Abstract
Neuroendocrinetumour (NET) of the gallbladder is an extremely rare tumour and with coexisting adenocarcinoma an even rarer occurrence. Mixed NETs have the tendency to invade the lymph nodes and the hepatic tissue from their high malignant potential, leading to poor prognosis. Survival rates of the patients with mixed NET can be improved with wide excision, adjuvant chemotherapy and radiation. We present a case of 62-year-old woman with history of hepatitis C infection, a risk factor for both hepatic and extrahepatic gastrointestinal malignancies. Patient underwent exploratory laparotomy with resection of the gallbladder and partial hepatectomy. Pathology showed high-grade larger cell neuroendocrine carcinoma 5×4×3 cm along with two separate lesions found out to be adenocarcinomas. In our patient, hepatitis C infection can be an inciting factor for the development of these carcinomas. We will discuss the presentation, treatment modalities and outcomes with this kind of coexisting tumours.
Keywords: hepatitis C, endocrine cancer
Background
Presence of concomitant neuroendocrine tumour (NET) and adenocarcinoma of the gallbladder is a rare entity. Neuroendocrine tumours take their origin from the enterochromaffin cells of the body. They have the potential to generate hormones, but a large proportion of cases encountered present with indolent course. They are commonly recognised as masses on imaging studies such ultrasound, CT and MRI. Biopsy is quintessential for definitive diagnoses as with all cancers. Surgical resection is the mainstay of therapy in cases of non-metastatic disease.
Case presentation
A 62-year-old woman presented with a medical history of chronic kidney disease, liver cirrhosis secondary to untreated hepatitis C, hypertension, diabetes mellitus, chronic obstructive pulmonary disease and opiate abuse on methadone. She presented with right upper quadrant, sharp abdominal pain, radiating to the epigastric region and left upper quadrant, associated with nausea and non-bloody diarrhoea for 3 days. On physical examination, the abdomen was distended, with tenderness in the right upper quadrant. The rest of the examination was unremarkable. Her laboratories on admission were significant for haemoglobin of 9.4 g/dL, creatinine of 5.17 suggestive of acute kidney injury as her baseline creatinine was 2.82, alkaline phosphatase of 116, Aspartate Transaminase (AST) of 29, Alanine Transaminase (ALT) of 13 and total bilirubin of 0.4 mg/dL. Right upper quadrant ultrasound showed an echogenic material in the gallbladder, measuring 7×4×3 cm in size without any gallstones (figure 1); no evidence of bile duct dilatation was noticed. MRI abdomen without intravenous contrast showed a mass arising from the gallbladder, extending transmurally with both intraluminal and extraluminal components. MRI also showed a smaller mass or polyps arising from the fundal wall measuring about 1×2 cm, with mildly distended gallbladder. Staging CT abdomen, pelvis and chest showed gallbladder mass with local extension, malignant appearing, without any evidence of metastasis or localised lymphadenopathy (figure 2). Blood work revealed an elevated Carcinoembryonic Antigen (CEA) of 6.8 with normal Aplha fetoprotein (AFP) of 6.4 IU/mL and Cancer Antigen (CA) 19-9 of 1.3 U/mL. The patient underwent radical cholecystectomy with partial hepatectomy and regional lymphadenectomy (figure 3). The patient’s hepatitis C viral load was 442 462 IU/mL and she was started on elbasvir–grazoprevir combination. Postoperative pathological findings revealed an invasive high-grade large cell NET (figure 4), measuring 5.3 cm in greatest dimension and extending to the serosa, but the overall margins of resection were negative. There were two adjacent papillary adenocarcinomas arising in villous adenomas (figure 5). Resected lymph nodes and liver specimen were negative for metastasis, but liver pathology was suggestive of cirrhosis with microscopic Von Meyenburg’s complexes. Immunohistochemical analysis of the tumour tissue was positive for Cluster of Differentiation (CD)56: 2+, synaptophysin: 4+ (figure 6), chromogranin: 3+, Ki-67: 80%, AE1/AE3: 1+ highly suggestive for stage III, high-grade NET. Given the size of the tumour and the transmural invasion and histology, the patient was started on radiation followed by chemotherapy with cisplatin and etoposide.
