MGF (5.6 ± 0.2 μg/mL) were. In all ethanolic extracts, we identified two flavonoid glycosides, namely rutin and isoquercitrin. Rutin was more abundant in MF (73 ± 1.6 μg/mL) than in MRF (26.1 ± 0.0 μg/mL) and MGF (12.9 ± 0.0 μg/mL). Isoquercitrin was higher in MGF (37.3 ± 0.2 μg/mL) than in MF (29.7 ± 0.7 μg/mL) and MRF (32.4 ± 0.3 μg/mL). In MF, MGF, and MRF, the tested alkaloids were in concentrations under LOD.
Table 1.
HPLC analysis of the M. aquifolium flower, green fruit, and ripe fruit extracts.
| Number | Compounds |
t
elution
(min) |
R2 | LOD (μg/mL) |
LOQ (μg/mL) |
Sample1 MF (μg/mL) |
Sample2 MRF (μg/mL) |
Sample3 MGF (μg/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic ac. | 5.41 | 0.9991 | 3.2 | 9.8 | 2013 ± 2 | 944 ± 22 | 1763 ± 7 |
| 2 | p-Coumaric ac. | 9.17 | 0.9999 | 1.3 | 4.0 | 7.2 ± 0.1 | 4.5 ± 2.0 | <LOQ |
| 3 | Ferulic ac. | 10.07 | 0.9998 | 1.4 | 4.2 | 10.0 ± 0.3 | 7.8 ± 0. | 5.6 ± 0.2 |
| 4 | Rutin | 14.55 | 0.9996 | 2.7 | 8.1 | 73 ± 1.6 | 26.1 ± 0.0 | 12.9 ± 0.0 |
| 5 | Isoquercitrin | 15.34 | 0.9995 | 1.7 | 5.2 | 29.7 ± 0.7 | 32.4 ± 0.3 | 37.3 ± 0.2 |
| 6 | Quercetin | 24.1 | 0.9949 | 13.7 | 41.6 | <LOD | <LOD | <LOD |
| 7 | Berbamine | 25.5 | 0.9997 | 2.4 | 7.3 | <LOD | <LOD | <LOD |
| 8 | Jatrorrhizine | 30.57 | 0.9994 | 2.7 | 8.3 | <LOD | <LOD | <LOD |
| 9 | Palmatine | 34.96 | 0.9998 | 1.7 | 5.2 | <LOD | <LOD | <LOD |
| 10 | Berberine | 36.08 | 0.9996 | 2.1 | 6.4 | <LOD | <LOD | <LOD |
MF—M. aquifolium flowers, MGF—M. aquifolium green fruits, MRF—M. aquifolium ripe fruits, LOD—limit of detection, LOQ—limit of quantification, and R2—coefficient of determination for the calibration curves (at six levels of concentrations). Indicated intervals represent the average ± standard deviation (n = 3).
Figure 1.
(a) Chromatograms at 280 nm of the M. aquifolium flower, green fruit, and ripe fruit extracts. The ten standards are indicated by arrows and numbers. Berbamine (7∗) is barely visible in the 280 nm chromatogram, but it is much better detected and quantified separately from the 220 nm chromatogram. (b) HPLC-DAD registered absorption molecular spectra in the UV-vis domain for the polyphenolic standards at 350 μg/mL. (c) HPLC-DAD registered absorption molecular spectra in the UV-vis domain for the alkaloid standards at 350 μg/mL. 1—Chlorogenic acid, 2—p-coumaric acid, 3—ferulic acid, 4—rutin, 5—isoquercitrin, 6—quercetin, 7—berbamine, 8—jatrorrhizine, 9—palmatine, and 10—berberine. Sample 1—Mahonia aquifolium flowers, sample 2—Mahonia aquifolium ripe fruits, and sample 3—Mahonia aquifolium green fruits.
3.2. In Vitro Antioxidant Activity
The ethanolic extracts of M. aquifolium had a good DPPH radical scavenging activity (Table 2). Trolox IC50 was 11.2 μg/mL. Considering that an antioxidant activity with an IC50 between 50 and 100 μg/mL is good and one that has between 100 and 200 μg/mL is weak, MF IC50 (60.82 μg/mL) and MGF IC 50 (81.6 μg/mL) had a good antioxidant activity and MRF IC50 (135.74 μg/mL) has a weak antioxidant activity.
Table 2.
In vitro DPPH radical scavenging activity of M. aquifolium flower, green fruit, and ripe fruit extracts.
| MGF | MRF | MF | |||
|---|---|---|---|---|---|
| (μg/mL) | (AA%) | (μg/mL) | (AA%) | (μg/mL) | (AA%) |
| 750 | 94.6 | 1000 | 83.1 | 375 | 88 |
| 562.5 | 86.65 | 750 | 73.13 | 250 | 79.95 |
| 375 | 76.65 | 500 | 67.82 | 125 | 65.6 |
| 187.5 | 65.17 | 250 | 61 | ||
MGF—M. aquifolium green fruits, MRF—M. aquifolium ripe fruits, MF—M. aquifolium flowers, and AA%—percentage of radical scavenging activity.
3.3. In Vivo Anti-Inflammatory and Antioxidant Effects
TNF-alpha was increased in the inflammation group (p < 0.01). TNF-alpha was reduced exclusively by MGF50 and MF25 (p < 0.05) and no significant effect was found for the rest of the tested extracts (p > 0.05) (Table 3).
Table 3.
In vivo anti-inflammatory effects of M. aquifolium flower, green fruit, and ripe fruit extracts.
| NOx | (μmol/L) | TNF | (pg/mL) | |
|---|---|---|---|---|
| CONTROL | 48,489 ± 12,975 | 116,634 ± 0.678 | ||
| INFLAM | 75,234 ± 12,136∗∗ | 140,594 ± 15,960∗∗∗ | ||
| DICLO | 52,318 ± 5389∗∗ | 127,228 ± 10,332 | ||
| MGF100% | 72,406 ± 9292∗ | 143,564 ± 16,419 | ||
| MGF50% | 57,473 ± 9110∗ | 118,317 ± 13,854∗ | ||
| MGF25% | 62,686 ± 10,155∗ | 133,168 ± 15,522 | ||
| MRF100% | 46,898 ± 6766∗∗∗ | 130,941 ± 17,836 | ||
| MRF50% | 53,172 ± 3981∗∗ | 146,287 ± 15,092 | ||
| MRF25% | 53,526 ± 7582∗∗ | 121,782 ± 15,522 | ||
| MF100% | 59,623 ± 10,224∗ | 132,178 ± 21,138 | ||
| MF50% | 69,638 ± 7507 | 129,951 ± 16,419 | ||
| MF25% | 62,421 ± 7539 | 115,842 ± 10,632∗ | ||
3NT—3-nitrithyrosine; M. aquifolium: MGF—green fruits, MRF—ripe fruits, and MF—flowers; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
NOx was significantly increased in the inflammation group (p < 0.01) and an important reduction was found in the group where diclofenac was administered (p < 0.01). Compared to the inflammation group, MRF extracts reduced NOx significantly (p < 0.01), MRF100 being the most efficient. MRF effects were comparable to that of diclofenac (p > 0.05). There was no significant inhibitory activity on NOx in all MF dilutions (p > 0.05). From the MGF samples, only MGF50 had a small inhibitory effect on NOx (p < 0.05) (Table 3).
TOS analysis showed that inflammation caused an important increase (p < 0.01) and diclofenac caused a significant reduction (p < 0.001). All MGF extracts reduced TOS (p < 0.01), but not as much as diclofenac (p > 0.05). MRF extracts did not influence TOS significantly (p > 0.05). MF reduced TOS, but only MF100 had a significant inhibitory effect (p < 0.01).
TAR was reduced in the inflammation group (p < 0.05), and it was slightly increased when diclofenac was administered (p < 0.05). MGF increased TAR, MGF25 being the better stimulator (p < 0.001). The MGF effect was as good as that of diclofenac (p > 0.05). MF100 and MF50 increased TAR (p < 0.05), but this effect was smaller than diclofenac (p < 0.001). MRF extracts had no significant effects on TAR (p > 0.05) (Table 4).
Table 4.
In vivo antioxidant effects of M. aquifolium flower, green fruit, and ripe fruit extracts.
| (μmol H2O2 TOS equiv./L) | (mmol TA Trolox R equiv./L) | OSI | 3NT | MD (nmol A MDA/L) | (mmol SH GSH/L) | |
|---|---|---|---|---|---|---|
| CONTROL | 29.58 ± 1.85 | 1.09 ± 0.001 | 27.13 ± 1.67 | 0.34 ± 0.06 | 4.31 ± 0.89 | 0.67 ± 0.09 |
| INFLAM | 41.58 ± 6.55∗∗ | 1.08 ± 0.0007∗ | 38.20 ± 6.009∗∗ | 0.67 ± 0.18∗∗ | 7.62 ± 0.62∗∗∗ | 0.42 ± 0.06∗∗ |
| DICLO | 24.92 ± 3.02∗∗∗ | 1.08 ± 0.0004∗ | 22.87 ± 2.77∗∗∗ | 0.29 ± 0.02∗∗∗ | 5.49 ± 0.72∗∗∗ | 0.56 ± 0.05∗∗ |
| MGF100% | 26.19 ± 7.37∗∗ | 1.09 ± 0.0011∗∗ | 24.03 ± 6.74∗∗ | 0.29 ± 0.04∗∗∗ | 6.90 ± 0.47 | 0.66 ± 0.11∗∗ |
| MGF50% | 32.09 ± 5.63∗ | 1.09 ± 0.0006∗∗ | 29.45 ± 5.15∗ | 0.32 ± 0.02∗∗ | 6.97 ± 0.54 | 0.67 ± 0.10∗∗ |
| MGF25% | 30.60 ± 4.59∗∗ | 1.09 ± 0.0008∗∗∗ | 28.07 ± 4.19∗∗ | 0.38 ± 0.14∗ | 6.90 ± 0.47 | 0.59 ± 0.11∗∗ |
| MRF100% | 39.21 ± 5.31 | 1.08 ± 0.0008 | 36.02 ± 4.86 | 0.41 ± 0.27 | 6.00 ± 0.66∗∗ | 0.77 ± 0.14∗∗ |
| MRF50% | 34.49 ± 6.64 | 1.08 ± 0.0006 | 31.70 ± 6.10 | 0.42 ± 0.26 | 6.54 ± 0.86∗ | 0.83 ± 0.20∗∗ |
| MRF25% | 34.85 ± 7.85 | 1.08 ± 0.0003 | 32.03 ± 7.21 | 0.29 ± 0.03∗∗∗ | 7.73 ± 0.29 | 0.67 ± 0.14∗∗ |
| MF100% | 30.18 ± 4.74∗∗ | 1.08 ± 0.0003∗ | 27.76 ± 4.36∗∗ | 0.32 ± 0.06∗∗ | 7.15 ± 0.97 | 0.66 ± 0.10∗∗ |
| MF50% | 39.65 ± 4.51 | 1.08 ± 0.001 | 36.43 ± 4.12 | 0.45 ± 0.25 | 7.12 ± 0.82 | 0.77 ± 0.05∗∗ |
| MF25% | 32.79 ± 6.77 | 1.08 ± 0.001 | 30.12 ± 6.21 | 0.28 ± 0.03∗∗∗ | 7.68 ± 0.97 | 0.75 ± 0.14∗∗ |
TOS—total oxidative status, TAR—total antioxidant reactivity, OSI—oxidative stress index, 3NT—3-nitrithyrosine, MDA—malondialdehyde, SH—total thiols, MGF—M. aquifolium green fruits, MRF—M. aquifolium ripe fruits, and MF—M. aquifolium flowers; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
OSI was increased in the inflammation group (p < 0.01) and decreased when diclofenac was administrated (p < 001). Only MF100 extracts decreased OSI (p < 0.05), but the effect was smaller than that of diclofenac (p < 0.05). MGF extracts were good inhibitors of OSI (p < 0.01), but the effect was smaller than that of diclofenac (p > 0.05). The MRF extract had no significant effect on OSI (p > 0.05) (Table 4).