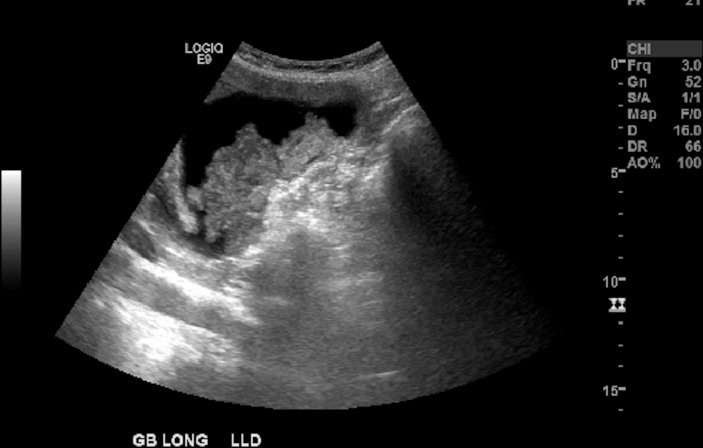
Figure 1.
Right upper quadrant ultrasound showed an echogenic material in the gallbladder, measuring 7×4×3 cm in size without any gallstones.
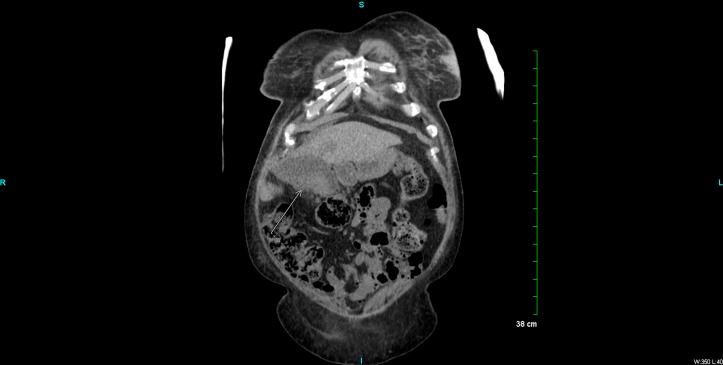
Figure 2.
CT abdomen coronal plane, showing gallbladder mass with local extension of malignant appearing process.
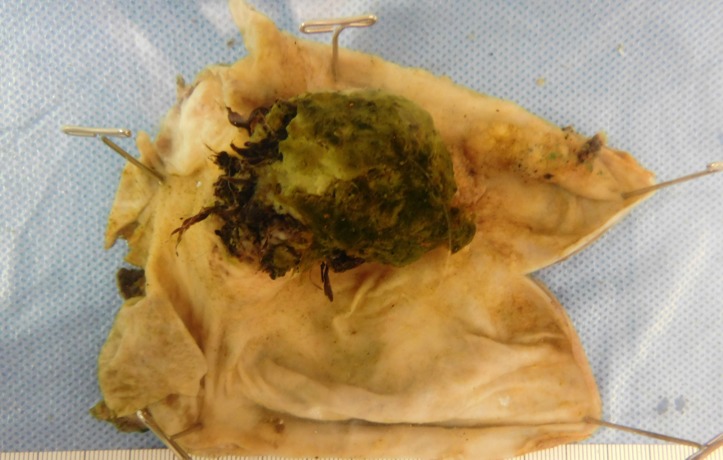
Figure 3.
Gross pathological view of the neuroendocrine tumour with adjacent papillary adenocarcinomas.
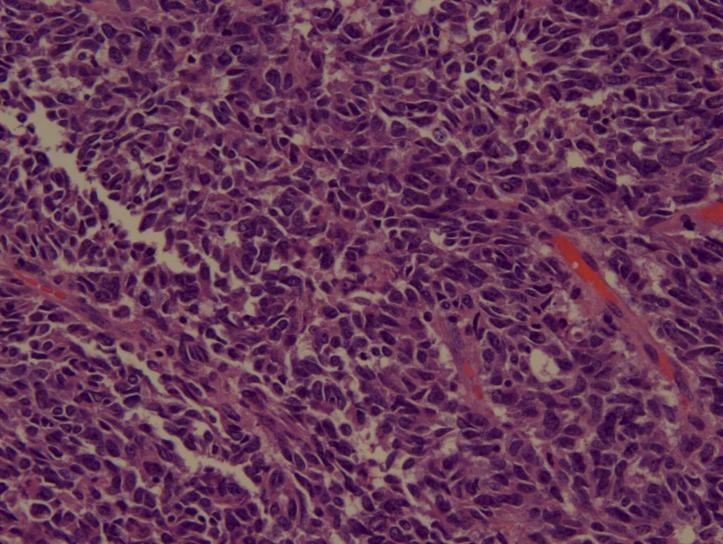
Figure 4.
Histopathological examination revealing high-grade neuroendocrine tumour.