3NT was increased in the inflammation group (p < 0.01) and was reduced significantly after diclofenac (p < 0.001). MGF were also good inhibitors of 3NT, MGF100 being more efficient (p < 0.001) than MGF50 (p < 0.01) and MGF25 (p < 0.05). From the MRF, only MRF25 reduced 3NT (p < 0.001). MF extracts decreased 3NT significantly (p < 0.01) (Table 4). MDA production was significantly increased in the inflammation group (p < 0.001), and diclofenac treatment caused an important decrease of MDA (p < 0.001). Only MRF100 (p < 0.01) and MRF50 (p < 0.05) reduced MDA. MF and MGF did not influence significantly the production of MDA (p > 0.05) (Table 4).
Inflammation caused a reduction of SH (p < 0.001), and diclofenac caused an important increase (p < 0.01). SH was increased by all M. aquifolium extracts (p < 0.01–0.001), and the effects were better than the effect of diclofenac (Table 4).
4. Discussion
In the present study, a phytochemical analysis of the ethanolic M. aquifolium flower, green fruit, and ripe fruit extracts, was performed for the first time. The antioxidant activity and anti-inflammatory effects were proved.
From the three hydroxycinnamic acid derivates measured in the ethanolic M. aquifolium extracts, the main component was chlorogenic acid. MF had a higher content of chlorogenic acid than MGF and MRF. This was an important result because chlorogenic acid improves the risk of cardiovascular and associated diseases by reducing the levels of free fatty acids and triglycerides [33]. Due to its antioxidant properties, chlorogenic acid also has a protective role in cardiovascular diseases by increasing NO production [34]. In hypertension, it has a vasodilator effect and reduces ROS production [35]. Chlorogenic acid also inhibits platelet aggregation and reduces blood viscosity [36].
Ferulic acid was found in much lesser concentrations. It was higher in MF than in MRF and MGF. Ferulic acid has antioxidant properties [37, 38] by scavenging ROS and activating DNA repair [39]. It interferes with insulin, ghrelin, and leptin to prevent weight gain and the accumulation of intra-abdominal fat [40]. Ferulic acid also has anti-inflammatory, anticancer, and cardioprotective effects [41]. Due to its antioxidant properties, it is beneficial in epilepsy [42] and other chronic neurological conditions [43]. It is also an antidepressant [44], and it improves memory [45].
The p-coumaric acid was found only in MRF and MF. It is a phenolic acid with antioxidant [46], anti-inflammatory, cardioprotective [47], hepatoprotective, and nefroprotective properties [48]. It reduces the lipoprotein peroxidation process and reduces the free radicals [46, 49]. The p-coumaric acid has anticancer activity by inducing apoptosis and cell cycle arrest [48].
From the three measured flavonoid glycosides, only rutin and isoquercitrin were found in MF, MGF, and MRF. Quercetin was under the LOD.
Rutin is a flavonoid with antioxidant, anti-inflammatory, antidiabetic, and antiobesity properties [50, 51]. Due to its antioxidant capacity, rutin has cardioprotective effects, reducing LDH, CK-MB, ROS, and apoptosis [52, 53]. Rutin protects against endothelial damage and reduces the occurrence of chronic complications in type 2 diabetes [42, 54]. The higher level of rutin concentration in MF was correlated with a good DPPH scavenging activity. Isoquercitrin is a glycated flavonoid [55] with a strong antioxidant activity due to its capacity to scavenge free radicals [56] and to inhibit arginase, favouring NO production [57]. This phenolic compound has anticancer [58] and antiapoptotic effects [59].
The isoquinoline alkaloids are the major subclass of alkaloids of the genus Mahonia [26]. The previously identified alkaloids belong to three major classes: protoberberines, aporphines, and bisbenzylisoquinolines. Berberine is the most widely distributed alkaloid in the Mahonia species [60], but other protoberberines, including palmatine, jatrorrhizine, berbamine, columbamine, and coptisine were also found in these species [20]. The analysis of M. aquifolium extracts looked for the presence of berberine, palmatine, jatrorrhizine, and berbamine but due to the trace amounts of these alkaloids they were under LOD.
The phytochemical analyses suggested the possible anti-inflammatory and antioxidant effects of the M. aquifolium fruit and flower extracts.