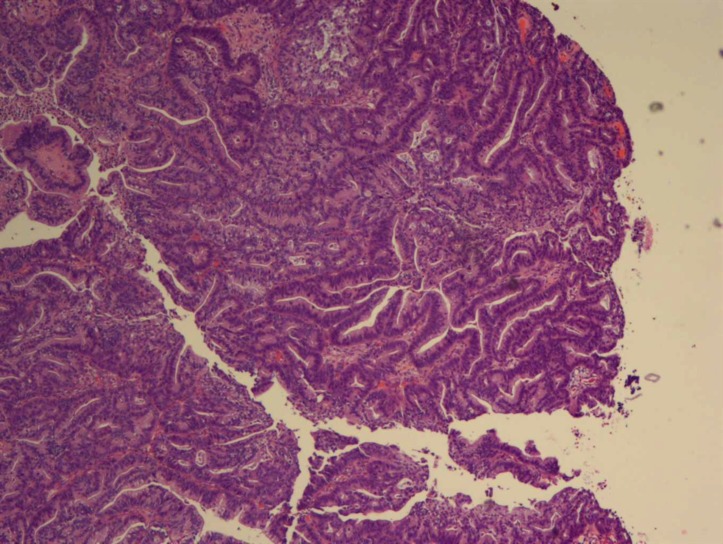
Figure 5.
Histopathological examination showing papillary adenocarcinoma.
Figure 6.
Immunohistochemical staining for synaptophysin, cells positive for synaptophysin.
Outcome and follow-up
Two months later, she was admitted for dialysis, secondary to blood urea nitrogen level of 110 mg/dL and creatinine of 10 mg/dL. Concurrent CT contrast imaging of the neck and chest suggested a chronic-appearing right internal jugular vein thrombus suggesting a central venous compromise. Her mental status rapidly deteriorated during dialysis, and she was transferred to the intensive care unit for poor respiratory effort and persistent hypotension. She was intubated and started on norepinephrine and epinephrine, which failed to raise the blood pressure; the patient expired secondary to refractory shock.
Discussion
Malignant gallbladder lesions are mostly adenocarcinomas, accounting for 1.25% of all malignant tumours. Out of these, less than 2% are found in the gallbladder itself.1–4
NETs can also be classified based on cellular differentiation and how much neoplastic cells resemble normal cells.5 Well-differentiated NETs have a typical organoid arrangement of cells with trabecular, nesting or gyriform patterns and produce a large number of secretory granules with diffuse immunoexpression of neuroendocrine markers.5 In contrast, poorly differentiated NETs have atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules and limited biomarker immunoexpression.5
Similarly, based on the histopathological features, WHO classified gastrointestinal and pancreatobiliary NETs into three categories (2010), which also indicates the prognosis: (1) well differentiated (G1) with monomorphic small population of round cells, low mitotic index (<2), low Ki-67 index (<3%) and absent necrosis; (2) moderately differentiated (G2) with 2%–20% mitotic rate and 2%–20% Ki-67 index; (3) poorly differentiated (G3) with cellular pleomorphism with >20% mitotic rate, >20% Ki-67 index and/or presence of necrosis. G1 tumours have prolonged survival, G2 intermediate and G3 poor survival.6 7
Neuroendocrine carcinoma is frequently combined with other carcinomas such as adenocarcinoma or squamous cell carcinoma in the gastrointestinal tract. Mixed adeno-neuroendocrine carcinoma is diagnosed only when both portions are more than 30% in the pathological examinations.8 Due to the high malignant potential, these tumours have the tendency to invade lymph nodes and adjacent hepatic tissues, resulting in poor prognosis even after complete resection.9 10 Prognosis is even worse with high Ki-67 index, which is another marker for high mitotic rate.
Right upper quadrant ultrasound, abdominopelvic CT and endoscopic ultrasonography (EUS) are widely used in diagnosing pancreatic and gallbladder diseases. EUS especially has higher spatial resolution than other imaging methods. Malignant masses tended to be shown homogeneously on the conventional EUS; the sensitivity and specificity were 77.1% and 82.7%, respectively.11
Whenever possible, the gold standard treatment for NET of gallbladder is complete surgical resection, which is extrapolated from the management of gallbladder adenocarcinoma as it is difficult to have a preoperative pathological diagnosis in these patients. For preinvasive and early-detected cancer (Tis and T1), simple cholecystectomy is probably an adequate therapy. For advanced lesions, a more aggressive radical surgery, including radical cholecystectomy, regional lymphadenectomy combined with a hepatic resection to achieve adequate free margin, is required.12 For patients with high-grade NET, cisplatin, carboplatin and etoposide are considered one of the first-line chemotherapeutic agents after surgery.13 Evolving data suggest that the patient with small cells or high Ki-67 index have a better response to the chemotherapy. Iype et al collected 29 cases of high-grade neuroendocrine carcinomas and concluded that the large cell subtype has a worse prognosis than the small cell variety and chemotherapy was more effective for small cell subtype.14 Immunotherapeutic agents such as anti PD-1 inhibitors are being considered as possible future therapies for metastatic NETs. A big nationwide population study from Taiwan showed that hepatitis C viral infection is a significant risk factor for gallbladder and extrahepatic bile duct cancers (HR 2.60, 95 % CI 1.42 to 4.73).15
Learning points.