The in vitro antioxidant activity was assessed with the DPPH test. MF and MGF proved to have a good antioxidant effect, but MRF had just a weak antioxidant activity. For MF, DPPH correlated with the high chlorogenic acid, feluric acid, and rutin content and for MGF with the high chlorogenic acid and isoquercitrin content.
Furthermore, the present study evaluated the in vivo anti-inflammatory and antioxidant effects in an experimental rat acute inflammation induced by turpentine oil, a nonantigenic inflammatory stimulus [61] that activates inflammatory cytokines and NO release. High serum levels of TNF alpha and NOx were positive markers of the inflammatory response. Only MF and MGF proved to have important anti-inflammatory effects by reducing TNF alpha.
NO is the product with the smallest molecular mass secreted in the mammalian cells. It has a high chemical reactivity and a short lifetime and specificity [62]. NO is generated by 3 isoforms of NOS, respectively, inducible NOS (iNOS), neuronal NOS (nNOS), and endothelial NOS (eNOS) [63]. The nNOS and eNOS are expressed constitutively and produce small quantities of NO. The iNOS is expressed after immunological and inflammatory stimuli and acts like a protector agent [64]. Low levels of NO induce normal physiological signalling, leading to antioxidant reactions. In intermediate concentrations, NO stimulates anti-inflammatory and immunosuppressive responses, has antiapoptotic, progrowth, and angiogenic effects. High NO levels have antiproliferative effects and induce cell cycle delay. Moreover, if there is a prolonged NO increase, it may induce apoptosis [65]. When iNOS is synthesised in high quantities, NO also reacts fast with superoxide anion and will form peroxynitrite (ONOO−), a nonradical reactive species responsible for most of the NO pathological effects [66–68]. Previous studies suggest that plants play an important anti-inflammatory role, because they inhibit NO synthesis [24]. In the present study, only MRF100 proved to have important anti-inflammatory effects by reducing NO synthesis and it was comparable with that induced by diclofenac. Treatment with MGF had a weak inhibitory effect, and MF had no important effect upon NOx. Because the MRF effect on NOx was not correlated with the in vitro DPPH test, it may be presumed that the antioxidant activity was not significantly involved. In some human diseases, antioxidant therapy failure was called the antioxidant paradox [69]. Due to the fact that overproduction of ROS can induce an inflammatory response, and inflammatory mediators can induce an oxidative stress, it was generally accepted that oxidation and inflammation are interlinked processes [70]. The latest explanation of antioxidant therapy failure comes from the finding that antioxidants do not inhibit oxidative stress and the associated inflammation at the same time [71].
Oxidative stress is destructive and it represents the dysbalance between antioxidants and oxidants, in favour of the last ones [72, 73]. The most important oxidants are the reactive oxygen species (ROS), which include superoxide anion, hydrogen peroxide, and hydroxyl radicals [2]. In small to moderate concentrations, ROS have physiologic roles [3], acting as signalling molecules, being involved in cell growth, intercellular adhesion, cellular differentiation, and apoptosis [73, 74]. In high concentrations, ROS are highly reactive molecules that may damage proteins, lipids, and DNA [2, 3, 75] and favour atherosclerosis, cancer, and ageing [76]. The measurement of the stable markers in circulation during oxidative stress [77–79] is a helpful way to appreciate plant extract effects. The oxidative stress biomarkers may be classified as molecules that are modified by interactions with ROS (e.g., DNA, lipids, proteins and carbohydrates) and molecules of the antioxidant system that change in response to increased redox stress [77]. For total oxidative status (TOS) evaluation, diverse methods were developed [28]. In our study, serum TOS was higher in the inflammation group. MF and MGF lowered serum TOS more than MRF. These results positively correlated with the DPPH test.
The antioxidant mechanisms are given in terms of the capacity to scavenge free radicals, to chelate metals, and to act in a synergic manner with other antioxidants. There are two groups of methods used for the determination of the total antioxidant capacity: those based on single-electron transfer monitored spectrophotometrically by a color change due to the free radical reduction, and those based on hydrogen atom transfer measured by the elimination of peroxyl radicals [80]. The antioxidant capacity of the plasma is represented by the thiol groups of proteins and uric acid [81]. Serum TAR [82] was lower in the inflammation group, and only the treatments with MF and MGF extracts increased TAR. In these extracts, we found higher levels of chlorogenic acid, rutin, and isoquercitrin, compounds known to have antioxidant activities.
OSI assesses the global oxidant/antioxidant balance in the living organisms [83]. It was decreased by the MGF and MF treatments, and this was correlated positively with TOS and negatively to TAR.
3NT is a product of protein tyrosine nitration mediated by peroxynitrite anion and nitrogen dioxide. Therefore, it is a good marker of oxidative cell injury and inflammation, as well as NO production [84]. Increased oxidative stress increases ROS and 3NT and consumes NO. Increased levels of 3NT were associated with atherosclerosis [85] and observed in patients with coronary dysfunction, as well as after the removal of the cardiovascular risk factors and normalization of C reactive protein [77]. All tested M. aquifolium extracts reduced 3NT, but MF and MGF were more effective than MRF. The inhibitory effect on 3NT was correlated positively with TOS reduction and negatively with TAR elevation. These results could be explained by the better MF and MGF in vitro DPPH tests.
Malondialdehyde (MDA) results from arachidonic acid, a polyunsaturated fatty acid, and it is a secondary product of lipid peroxidation [86]. Aldehydes are toxic because they interact with DNA and proteins favouring mutations, which are risk factors for cancer, atherosclerosis, and other cardiovascular diseases [87]. The link between MDA and atherosclerosis is its reaction with lipoproteins and the formation of arterial foam cells [88, 89]. Collagen has a high reactivity for MDA, and this will increase heart and vessel rigidity [87]. High levels of MDA were correlated also with myocardial infarction [90], cardiovascular complications of haemodialysis, diabetes, preeclampsia [87], congestive heart failure, and with the severity of the disease [91]. Only treatment with MRF decreased MDA, and it was negatively correlated with TAR.
Protein thiol groups are important determinants of the total antioxidant capacity. They contain a sulfhydryl group which can transform into disulfide bonds when oxidized by the oxygen molecules. This reaction is reversible, so the disulfide bonds can turn back into thiols [92]. The chemical versatility allows them to participate in different processes like signalling, antioxidant defence, and structural stabilization [93]. Studies show that albumin, the most thiol-abundant protein, is implied in the reduction of blood pressure and cardiovascular risk [94]. All tested M. aquifolium ethanolic extracts increased serum SH, proving that these extracts may improve the antioxidant defence.
5. Conclusion
Considering the study results, we concluded that M. aquifolium flower, green fruit, and ripe fruit ethanol extracts have good in vitro and in vivo antioxidant activities, and good anti-inflammatory effects. The efficiency varies with plant organ phytochemical composition. M. aquifolium flower, green fruit, and ripe fruit extracts may be considered for therapeutic interventions needing simultaneous antioxidant and anti-inflammatory effects.
Acknowledgments
The study was partly supported by research grants from the “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania (PCD 7690/19/15.04.2016; PCD 2017 1300/12/13.01.2017).
Data Availability
The data sets for this manuscript will not be publicly available until an associated PhD thesis is published. Requests to access these data sets should be directed to Andra-Diana Andreicut at andra_cecan@yahoo.com.
Ethical Approval
The study protocol was approved by the Institutional Animal Ethical Committee (IAEC) of the “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania (number 18/13.12.2016).
Conflicts of Interest
None of the authors has any conflict of interest that could affect the performance of the work or the interpretation of the data.
Authors' Contributions
Andra-Diana Andreicut, Alexandru Irimie, Alina Elena Pârvu, and Marcel Pârvu contributed to the study conception and design, and interpreted the data. Augustin Cătălin Mot was responsible for the phytochemical analysis. Andra-Diana Andreicut and Mihai Cecan were responsible for the experimental study. Adriana Florinela Cătoi was responsible for data acquisition and statistical analysis. Eva Fischer Fodor and Vasile Feldrihan performed biochemical and ELISA tests. All authors have reviewed and approved the final submitted version.
References
- 1.Jeung I. C., Jee D., Rho C.-R., Kang S. Melissa officinalis L. extracts protect human retinal pigment epithelial cells against oxidative stress-induced apoptosis. International Journal of Medical Sciences. 2016;13(2):139–146. doi: 10.7150/ijms.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieber M., Chandel N. S. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C. J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends in Pharmacological Sciences. 2017;38(7):592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Shoaib M., Ali Shah S. W., Ali N., et al. In vitro enzyme inhibition potentials and antioxidant activity of synthetic flavone derivatives. Journal of Chemistry. 2015;2015:7. doi: 10.1155/2015/516878.516878 [DOI] [Google Scholar]
- 5.Pyrkosz-Biardzka K., Kucharska A. Z., Sokół-Łȩtowska A., Strugała P., Gabrielska J. A comprehensive study on antioxidant properties of crude extracts from fruits of Berberis vulgaris L., Cornus mas L. and Mahonia aquifolium Nutt. Polish Journal of Food and Nutrition Sciences. 2014;64(2):91–99. doi: 10.2478/v10222-012-0097-x. [DOI] [Google Scholar]
- 6.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. The American Journal of Clinical Nutrition. 2005;81(1):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 7.Croft K. D. The chemistry and biological effects of flavonoids and phenolic acids. Annals of the New York Academy of Sciences. 1998;854(1):435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 8.Scalbert A., Johnson I. T., Saltmarsh M. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 2005;81(1):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 9.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition. 2005;45(4):287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 10.Tresserra-Rimbau A., Rimm E. B., Medina-Remón A., et al. Polyphenol intake and mortality risk: a re-analysis of the PREDIMED trial. BMC Medicine. 2014;12(1):1–11. doi: 10.1186/1741-7015-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velioglu Y. S., Mazza G., Gao L., Oomah B. D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 12.Christy Tangney H. E. R. NIH public access. Current Atherosclerosis Reports. 2008;6(9):2166–2171. [Google Scholar]
- 13.Damjanović A. D., Zdunić G., Šavikin K., et al. Evaluation of the anti-cancer potential of Mahonia aquifolium extracts via apoptosis and anti-angiogenesis. Bangladesh Journal of Pharmacology. 2016;11(3):741–749. doi: 10.3329/bjp.v11i3.27103. [DOI] [Google Scholar]
- 14.Coklar H., Akbulut M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. Journal of Functional Foods. 2017;35:166–174. doi: 10.1016/j.jff.2017.05.037. [DOI] [Google Scholar]