Gallbladder neuroendocrine tumours (NETs) are extremely rare tumours and the coexistence of adenocarcinoma as separate lesions in the same organ is an extremely rare phenomenon.
Our patient’s underlying chronic uncontrolled hepatitis C infection can be a key inciting factor for both carcinomas.
Early detection, complete resection with adjuvant chemotherapy and radiation are the keys for better survival outcomes.
Coexisting two different carcinomas in one organ, the histology of NET being high grade and high Ki-67 positivity, along with other comorbidities like hepatitis C infection and chronic kidney disease are all suggestive of poor prognosis.
Acknowledgments
Dr. Kelley Kozma, DO
Footnotes
Contributors: SS was involved in writing the first draft and BSP, PTM and ASD were involved in conception and design, acquisition of data or analysis and interpretation of data, and revising them critically for important intellectual context.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kim DI, Seo HI, Lee JY, et al. . Curative resection of combined neuroendocrine carcinoma and adenocarcinoma of the gallbladder. Tumori 2011;97:815–8. 10.1177/030089161109700623 [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Wang L, He XD, Xd H, et al. . Mixed large cell neuroendocrine carcinoma and adenocarcinoma of the gallbladder: a case report and brief review of the literature. World J Surg Oncol 2015;13:114–102. 10.1186/s12957-015-0533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav R, Jain D, Mathur SR, et al. . Cytomorphology of neuroendocrine tumours of the gallbladder. Cytopathology 2016;27:97–102. 10.1111/cyt.12239 [DOI] [PubMed] [Google Scholar]
- 4.Albores-Saavedra J, Batich K, Hossain S, et al. . Carcinoid tumors and small-cell carcinomas of the gallbladder and extrahepatic bile ducts: a comparative study based on 221 cases from the Surveillance, Epidemiology, and End Results Program. Ann Diagn Pathol 2009;13:378–83. 10.1016/j.anndiagpath.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, Anthony LB, Bushnell DL, et al. . NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735–52. 10.1097/MPA.0b013e3181ebb168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev 2011;30(suppl 1):3–7. 10.1007/s10555-011-9292-1 [DOI] [PubMed] [Google Scholar]
- 7.Klimstra DS, Modlin IR, Coppola D, et al. . The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707–12. 10.1097/MPA.0b013e3181ec124e [DOI] [PubMed] [Google Scholar]
- 8.Deehan DJ, Heys SD, Kernohan N, et al. . Carcinoid tumour of the gall bladder: two case reports and a review of published works. Gut 1993;34:1274–6. 10.1136/gut.34.9.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu T, Tajiri T, Akimaru K, et al. . Combined neuroendocrine cell carcinoma and adenocarcinoma of the gallbladder: report of a case. J Nippon Med Sch 2006;73:101–5. 10.1272/jnms.73.101 [DOI] [PubMed] [Google Scholar]
- 10.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59. 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 11.Leem G, Chung MJ, Park JY, et al. . Clinical value of contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of pancreatic and gallbladder masses. Clin Endosc 2018;51:80–8. 10.5946/ce.2017.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltawil KM, Gustafsson BI, Kidd M, et al. . Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol 2010;44:687–95. 10.1097/MCG.0b013e3181d7a6d4 [DOI] [PubMed] [Google Scholar]
- 13.Iwasa S, Morizane C, Okusaka T, et al. . Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010;40:313–8. 10.1093/jjco/hyp173 [DOI] [PubMed] [Google Scholar]
- 14.Iype S, Mirza TA, Propper DJ, et al. . Neuroendocrine tumours of the gallbladder: three cases and a review of the literature. Postgrad Med J 2009;85:213–8. 10.1136/pgmj.2008.070649 [DOI] [PubMed] [Google Scholar]
- 15.Kamiza AB, Su FH, Wang WC, et al. . Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer 2016;16:861 10.1186/s12885-016-2918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]