- 15.Wong B. S., Hsiao Y. C., Lin T. W., et al. The in vitro and in vivo apoptotic effects of Mahonia oiwakensis on human lung cancer cells. Chemico-Biological Interactions. 2009;180(2):165–174. doi: 10.1016/j.cbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Gunduz K. Morphological and phytochemical properties of Mahonia aquifolium from Turkey. Pakistan Journal of Agricultural Sciences. 2013;50(3):439–443. [Google Scholar]
- 17.Singh A., Bajpai V., Kumar S., Singh Rawat A. K., Kumar B. Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MSn and UHPLC-QqQLIT-MS/MS. Journal of Pharmaceutical Analysis. 2017;7(2):77–86. doi: 10.1016/j.jpha.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotníková Ruz̆ena, Ko s̆t’álová D., Vaverková s̆tefánia. Effect of bisbenzylisoquinoline alkaloids from Mahonia aquifolium on the isolated rat aorta. General Pharmacology: The Vascular System. 1994;25(7):1405–1410. doi: 10.1016/0306-3623(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 19.Sotníková R., Kettmann V., Kostálová D., Táborská E. Relaxant properties of some aporphine alkaloids from Mahonia aquifolium. Methods and Findings in Experimental and Clinical Pharmacology. 1997;19(9):589–597. [PubMed] [Google Scholar]
- 20.He J. M., Mu Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: an ethnopharmacological, phytochemical and pharmacological review. Journal of Ethnopharmacology. 2015;175:668–683. doi: 10.1016/j.jep.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Parvu A. E., Parvu M., Vlase L., Miclea P., Mot A. C., Silaghi-Dumitrescu R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. Journal of Physiology and Pharmacology. 2014;65(2) [PubMed] [Google Scholar]
- 22.Benedec D., Hanganu D., Oniga I., et al. Achillea schurii flowers: chemical, antioxidant, and antimicrobial investigations. Molecules. 2016;21(8) doi: 10.3390/molecules21081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barcelos R. P., Bresciani G., Cuevas M. J., Martínez-Flórez S., Soares F. A. A., González-Gallego J. Diclofenac pretreatment modulates exercise-induced inflammation in skeletal muscle of rats through the TLR4/NF-κB pathway. Applied Physiology, Nutrition, and Metabolism. 2017;42(7):757–764. doi: 10.1139/apnm-2016-0593. [DOI] [PubMed] [Google Scholar]
- 24.Pârvu A. E., Ţălu Ş., Taulescu M. A., et al. Fractal analysis of ibuprofen effect on experimental dog peri-implantitis. Implant Dentistry. 2014;23(3):295–304. doi: 10.1097/ID.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 25.Francischi J. N., Frade T. I. C., Almeida M. P. A. d., Queiroz B. F. G. d., Bakhle Y. S. Ketamine-xylazine anaesthesia and orofacial administration of substance P: a lethal combination in rats. Neuropeptides. 2017;62:21–26. doi: 10.1016/j.npep.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Andreicuţ A. D., Pârvu A. E., Moț A. C., et al. Anti-inflammatory and antioxidant effects of Mahonia aquifolium leaves and bark extracts. Farmacia. 2018;66(1) doi: 10.1155/2018/2879793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda K. M., Espey M. G., Wink D. A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 28.Erel O. A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry. 2005;38(12):1103–11111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical Biochemistry. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Harma M., Harma M., Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Medical Weekly. 2003;133(41-42):563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 31.Draper H. H., Squires E. J., Mahmoodi H., Wu J., Agarwal S., Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radical Biology & Medicine. 1993;15(4):353–363. doi: 10.1016/0891-5849(93)90035-S. [DOI] [PubMed] [Google Scholar]
- 32.Mitev D., Gradeva H., Stoyanova Z., et al. Evaluation of thiol compounds and lipid peroxidative products in plasma of patients with COPD. Trakia Journal of Sciences. 2010;8:306–314. [Google Scholar]
- 33.Sudeep H. V., K V., Patel D., K S. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complementary and Alternative Medicine. 2016;16(1):274–278. doi: 10.1186/s12906-016-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang R., Hodgson J. M., Mas E., Croft K. D., Ward N. C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. The Journal of Nutritional Biochemistry. 2016;27:53–60. doi: 10.1016/j.jnutbio.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Tsang M. S. M., Jiao D., Chan B., et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules. 2016;21(4) doi: 10.3390/molecules21040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M., Hu X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomedical Reports. 2016;5(1):57–62. doi: 10.3892/br.2016.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin F. H., Lin J. Y., Gupta R. D., et al. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. The Journal of Investigative Dermatology. 2005;125(4):826–832. doi: 10.1111/j.0022-202X.2005.23768.x. [DOI] [PubMed] [Google Scholar]
- 38.Sultana R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822(5):748–752. doi: 10.1016/j.bbadis.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Das U., Manna K., Khan A., et al. Ferulic acid (FA) abrogates γ-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radical Research. 2016;51(1):47–63. doi: 10.1080/10715762.2016.1267345. [DOI] [PubMed] [Google Scholar]
- 40.de Melo T. S., Lima P. R., Carvalho K. M. M. B., et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Brazilian Journal of Medical and Biological Research. 2017;50, article e5630:1–8. doi: 10.1590/1414-431X20165630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh S., Basak P., Dutta S., Chowdhury S., Sil P. C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food and Chemical Toxicology. 2017;103:41–55. doi: 10.1016/j.fct.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 42.Aitken J. F., Loomes K. M., Riba-Garcia I., et al. Rutin suppresses human-amylin/hIAPP misfolding and oligomer formation in-vitro, and ameliorates diabetes and its impacts in human-amylin/hIAPP transgenic mice. Biochemical and Biophysical Research Communications. 2017;482(4):625–631. doi: 10.1016/j.bbrc.2016.11.083. [DOI] [PubMed] [Google Scholar]
- 43.Asano T., Matsuzaki H., Iwata N., et al. Protective effects of ferulic acid against chronic cerebral hypoperfusion-induced swallowing dysfunction in rats. International Journal of Molecular Sciences. 2017;18(3) doi: 10.3390/ijms18030550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y. M., Hu C. Y., Shen J. D., Wu S. H., Li Y. C., Yi L. T. Elevation of synaptic protein is associated with the antidepressant-like effects of ferulic acid in a chronic model of depression. Physiology & Behavior. 2017;169:184–188. doi: 10.1016/j.physbeh.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Mhillaj E., Catino S., Miceli F. M., et al. Ferulic acid improves cognitive skills through the activation of the heme oxygenase system in the rat. Molecular Neurobiology. 2018;55(2):905–916. doi: 10.1007/s12035-017-0381-1. [DOI] [PubMed] [Google Scholar]
- 46.Kiliç I., Yeşiloǧlu Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;115:719–724. doi: 10.1016/j.saa.2013.06.110. [DOI] [PubMed] [Google Scholar]
- 47.Aguilar-Hernández I., Afseth N. K., López-Luke T., Contreras-Torres F. F., Wold J. P., Ornelas-Soto N. Surface enhanced Raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vibrational Spectroscopy. 2017;89:113–122. doi: 10.1016/j.vibspec.2017.02.002. [DOI] [Google Scholar]
- 48.Sharma S. H., Chellappan D. R., Chinnaswamy P., Nagarajan S. Protective effect of p-coumaric acid against 1,2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomedicine & Pharmacotherapy. 2017;94:577–588. doi: 10.1016/j.biopha.2017.07.146. [DOI] [PubMed] [Google Scholar]
- 49.Heleno S. A., Martins A., Queiroz M. J. R. P., Ferreira I. C. F. R. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chemistry. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 50.Aksu E. H., Kandemir F. M., Özkaraca M., Ömür A. D., Küçükler S., Çomaklı S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49(1) doi: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- 51.Yuan X., Wei G., You Y., et al. Rutin ameliorates obesity through brown fat activation. The FASEB Journal. 2017;31(1):333–345. doi: 10.1096/fj.201600459RR. [DOI] [PubMed] [Google Scholar]
- 52.Imam F., Al-Harbi N. O., Al-Harbia M. M., et al. Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovascular Toxicology. 2017;17(1):58–66. doi: 10.1007/s12012-015-9356-5. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Zhang Y., Sun B., Tong Q., Ren L. Rutin protects against pirarubicin-induced cardiotoxicity through TGF-β1-p38 MAPK signaling pathway. Evidence-based Complementary and Alternative Medicine. 2017;2017:10. doi: 10.1155/2017/1759385.1759385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W., Wu Q. H., Sui Y., Wang Y., Qiu X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomedicine & Pharmacotherapy. 2017;86:32–40. doi: 10.1016/j.biopha.2016.11.134. [DOI] [PubMed] [Google Scholar]
- 55.Kotsiou A., Seferos N., Mikail H. G., Tesseromatis C. Pleiotropic activity of Hypericum perforatum L. Journal of Medicinal Plants Studies. 2016;4(4):256–258. [Google Scholar]
- 56.Wu P., Li F., Zhang J., Yang B., Ji Z., Chen W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complementary and Alternative Medicine. 2017;17(1):151–157. doi: 10.1186/s12906-017-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akomolafe S. F., Oboh G., Oyeleye S. I., Boligon A. A. Aqueous extract from Ficus capensis leaves inhibits key enzymes linked to erectile dysfunction and prevent oxidative stress in rats’ penile tissue. NFS Journal. 2016;4:15–21. doi: 10.1016/j.nfs.2016.06.001. [DOI] [Google Scholar]
- 58.Kim D.-H., Cho J.-H., Cho Y.-J. Anti-inflammatory activity of extracts from ultra-fine ground Saururus chinensis leaves in lipopolysaccharide-stimulated Raw 264.7 cells. Journal of Applied Biological Chemistry. 2016;59(1):37–43. doi: 10.3839/jabc.2016.008. [DOI] [Google Scholar]
- 59.Liang Y., Li J., Lin Q., et al. Research progress on signaling pathway-associated oxidative stress in endothelial cells. Oxidative Medicine and Cellular Longevity. 2017;2017:8. doi: 10.1155/2017/7156941.7156941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu S., Cao B., Sun R., et al. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Molecular BioSystems. 2015;11(2):463–474. doi: 10.1039/C4MB00500G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad V. G. N. V., Vivek C., Anand Kumar P., Ravi Kumar P., Rao G. S. Turpentine oil induced inflammation decreases absorption and increases distribution of phenacetin without altering its elimination process in rats. European Journal of Drug Metabolism and Pharmacokinetics. 2015;40(1):23–28. doi: 10.1007/s13318-013-0172-7. [DOI] [PubMed] [Google Scholar]
- 62.Lei J., Vodovotz Y., Tzeng E., Billiar T. R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide. 2013;35:175–185. doi: 10.1016/j.niox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Yuan S., Patel R. P., Kevil C. G. Working with nitric oxide and hydrogen sulfide in biological systems. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308(5):L403–L415. doi: 10.1152/ajplung.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Predonzani A., Calì B., Agnellini A. H., Molon B. Spotlights on immunological effects of reactive nitrogen species: when inflammation says nitric oxide. World Journal of Experimental Medicine. 2015;5(2):64–76. doi: 10.5493/wjem.v5.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas D. D., Heinecke J. L., Ridnour L. A., et al. Signaling and stress: the redox landscape in NOS2 biology. Free Radical Biology & Medicine. 2015;87(301):204–225. doi: 10.1016/j.freeradbiomed.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bondonno C. P., Croft K. D., Ward N., Considine M. J., Hodgson J. M. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutrition Reviews. 2015;73(4):216–235. doi: 10.1093/nutrit/nuu014. [DOI] [PubMed] [Google Scholar]
- 67.Lorin J., Zeller M., Guilland J. C., Cottin Y., Vergely C., Rochette L. Arginine and nitric oxide synthase: regulatory mechanisms and cardiovascular aspects. Molecular Nutrition & Food Research. 2014;58(1):101–116. doi: 10.1002/mnfr.201300033. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh H.-J., Liu C.-A., Huang B., Tseng A. H. H., Wang D. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. Journal of Biomedical Science. 2014;21(1):3–15. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halliwell B. The antioxidant paradox: less paradoxical now? British Journal of Clinical Pharmacology. 2013;75(3):637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauer M. E., Fuente M. D. l. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mechanisms of Ageing and Development. 2016;158:27–37. doi: 10.1016/j.mad.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Biswas S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Medicine and Cellular Longevity. 2016;2016:9. doi: 10.1155/2016/5698931.5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sies H. Physiological Society symposium: impaired endothelial and smooth muscle cell function in oxidative stress oxidative stress: oxidants and antioxidants. Experimental Physiology. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 73.Finkel T. Oxidant signals and oxidative stress. Current Opinion in Cell Biology. 2003;15(2):247–254. doi: 10.1016/S0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 74.Mittal M., Siddiqui M. R., Tran K., Reddy S. P., Malik A. B. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sevgi K., Tepe B., Sarikurkcu C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food and Chemical Toxicology. 2015;77:12–21. doi: 10.1016/j.fct.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Milne G. L., Sanchez S. C., Musiek E. S., Morrow J. D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature Protocols. 2007;2(1):221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 77.Ho E., Karimi Galougahi K., Liu C.-C., Bhindi R., Figtree G. A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biology. 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cǎtoi A. F., Pârvu A., Galea R. F., Pop I. D., Mureşan A., Cǎtoi C. Nitric oxide, oxidant status and antioxidant response in morbidly obese patients: the impact of 1-year surgical weight loss. Obesity Surgery. 2013;23(11):1858–1863. doi: 10.1007/s11695-013-0968-1. [DOI] [PubMed] [Google Scholar]
- 79.Cătoi A. F., Pârvu A., Mureşan A., Busetto L. Metabolic mechanisms in obesity and type 2 diabetes: insights from bariatric/metabolic surgery. Obesity Facts. 2015;8(6):350–363. doi: 10.1159/000441259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barcia M. T., Pertuzatti P. B., Bochi V. C., Hermosín-Gutiérrez I., Godoy H. T. Vinification by-products and their phenolic compounds. American Journal of Food Science and Technology. 2015;3(4A):18–23. [Google Scholar]
- 81.Ghiselli A., Serafini M., Natella F., Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biology & Medicine. 2000;29(11):1106–1114. doi: 10.1016/S0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 82.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochemistry. 2004;37(2):112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Yalcin F. K., Er M., Hasanoglu H. C., et al. Deteriorations of pulmonary function, elevated carbon monoxide levels and increased oxidative stress amongst water-pipe smokers. International Journal of Occupational Medicine and Environmental Health. 2017;30(5):731–742. doi: 10.13075/ijomeh.1896.00912. [DOI] [PubMed] [Google Scholar]
- 84.Butterfield D. A., Reed T., Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radical Research. 2010;45(1):59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- 85.Shishehbor M. H., Aviles R. J., Brennan M. L., et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289(13):1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 86.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Analytical Biochemistry. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Del Rio D., Stewart A. J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism, and Cardiovascular Diseases. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Uchida K. Forum. Role of oxidation in atherosclerosis. Role of reactive aldehyde in cardiovascular diseases. Free Radical Biology & Medicine. 2000;28(12):1685–1696. doi: 10.1016/S0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 89.Pirinccioglu A. G., Gökalp D., Pirinccioglu M., Kizil G., Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clinical Biochemistry. 2010;43(15):1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 90.Boaz M., Matas Z., Biro A., et al. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney International. 1999;56(3):1078–1083. doi: 10.1046/j.1523-1755.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 91.Polidori M. C., Savino K., Alunni G., et al. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity. Free Radical Biology and Medicine. 2002;32(2):148–152. doi: 10.1016/S0891-5849(01)00782-1. [DOI] [PubMed] [Google Scholar]
- 92.Elbay A., Ozer O. F., Altinisik M., et al. A novel tool reflecting the role of oxidative stress in the cataracts: thiol/disulfide homeostasis. Scandinavian Journal of Clinical and Laboratory Investigation. 2017;77(3):223–227. doi: 10.1080/00365513.2017.1292539. [DOI] [PubMed] [Google Scholar]
- 93.Gilmore J. L., Yi X., Quan L., Kabanov A. V. Novel nanomaterials for clinical neuroscience. Journal of Neuroimmune Pharmacology. 2008;3(2):83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prakash M., Shetty M. S., Tilak P., Anwar N. Total thiols: biomedical importance and their alteration in various disorders. Online Journal of Health and Allied Sciences. 2009;8(2):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets for this manuscript will not be publicly available until an associated PhD thesis is published. Requests to access these data sets should be directed to Andra-Diana Andreicut at andra_cecan@yahoo.com.